Enrichment of Anaerobic Microbial Communities from Midgut and Hindgut of Sun Beetle Larvae (Pachnoda marginata) on Wheat Straw: Effect of Inoculum Preparation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Medium and Wheat Straw Batch Cultivation
2.2. Inoculum Preparation
2.3. Analytical Methods
2.4. Microbial Community Analysis
3. Results and Discussion
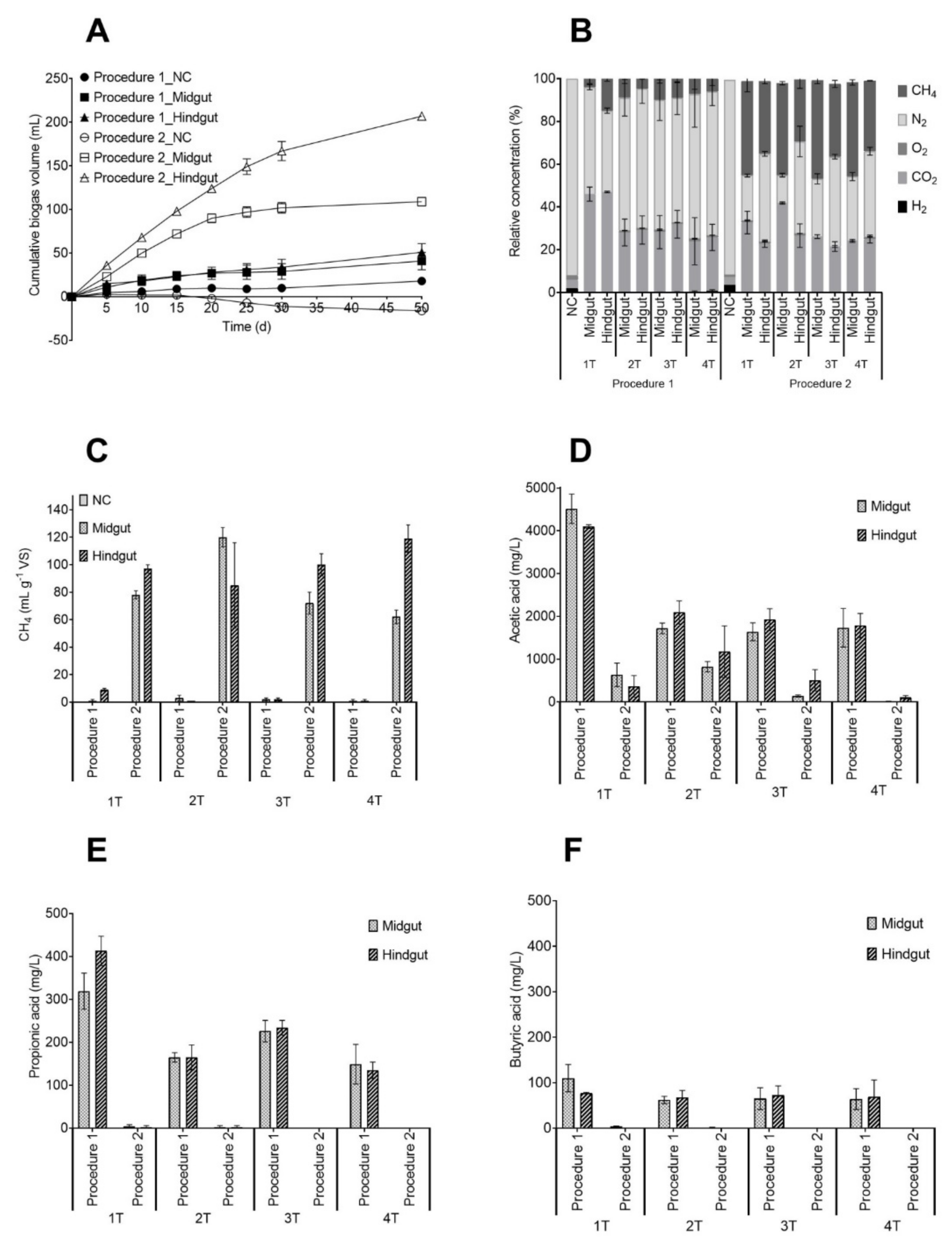
3.1. Physical-Chemical Characterization of the Inoculum and Enrichment Cultures
3.2. Bacterial Community Structure
3.3. Methanogenic Community Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dessie, W.; Luo, X.; Wang, M.; Feng, L.; Liao, Y.; Wang, Z.; Yong, Z.; Qin, Z. Current advances on waste biomass transformation into value-added products. Appl. Microbiol. Biotechnol. 2020, 104, 4757–4770. [Google Scholar] [CrossRef] [PubMed]
- Wenger, J.; Stern, T. Reflection on the research on and implementation of biorefinery systems—A systematic literature review with a focus on feedstock. Biofuels Bioprod. Biorefining 2019, 13, 1347–1364. [Google Scholar] [CrossRef] [Green Version]
- Langeveld, J.W.A.; Peterson, E.C. Feedstocks for Biogas Production: Biogas and Electricity Generation Potentials; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9783319773353. [Google Scholar]
- Ozbayram, E.G.; Kleinsteuber, S.; Nikolausz, M. Biotechnological utilization of animal gut microbiota for valorization of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2019, 104, 489–508. [Google Scholar] [CrossRef] [PubMed]
- Formann, S.; Hahn, A.; Janke, L.; Stinner, W.; Sträuber, H.; Logroño, W.; Nikolausz, M. Beyond Sugar and Ethanol Production: Value Generation Opportunities Through Sugarcane Residues. Front. Energy Res. 2020, 8, 267. [Google Scholar] [CrossRef]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef] [Green Version]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef] [Green Version]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [Green Version]
- Feofilova, E.P.; Mysyakina, I.S. Lignin: Chemical structure, biodegradation, and practical application (a review). Appl. Biochem. Microbiol. 2016, 52, 573–581. [Google Scholar] [CrossRef]
- Saha, B.C.; Cotta, M.A. Ethanol Production from Alkaline Peroxide Pretreated Enzymatically Saccharified Wheat Straw. Biotechnol. Prog. 2006, 22, 449–453. [Google Scholar] [CrossRef]
- Paul, S.; Dutta, A. Challenges and opportunities of lignocellulosic biomass for anaerobic digestion. Resour. Conserv. Recycl. 2018, 130, 164–174. [Google Scholar] [CrossRef]
- Shah, T.A.; Ullah, R. Pretreatment of wheat straw with ligninolytic fungi for increased biogas productivity. Int. J. Environ. Sci. Technol. 2019, 16, 7497–7508. [Google Scholar] [CrossRef]
- Nyns, E.-J.; Nikolausz, M.; Leibtrau, J. Biogas. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; ISBN 9783527306732. [Google Scholar] [CrossRef]
- Ghasemi Ghodrat, A.; Tabatabaei, M.; Aghbashlo, M. Waste Management Strategies. In The State of the Art; Biogas. Biofuel and Biorefinery Technologies; Tabatabaei, M., Ghanavati, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9783319773353. [Google Scholar]
- Porsch, K.; Wirth, B.; Tóth, E.M.; Schattenberg, F.; Nikolausz, M. Characterization of wheat straw-degrading anaerobic alkali-tolerant mixed cultures from soda lake sediments by molecular and cultivation techniques. Microb. Biotechnol. 2015, 8, 801–814. [Google Scholar] [CrossRef]
- Cazemier, A.E.; Camp, H.J.D.; Hackstein, J.H.; Vogels, G.D. Fibre Digestion in Arthropods. Comp. Biochem. Physiol. Part A Physiol. 1997, 118, 101–109. [Google Scholar] [CrossRef]
- Egert, M.; Wagner, B.; Lemke, T.; Brune, A.; Friedrich, M.W. Microbial Community Structure in Midgut and Hindgut of the Humus-Feeding Larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Appl. Environ. Microbiol. 2003, 69, 6659–6668. [Google Scholar] [CrossRef] [Green Version]
- Brune, A.; Dietrich, C. The Gut Microbiota of Termites: Digesting the Diversity in the Light of Ecology and Evolution. Annu. Rev. Microbiol. 2015, 69, 145–166. [Google Scholar] [CrossRef]
- Mikaelyan, A.; Strassert, J.F.H.; Tokuda, G.; Brune, A. The fibre-associated cellulolytic bacterial community in the hindgut of wood-feeding higher termites (Nasutitermes spp.). Environ. Microbiol. 2014, 16, 2711–2722. [Google Scholar] [CrossRef]
- Auer, L.; Lazuka, A.; Sillam-Dussès, D.; Miambi, E.; O’Donohue, M.; Hernandez-Raquet, G. Uncovering the Potential of Termite Gut Microbiome for Lignocellulose Bioconversion in Anaerobic Batch Bioreactors. Front. Microbiol. 2017, 8, 2623. [Google Scholar] [CrossRef] [Green Version]
- Gales, A.; Chatellard, L.; Abadie, M.; Bonnafous, A.; Auer, L.; Carrere, H.; Godon, J.-J.; Hernandez-Raquet, G.; Dumas, C. Screening of Phytophagous and Xylophagous Insects Guts Microbiota Abilities to Degrade Lignocellulose in Bioreactor. Front. Microbiol. 2018, 9, 2222. [Google Scholar] [CrossRef]
- Lazuka, A.; Auer, L.; Bozonnet, S.; Morgavi, D.P.; O’Donohue, M.; Hernandez-Raquet, G. Efficient anaerobic transformation of raw wheat straw by a robust cow rumen-derived microbial consortium. Bioresour. Technol. 2015, 196, 241–249. [Google Scholar] [CrossRef]
- Ozbayram, E.G.; Kleinsteuber, S.; Nikolausz, M.; Ince, B.; Ince, O. Enrichment of lignocellulose-degrading microbial communities from natural and engineered methanogenic environments. Appl. Microbiol. Biotechnol. 2018, 102, 1035–1043. [Google Scholar] [CrossRef]
- Bayané, A.; Guiot, S.R. Animal digestive strategies versus anaerobic digestion bioprocesses for biogas production from lignocellulosic biomass. Rev. Environ. Sci. Bio/Technol. 2011, 10, 43–62. [Google Scholar] [CrossRef] [Green Version]
- Micó, E.; Morón, M.; Šípek, P.; Galante, E. Larval morphology enhances phylogenetic reconstruction in Cetoniidae (Coleoptera: Scarabaeoidea) and allows the interpretation of the evolution of larval feeding habits. Syst. Éntomol. 2009, 33, 128–144. [Google Scholar] [CrossRef]
- Orozco, J.; Philips, T.K. Pachnoda marginata (Drury) (Coleoptera: Scarabaeidae: Cetoniinae) Developing in Bat Guano in a West African Cave. Coleopt. Bull. 2012, 66, 378–379. [Google Scholar] [CrossRef]
- Zverlov, V.; Höll, W.; Schwarz, W. Enzymes for digestion of cellulose and other polysaccharides in the gut of longhorn beetle larvae, Rhagium inquisitor L. (Col., Cerambycidae). Int. Biodeterior. Biodegrad. 2003, 51, 175–179. [Google Scholar] [CrossRef]
- Werner, E. Die Ernärung Der Larve von Potosia Cuprea Fbr.(Cetonia Floricola Hbst.). Ein Beitrag Zum Problem Der Celluloseverdauung Bei Insectlarven. Z. Morphol. Okol. Tiere 1926, 6, 150–206. [Google Scholar] [CrossRef]
- Lemke, T.; Stingl, U.; Egert, M.; Friedrich, M.W.; Brune, A. Physicochemical Conditions and Microbial Activities in the Highly Alkaline Gut of the Humus-Feeding Larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Appl. Environ. Microbiol. 2003, 69, 6650–6658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazemier, A.E.; Verdoes, J.C.; Reubsaet, F.A.; Hackstein, J.H.; Van Der Drift, C.; Camp, H.J.O.D. Promicromonospora pachnodae sp. nov., a member of the (hemi) cellulolytic hindgut flora of larvae of the scarab beetle Pachnoda marginata. Antonie van Leeuwenhoek 2003, 83, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Cazemier, A.; Hackstein, J.; Camp, H.O.D.; Rosenberg, J.; van der Drift, C. Bacteria in the Intestinal Tract of Different Species of Arthropods. Microb. Ecol. 1997, 33, 189–197. [Google Scholar] [CrossRef]
- Huang, S.; Sheng, P.; Zhang, H. Isolation and Identification of Cellulolytic Bacteria from the Gut of Holotrichia parallela Larvae (Coleoptera: Scarabaeidae). Int. J. Mol. Sci. 2012, 13, 2563–2577. [Google Scholar] [CrossRef]
- Sheng, P.; Huang, J.; Zhang, Z.; Wang, D.; Tian, X.; Ding, J. Construction and Characterization of a Cellulolytic Consortium Enriched from the Hindgut of Holotrichia parallela Larvae. Int. J. Mol. Sci. 2016, 17, 1646. [Google Scholar] [CrossRef] [Green Version]
- Logroño, W.; Popp, D.; Kleinsteuber, S.; Sträuber, H.; Harms, H.; Nikolausz, M. Microbial Resource Management for Ex Situ Biomethanation of Hydrogen at Alkaline pH. Microorganisms 2020, 8, 614. [Google Scholar] [CrossRef] [Green Version]
- Logroño, W.; Popp, D.; Nikolausz, M.; Kluge, P.; Harms, H.; Kleinsteuber, S. Microbial Communities in Flexible Biomethanation of Hydrogen Are Functionally Resilient Upon Starvation. Front. Microbiol. 2021, 12, 619632. [Google Scholar] [CrossRef]
- Brune, A.; Emerson, D.; Breznak, J.A. The Termite Gut Microflora as an Oxygen Sink: Microelectrode Determination of Oxygen and pH Gradients in Guts of Lower and Higher Termites. Appl. Environ. Microbiol. 1995, 61, 2681–2687. [Google Scholar] [CrossRef] [Green Version]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Steinberg, L.M.; Regan, J.M. Phylogenetic Comparison of the Methanogenic Communities from an Acidic, Oligotrophic Fen and an Anaerobic Digester Treating Municipal Wastewater Sludge. Appl. Environ. Microbiol. 2008, 74, 6663–6671. [Google Scholar] [CrossRef] [Green Version]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [Green Version]
- Popp, D.; Plugge, C.M.; Kleinsteuber, S.; Harms, H.; Sträuber, H. Inhibitory Effect of Coumarin on Syntrophic Fatty Acid-Oxidizing and Methanogenic Cultures and Biogas Reactor Microbiomes. Appl. Environ. Microbiol. 2017, 83, e00438-17. [Google Scholar] [CrossRef] [Green Version]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Lian, S.; Nikolausz, M.; Nijenhuis, I.; da Rocha, U.N.; Liu, B.; Corrêa, F.B.; Saraiva, J.P.; Richnow, H.H. Biotransformation of hexachlorocyclohexanes contaminated biomass for energetic utilization demonstrated in continuous anaerobic digestion system. J. Hazard. Mater. 2020, 384, 121448. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA); Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–15. [Google Scholar] [CrossRef]
- Rosado, P.M.; Leite, D.C.D.A.; Duarte, G.A.S.; Chaloub, R.M.; Jospin, G.; da Rocha, U.N.; Saraiva, J.P.; Dini-Andreote, F.; Eisen, J.A.; Bourne, D.G.; et al. Marine probiotics: Increasing coral resistance to bleaching through microbiome manipulation. ISME J. 2019, 13, 921–936. [Google Scholar] [CrossRef] [Green Version]
- Ozbayram, E.G.; Kleinsteuber, S.; Nikolausz, M.; Ince, B.; Ince, O. Effect of bioaugmentation by cellulolytic bacteria enriched from sheep rumen on methane production from wheat straw. Anaerobe 2017, 46, 122–130. [Google Scholar] [CrossRef]
- Ozbayram, E.G.; Kleinsteuber, S.; Nikolausz, M.; Ince, B.; Ince, O. Bioaugmentation of anaerobic digesters treating lignocellulosic feedstock by enriched microbial consortia. Eng. Life Sci. 2018, 18, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Bayon, C. Volatile fatty acids and methane production in relation to anaerobic carbohydrate fermentation in Oryctes nasicornis larvae (Coleoptera: Scarabaeidae). J. Insect Physiol. 1980, 26, 819–828. [Google Scholar] [CrossRef]
- Hackstein, J.H.; Stumm, C.K. Methane production in terrestrial arthropods. Proc. Natl. Acad. Sci. USA 1994, 91, 5441–5445. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Chen, X.; Liu, Z.; Zhou, X.; Zhang, Y. Effect of inoculum sources on the anaerobic digestion of rice straw. Bioresour. Technol. 2014, 158, 149–155. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.; Vasileiou, S.; Lyberatos, G.; Angelidaki, I. Effect of micro-aeration and inoculum type on the biodegradation of lignocellulosic substrate. Bioresour. Technol. 2017, 225, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Hornbak, T. The effect of inoculum age and solid versus liquid propagation on inoculum quality of an industrial Bacillus licheniformis strain. FEMS Microbiol. Lett. 2004, 236, 145–151. [Google Scholar] [CrossRef]
- Karakashev, D.B.; Galabova, D.; Simeonov, I. A simple and rapid test for differentiation of aerobic from anaerobic bacteria. World J. Microbiol. Biotechnol. 2003, 19, 233–238. [Google Scholar] [CrossRef]
- Achinas, S.; Euverink, G.J.W. Effect of Combined Inoculation on Biogas Production from Hardly Degradable Material. Energies 2019, 12, 217. [Google Scholar] [CrossRef] [Green Version]
- Lawal, A.; Dzivama, A.; Wasinda, M. Effect of inoculum to substrate ratio on biogas production of sheep paunch manure. Res. Agric. Eng. 2016, 62, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Elsayed, M.; Andres, Y.; Blel, W.; Hassan, R.; Ahmed, A. Effect of inoculum VS, organic loads and I/S on the biochemical methane potential of sludge, buckwheat husk and straw. Desalination Water Treat. 2019, 157, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Kaur, H.; Kommalapati, R.R. Effect of Inoculum Concentration and Pretreatment on Biomethane Recovery from Cotton Gin Trash. J. Agric. Sci. 2021, 13, 15. [Google Scholar] [CrossRef]
- Furchtenicht, J.E.; Broderick, G.A. Effect of Inoculum Preparation and Dietary Energy on Microbial Numbers and Rumen Protein Degradation Activity. J. Dairy Sci. 1987, 70, 1404–1410. [Google Scholar] [CrossRef]
- Olubobokun, J.A.; Craig, W.M. Quantity and characteristics of microorganisms associated with ruminal fluid or particles. J. Anim. Sci. 1990, 68, 3360–3370. [Google Scholar] [CrossRef]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Zhang, H.; Jackson, T. Autochthonous bacterial flora indicated by PCR-DGGE of 16S rRNA gene fragments from the alimentary tract of Costelytra zealandica (Coleoptera: Scarabaeidae). J. Appl. Microbiol. 2008, 105, 1277–1285. [Google Scholar] [CrossRef]
- Friedman, D.I. Pseudotumor cerebri. Neurol. Clin. 2016, 22, 99–131. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, H. The Impact of Environmental Heterogeneity and Life Stage on the Hindgut Microbiota of Holotrichia parallela Larvae (Coleoptera: Scarabaeidae). PLoS ONE 2013, 8, e57169. [Google Scholar] [CrossRef]
- Sheng, P.; Li, Y.; Marshall, S.D.G.; Zhang, H. High Genetic Diversity of Microbial Cellulase and Hemicellulase Genes in the Hindgut of Holotrichia parallela Larvae. Int. J. Mol. Sci. 2015, 2, 16545–16559. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.-Y.; Yuan, Y.; Ali, M.W.; Peng, T.; Peng, W.; Raza, M.F.; Zhao, Y.; Zhang, H. Cultivable anaerobic and aerobic bacterial communities in the fermentation chambers of Holotrichia parallela (Coleoptera: Scarabaeidae) larvae. PLoS ONE 2018, 13, e0190663. [Google Scholar] [CrossRef]
- Andert, J.; Marten, A.; Brandl, R.; Brune, A. Inter- and intraspecific comparison of the bacterial assemblages in the hindgut of humivorous scarab beetle larvae (Pachnoda spp.). FEMS Microbiol. Ecol. 2010, 74, 439–449. [Google Scholar] [CrossRef]
- Li, X.; Brune, A. Digestion of microbial biomass, structural polysaccharides, and protein by the humivorous larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Soil Biol. Biochem. 2005, 37, 107–116. [Google Scholar] [CrossRef]
- Sheng, P.; Huang, S.; Wang, Q.; Wang, A.; Zhang, H. Isolation, Screening, and Optimization of the Fermentation Conditions of Highly Cellulolytic Bacteria from the Hindgut of Holotrichia parallela Larvae (Coleoptera: Scarabaeidae). Appl. Biochem. Biotechnol. 2012, 167, 270–284. [Google Scholar] [CrossRef]
- Trabalza-Marinucci, M.; Poncet, C.; Delval, E.; Fonty, G. Evaluation of techniques to detach particle-associated microorganisms from rumen contents. Anim. Feed Sci. Technol. 2006, 125, 1–16. [Google Scholar] [CrossRef]
- Barkovskii, A.; Fukui, H. A simple method for differential isolation of freely dispersed and particle-associated peat microorganisms. J. Microbiol. Methods 2003, 56, 93–105. [Google Scholar] [CrossRef]
- Craig, W.M.; Broderick, G.A.; Ricker, D.B. Quantitation of Microorganisms Associated with the Particulate Phase of Ruminal Ingesta. J. Nutr. 1987, 117, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.M.; Brown, D.R.; Broderick, G.A.; Ricker, D.B. Post-Prandial Compositional Changes of Fluid- and Particle-Associated Ruminal Microorganisms. J. Anim. Sci. 1987, 65, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Lazuka, A.; Auer, L.; O’Donohue, M.; Hernandez-Raquet, G. Anaerobic lignocellulolytic microbial consortium derived from termite gut: Enrichment, lignocellulose degradation and community dynamics. Biotechnol. Biofuels 2018, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.A.; Zeshan; Visvanathan, C. Effect of thermal pretreatment on chemical composition, physical structure and biogas production kinetics of wheat straw. J. Environ. Manag. 2018, 221, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yu, Y.; Wang, X.; Qu, Y.; Li, D.; He, W.; Kim, B.H. Degradation of raw corn stover powder (RCSP) by an enriched microbial consortium and its community structure. Bioresour. Technol. 2011, 102, 742–747. [Google Scholar] [CrossRef]
- Terra, W.R.; Ferreira, C. Insect digestive enzymes: Properties, compartmentalization and function. Comp. Biochem. Physiol. Part B Comp. Biochem. 1994, 109, 1–62. [Google Scholar] [CrossRef]
- Elpidina, E.N.; Vinokurov, K.S.; Gromenko, V.A.; Rudenskaya, Y.A.; Dunaevsky, Y.E.; Zhuzhikov, D.P. Compartmentalization of proteinases and amylases in Nauphoeta cinerea midgut. Arch. Insect Biochem. Physiol. 2001, 48, 206–216. [Google Scholar] [CrossRef]
- Oppert, B.; Walters, P.; Zuercher, M. Digestive proteinases of the larger black flour beetle, Cynaeus angustus (Coleoptera: Tenebrionidae). Bull. Éntomol. Res. 2006, 96, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-W.; Zhang, H.-Y.; Marshall, S.; Jackson, T.A. The scarab gut: A potential bioreactor for bio-fuel production. Insect Sci. 2010, 17, 175–183. [Google Scholar] [CrossRef]
- Takeishi, H.; Anzai, H.; Urai, M.; Aizawa, T.; Wada, N.; Iwabuchi, N.; Sunairi, M.; Nakajima, M. Xylanolytic and Alkaliphilic Dietzia sp. Isolated from Larvae of the Japanese Horned Beetle, Trypoxylus dichotomus. Actinomycetologica 2006, 20, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Wada, N.; Sunairi, M.; Anzai, H.; Iwata, R.; Yamane, A.; Nakajima, M. Glycolytic Activities in the Larval Digestive Tract of Trypoxylus dichotomus (Coleoptera: Scarabaeidae). Insects 2014, 5, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Bhawane, G.P.; Bhanot, R.K. Digestive Enzymes of White Grubs, Leucopholis lepidophora Bl. and Holotrichia fissa Br. (Coleoptera; Scarabaeidae: Melolonthinae). Biosci. Discov. 2017, 8, 880–890. [Google Scholar]
- Felton, G.W.; Duffey, S.S. Reassessment of the role of gut alkalinity and detergency in insect herbivory. J. Chem. Ecol. 1991, 17, 1821–1836. [Google Scholar] [CrossRef]
- Mancini, G.; Papirio, S.; Lens, P.N.L.; Esposito, G. Increased biogas production from wheat straw by chemical pretreatments. Renew. Energy 2018, 119, 608–614. [Google Scholar] [CrossRef]
- Rajeswari, G.; Jacob, S.; Chandel, A.K.; Kumar, V. Unlocking the potential of insect and ruminant host symbionts for recycling of lignocellulosic carbon with a biorefinery approach: A review. Microb. Cell Factories 2021, 20, 107. [Google Scholar] [CrossRef]
- Jiménez, D.J.; Dini-Andreote, F.; Van Elsas, J.D. Metataxonomic profiling and prediction of functional behaviour of wheat straw degrading microbial consortia. Biotechnol. Biofuels 2014, 7, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trifonova, R.; Postma, J.; Ketelaars, J.J.M.H.; van Elsas, J.D. Thermally Treated Grass Fibers as Colonizable Substrate for Beneficial Bacterial Inoculum. Microb. Ecol. 2008, 56, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, D.J.; Korenblum, E.; Van Elsas, J.D. Novel multispecies microbial consortia involved in lignocellulose and 5-hydroxymethylfurfural bioconversion. Appl. Microbiol. Biotechnol. 2013, 98, 2789–2803. [Google Scholar] [CrossRef] [Green Version]
- Cazemier, A.E. (Hemi)Cellulose Degradation by Microorganisms from the Intestinal Tract of Arthropods; Radboud University Nijmegen: Nijmegen, The Netherlands, 1997. [Google Scholar]
- Franzini, P.Z.N.; Ramond, J.-B.; Scholtz, C.H.; Sole, C.L.; Ronca, S.; Cowan, D.A. The Gut Microbiomes of Two Pachysoma MacLeay Desert Dung Beetle Species (Coleoptera: Scarabaeidae: Scarabaeinae) Feeding on Different Diets. PLoS ONE 2016, 11, e0161118. [Google Scholar] [CrossRef]
- Suárez-Moo, P.; Cruz-Rosales, M.; Ibarra-Laclette, E.; Desgarennes, D.; Huerta, C.; Lamelas, A. Diversity and Composition of the Gut Microbiota in the Developmental Stages of the Dung Beetle Copris incertus Say (Coleoptera, Scarabaeidae). Front. Microbiol. 2020, 11, 1698. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.S.; Newton, I.L.; Moczek, A.P. (My Microbiome) Would Walk 10,000 miles: Maintenance and Turnover of Microbial Communities in Introduced Dung Beetles. Microb. Ecol. 2020, 80, 435–446. [Google Scholar] [CrossRef]
- De León, A.V.-P.; Jahnes, B.C.; Duan, J.; Camuy-Vélez, L.A.; Sabree, Z.L. Cultivable, Host-Specific Bacteroidetes Symbionts Exhibit Diverse Polysaccharolytic Strategies. Appl. Environ. Microbiol. 2020, 86, e00091-20. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Zhang, N.; Ji, S.-Q.; Lan, X.; Zhang, K.-D.; Shen, Y.-L.; Li, F.-L.; Ni, J.-F. Dysgonomonas macrotermitis sp. nov., isolated from the hindgut of a fungus-growing termite. Int. J. Syst. Evol. Microbiol. 2014, 64, 2956–2961. [Google Scholar] [CrossRef]
- Bridges, C.M.; Nelson, M.C.; Graf, J.; Gage, D.J. Draft Genome Sequences of Dysgonomonas sp. Strains BGC7 and HGC4, Isolated from the Hindgut of a Lower Termite. Microbiol. Resour. Announc. 2021, 10, 18–20. [Google Scholar] [CrossRef]
- Mikaelyan, A.; Thompson, C.L.; Hofer, M.J.; Brune, A. Deterministic Assembly of Complex Bacterial Communities in Guts of Germ-Free Cockroaches. Appl. Environ. Microbiol. 2016, 82, 1256–1263. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Yang, Y.; Zhang, N.; Shen, Y.; Ni, J. Draft Genome Sequence of Dysgonomonas macrotermitis Strain JCM 19375 T, Isolated from the Gut of a Termite. Genome Announc. 2015, 3, 61739. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Nguyen, D.; Lam, T.Y.; Zhuang, H.; Shrestha, S.; Raskin, L.; Khanal, S.K.; Lee, P.-H. Synergistic association between cytochrome bd-encoded Proteiniphilum and reactive oxygen species (ROS)-scavenging methanogens in microaerobic-anaerobic digestion of lignocellulosic biomass. Water Res. 2020, 190, 116721. [Google Scholar] [CrossRef]
- Hahnke, S.; Langer, T.; Koeck, D.E.; Klocke, M. Description of Proteiniphilum saccharofermentans sp. nov., Petrimonas mucosa sp. nov. and Fermentimonas caenicola gen. nov., sp. nov., isolated from mesophilic laboratory-scale biogas reactors, and emended description of the genus Proteiniphilum. Int. J. Syst. Evol. Microbiol. 2016, 66, 1466–1475. [Google Scholar] [CrossRef]
- Pramono, A.; Sakamoto, M.; Iino, T.; Hongoh, Y.; Ohkuma, M. Dysgonomonas termitidis sp. nov., isolated from the gut of the subterranean termite Reticulitermes speratus. Int. J. Syst. Evol. Microbiol. 2015, 65, 681–685. [Google Scholar] [CrossRef]
- Chen, S.; Dong, X. Proteiniphilum acetatigenes gen. nov., sp. nov., from a UASB reactor treating brewery wastewater. Int. J. Syst. Evol. Microbiol. 2005, 55, 2257–2261. [Google Scholar] [CrossRef] [Green Version]
- Hofstad, T.; Olsen, I.; Eribe, E.R.; Falsen, E.; Collins, M.D.; Lawson, P.A. Dysgonomonas gen. nov. to accommodate Dysgonomonas gadei sp. nov., an organism isolated from a human gall bladder, and Dysgonomonas capnocytophagoides (formerly CDC group DF-3). Int. J. Syst. Evol. Microbiol. 2000, 50, 2189–2195. [Google Scholar] [CrossRef]
- Chouaia, B.; Goda, N.; Mazza, G.; Alali, S.; Florian, F.; Gionechetti, F.; Callegari, M.; Gonella, E.; Magoga, G.; Fusi, M.; et al. Developmental stages and gut microenvironments influence gut microbiota dynamics in the invasive beetle Popillia japonica Newman (Coleoptera: Scarabaeidae). Environ. Microbiol. 2019, 21, 4343–4359. [Google Scholar] [CrossRef]
- Bourguignon, T.; Lo, N.; Dietrich, C.; Šobotník, J.; Sidek, S.; Roisin, Y.; Brune, A.; Evans, T. Rampant Host Switching Shaped the Termite Gut Microbiome. Curr. Biol. 2018, 28, 649–654.e2. [Google Scholar] [CrossRef] [Green Version]
- Murakami, T.; Segawa, T.; Takeuchi, N.; Sepúlveda, G.B.; Labarca, P.; Kohshima, S.; Hongoh, Y. Metagenomic analyses highlight the symbiotic association between the glacier stonefly Andiperla willinki and its bacterial gut community. Environ. Microbiol. 2018, 20, 4170–4183. [Google Scholar] [CrossRef]
- Meehan, C.; Beiko, R.G. A Phylogenomic View of Ecological Specialization in the Lachnospiraceae, a Family of Digestive Tract-Associated Bacteria. Genome Biol. Evol. 2014, 6, 703–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagen, E.J.; Padmanabha, J.; Denman, S.; McSweeney, C.S. Hydrogenotrophic culture enrichment reveals rumen Lachnospiraceae and Ruminococcaceae acetogens and hydrogen-responsive Bacteroidetes from pasture-fed cattle. FEMS Microbiol. Lett. 2015, 362, fnv104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiß, S.; Zankel, A.; Lebuhn, M.; Petrak, S.; Somitsch, W.; Guebitz, G. Investigation of mircroorganisms colonising activated zeolites during anaerobic biogas production from grass silage. Bioresour. Technol. 2011, 102, 4353–4359. [Google Scholar] [CrossRef] [PubMed]
- Dröge, S.; Limper, U.; Emtiazi, F.; Schönig, I.; Pavlus, N.; Drzyzga, O.; Fischer, U.; König, H. In vitro and in vivo sulfate reduction in the gut contents of the termite Mastotermes darwiniensis and the rose-chafer Pachnoda marginata. J. Gen. Appl. Microbiol. 2005, 51, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Sass, H.; Berchtold, M.; Branke, J.; König, H.; Cypionka, H.; Babenzien, H.-D. Psychrotolerant Sulfate-reducing Bacteria from an Oxic Freshwater Sediment Description of Desulfovibrio cuneatus sp. nov. and Desulfovibrio litoralis sp. nov. Syst. Appl. Microbiol. 1998, 21, 212–219. [Google Scholar] [CrossRef]
- Cypionka, H. Oxygen Respiration by Desulfovibrio Species. Annu. Rev. Microbiol. 2000, 54, 827–848. [Google Scholar] [CrossRef]
- Dilling, W.; Cypionka, H. Aerobic respiration in sulfate-reducing bacteria. FEMS Microbiol. Lett. 1990, 71, 123–127. [Google Scholar] [CrossRef]
- Egert, M.; Stingl, U.; Bruun, L.D.; Pommerenke, B.; Brune, A.; Friedrich, M.W. Structure and Topology of Microbial Communities in the Major Gut Compartments of Melolontha melolontha Larvae (Coleoptera: Scarabaeidae). Appl. Environ. Microbiol. 2005, 71, 4556–4566. [Google Scholar] [CrossRef] [Green Version]
- Ebert, K.M.; Arnold, W.G.; Ebert, P.R.; Merritt, D.J. Hindgut Microbiota Reflects Different Digestive Strategies in Dung Beetles (Coleoptera: Scarabaeidae: Scarabaeinae). Appl. Environ. Microbiol. 2021, 87, e02100-20. [Google Scholar] [CrossRef]
- Kuhnigk, T.; Branke, J.; Krekeler, D.; Cypionka, H.; König, H. A Feasible Role of Sulfate-Reducing Bacteria in the Termite Gut. Syst. Appl. Microbiol. 1996, 19, 139–149. [Google Scholar] [CrossRef]
- Schauer, C.; Thompson, C.L.; Brune, A. The Bacterial Community in the Gut of the Cockroach Shelfordella lateralis Reflects the Close Evolutionary Relatedness of Cockroaches and Termites. Appl. Environ. Microbiol. 2012, 78, 2758–2767. [Google Scholar] [CrossRef] [Green Version]
- Dumond, L.; Lam, P.Y.; van Erven, G.; Kabel, M.; Mounet, F.; Grima-Pettenati, J.; Tobimatsu, Y.; Hernandez-Raquet, G. Termite Gut Microbiota Contribution to Wheat Straw Delignification in Anaerobic Bioreactors. ACS Sustain. Chem. Eng. 2021, 9, 2191–2202. [Google Scholar] [CrossRef]
- Mathews, S.L.; Epps, M.J.; Blackburn, R.K.; Goshe, M.B.; Grunden, A.; Dunn, R.R. Public questions spur the discovery of new bacterial species associated with lignin bioconversion of industrial waste. R. Soc. Open Sci. 2019, 6, 6180748. [Google Scholar] [CrossRef] [Green Version]
- Bugg, T.D.; Ahmad, M.; Hardiman, E.M.; Singh, R. The emerging role for bacteria in lignin degradation and bio-product formation. Curr. Opin. Biotechnol. 2011, 22, 394–400. [Google Scholar] [CrossRef]
- Geib, S.M.; Filley, T.R.; Hatcher, P.G.; Hoover, K.; Carlson, J.E.; Jimenez-Gasco, M.D.M.; Nakagawa-Izumi, A.; Sleighter, R.L.; Tien, M. Lignin degradation in wood-feeding insects. Proc. Natl. Acad. Sci. USA 2008, 105, 12932–12937. [Google Scholar] [CrossRef] [Green Version]
- Scully, E.D.; Geib, S.M.; Hoover, K.; Tien, M.; Tringe, S.G.; Barry, K.W.; del Rio, T.G.; Chovatia, M.; Herr, J.R.; Carlson, J.E. Metagenomic Profiling Reveals Lignocellulose Degrading System in a Microbial Community Associated with a Wood-Feeding Beetle. PLoS ONE 2013, 8, e73827. [Google Scholar] [CrossRef] [Green Version]
- Scully, E.D.; Geib, S.M.; Carlson, J.E.; Tien, M.; McKenna, D.; Hoover, K. Functional genomics and microbiome profiling of the Asian longhorned beetle (Anoplophora glabripennis) reveal insights into the digestive physiology and nutritional ecology of wood feeding beetles. BMC Genom. 2014, 15, 1096. [Google Scholar] [CrossRef] [Green Version]
- Bayon, C.; Mathelin, J. Carbohydrate fermentation and by-product absorption studied with labelled cellulose in Oryctes nasicornis Larvae (Coleoptera: Scarabaeidae). J. Insect Physiol. 1980, 26, 833–840. [Google Scholar] [CrossRef]
- Liu, Y.; Whitman, W.B. Metabolic, Phylogenetic, and Ecological Diversity of the Methanogenic Archaea. Ann. N. Y. Acad. Sci. 2008, 1125, 171–189. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Abbas, B.; Geleijnse, M.; Pimenov, N.V.; Sukhacheva, M.V.; van Loosdrecht, M. Methanogenesis at extremely haloalkaline conditions in the soda lakes of Kulunda Steppe (Altai, Russia). FEMS Microbiol. Ecol. 2015, 91, fiv016. [Google Scholar] [CrossRef] [Green Version]
- Sanz, J.L.; Rodriguez, N.; Díaz, E.E.; Amils, R. Methanogenesis in the sediments of Rio Tinto, an extreme acidic river. Environ. Microbiol. 2011, 13, 2336–2341. [Google Scholar] [CrossRef]
- Lv, Z.; Leite, A.F.; Harms, H.; Glaser, K.; Liebetrau, J.; Kleinsteuber, S.; Nikolausz, M. Microbial community shifts in biogas reactors upon complete or partial ammonia inhibition. Appl. Microbiol. Biotechnol. 2019, 103, 519–533. [Google Scholar] [CrossRef]
- Sousa, D.Z.; Smidt, H.; Alves, M.M.; Stams, A. Syntrophomonas zehnderi sp. nov., an anaerobe that degrades long-chain fatty acids in co-culture with Methanobacterium formicicum. Int. J. Syst. Evol. Microbiol. 2007, 57, 609–615. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Guo, R.; Xu, X.; Wang, L.; Dai, M. Enhanced methane production via repeated batch bioaugmentation pattern of enriched microbial consortia. Bioresour. Technol. 2016, 216, 471–477. [Google Scholar] [CrossRef]
- Allison, M.J.; MacGregor, B.J.; Stahl, D.A. Synergistes. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Westerholm, M.; Müller, B.; Singh, A.; Lindsjö, O.K.; Schnürer, A. Detection of novel syntrophic acetate-oxidizing bacteria from biogas processes by continuous acetate enrichment approaches. Microb. Biotechnol. 2018, 11, 680–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferry, J.G. Methanogenesis; Springer: Berlin/Heidelberg, Germany, 1993; ISBN 9781461360131. [Google Scholar]
- Honda, T.; Fujita, T.; Tonouchi, A. Aminivibrio pyruvatiphilus gen. nov., sp. nov., an anaerobic, amino-acid-degrading bacterium from soil of a Japanese rice field. Int. J. Syst. Evol. Microbiol. 2013, 63, 3679–3686. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Sun, L.; Schnürer, A. First insights into the syntrophic acetate-oxidizing bacteria—A genetic study. Microbiol. Open 2013, 2, 35–53. [Google Scholar] [CrossRef] [PubMed]





| Strategy | Gut Compartment | Culture | ASVs | Relative Abundance (%) | Family | Relative Abundance (%) |
|---|---|---|---|---|---|---|
| P1 | Midgut | Inoculum | Pseudoxanthomonas sp. (Xanthomonadaceae) | 14 | Bacillaceae | 22 |
| Bacillus niacini (Bacillaceae) | 5 | Xanthomonadaceae | 4 | |||
| Bacillus drentensis (Bacillaceae) | 4 | Promicromonosporaceae | 4 | |||
| Xylanimicrobium pachnodae (Promicromonosporaceae) | 4 | Ruminococcaceae | 2 | |||
| Bacillus sp. (Bacillaceae) | 3 | Enterococcaceae | 1 | |||
| Enrichment | Dysgonomonas sp. (Dysgonomonadaceae) | 32 | Dysgonomonadaceae | 47 | ||
| Hydrogenispora sp. (Heliobacteriaceae) | 18 | Heliobacteriaceae | 17 | |||
| Dysgonomonas sp. (Dysgonomonadaceae) | 9 | Family_XI | 8 | |||
| Desulfovibrio sp. (Desulfovibrionaceae) | 6 | Desulfovibrionaceae | 5 | |||
| Dysgonomonas gadei (Dysgonomonadaceae) | 6 | Lachnospiraceae | 4 | |||
| Hindgut | Inoculum | Desulfovibrio sp. (Desulfovibrionaceae) | 5 | Ruminococcaceae | 28 | |
| Proteiniphilum sp. (Dysgonomonadaceae) | 5 | Christensenellaceae | 13 | |||
| Desulfovibrio sp. (Desulfovibrionaceae) | 3 | Desulfovibrionaceae | 12 | |||
| Bacteroides sp. (Bacteroidaceae) | 3 | Dysgonomonadaceae | 11 | |||
| Parabacteroides sp. (Tannerellaceae) | 2 | Lachnospiraceae | 10 | |||
| Enrichment | Dysgonomonas sp. (Dysgonomonadaceae) | 16 | Dysgonomonadaceae | 31 | ||
| Hydrogenispora sp. (Heliobacteriaceae) | 12 | Heliobacteriaceae | 21 | |||
| Hydrogenispora sp. (Heliobacteriaceae) | 11 | Ruminococcaceae | 10 | |||
| Dysgonomonas sp. (Dysgonomonadaceae) | 9 | Lachnospiraceae | 7 | |||
| Dysgonomonas sp. (Dysgonomonadaceae) | 5 | Desulfovibrionaceae | 10 | |||
| P2 | Midgut | Inoculum | Xylanimicrobium pachnodae (Promicromonosporaceae) | 7 | Promicromonosporaceae | 12 |
| Cellulosimicrobium sp. (Promicromonosporaceae) | 3 | Bacillaceae | 6 | |||
| Luteimonas sp. (Xanthomonadaceae) | 3 | Microbacteriaceae | 4 | |||
| Lactobacillales (order) (Bacilli) | 2 | Enterococcaceae | 4 | |||
| Agromyces sp. (Microbacteriaceae) | 2 | Xanthomonadaceae | 4 | |||
| Enrichment | Proteiniphilum sp. (Dysgonomonadaceae) | 10 | Marinilabiliacea | 28 | ||
| Proteiniphilum saccharofermentans (Dysgonomonadaceae) | 8 | Dysgonomonadaceae | 22 | |||
| Ruminofilibacter sp. (Marinilabiliaceae) | 6 | Ruminococcaceae | 10 | |||
| Ruminofilibacter xylanolyticum (Marinilabiliaceae) | 6 | Heliobacteriaceae | 7 | |||
| Proteiniphilum acetatigenes (Dysgonomonadaceae) | 6 | Clostridiaceae | 7 | |||
| Hindgut | Inoculum | Eubacterium sp. (Eubacteriaceae) | 10 | Christensenellaceae | 16 | |
| Sebaldella termitidis (Leptotrichiaceae) | 4 | Ruminococcaceae | 15 | |||
| Enterococcus pallens (Enterococcaceae) | 3 | Eubacteriaceae | 10 | |||
| Lactobacillales (order) (Bacilli) | 3 | Enterococcaceae | 7 | |||
| Xylanimicrobium pachnodae (Promicromonosporaceae) | 3 | Lachnospiraceae | 5 | |||
| Enrichment | Hydrogenispora sp. (Heliobacteriaceae) | 16 | Dysgonomonadaceae | 22 | ||
| Ruminofilibacter sp. (Marinilabiliaceae) | 6 | Marinilabiliaceae | 19 | |||
| NA. (Marinilabiliaceae) | 5 | Ruminococcaceae | 12 | |||
| Lentimicrobium sp. (Lentimicrobiaceae) | 5 | Heliobacteriaceae | 10 | |||
| Lutispora sp. (Gracilibacteraceae) | 4 | Lentimicrobiaceae | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schroeder, B.G.; Logroño, W.; Rocha, U.N.d.; Harms, H.; Nikolausz, M. Enrichment of Anaerobic Microbial Communities from Midgut and Hindgut of Sun Beetle Larvae (Pachnoda marginata) on Wheat Straw: Effect of Inoculum Preparation. Microorganisms 2022, 10, 761. https://doi.org/10.3390/microorganisms10040761
Schroeder BG, Logroño W, Rocha UNd, Harms H, Nikolausz M. Enrichment of Anaerobic Microbial Communities from Midgut and Hindgut of Sun Beetle Larvae (Pachnoda marginata) on Wheat Straw: Effect of Inoculum Preparation. Microorganisms. 2022; 10(4):761. https://doi.org/10.3390/microorganisms10040761
Chicago/Turabian StyleSchroeder, Bruna Grosch, Washington Logroño, Ulisses Nunes da Rocha, Hauke Harms, and Marcell Nikolausz. 2022. "Enrichment of Anaerobic Microbial Communities from Midgut and Hindgut of Sun Beetle Larvae (Pachnoda marginata) on Wheat Straw: Effect of Inoculum Preparation" Microorganisms 10, no. 4: 761. https://doi.org/10.3390/microorganisms10040761
APA StyleSchroeder, B. G., Logroño, W., Rocha, U. N. d., Harms, H., & Nikolausz, M. (2022). Enrichment of Anaerobic Microbial Communities from Midgut and Hindgut of Sun Beetle Larvae (Pachnoda marginata) on Wheat Straw: Effect of Inoculum Preparation. Microorganisms, 10(4), 761. https://doi.org/10.3390/microorganisms10040761







