Preclinical Testing of Boron-Doped Diamond Electrodes for Root Canal Disinfection—A Series of Preliminary Studies
Abstract
:1. Introduction
2. Materials and Methods
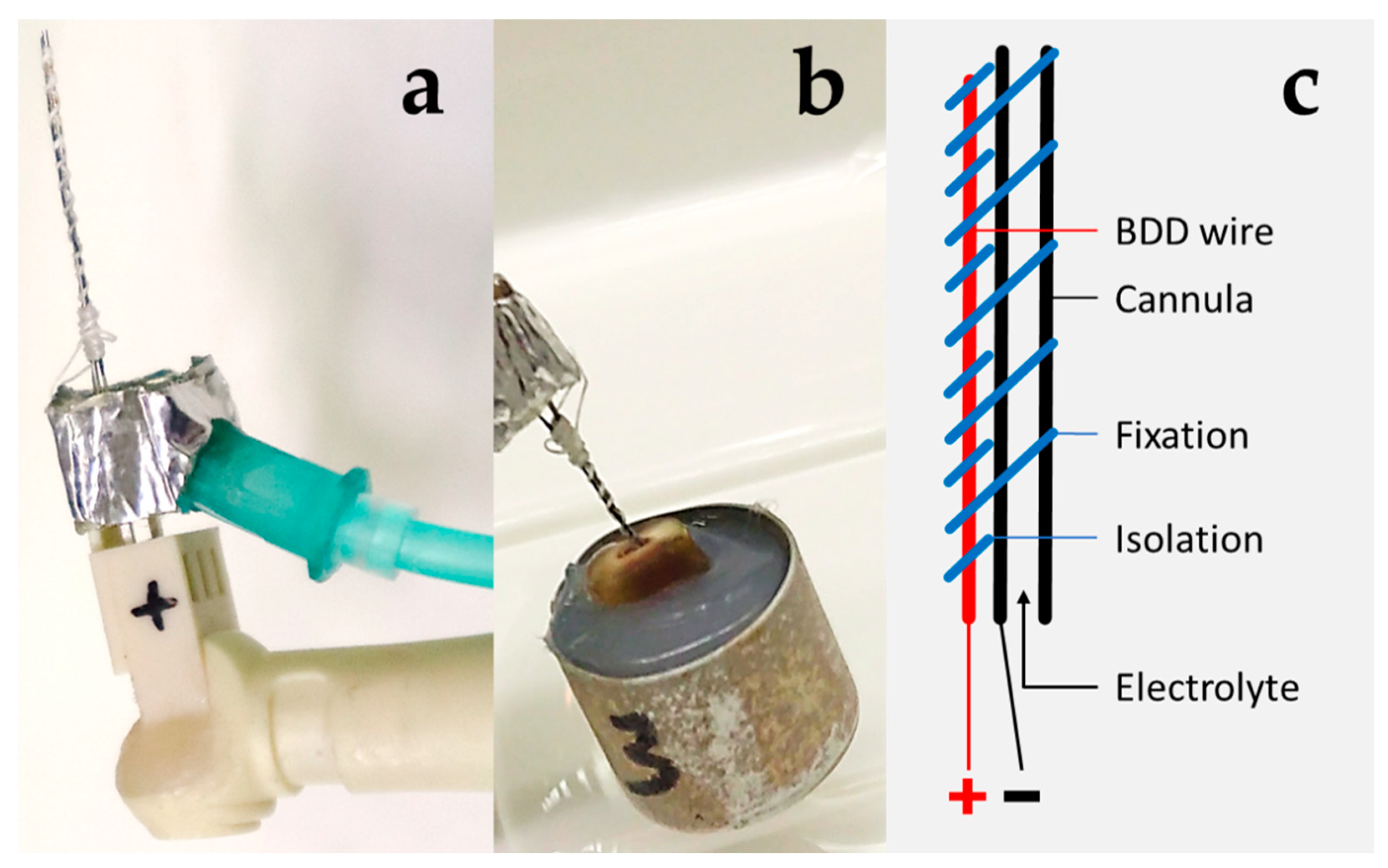
2.1. BDD Electrode Treatment Systems
2.2. Determination of Disinfection Parameters
2.3. Analysis of Multispecies Biofilm Formation
2.4. Disinfection of Root Canals Colonized by Multispecies Biofilm
2.5. Canine Tooth Model
3. Results
3.1. Determination of Disinfection Parameters
3.2. Multispecies Biofilm Formation
3.3. Elimination of Multispecies Biofilm
3.4. Canine Tooth Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergenholtz, G.; Spångberg, L. Controversies in endodontics. Crit. Rev. Oral Biol. Med. 2004, 15, 99–114. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.S.; Kim, J.R.; Ling, J.; Choi, K.K.; Pashley, D.H.; Tay, F.R. Review of contemporary irrigant agitation techniques and devices. J. Endod. 2009, 35, 791–804. [Google Scholar] [CrossRef]
- Li, G.H.; Niu, L.N.; Zhang, W.; Olsen, M.; De-Deus, G.; Eid, A.A.; Chen, J.H.; Pashley, D.H.; Tay, F.R. Ability of new obturation materials to improve the seal of the root canal system: A review. Acta Biomater. 2014, 10, 1050–1063. [Google Scholar] [CrossRef] [Green Version]
- Briseño-Marroquín, B.; Paqué, F.; Maier, K.; Willershausen, B.; Wolf, T.G. Root canal morphology and configuration of 179 maxillary first molars by means of micro-computed tomography: An ex vivo study. J. Endod. 2015, 41, 2008–2013. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.J.; Castro, R.W.; Nejaim, Y.; Silva, A.I.; Haiter-Neto, F.; Silberman, A.; Cohenca, N. Evaluation of root canal configuration of maxillary and mandibular anterior teeth using cone beam computed tomography: An in-vivo study. Quintessence Int. 2016, 47, 19–24. [Google Scholar]
- Gokturk, H.; Ozkocak, I.; Buyukgebiz, F.; Demir, O. Effectiveness of various irrigation protocols for the removal of calcium hydroxide from artificial standardized grooves. J. Appl. Oral Sci. 2017, 25, 290–298. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Shen, Y.; Haapasalo, M. A comparative study of biofilm removal with hand, rotary nickel-titanium, and self-adjusting file instrumentation using a novel in vitro biofilm model. J. Endod. 2013, 39, 658–663. [Google Scholar] [CrossRef]
- Burkovski, A.; Karl, M. Lack of evidence for the necessity of root canal obturation. Quintessence Int. 2019, 50, 22–28. [Google Scholar]
- Gänsbauer, M.; Burkovski, A.; Karl, M.; Grobecker-Karl, T. Comparison of simplistic biofilm models for evaluating irrigating solutions. Quintessence Int. 2017, 48, 521–526. [Google Scholar]
- Signoretti, F.G.; Endo, M.S.; Gomes, B.P.; Montagner, F.; Tosello, F.B.; Jacinto, R.C. Persistent extraradicular infection in root-filled asymptomatic human tooth: Scanning electron microscopic analysis and microbial investigation after apical microsurgery. J. Endod. 2011, 37, 1696–1700. [Google Scholar] [CrossRef]
- del Carpio-Perochena, A.; Bramante, C.M.; de Andrade, F.B.; Maliza, A.G.; Cavenago, B.C.; Marciano, M.A.; Amoroso-Silva, P.; Duarte, M.H. Antibacterial and dissolution ability of sodium hypochlorite in different pHs on multi-species biofilms. Clin. Oral Investig. 2015, 19, 2067–2073. [Google Scholar] [CrossRef]
- Rôças, I.N.; Hülsmann, M.; Siqueira, J.F., Jr. Microorganisms in root canal-treated teeth from a German population. J. Endod. 2008, 34, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Pelz, K.; Schirrmeister, J.F.; Hellwig, E.; Pukall, R. Characterization of the first oral Vagococcus isolate from a root-filled tooth with periradicular lesions. Curr. Microbiol. 2008, 57, 235–238. [Google Scholar] [CrossRef]
- Haapasalo, M.; Shen, Y.; Qian, W.; Gao, Y. Irrigation in endodontics. Dent. Clin. N. Am. 2010, 54, 291–312. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, M.; Lu, Y.; Guo, X.; Qiao, F.; Wu, L. Antibacterial and residual antimicrobial activities against Enterococcus faecalis biofilm: A comparison between EDTA, chlorhexidine, cetrimide, MTAD and QMix. Sci. Rep. 2015, 5, 12944. [Google Scholar] [CrossRef] [Green Version]
- Kanaan, C.G.; Pelegrine, R.A.; da Silveira Bueno, C.E.; Shimabuko, D.M.; Valamatos Pinto, N.M.; Kato, A.S. Can irrigant agitation lead to the formation of a smear layer? J. Endod. 2020, 46, 1120–1124. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Y.; Haapasalo, M. Effectiveness of endodontic disinfecting solutions against young and old Enterococcus faecalis biofilms in dentin canals. J. Endod. 2012, 38, 1376–1379. [Google Scholar] [CrossRef]
- Layton, G.; Wu, W.I.; Selvaganapathy, P.R.; Friedman, S.; Kishen, A. Fluid dynamics and biofilm removal generated by syringe-delivered and 2 ultrasonic-assisted irrigation methods: A novel experimental approach. J. Endod. 2015, 41, 884–889. [Google Scholar] [CrossRef]
- Castelo-Baz, P.; Lozano, F.J.R.; Ginzo-Villamayor, M.J.; Vila, R.M.; Seoane-Romero, J.; Martín-Cruces, J.; Martín-Biedma, B. Efficacy of continuous apical negative ultrasonic irrigation (CANUI) in penetration of simulated lateral canals in extracted teeth. Sci. Rep. 2021, 11, 10908. [Google Scholar] [CrossRef]
- Lendini, M.; Alemanno, E.; Migliaretti, G.; Berutti, E. The effect of high-frequency electrical pulses on organic tissue in root canals. Int. Endod. J. 2005, 38, 531–538. [Google Scholar] [CrossRef]
- Tronstad, L.; Trope, M.; Hammond, B.F. Effect of electric current and silver electrodes on oral bacteria. Endod. Dent. Traumatol. 1985, 1, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Sahar-Helft, S.; Stabholtz, A.; Moshonov, J.; Gutkin, V.; Redenski, I.; Steinberg, D. Effect of Er:YAG laser-activated irrigation solution on Enterococcus faecalis biofilm in an ex-vivo root canal model. Photomed. Laser Surg. 2013, 31, 334–341. [Google Scholar] [CrossRef]
- Virtej, A.; MacKenzie, C.R.; Raab, W.H.; Pfeffer, K.; Barthel, C.R. Determination of the performance of various root canal disinfection methods after in situ carriage. J. Endod. 2007, 33, 926–929. [Google Scholar] [CrossRef]
- Meto, A.; Colombari, B.; Sala, A.; Pericolini, E.; Meto, A.; Peppoloni, S.; Blasi, E. Antimicrobial and antibiofilm efficacy of a copper/calcium hydroxide-based endodontic paste against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. Dent. Mater. J. 2019, 38, 591–603. [Google Scholar] [CrossRef] [Green Version]
- Meto, A.; Droboniku, E.; Blasi, E.; Colombari, B.; Tragaj, E.; Cervino, G.; Fiorillo, L.; Meto, A. Copper-Calcium Hydroxide and Permanent Electrophoretic Current for Treatment of Apical Periodontitis. Materials 2021, 14, 678. [Google Scholar] [CrossRef]
- Peters, L.B.; Wesselink, P.R.; Moorer, W.R. The fate and the role of bacteria left in root dentinal tubules. Int. Endod. J. 1995, 28, 95–99. [Google Scholar] [CrossRef]
- Cueva-Goig, R.; Forner-Navarro, L.; Llena-Puy, M.C. Microscopic assessment of the sealing ability of three endodontic filling techniques. J. Clin. Exp. Dent. 2016, 8, e27–e31. [Google Scholar] [CrossRef] [Green Version]
- Roth, K.A.; Friedman, S.; Lévesque, C.M.; Basrani, B.R.; Finer, Y. Microbial biofilm proliferation within sealer-root dentin interfaces is affected by sealer type and aging period. J. Endod. 2012, 38, 1253–1256. [Google Scholar] [CrossRef] [Green Version]
- Diesendorf, N.; Köhler, S.; Geißdörfer, W.; Grobecker-Karl, T.; Karl, M.; Burkovski, A. Characterisation of Roseomonas mucosa isolated from the root canal of an infected tooth. BMC Res. Notes 2017, 10, 212. [Google Scholar] [CrossRef]
- Yücel, A.C.; Güler, E.; Güler, A.U.; Ertaş, E. Bacterial penetration after obturation with four different root canal sealers. J. Endod. 2006, 32, 890–893. [Google Scholar] [CrossRef]
- Ebert, J.; Roggendorf, M.J.; Frank, K.; Petschelt, A. Antimicrobial activity of various ‘active’ gutta-percha points against Enterococcus faecalis in simulated root canals. Int. Endod. J. 2008, 41, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Böhm, A.L.; Koch, M.; Rosiwal, S.; Burkovski, A.; Karl, M.; Grobecker-Karl, T. Electrochemical disinfection of experimentally infected teeth by boron-doped diamond electrode treatment. J. Clin. Med. 2019, 8, 2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, M.; Göltz, M.; Xiangjun, M.; Karl, M.; Rosiwal, S.; Burkovski, A. Electrochemical disinfection of dental implants experimentally contaminated with microorganisms as a model for periimplantitis. J. Clin. Med. 2020, 9, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, M.; Burkovski, A.; Zulla, M.; Rosiwal, S.; Geißdörfer, W.; Dittmar, R.; Grobecker-Karl, T. Pilot study on the use of a laser structured double diamond electrode (DDE) for biofilm removal from dental implant surfaces. J. Clin. Med. 2020, 9, 3036. [Google Scholar] [CrossRef]
- Göltz, M.; Koch, M.; Detsch, R.; Karl, M.; Burkovski, A.; Rosiwal, S. Influence of in-situ electrochemical oxidation on implant surface and colonizing microorganisms evaluated by scatter electron microscopy. Materials 2019, 12, 3977. [Google Scholar] [CrossRef] [Green Version]
- Hage, W.; De Moor, R.J.G.; Hajj, D.; Sfeir, G.; Sarkis, D.K.; Zogheib, C. Impact of different irrigant agitation methods on bacterial elimination from infected root canals. Dent. J. 2019, 7, 64. [Google Scholar] [CrossRef] [Green Version]
- Karsa, D.R. Biocides. In Handbook for Cleaning/Decontamination of Surfaces; Johansson, I., Somasundaran, P., Eds.; Elsevier, B.V.: Amsterdam, The Netherlands, 2007; pp. 593–623. [Google Scholar]
- Koch, M.; Palarie, V.; Götz, M.; Willner, M.; Burkovski, A.; Karl, M. Root canal obstruation by electrochemical precipitation of calcium phosphates. Appl. Sci. 2022, 12, 2956. [Google Scholar] [CrossRef]
- Rosen, E.; Tsesis, I.; Elbahary, S.; Storzi, N.; Kolodkin-Gal, I. Eradication of Enterococcus faecalis biofilms on human dentin. Front. Microbiol. 2016, 7, 2055. [Google Scholar] [CrossRef]
- Haapasalo, M.; Shen, Y.; Wang, Z.; Gao, Y. Irrigation in endodontics. Br. Dent. J. 2014, 216, 299–303. [Google Scholar] [CrossRef]





| Group | Treatment |
|---|---|
| control | irrigation with saline (0.9% NaCl) |
| CHX | irrigation with chlorhexidine (0.2%) |
| BDD 4.5 V/0.5 mA | electrochemical disinfection and irrigation with saline |
| BDD 5.5 V/3.0 mA | electrochemical disinfection and irrigation with saline |
| BDD 6.5 V/10.0 mA | electrochemical disinfection and irrigation with saline |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, M.; Palarie, V.; Koch, L.; Burkovski, A.; Zulla, M.; Rosiwal, S.; Karl, M. Preclinical Testing of Boron-Doped Diamond Electrodes for Root Canal Disinfection—A Series of Preliminary Studies. Microorganisms 2022, 10, 782. https://doi.org/10.3390/microorganisms10040782
Koch M, Palarie V, Koch L, Burkovski A, Zulla M, Rosiwal S, Karl M. Preclinical Testing of Boron-Doped Diamond Electrodes for Root Canal Disinfection—A Series of Preliminary Studies. Microorganisms. 2022; 10(4):782. https://doi.org/10.3390/microorganisms10040782
Chicago/Turabian StyleKoch, Maximilian, Victor Palarie, Lisa Koch, Andreas Burkovski, Manuel Zulla, Stefan Rosiwal, and Matthias Karl. 2022. "Preclinical Testing of Boron-Doped Diamond Electrodes for Root Canal Disinfection—A Series of Preliminary Studies" Microorganisms 10, no. 4: 782. https://doi.org/10.3390/microorganisms10040782
APA StyleKoch, M., Palarie, V., Koch, L., Burkovski, A., Zulla, M., Rosiwal, S., & Karl, M. (2022). Preclinical Testing of Boron-Doped Diamond Electrodes for Root Canal Disinfection—A Series of Preliminary Studies. Microorganisms, 10(4), 782. https://doi.org/10.3390/microorganisms10040782








