Evaluation of Antimicrobial Resistance in Salmonella Strains Isolated from Food, Animal and Human Samples between 2017 and 2021 in Southern Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Salmonella Isolation and Typing
2.3. Antimicrobial Susceptibility Testing
3. Results
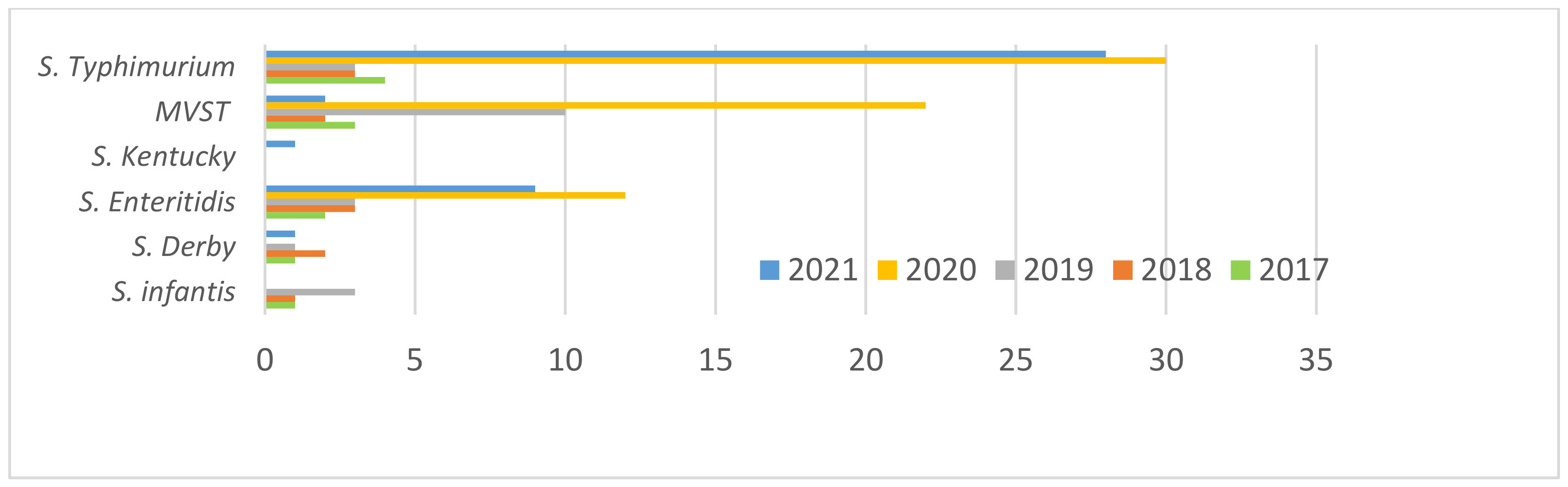
3.1. Salmonella Strains Collected and Typed
3.2. Distribution of Sample
3.3. Antimicrobial Susceptibility Testing
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA J. 2019, 17, 5598. [Google Scholar]
- Leati, M.; Zaccherini, A.; Ruocco, L.; D’Amato, S.; Busani, L.; Villa, L.; Barco, L.; Ricci, A.; Cibin, V. The challenging task to select Salmonella target serovars in poultry: The Italian point of view. Epidemiol. Infect. 2021, 149, e160. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on Official Controls and Other Official Activities Performed to Ensure the Application of Food and Feed Law, Rules on Animal Health and Welfare, Plant Health and Plant Protection Products. 2017. Available online: http://data.europa.eu/eli/reg/2017/625/oj (accessed on 27 December 2021).
- European Commission. Regulation (EC) No 2160/2003 of the European Parliament and of the Council of 17 November 2003 on the control of salmonella and other specified food-borne zoonotic agents. Off. J. Eur. Union 2003, 50, 1–15. [Google Scholar]
- Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying Down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying Down Procedures in Matters of Food Safety. 2002. Available online: http://data.europa.eu/eli/reg/2002/178/oj (accessed on 27 December 2021).
- Commission Implementing Decision (EU) 2020/1729 of 17 November 2020 on the Monitoring and Reporting of Antimicrobial Resistance in Zoonotic and Commensal Bacteria and Repealing Implementing Decision 2013/652/EU. 2020. Available online: http://data.europa.eu/eli/dec_impl/2020/1729/oj (accessed on 27 December 2021).
- CLSI M100-ED31:2021 Performance Standards for Antimicrobial Susceptibility Testing, 31st Ed. 2021. Available online: http://em100.edaptivedocs.net/GetDoc.aspx?doc=CLSI%20M100%20ED31:2021&scope=user (accessed on 1 December 2021).
- European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 6406. [Google Scholar]
- Franco, A.; Leekitcharoenphon, P.; Feltrin, F.; Alba, P.; Cordaro, G.; Iurescia, M.; Tolli, R.; D’Incau, M.; Staffolani, M.; Di Giannatale, E.; et al. Emergence of a Clonal Lineage of Multidrug-Resistant ESBL-Producing Salmonella Infantis Transmitted from Broilers and Broiler Meat to Humans in Italy between 2011 and 2014. PLoS ONE 2016, 10, e0144802. [Google Scholar] [CrossRef] [Green Version]
- Alba, P.; Leekitcharoenphon, P.; Carfora, V.; Amoruso, R.; Cordaro, G.; Di Matteo, P.; Ianzano, A.; Iurescia, M.; Diaconu, E.L.; ENGAGE-EURL-AR Network Study Group; et al. Molecular epidemiology of Salmonella Infantis in Europe: Insights into the success of the bacterial host and its parasitic pESI-like megaplasmid. Microb. Genom. 2020, 6, e000365. [Google Scholar] [CrossRef]
- Martínez-Puchol, S.; Riveros, M.; Ruidias, K.; Granda, A.; Ruiz-Roldán, L.; Zapata-Cachay, C.; Ochoa, T.J.; Pons, M.J.; Ruiz, J. Dissemination of a multidrug resistant CTX-M-65 producer Salmonella enterica serovar Infantis clone between marketed chicken meat and children. Int. J. Food Microbiol. 2021, 344, 109109. [Google Scholar] [CrossRef]
- Fortini, D.; Owczarek, S.; Dionisi, A.M.; Lucarelli, C.; Arena, S.; Carattoli, A.; Enter-Net Italia Colistin Resistance Study Group; Villa, L.; García-Fernández, A. Colistin Resistance Mechanisms in Human Salmonella enterica Strains Isolated by the National Surveillance Enter-Net Italia (2016–2018). Antibiotics 2022, 11, 102. [Google Scholar] [CrossRef]
- Alessiani, A.; Sacchini, L.; Pontieri, E.; Gavini, J.; Di Giannatale, E. Molecular typing of Salmonella enterica subspecies enterica serovar Typhimurium isolated in Abruzzo region (Italy) from 2008 to 2010. Vet. Ital. 2014, 50, 31–39. [Google Scholar]
- De Vito, D.; Monno, R.; Nuccio, F.; Legretto, M.; Oliva, M.; Coscia, M.F.; Dionisi, A.M.; Calia, C.; Capolongo, C.; Pazzani, C. Diffusion and Persistence of Multidrug Resistant Salmonella Typhimurium Strains Phage Type DT120 in Southern Italy. BioMed Res. 2015, 2015, 265042. [Google Scholar]
- Cito, F.; Baldinelli, F.; Calistri, P.; Di Giannatale, E.; Scavia, G.; Orsini, M.; Iannetti, S.; Sacchini, L.; Mangone, I.; Candeloro, L.; et al. Outbreak of unusual Salmonella enterica serovar Typhimurium monophasic variant 1,4 (5),12:i:-, Italy, June 2013 to September 2014. Eurosurveillance 2016, 21, 30194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Tela, I.; Peruzy, M.F.; D’Alessio, N.; Di Nocera, F.; Casalinuovo, F.; Carullo, M.R.; Cardinale, D.; Cristiano, D.; Capuano, F. Serotyping and Evaluation of Antimicrobial Resistance of Salmonella Strains Detected in Wildlife and Natural Environments in Southern Italy. Antibiotics 2021, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Gargano, V.; Sciortino, S.; Gambino, D.; Costa, A.; Agozzino, V.; Reale, S.; Alduina, R.; Vicari, D. Antibiotic Susceptibility Profile and Tetracycline Resistance Genes Detection in Salmonella spp. Strains Isolated from Animals and Food. Antibiotics 2021, 10, 809. [Google Scholar] [CrossRef] [PubMed]
- Ferrari Rafaela, G.; Panzenhagen Pedro, H.N.; Conte-Junior Carlos, A. Phenotypic and Genotypic Eligible Methods for Salmonella Typhimurium Source Tracking. Front. Microbiol. 2017, 8, 2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palma, F.; Manfreda, G.; Silva, M.; Parisi, A.; Barker, D.; Taboada, E.; Pasquali, F.; Rossi, M. Genome-wide identification of geographical segregated genetic markers in Salmonella enterica serovar Typhimurium variant 4,[1],12:i:-. Sci. Rep. 2018, 8, 15251. [Google Scholar] [CrossRef]
- El-Tawab, A.A.A.; Rizk, A.M.; Homouda, S.N.; El-Mougy, E.E.; Gouda, A.M. Molecular Characterization and Antimicrobial Resistance Gene of E. coli and Salmonella Kentucky Isolated from Turkeys in Egypt. Adv. Anim. Vet. Sci. 2020, 8, 742–747. [Google Scholar] [CrossRef]
- El Hage, R.; Losasso, C.; Longo, A.; Petrin, S.; Ricci, A.; Mathieu, F.; Khattar, Z.A.; El Rayess, Y. Whole-genome characterisation of TEM-1 and CMY-2 beta-lactamase-producing Salmonella Kentucky ST198 in Lebanese broiler chain. J. Glob. Antimicrob. Resist. 2020, 23, 408–416. [Google Scholar] [CrossRef]
- Coipan, C.E.; Westrell, T.; van Hoek, A.H.; Alm, E.; Kotila, S.; Berbers, B.; de Keersmaecker, S.C.J.; Ceyssens, P.-J.; Borg, M.L.; Chattaway, M.; et al. Genomic epidemiology of emerging ESBL-producing Salmonella Kentucky blaCTX-M-14b in Europe. Emerg. Microbes Infect. 2020, 9, 2124–2135. [Google Scholar] [CrossRef]
- European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2018/2019. EFSA J. 2021, 19, 6490. [Google Scholar]
- Rakitin, A.L.; Yushina, Y.K.; Zaiko, E.V.; Bataeva, D.S.; Kuznetsova, O.A.; Semenova, A.A.; Ermolaeva, S.A.; Beletskiy, A.V.; Kolganova, T.V.; Mardanov, A.V.; et al. Evaluation of Antibiotic Resistance of Salmonella Serotypes and Whole-Genome Sequencing of Multiresistant Strains Isolated from Food Products in Russia. Antibiotics 2021, 11, 1. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Q.; Cao, C.; Cui, S.; Wu, Y.; Yang, H.; Xiao, Y.; Yang, B. Prevalence and characteristics of Salmonella isolates recovered from retail raw chickens in Shaanxi Province, China. Poult. Sci. 2020, 99, 6031–6044. [Google Scholar] [CrossRef] [PubMed]

| Antimicrobial Agents | Human-Resistant Strains/TOT | Food–Animal-Resistant Strains/TOT |
|---|---|---|
| Sulfamethoxazole (SUL) | 100.0% | 100.0% |
| Nalidixic Acid (NAL) | 8.2% | 63.6% |
| Ciprofloxacin (CIP) | 8.9% | 60.7% |
| Tetracycline (TET) | 50.7% | 60.7% |
| Trimethoprim (TRI) | 6.2% | 42.7% |
| Ampicillin (AMP) | 57.5% | 41.3% |
| Cefotaxime (FOT) | 4.1% | 17.5% |
| Ceftazidime (TAZ) | 3.4% | 14.1% |
| Tigecycline (TGC) | 2.7% | 7.8% |
| Azithromycin (AZM) | 36.3% | 7.3% |
| Chloramphenicol (CLO) | 9.6% | 6.3% |
| Amikacin (AMI) | 3.4% | 4.4% |
| Colistin (COL) | 2.7% | 2.9% |
| Gentamicin (GEN) | 0.0% | 1.5% |
| Meropenem (MERO) | 0.0% | 0.0% |
| Antimicrobial Agents | S. Infantis | S. Derby | S. Enteritidis | S. Kentucky | VMST | S. Typhimurium |
|---|---|---|---|---|---|---|
| Ampicillin | 44.0% | 46.2% | 40.0% | 14.3% | 63.0% | 42.3% |
| Meropenem | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Ciprofloxacin | 69.0% | 76.9% | 80.0% | 77.1% | 18.5% | 38.5% |
| Azithromycin | 9.0% | 0.0% | 0.0% | 5.7% | 3.7% | 11.5% |
| Amikacin | 5.0% | 15.4% | 0.0% | 2.9% | 3.7% | 0.0% |
| Gentamicin | 0.0% | 7.7% | 0.0% | 0.0% | 7.4% | 0.0% |
| Tigecycline | 11.0% | 7.7% | 0.0% | 2.9% | 11.1% | 0.0% |
| Ceftazidime | 19.0% | 30.8% | 20.0% | 5.7% | 3.7% | 7.7% |
| Cefotaxime | 23.0% | 30.8% | 40.0% | 11.4% | 0.0% | 11.5% |
| Chloramphenicol | 5.0% | 0.0% | 0.0% | 2.9% | 11.1% | 15.4% |
| Colistin | 2.0% | 7.7% | 0.0% | 2.9% | 0.0% | 7.7% |
| Nalidixic Acid | 70.0% | 69.23% | 80.0% | 85.7% | 18.5% | 50.0% |
| Tetracycline | 73.0% | 53.9% | 60.0% | 34.3% | 70.4% | 42.3% |
| Trimethoprim | 55.0% | 61.5% | 60.0% | 25.7% | 29.6% | 19.2% |
| Sulfamethoxazole | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% |
| Antimicrobial Agents | S. Infantis | S. Derby | S. Enteritidis | S. Kentucky | MVST | S. Typhimurium |
|---|---|---|---|---|---|---|
| Ampicillin | 85.7% | 33.3% | 3.7% | 0.0% | 63.6% | 75.0% |
| Meropenem | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Ciprofloxacin | 85.7% | 16.7% | 0.0% | 0.0% | 6.1% | 5.6% |
| Azithromycin | 85.7% | 66.7% | 14.8% | 0.0% | 42.4% | 34.7% |
| Amikacin | 14.3% | 16.7% | 3.7% | 0.0% | 3.0% | 1.4% |
| Gentamicin | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Tigecycline | 28.6% | 0.0% | 3.7% | 0.0% | 0.0% | 1.4% |
| Ceftazidime | 57.1% | 16.7% | 0.0% | 0.0% | 0.0% | 0.0% |
| Cefotaxime | 57.1% | 16.7% | 0.0% | 0.0% | 0.0% | 1.4% |
| Chloramphenicol | 14.3% | 16.7% | 7.4% | 0.0% | 6.1% | 11.1% |
| Colistin | 14.3% | 0.0% | 7.4% | 0.0% | 0.0% | 1.4% |
| Nalidixic Acid | 71.4% | 0.0% | 7.4% | 0.0% | 9.1% | 2.8% |
| Tetracycline | 71.4% | 33.3% | 3.7% | 0.0% | 57.6% | 65.3% |
| Trimethoprim | 85.7% | 0.0% | 0.0% | 0.0% | 3.0% | 2.8% |
| Sulfamethoxazole | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alessiani, A.; Goffredo, E.; Mancini, M.; Occhiochiuso, G.; Faleo, S.; Didonna, A.; Fischetto, R.; Suglia, F.; De Vito, D.; Stallone, A.; et al. Evaluation of Antimicrobial Resistance in Salmonella Strains Isolated from Food, Animal and Human Samples between 2017 and 2021 in Southern Italy. Microorganisms 2022, 10, 812. https://doi.org/10.3390/microorganisms10040812
Alessiani A, Goffredo E, Mancini M, Occhiochiuso G, Faleo S, Didonna A, Fischetto R, Suglia F, De Vito D, Stallone A, et al. Evaluation of Antimicrobial Resistance in Salmonella Strains Isolated from Food, Animal and Human Samples between 2017 and 2021 in Southern Italy. Microorganisms. 2022; 10(4):812. https://doi.org/10.3390/microorganisms10040812
Chicago/Turabian StyleAlessiani, Alessandra, Elisa Goffredo, Maria Mancini, Gilda Occhiochiuso, Simona Faleo, Antonella Didonna, Rita Fischetto, Federica Suglia, Danila De Vito, Antonella Stallone, and et al. 2022. "Evaluation of Antimicrobial Resistance in Salmonella Strains Isolated from Food, Animal and Human Samples between 2017 and 2021 in Southern Italy" Microorganisms 10, no. 4: 812. https://doi.org/10.3390/microorganisms10040812
APA StyleAlessiani, A., Goffredo, E., Mancini, M., Occhiochiuso, G., Faleo, S., Didonna, A., Fischetto, R., Suglia, F., De Vito, D., Stallone, A., D’Attoli, L., & Donatiello, A. (2022). Evaluation of Antimicrobial Resistance in Salmonella Strains Isolated from Food, Animal and Human Samples between 2017 and 2021 in Southern Italy. Microorganisms, 10(4), 812. https://doi.org/10.3390/microorganisms10040812







