Update on the Epidemiological Features and Clinical Implications of Human Papillomavirus Infection (HPV) and Human Immunodeficiency Virus (HIV) Coinfection
Abstract
1. Introduction
2. Methods and Search Strategy
3. Human Papillomavirus
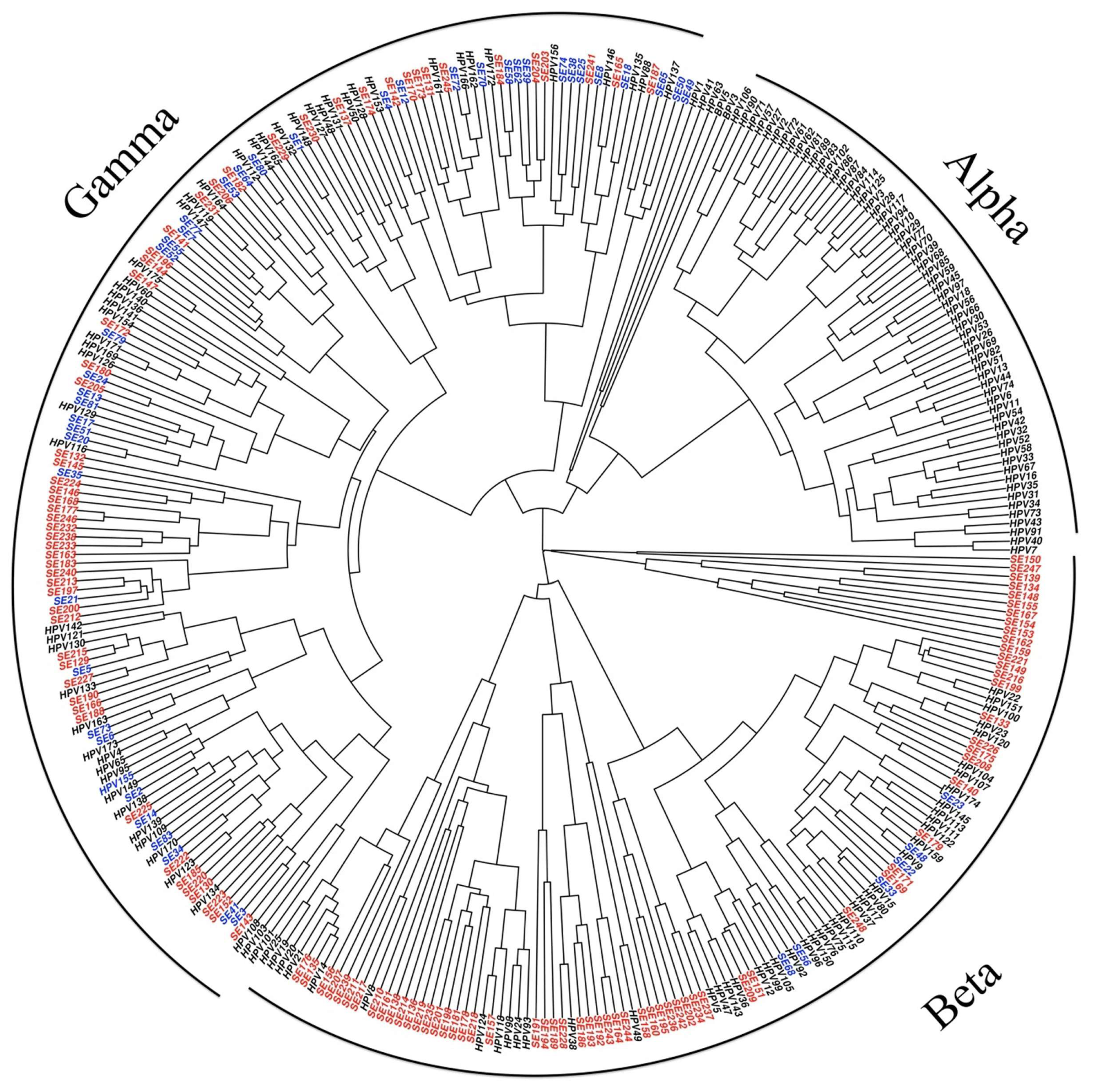
Tropism and Genotypes
4. Natural History of HPV Infection
5. Relationship between HIV and HPV
5.1. Clinical Implications of HIV–HPV Coinfection
5.2. HPV-Related Cancers in People Living with HIV
6. Relationship between Antiretroviral Therapy and HPV
6.1. Relationship between ART and HPV Prevalence
6.2. Cervical HPV Infection
6.3. Anal HPV Infection
6.4. HPV Infection of the Male Genitals
6.5. Oral HPV Infection
6.6. Relationship between Antiretroviral Therapy and HPV-Related Dysplasia
6.7. Relationship between the CD4 Lymphocyte Count and HPV Infection
6.8. Relationship between the CD4 Lymphocyte Count and HPV-Related Dysplasia
7. Dysplasia Screening Programs in People Living with HIV
8. Microbiomes and HPV and HIV
8.1. The Cervical Microbiome, HPV, and HIV
8.2. The Anal Microbiome and HIV
8.3. The Penile Microbiome, Circumcision, and HPV–HIV Coinfection
8.4. The Oral microbiome, HIV, and HPV
9. Sexually Transmitted Infections and HPV and HIV
10. HPV Vaccination in People Living with HIV
10.1. The Impact of HPV Vaccination on Dysplasia
10.2. Recommendations for HPV Vaccination
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gravitt, P.E.; Winer, R.L. Natural History of HPV Infection across the Lifespan: Role of Viral Latency. Viruses 2017, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Béziat, V.; Casanova, J.-L.; Jouanguy, E. Human Genetic and Immunological Dissection of Papillomavirus-Driven Diseases: New Insights into Their Pathogenesis. Curr. Opin. Virol. 2021, 51, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Moscicki, A.-B.; Flowers, L.; Huchko, M.J.; Long, M.E.; MacLaughlin, K.L.; Murphy, J.; Spiryda, L.B.; Gold, M.A. Guidelines for Cervical Cancer Screening in Immunosuppressed Women Without HIV Infection. J. Low. Genit. Tract Dis. 2019, 23, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Wielgos, A.; Pietrzak, B.; Suchonska, B.; Sikora, M.; Rudnicka, L.; Wielgos, M. A Six-Year Gynecological Follow-Up of Immunosuppressed Women with a High-Risk Human Papillomavirus Infection. Int. J. Environ. Res. Public Health 2022, 19, 3531. [Google Scholar] [CrossRef]
- Bzhalava, D.; Mühr, L.S.A.; Lagheden, C.; Ekström, J.; Forslund, O.; Dillner, J.; Hultin, E. Deep Sequencing Extends the Diversity of Human Papillomaviruses in Human Skin. Sci. Rep. 2014, 4, 5807. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Biological Agents. A Review of Human Carcinogens. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 1–441. [Google Scholar]
- Shiels, M.S.; Pfeiffer, R.M.; Gail, M.H.; Hall, H.I.; Li, J.; Chaturvedi, A.K.; Bhatia, K.; Uldrick, T.S.; Yarchoan, R.; Goedert, J.J.; et al. Cancer Burden in the HIV-Infected Population in the United States. J. Natl. Cancer Inst. 2011, 103, 753–762. [Google Scholar] [CrossRef]
- Massad, L.S.; Xie, X.; Greenblatt, R.M.; Minkoff, H.; Sanchez-Keeland, L.; Watts, D.H.; Wright, R.L.; D’Souza, G.; Merenstein, D.; Strickler, H. Effect of Human Immunodeficiency Virus Infection on the Prevalence and Incidence of Vaginal Intraepithelial Neoplasia. Obstet. Gynecol. 2012, 119, 582–589. [Google Scholar] [CrossRef]
- Vuyst, H.D.; Clifford, G.M.; Nascimento, M.C.; Madeleine, M.M.; Franceschi, S. Prevalence and Type Distribution of Human Papillomavirus in Carcinoma and Intraepithelial Neoplasia of the Vulva, Vagina and Anus: A Meta-Analysis. Int. J. Cancer 2009, 124, 1626–1636. [Google Scholar] [CrossRef]
- Pytynia, K.B.; Dahlstrom, K.R.; Sturgis, E.M. Epidemiology of HPV-Associated Oropharyngeal Cancer. Oral Oncol. 2014, 50, 380–386. [Google Scholar] [CrossRef]
- Palmer, T.; Wallace, L.; Pollock, K.G.; Cuschieri, K.; Robertson, C.; Kavanagh, K.; Cruickshank, M. Prevalence of Cervical Disease at Age 20 after Immunisation with Bivalent HPV Vaccine at Age 12–13 in Scotland: Retrospective Population Study. BMJ 2019, 365, l1161. [Google Scholar] [CrossRef]
- Shiels, M.S.; Islam, J.Y.; Rosenberg, P.S.; Hall, H.I.; Jacobson, E.; Engels, E.A. Projected Cancer Incidence Rates and Burden of Incident Cancer Cases in HIV-Infected Adults in the United States Through 2030. Ann. Intern. Med. 2018, 168, 866–873. [Google Scholar] [CrossRef]
- Duan, R.; Xu, K.; Huang, L.; Yuan, M.; Wang, H.; Qiao, Y.; Zhao, F. Temporal Trends and Projection of Cancer Attributable to Human Papillomavirus Infection in China, 2007–2030. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1130–1136. [Google Scholar] [CrossRef]
- Guisado, Y.M.; Sotomayor, C.; Fontillón, M.; Castaño, A.D.; Espinosa, N.; Roca, C.; López-Cortés, L.F.; Viciana, P.; Neukam, K. Incidence Rate and Risk Factors for Anal Squamous Cell Carcinoma in a Cohort of People Living with HIV from 2004 to 2017: Implementation of a Screening Program. Dis. Colon Rectum 2022, 65, 28–39. [Google Scholar] [CrossRef]
- Chipollini, J.; Pollock, G.; Hsu, C.H.; Batai, K.; Recio-Boiles, A.; Lee, B.R. National Trends and Survival Outcomes of Penile Squamous Cell Carcinoma Based on Human Papillomavirus Status. Cancer Med. 2021, 10, 7466–7474. [Google Scholar] [CrossRef]
- Owosho, A.A.; Velez, M.; Tyburski, A.; Hofheins, J.; Wiley, R.; Stansbury, T.; Gbadamosi, S.O.; Ryder, J.S. Epidemiological Trends of Oropharyngeal and Oral Cavity Squamous Cell Carcinomas in Northern New England, 2000–2013. Cancer Causes Control 2019, 30, 291–299. [Google Scholar] [CrossRef]
- Giacomet, V.; Penagini, F.; Trabattoni, D.; Viganò, A.; Rainone, V.; Bernazzani, G.; Bonardi, C.M.; Clerici, M.; Bedogni, G.; Zuccotti, G.V. Safety and Immunogenicity of a Quadrivalent Human Papillomavirus Vaccine in HIV-Infected and HIV-Negative Adolescents and Young Adults. Vaccine 2014, 32, 5657–5661. [Google Scholar] [CrossRef]
- McKenzie, N.D.; Kobetz, E.N.; Hnatyszyn, J.; Twiggs, L.B.; Lucci, J.A. Women with HIV Are More Commonly Infected with Non-16 and -18 High-Risk HPV Types. Gynecol. Oncol. 2010, 116, 572–577. [Google Scholar] [CrossRef]
- Burk, R.D.; Harari, A.; Chen, Z. Human Papillomavirus Genome Variants. Virology 2013, 445, 232–243. [Google Scholar] [CrossRef]
- Sias, C.; Salichos, L.; Lapa, D.; Nonno, F.D.; Baiocchini, A.; Capobianchi, M.R.; Garbuglia, A.R. Alpha, Beta, Gamma Human PapillomaViruses (HPV) Detection with a Different Sets of Primers in Oropharyngeal Swabs, Anal and Cervical Samples. Virol. J. 2019, 16, 27. [Google Scholar] [CrossRef]
- Béziat, V. Human Genetic Dissection of Papillomavirus-Driven Diseases: New Insight into Their Pathogenesis. Hum. Genet. 2020, 139, 919–939. [Google Scholar] [CrossRef]
- Basukala, O.; Banks, L. The Not-So-Good, the Bad and the Ugly: HPV E5, E6 and E7 Oncoproteins in the Orchestration of Carcinogenesis. Viruses 2021, 13, 1892. [Google Scholar] [CrossRef]
- Chen, X.S.; Garcea, R.L.; Goldberg, I.; Casini, G.; Harrison, S.C. Structure of Small Virus-like Particles Assembled from the L1 Protein of Human Papillomavirus 16. Mol. Cell 2000, 5, 557–567. [Google Scholar] [CrossRef]
- Yang, R.; Yutzy, W.H., IV; Viscidi, R.P.; Roden, R.B.S. Interaction of L2 with β-Actin Directs Intracellular Transport of Papillomavirus and Infection. J. Biol. Chem. 2003, 278, 12546–12553. [Google Scholar] [CrossRef]
- Liblekas, L.; Piirsoo, A.; Laanemets, A.; Tombak, E.-M.; Laaneväli, A.; Ustav, E.; Ustav, M.; Piirsoo, M. Analysis of the Replication Mechanisms of the Human Papillomavirus Genomes. Front. Microbiol. 2021, 12, 738125. [Google Scholar] [CrossRef]
- Cogliano, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; Ghissassi, F. El Carcinogenicity of Human Papillomaviruses. Lancet Oncol. 2005, 6, 204. [Google Scholar] [CrossRef]
- Kricker, A.; Weber, M.F.; Pawlita, M.; Sitas, F.; Hodgkinson, V.S.; Rahman, B.; Kemenade, C.H.V.; Armstrong, B.K.; Waterboer, T. Cutaneous β HPVs, Sun Exposure, and Risk of Squamous and Basal Cell Skin Cancers in Australia. Cancer Epidemiol. Biomark. Prev. 2022, 31, 614–624. [Google Scholar] [CrossRef]
- Bolatti, E.M.; Hošnjak, L.; Chouhy, D.; Casal, P.E.; Re-Louhau, M.F.; Bottai, H.; Komloš, K.F.; Poljak, M.; Giri, A.A. Assessing Gammapapillomavirus Infections of Mucosal Epithelia with Two Broad-Spectrum PCR Protocols. BMC Infect. Dis. 2020, 20, 274. [Google Scholar] [CrossRef]
- Galati, L.; Brancaccio, R.N.; Gupta, P.; Lohmann, E.; Robitaille, A.; Mandishora, R.S.D.; Cuenin, C.; Filotico, R.; Combes, J.-D.; Giuliano, A.R.; et al. Diversity of Human Papillomavirus in the Anal Canal of HIV-Positive and HIV-Negative Men. J. Infect. 2021, 82, 112–116. [Google Scholar] [CrossRef]
- World Health Organization. Human Papillomaviruses; World Health Organization: Lyon, France, 2006; ISBN 9789283212904. [Google Scholar]
- Barillari, G.; Bei, R.; Manzari, V.; Modesti, A. Infection by High-Risk Human Papillomaviruses, Epithelial-to-Mesenchymal Transition and Squamous Pre-Malignant or Malignant Lesions of the Uterine Cervix: A Series of Chained Events? Int. J. Mol. Sci. 2021, 22, 13543. [Google Scholar] [CrossRef]
- Rashid, N.N.; Yong, Z.L.; Yusof, R.; Watson, R.J. HPV 16E7 and 48E7 Proteins Use Different Mechanisms to Target P130 to Overcome Cell Cycle Block Other Viruses (e.g., Pox, Papilloma, Parvo, Reoviridae). Virol. J. 2016, 13, 2. [Google Scholar] [CrossRef]
- Litviakov, N.V.; Ibragimova, M.K.; Tsyganov, M.M.; Shpileva, O.V.; Churuksaeva, O.N.; Kolomiets, L.A. Changes in the Genetic Landscape during the Malignization of High Grade Squamous Intraepithelial Lesion into Cervical Cancer. Curr. Probl. Cancer 2020, 44, 100567. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Piskorz, A.M.; Bosse, T.; Jimenez-Linan, M.; Rous, B.; Brenton, J.D.; Gilks, C.B.; Köbel, M. P53 Immunohistochemistry Is an Accurate Surrogate for TP53 Mutational Analysis in Endometrial Carcinoma Biopsies. J. Pathol. 2020, 250, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Blakely, M.; Sigel, K.; Thin, T.H.; Hui, P.; Donovan, M.; Gaisa, M.M. Biomarker P16 Predicts Progression Risk of Anal Low-Grade Squamous Intraepithelial Lesions. AIDS 2018, 32, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Song, L.; Zhao, W.; Li, X.; Gao, W.; Qi, Z.; Wang, J. Predictive Value of P16INK4a, Ki-67 and ProExC Immuno-qualitative Features in LSIL Progression into HSIL. Exp. Ther. Med. 2020, 19, 2457–2466. [Google Scholar] [CrossRef]
- Siegel, E.M.; Ajidahun, A.; Berglund, A.; Guerrero, W.; Eschrich, S.; Putney, R.M.; Magliocco, A.; Riggs, B.; Winter, K.; Simko, J.P.; et al. Genome-Wide Host Methylation Profiling of Anal and Cervical Carcinoma. PLoS ONE 2021, 16, e0260857. [Google Scholar] [CrossRef]
- Darragh, T.M.; Colgan, T.J.; Cox, J.T.; Heller, D.S.; Henry, M.R.; Luff, R.D.; McCalmont, T.; Nayar, R.; Palefsky, J.M.; Stoler, M.H.; et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions. Int. J. Gynecol. Pathol. 2013, 32, 76–115. [Google Scholar] [CrossRef]
- Bogani, G.; Martinelli, F.; Ditto, A.; Taverna, F.; Lombardo, C.; Signorelli, M.; Chiappa, V.; Maggiore, U.L.R.; Fontanella, C.; Sabatucci, I.; et al. Human Papillomavirus (HPV) Persistence and HPV 31 Predict the Risk of Recurrence in High-Grade Vaginal Intraepithelial Neoplasia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 210, 157–165. [Google Scholar] [CrossRef]
- Kreuter, A.; Brockmeyer, N.H.; Weissenborn, S.J.; Gambichler, T.; Stücker, M.; Altmeyer, P.; Pfister, H.; Wieland, U. Penile Intraepithelial Neoplasia Is Frequent in HIV-Positive Men with Anal Dysplasia. J. Investig. Dermatol. 2008, 128, 2316–2324. [Google Scholar] [CrossRef]
- Hübbers, C.U.; Akgül, B. HPV and Cancer of the Oral Cavity. Virulence 2015, 6, 244–248. [Google Scholar] [CrossRef]
- Odell, E.; Kujan, O.; Warnakulasuriya, S.; Sloan, P. Oral Epithelial Dysplasia: Recognition, Grading and Clinical Significance. Oral Dis. 2021, 27, 1947–1976. [Google Scholar] [CrossRef]
- Nyagol, J.; Leucci, E.; Onnis, A.; Falco, G.D.; Tigli, C.; Sanseverino, F.; Torriccelli, M.; Palummo, N.; Pacenti, L.; Santopietro, R.; et al. The Effects of HIV-1 Tat Protein on Cell Cycle during Cervical Carcinogenesis. Cancer Biol. Ther. 2006, 5, 684–690. [Google Scholar] [CrossRef]
- Barillari, G.; Palladino, C.; Bacigalupo, I.; Leone, P.; Falchi, M.; Ensoli, B. Entrance of the Tat Protein of HIV-1 into Human Uterine Cervical Carcinoma Cells Causes Upregulation of HPV-E6 Expression and a Decrease in P53 Protein Levels. Oncol. Lett. 2016, 12, 2389–2394. [Google Scholar] [CrossRef]
- Kim, R.H.; Yochim, J.M.; Kang, M.K.; Shin, K.-H.; Christensen, R.; Park, N.-H. HIV-1 Tat Enhances Replicative Potential of Human Oral Keratinocytes Harboring HPV-16 Genome. Int. J. Oncol. 2008, 33, 777–782. [Google Scholar]
- Hladíková, K.; Koucký, V.; Bouček, J.; Laco, J.; Grega, M.; Hodek, M.; Zábrodský, M.; Vošmik, M.; Rozkošová, K.; Vošmiková, H.; et al. Tumor-Infiltrating B Cells Affect the Progression of Oropharyngeal Squamous Cell Carcinoma via Cell-to-Cell Interactions with CD8+ T Cells. J. ImmunoTherapy Cancer 2019, 7, 1–16. [Google Scholar] [CrossRef]
- Wu, Y.; Meng, L.; Cai, K.; Zhao, J.; He, S.; Shen, J.; Wei, Q.; Wang, Z.; Sooranna, S.; Li, H.; et al. A Tumor-Infiltration CD8+ T Cell-Based Gene Signature for Facilitating the Prognosis and Estimation of Immunization Responses in HPV+ Head and Neck Squamous Cell Cancer. Front. Oncol. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Hu, S.; Zhao, X.; Zhang, Y.; Qiao, Y.; Zhao, F. Interpretation of “WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention, Second Edition”. Zhonghua Yi Xue Za Zhi 2021, 101, 1–5. [Google Scholar]
- Castle, P.E.; Einstein, M.H.; Sahasrabuddhe, V.V. Cervical Cancer Prevention and Control in Women Living with Human Immunodeficiency Virus. CA Cancer J. Clin. 2021, 71, 505–526. [Google Scholar] [CrossRef]
- Wei, F.; Gaisa, M.M.; D’Souza, G.; Xia, N.; Giuliano, A.R.; Hawes, S.E.; Gao, L.; Cheng, S.-H.; Donà, M.G.; Goldstone, S.E.; et al. Epidemiology of Anal Human Papillomavirus Infection and High-Grade Squamous Intraepithelial Lesions in 29,900 Men According to HIV Status, Sexuality, and Age: A Collaborative Pooled Analysis of 64 Studies. Lancet HIV 2021, 8, 531–543. [Google Scholar] [CrossRef]
- Hernández-Ramírez, R.U.; Shiels, M.S.; Dubrow, R.; Engels, E.A. Cancer Risk in HIV-Infected People in the USA from 1996 to 2012: A Population-Based, Registry-Linkage Study. Lancet HIV 2017, 4, e495–e504. [Google Scholar] [CrossRef]
- Mazul, A.L.; Hartman, C.; Kramer, J.; White, D.L.; Royse, K.; Raychaudhury, S.; Sandulache, V.; Ahmed, S.T.; Richardson, P.; Sikora, A.G.; et al. Incidence and Survival for Oropharynx and Non-oropharynx Head and Neck Cancers among Veterans Living with HIV. Cancer Med. 2020, 9, 9326–9335. [Google Scholar] [CrossRef]
- Ortiz, A.P.; Engels, E.A.; Nogueras-González, G.M.; Colón-López, V.; Soto-Salgado, M.; Vargas, A.; Machin, M.; Shiels, M.S. Disparities in Human Papillomavirus–Related Cancer Incidence and Survival among Human Immunodeficiency Virus–Infected Hispanics Living in the United States. Cancer 2018, 124, 4520–4528. [Google Scholar] [CrossRef]
- Pokomandy, A.D.; Rouleau, D.; Ghattas, G.; Vézina, S.; Coté, P.; Macleod, J.; Allaire, G.; Franco, E.L.; Coutlée, F.; HIPVIRG Study Group. Prevalence, Clearance, and Incidence of Anal Human Papillomavirus Infection in HIV-Infected Men: The HIPVIRG Cohort Study. J. Infect. Dis. 2009, 199, 965–973. [Google Scholar] [CrossRef]
- Maugin, F.; Lesage, A.-C.; Hoyeau, N.; Fléjou, J.-F.; Zuber, K.; Atienza, P.; Etienney, I. Early Detection of Anal High-Grade Squamous Intraepithelial Lesion: Do We Have an Impact on Progression to Invasive Anal Carcinoma? J. Low. Genit. Tract Dis. 2020, 24, 82–86. [Google Scholar] [CrossRef]
- Brenner, D.R.; Weir, H.K.; Demers, A.A.; Ellison, L.F.; Louzado, C.; Shaw, A.; Turner, D.; Woods, R.R.; Smith, L.M.; Canadian Cancer Statistics Advisory Committee. Projected Estimates of Cancer in Canada in 2020. CMAJ 2020, 192, E199–E205. [Google Scholar] [CrossRef]
- Stelzle, D.; Tanaka, L.F.; Lee, K.K.; Khalil, A.I.; Baussano, I.; Shah, A.S.V.; McAllister, D.A.; Gottlieb, S.L.; Klug, S.J.; Winkler, A.S.; et al. Estimates of the Global Burden of Cervical Cancer Associated with HIV. Lancet Glob. Health 2021, 9, e161–e169. [Google Scholar] [CrossRef]
- Simo, R.T.; Nono, A.G.D.; Dongmo, H.P.F.; Etet, P.F.S.; Fonyuy, B.K.; Kamdje, A.H.N.; Yanou, N.N.; Telefo, P.B. Prevalence of Precancerous Cervical Lesions and High-Risk Human Papillomavirus Types in Yaounde, Cameroon. J. Infect. Dev. Ctries. 2021, 15, 1339–1345. [Google Scholar] [CrossRef]
- Deressa, B.T.; Assefa, M.; Tafesse, E.; Kantelhardt, E.J.; Soldatovic, I.; Cihoric, N.; Rauch, D.; Jemal, A. Contemporary Treatment Patterns and Survival of Cervical Cancer Patients in Ethiopia. BMC Cancer 2021, 21, 1102. [Google Scholar] [CrossRef]
- Stier, E.A.; Engels, E.; Horner, M.J.; Robinson, W.T.; Qiao, B.; Hayes, J.; Bayakly, R.; Anderson, B.J.; Gonsalves, L.; Pawlish, K.S.; et al. Cervical Cancer Incidence Stratified by Age in Women with HIV Compared with the General Population in the United States, 2002–2016. AIDS 2021, 35, 1851–1856. [Google Scholar] [CrossRef]
- Piechocki, M.; Koziołek, W.; Sroka, D.; Matrejek, A.; Miziołek, P.; Saiuk, N.; Sledzik, M.; Jaworska, A.; Bereza, K.; Pluta, E.; et al. Trends in Incidence and Mortality of Gynecological and Breast Cancers in Poland (1980–2018). Clin. Epidemiol. 2022, 14, 95–114. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- MacDuffie, E.; Sakamuri, S.; Luckett, R.; Wang, Q.; Bvochara-Nsingo, M.; Monare, B.; Bazzett-Matabele, L.; Moloi, T.; Ralefala, T.; Ramogola-Masire, D.; et al. Vulvar Cancer in Botswana in Women with and without HIV Infection: Patterns of Treatment and Survival Outcomes. Int. J. Gynecol. Cancer 2021, 31, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Lekoane, K.M.B.; Kuupiel, D.; Mashamba-Thompson, T.P.; Ginindza, T.G. The Interplay of HIV and Human Papillomavirus-Related Cancers in Sub-Saharan Africa: Scoping Review. Syst. Rev. 2020, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Olesen, T.B.; Sand, F.L.; Aalborg, G.L.; Munk, C.; Kjaer, S.K. Incidence of Penile Intraepithelial Neoplasia and Incidence and Survival of Penile Cancer in Denmark, 1997 to 2018. Cancer Causes Control 2022, 33, 117–123. [Google Scholar] [CrossRef]
- Hoekstra, R.J.; Trip, E.J.; Kate, F.J.W.T.; Horenblas, S.; Lock, M.T. Penile Intraepithelial Neoplasia: Nomenclature, Incidence and Progression to Malignancy in the Netherlands. Int. J. Urol. 2019, 26, 353–357. [Google Scholar] [CrossRef]
- Hessol, N.A.; Whittemore, H.; Vittinghoff, E.; Hsu, L.C.; Ma, D.; Scheer, S.; Schwarcz, S.K. Incidence of First and Second Primary Cancers Diagnosed among People with HIV, 1985–2013: A Population-Based, Registry Linkage Study. Lancet HIV 2018, 5, e647–e655. [Google Scholar] [CrossRef]
- Hiles, G.L.; Chang, K.-P.; Bellile, E.L.; Wang, C.-I.; Yen, W.-C.; Goudsmit, C.M.; Briggs, H.L.; Thomas, T.B.; Peters, L.; Afsari, M.A.; et al. Understanding the Impact of High-Risk Human Papillomavirus on Oropharyngeal Squamous Cell Carcinomas in Taiwan: A Retrospective Cohort Study. PLoS ONE 2021, 16, e0250530. [Google Scholar] [CrossRef]
- Chew, E.Y.; Hartman, C.M.; Richardson, P.A.; Zevallos, J.P.; Sikora, A.G.; Kramer, J.R.; Chiao, E.Y. Risk Factors for Oropharynx Cancer in a Cohort of HIV-Infected Veterans. Oral Oncol. 2017, 68, 60–66. [Google Scholar] [CrossRef]
- Kelly, H.; Weiss, H.A.; Benavente, Y.; Sanjose, S.D.; Mayaud, P.; Qiao, Y.L.; Feng, R.M.; DeVuyst, H.; Tenet, V.; Jaquet, A.; et al. Association of Antiretroviral Therapy with High-Risk Human Papillomavirus, Cervical Intraepithelial Neoplasia, and Invasive Cervical Cancer in Women Living with HIV: A Systematic Review and Meta-Analysis. Lancet HIV 2018, 5, e45–e58. [Google Scholar] [CrossRef]
- Ermel, A.; Tong, Y.; Tonui, P.; Orang’o, O.; Muthoka, K.; Wong, N.; Manai, T.; Kiptoo, S.; Loehrer, P.J.; Brown, D.R. Longer Duration of Anti-Retroviral Therapy Is Associated with Decreased Risk of Human Papillomaviruses Detection in Kenyan Women Living with HIV. Int. J. STD AIDS 2021, 32, 1212–1220. [Google Scholar] [CrossRef]
- Zeier, M.D.; Botha, M.H.; Engelbrecht, S.; Machekano, R.N.; Jacobs, G.B.; Isaacs, S.; Schalkwyk, M.V.; Merwe, H.V.D.; Mason, D.; Nachega, J.B. Combination Antiretroviral Therapy Reduces the Detection Risk of Cervical Human Papilloma Virus Infection in Women Living with HIV. Aids 2015, 29, 59–66. [Google Scholar] [CrossRef]
- Murenzi, G.; Kanyabwisha, F.; Murangwa, A.; Kubwimana, G.; Mutesa, L.; Burk, R.D.; Anastos, K.; Castle, P.E. Twelve-Year Trend in the Prevalence of High-Risk Human Papillomavirus Infection among Rwandan Women Living with HIV. J. Infect. Dis. 2020, 222, 74–81. [Google Scholar] [CrossRef]
- Kelly, H.; Chikandiwa, A.; Vilches, L.A.; Palefsky, J.M.; Sanjose, S.D.; Mayaud, P. Association of Antiretroviral Therapy with Anal High-Risk Human Papillomavirus, Anal Intraepithelial Neoplasia, and Anal Cancer in People Living with HIV: A Systematic Review and Meta-Analysis. Lancet HIV 2020, 7, e262–e278. [Google Scholar] [CrossRef]
- Hidalgo-Tenorio, C.; Gil-Anguita, C.; Ruz, M.A.L.; Omar, M.; López-Hidalgo, J.; Pasquau, J. ART Is Key to Clearing Oncogenic HPV Genotypes (HR-HPV) in Anal Mucosa of HIV-Positive MSM. PLoS ONE 2019, 14, e0224183. [Google Scholar] [CrossRef]
- Ucciferri, C.; Tamburro, M.; Falasca, K.; Sammarco, M.L.; Ripabelli, G.; Vecchiet, J. Prevalence of Anal, Oral, Penile and Urethral Human Papillomavirus in HIV Infected and HIV Uninfected Men Who Have Sex with Men. J. Med. Virol. 2018, 90, 358–366. [Google Scholar] [CrossRef]
- Aar, F.V.; Mooij, S.H.; Sande, M.A.B.V.D.; Speksnijder, A.G.C.L.; Stolte, I.G.; Meijer, C.J.L.M.; Verhagen, D.W.M.; King, A.J.; Vries, H.J.C.D.; Loeff, M.F.S.V.D. Anal and Penile High-Risk Human Papillomavirus Prevalence in HIV-Negative and HIV-Infected MSM. AIDS 2013, 27, 2921–2931. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Lee, A.; Chen, H.; Webster-Cyriaque, J.; Seaman, T.; Landovitz, R.J.; John, M.; Reilly, N.; Naini, L.; Palefsky, J.; et al. Human Papillomavirus Infection in the Oral Cavity of HIV Patients Is Not Reduced by Initiating Antiretroviral Therapy. AIDS 2016, 30, 1573–1582. [Google Scholar] [CrossRef]
- Liu, G.; Sharma, M.; Tan, N.; Barnabas, R. V HIV-Positive Women Have Higher Risk of Human Papilloma Virus Infection, Precancerous Lesions, and Cervical Cancer. AIDS 2018, 32, 795–808. [Google Scholar] [CrossRef]
- Clifford, G.M.; Franceschi, S.; Keiser, O.; Schöni-Affolter, F.; Lise, M.; Dehler, S.; Levi, F.; Mousavi, M.; Bouchardy, C.; Wolfensberger, A.; et al. Immunodeficiency and the Risk of Cervical Intraepithelial Neoplasia 2/3 and Cervical Cancer: A Nested Case-Control Study in the Swiss HIV Cohort Study. Int. J. Cancer 2016, 138, 1732–1740. [Google Scholar] [CrossRef]
- Piketty, C.; Selinger-Leneman, H.; Bouvier, A.-M.; Belot, A.; Mary-Krause, M.; Duvivier, C.; Bonmarchand, M.; Abramowitz, L.; Costagliola, D.; Grabar, S. Incidence of HIV-Related Anal Cancer Remains Increased despite Long-Term Combined Antiretroviral Treatment: Results from the French Hospital Database on HIV. J. Clin. Oncol. 2012, 30, 4360–4366. [Google Scholar] [CrossRef]
- Hidalgo-Tenorio, C.; Rivero-Rodriguez, M.; Gil-Anguita, C.; Hierro, M.L.D.; Palma, P.; Ramírez-Taboada, J.; Esquivias, J.; López-Ruz, M.A.; Javier-Martínez, R.; Pasquau-Liaño, J. Antiretroviral Therapy as a Factor Protective against Anal Dysplasia in HIV-Infected Males Who Have Sex with Males. PLoS ONE 2014, 9, e92376. [Google Scholar] [CrossRef][Green Version]
- Franceschi, S.; Lise, M.; Clifford, G.M.; Rickenbach, M.; Levi, F.; Maspoli, M.; Bouchardy, C.; Dehler, S.; Jundt, G.; Ess, S.; et al. Changing Patterns of Cancer Incidence in the Early-and Late-HAART Periods: The Swiss HIV Cohort Study. Br. J. Cancer 2010, 103, 416–422. [Google Scholar] [CrossRef]
- Duan, R.; Zhang, H.; Wu, A.; Li, C.; Li, L.; Xu, X.; Qiao, Y.; Zhao, F.; Clifford, G. Prevalence and Risk Factors for Anogenital HPV Infection and Neoplasia among Women Living with HIV in China. Sex. Transm. Infect. 2021, 98, 247–254. [Google Scholar] [CrossRef]
- Hidalgo-Tenorio, C.; Gil-Anguita, C.; Ramírez-Taboada, J.; Esquivias, J.; López-Ruz, M.A.; Balgahata, O.M.; Javier-Martinez, R.; Pasquau, J. Risk Factors for Infection by Oncogenic Human Papillomaviruses in HIV-Positive MSM Patients in the ART Era (2010–2016). Medicine 2017, 96, e8109. [Google Scholar] [CrossRef]
- Grinsztejn, B.; Veloso, V.G.; Levi, J.E.; Velasque, L.; Luz, P.M.; Friedman, R.K.; Andrade, A.C.; Moreira, R.I.; Russomano, F.; Pilotto, J.H.; et al. Factors Associated with Increased Prevalence of Human Papillomavirus Infection in a Cohort of HIV-Infected Brazilian Women. Int. J. Infect. Dis. 2009, 13, 72–80. [Google Scholar] [CrossRef]
- Camargo, M.; Río-Ospina, L.D.; León, S.C.S.-D.; Sánchez, R.; Pineda-Peña, A.C.; Sussmann, O.; Patarroyo, M.E.; Patarroyo, M.A. Association of HIV Status with Infection by Multiple HPV Types. Trop. Med. Int. Health 2018, 23, 1259–1268. [Google Scholar] [CrossRef]
- Giuliani, M.; Rollo, F.; Vescio, M.F.; Pichi, B.; Latini, A.; Benevolo, M.; Pellini, R.; Cristaudo, A.; Dona’, M.G. Oral Human Papillomavirus Infection in HIV-Infected and HIV-Uninfected MSM: The OHMAR Prospective Cohort Study. Sex. Transm. Infect. 2020, 96, 528–536. [Google Scholar] [CrossRef]
- Aar, F.V.; Mooij, S.H.; Sande, M.A.B.V.D.; Meijer, C.J.L.M.; King, A.J.; Verhagen, D.W.M.; Heijman, T.; Coutinho, R.A.; Loeff, M.F.S.S.V.D. Twelve-Month Incidence and Clearance of Oral HPV Infection in HIV-Negative and HIV-Infected Men Who Have Sex with Men: The H2M Cohort Study. BMC Infect. Dis. 2015, 14, 668. [Google Scholar] [CrossRef]
- Parisi, S.G.; Cruciani, M.; Scaggiante, R.; Boldrin, C.; Andreis, S.; Bello, F.D.; Pagni, S.; Barelli, A.; Sattin, A.; Mengoli, C.; et al. Anal and Oral Human Papillomavirus (HPV) Infection in HIV-Infected Subjects in Northern Italy: A Longitudinal Cohort Study among Men Who Have Sex with Men. BMC Infect. Dis. 2011, 11, 150. [Google Scholar] [CrossRef]
- Mooij, S.H.; Santen, D.K.V.; Geskus, R.B.; Sande, M.A.B.V.D.; Coutinho, R.A.; Stolte, I.G.; Snijders, P.J.F.; Meijer, C.J.L.M.; Speksnijder, A.G.C.L.; Vries, H.J.C.D.; et al. The Effect of HIV Infection on Anal and Penile Human Papillomavirus Incidence and Clearance: A Cohort Study among MSM. AIDS 2016, 30, 121–132. [Google Scholar] [CrossRef]
- Badial, R.M.; Dias, M.C.; Stuqui, B.; Melli, P.P.D.S.; Quintana, S.M.; Bonfim, C.M.D.; Cordeiro, J.A.; Rabachini, T.; Calmon, M.D.F.; Provazzi, P.J.S.; et al. Detection and Genotyping of Human Papillomavirus (HPV) in HIV-Infected Women and Its Relationship with HPV/HIV Co-Infection. Medicine 2018, 97, e9545. [Google Scholar] [CrossRef] [PubMed]
- Lehtovirta, P.; Paavonen, J.; Heikinheimo, O. Risk Factors, Diagnosis and Prognosis of Cervical Intraepithelial Neoplasia among HIV-Infected Women. Int. J. STD AIDS 2008, 19, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Okoye, J.O.; Erinle, C.; Ngokere, A.A.; Jimoh, A. Low CD4 Cells and Viral Co-Infection Increase the Risk of VaIN: Use of SCCA1 and Ki67 as Diagno-Prognostic Biomarkers. Pathophysiology 2018, 25, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Tenorio, C.; García-Martínez, C.M.; Pasquau, J.; Omar-Mohamed-Balgahata, M.; López-Ruz, M.; López-Hidalgo, J.; Gil-Anguita, C. Risk Factors for ≥high-Grade Anal Intraepithelial Lesions in MSM Living with HIV and the Response to Topical and Surgical Treatments. PLoS ONE 2021, 16, e0245870. [Google Scholar] [CrossRef]
- Heukelom, M.L.S.V.; Marra, E.; Vries, H.J.C.D.; Loeff, M.F.S.V.D.; Prins, J.M. Risk Factors for Anal High-Grade Squamous Intraepithelial Lesions in HIV-Positive MSM: Is Targeted Screening Possible? AIDS 2017, 31, 2295–2301. [Google Scholar] [CrossRef]
- Mathews, W.C.; Agmas, W.; Cachay, E.R.; Cosman, B.C.; Jackson, C. Natural History of Anal Dysplasia in an HIV-Infected Clinical Care Cohort: Estimates Using Multi-State Markov Modeling. PLoS ONE 2014, 9, e104116. [Google Scholar] [CrossRef]
- Geltzeiler, C.B.; Xu, Y.; Carchman, E.; Ghouse, Y.; Beczkiewicz, J.; Son, J.; Voils, C.I.; Striker, R. CD4/CD8 Ratio as a Novel Marker for Increased Risk of High-Grade Anal Dysplasia and Anal Cancer in HIV+ Patients: A Retrospective Cohort Study. Dis. Colon. Rectum 2020, 63, 1585–1592. [Google Scholar] [CrossRef]
- Sanger, C.B.; Xu, Y.; Carchman, E.; Lawson, E.H.; Heise, C.P.; Striker, R.; Voils, C.I. Prevalence of High-Grade Anal Dysplasia and Anal Cancer in Veterans Living with HIV and CD4/CD8 Ratio as a Marker For Increased Risk: A Regional Retrospective Cohort Study. Dis. Colon. Rectum 2021, 64, 805–811. [Google Scholar] [CrossRef]
- Ye, Y.; Burkholder, G.A.; Wiener, H.W.; Aslibekyan, S.; Khan, A.; Shrestha, S. CD4 Trajectory Models and Onset of Non-AIDS-Defining Anal Genital Warts, Precancer, and Cancer in People Living with HIV Infection-1. Sex. Transm. Dis. 2020, 47, 628–633. [Google Scholar] [CrossRef]
- Beachler, D.C.; Abraham, A.G.; Silverberg, M.J.; Jing, Y.; Fakhry, C.; Gill, M.J.; Dubrow, R.; Kitahata, M.M.; Klein, M.B.; Burchell, A.N.; et al. Incidence and Risk Factors of HPV-Related and HPV-Unrelated Head and Neck Squamous Cell Carcinoma in HIV-Infected Individuals. Oral Oncol. 2014, 50, 1169–1176. [Google Scholar] [CrossRef]
- Mapanga, W.; Girdler-Brown, B.; Feresu, S.A.; Chipato, T.; Singh, E. Prevention of Cervical Cancer in HIV-Seropositive Women from Developing Countries through Cervical Cancer Screening: A Systematic Review. Syst. Rev. 2018, 7, 198. [Google Scholar] [CrossRef]
- Cimic, A.; Wilkin, T.J.; Heymann, J.J.; Alperstein, S.; Ellsworth, G.; Siddiqui, M.T. Importance of Anal Cytology and Screening for Anal Dysplasia in Individuals Living with HIV with an Emphasis on Women. Cancer Cytopathol. 2019, 127, 407–413. [Google Scholar] [CrossRef]
- Pernot, S.; Boucheron, P.; Péré, H.; Lucas, M.L.; Veyer, D.; Fathallah, N.; Parades, V.D.; Pavie, J.; Netter, J.; Collias, L.; et al. Comparison of Anal Cancer Screening Strategies Including Standard Anoscopy, Anal Cytology, and HPV Genotyping in HIV-Positive Men Who Have Sex with Men. Br. J. Cancer 2018, 119, 381–386. [Google Scholar] [CrossRef]
- Mathews, C.; Caperna, J.; Cachay, E.R.; Cosman, B. Early Impact and Performance Characteristics of an Established Anal Dysplasia Screening Program: Program Evaluation Considerations. Open AIDS J. 2007, 1, 11–20. [Google Scholar] [CrossRef]
- Brogden, D.R.L.; Lupi, M.E.E.; Warren, O.J.; Kontovounisios, C.; Mills, S.C. Comparing and Contrasting Clinical Consensus and Guidelines for Anal Intraepithelial Neoplasia in Different Geographical Regions. Updates Surg. 2021, 73, 2047–2058. [Google Scholar] [CrossRef]
- Palefsky, J.M. Treatment of Anal High-Grade Squamous Intraepithelial Lesions to Prevent Anal Cancer. In Proceedings of the 2022 Conference on Retroviruses and Opportunistic Infections (Virtual CROI), Virtual, 22–24 February 2022. [Google Scholar]
- Paola, M.D.; Sani, C.; Clemente, A.M.; Iossa, A.; Perissi, E.; Castronovo, G.; Tanturli, M.; Rivero, D.; Cozzolino, F.; Cavalieri, D.; et al. Characterization of Cervico-Vaginal Microbiota in Women Developing Persistent High-Risk Human Papillomavirus Infection. Sci. Rep. 2017, 7, 10200. [Google Scholar] [CrossRef]
- Onywera, H.; Williamson, A.L.; Mbulawa, Z.Z.A.; Coetzee, D.; Meiring, T.L. The Cervical Microbiota in Reproductive-Age South African Women with and without Human Papillomavirus Infection. Papillomavirus Res. 2019, 7, 154–163. [Google Scholar] [CrossRef]
- Gao, W.; Weng, J.; Gao, Y.; Chen, X. Comparison of the Vaginal Microbiota Diversity of Women with and without Human Papillomavirus Infection: A Cross-Sectional Study. BMC Infect. Dis. 2013, 13, 271. [Google Scholar] [CrossRef]
- Wu, M.; Gao, J.; Wu, Y.; Li, Y.; Chen, Y.; Zhao, F.; Li, C.; Ying, C. Characterization of Vaginal Microbiota in Chinese Women with Cervical Squamous Intra-Epithelial Neoplasia. Int. J. Gynecol. Cancer 2020, 30, 1500–1504. [Google Scholar] [CrossRef]
- Wu, S.; Ding, X.; Kong, Y.; Acharya, S.; Wu, H.; Huang, C.; Liang, Y.; Nong, X.; Chen, H. The Feature of Cervical Microbiota Associated with the Progression of Cervical Cancer among Reproductive Females. Gynecol. Oncol. 2021, 163, 348–357. [Google Scholar] [CrossRef]
- Nieves-Ramírez, M.E.; Partida-Rodríguez, O.; Moran, P.; Serrano-Vázquez, A.; Pérez-Juárez, H.; Pérez-Rodríguez, M.E.; Arrieta, M.C.; Ximénez-García, C.; Finlay, B.B. Cervical Squamous Intraepithelial Lesions Are Associated with Differences in the Vaginal Microbiota of Mexican Women. Microbiol. Spectr. 2021, 9, e00143-21. [Google Scholar] [CrossRef]
- Nowak, R.G.; Bentzen, S.M.; Ravel, J.; Crowell, T.A.; Dauda, W.; Ma, B.; Liu, H.; Blattner, W.A.; Baral, S.D.; Charurat, M.E. Anal Microbial Patterns and Oncogenic Human Papillomavirus in a Pilot Study of Nigerian Men Who Have Sex with Men at Risk for or Living with HIV. AIDS Res. Hum. Retrovir. 2019, 35, 267–275. [Google Scholar] [CrossRef]
- Ron, R.; Cabello, A.; Gosalbes, M.J.; Sánchez-Conde, M.; Talavera-Rodríguez, A.; Zamora, J.; Monge-Maillo, B.; Jiménez, D.; Martínez-Sanz, J.; López, Y.; et al. Exploiting the Microbiota for the Diagnosis of Anal Precancerous Lesions in Men Who Have Sex with Men. J. Infect. Dis. 2021, 224, 1247–1256. [Google Scholar] [CrossRef]
- Serrano-Villar, S.; Vásquez-Domínguez, E.; Pérez-Molina, J.A.; Sainz, T.; Benito, A.D.; Latorre, A.; Moya, A.; Gosalbes, M.J.; Moreno, S. HIV, HPV, and Microbiota: Partners in Crime? AIDS 2017, 31, 591–594. [Google Scholar] [CrossRef]
- Bailey, R.C.; Moses, S.; Parker, C.B.; Agot, K.; Maclean, I.; Krieger, J.N.; Williams, C.F.; Campbell, R.T.; Ndinya-Achola, J.O. Male Circumcision for HIV Prevention in Young Men in Kisumu, Kenya: A Randomised Controlled Trial. Lancet 2007, 369, 643–656. [Google Scholar] [CrossRef]
- Smith, J.S.; Backes, D.M.; Hudgens, M.G.; Mei, W.; Chakraborty, H.; Rohner, E.; Moses, S.; Agot, K.; Meijer, C.J.L.M.; Bailey, R.C. Male Circumcision Reduces Penile HPV Incidence and Persistence: A Randomized Controlled Trial in Kenya. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1139–1148. [Google Scholar] [CrossRef]
- Onywera, H.; Williamson, A.L.; Cozzuto, L.; Bonnin, S.; Mbulawa, Z.Z.A.; Coetzee, D.; Ponomarenko, J.; Meiring, T.L. The Penile Microbiota of Black South African Men: Relationship with Human Papillomavirus and HIV Infection. BMC Microbiol. 2020, 20, 78. [Google Scholar] [CrossRef]
- Shannon, B.; Yi, T.J.; Perusini, S.; Gajer, P.; Ma, B.; Humphrys, M.S.; Thomas-Pavanel, J.; Chieza, L.; Janakiram, P.; Saunders, M.; et al. Association of HPV Infection and Clearance with Cervicovaginal Immunology and the Vaginal Microbiota. Mucosal Immunol. 2017, 10, 1310–1319. [Google Scholar] [CrossRef]
- Onywera, H.; Williamson, A.L.; Ponomarenko, J.; Meiring, T.L. The Penile Microbiota in Uncircumcised and Circumcised Men: Relationships with HIV and Human Papillomavirus Infections and Cervicovaginal Microbiota. Front. Med. 2020, 7, 383. [Google Scholar] [CrossRef]
- Zhang, Y.; D’Souza, G.; Fakhry, C.; Bigelow, E.O.; Usyk, M.; Burk, R.D.; Zhao, N. Oral HPV Associated with Differences in Oral Microbiota Beta Diversity and Microbiota Abundance. J. Infect. Dis. 2022, jiac010. [Google Scholar] [CrossRef]
- Sarkar, P.; Malik, S.; Laha, S.; Das, S.; Bunk, S.; Ray, J.G.; Chatterjee, R.; Saha, A. Dysbiosis of Oral Microbiota During Oral Squamous Cell Carcinoma Development. Front. Oncol. 2021, 11, 614448. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, K.; Carey, R.M.; Lin, X.; Seckar, T.D.; Wei, Z.; Chorath, K.; Newman, J.G.; O’Malley, B.W.; Weinstein, G.S.; Feldman, M.D.; et al. The Microbiome of HPV-Positive Tonsil Squamous Cell Carcinoma and Neck Metastasis. Oral Oncol. 2021, 117, 105305. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yu, Q.; Lin, Y.; Zhou, Y.; Lan, L.; Yang, S.; Wu, J. Global Burden and Trends of Sexually Transmitted Infections from 1990 to 2019: An Observational Trend Study. Lancet Infect. Dis. 2021, 22, 541–551. [Google Scholar] [CrossRef]
- Tuddenham, S.; Hamill, M.M.; Ghanem, K.G. Diagnosis and Treatment of Sexually Transmitted Infections: A Review. Jama 2022, 327, 161–172. [Google Scholar] [CrossRef]
- Jary, A.; Teguete, I.; Sidibé, Y.; Kodio, A.; Dolo, O.; Burrel, S.; Boutolleau, D.; Beauvais-Remigereau, L.; Sayon, S.; Kampo, M.; et al. Prevalence of Cervical HPV Infection, Sexually Transmitted Infections and Associated Antimicrobial Resistance in Women Attending Cervical Cancer Screening in Mali. Int. J. Infect. Dis. 2021, 108, 610–616. [Google Scholar] [CrossRef]
- Jongen, V.W.; Loeff, M.F.S.V.D.; Botha, M.H.; Sudenga, S.L.; Abrahamsen, M.E.; Giuliano, A.R. Incidence and Risk Factors of C. Trachomatis and N. Gonorrhoeae among Young Women from the Western Cape, South Africa: The EVRI Study. PLoS ONE 2021, 16, e0250871. [Google Scholar] [CrossRef]
- Goddard, S.L.; Poynten, I.M.; Petoumenous, K.; Jin, F.; Hillman, R.J.; Law, C.; Roberts, J.M.; Fairley, C.K.; Garland, S.M.; Grulich, A.E.; et al. Prevalence, Incidence and Predictors of Anal Chlamydia Trachomatis, Anal Neisseria Gonorrhoeae and Syphilis among Older Gay and Bisexual Men in the Longitudinal Study for the Prevention of Anal Cancer (SPANC). Sex. Transm. Infect. 2019, 95, 477–483. [Google Scholar] [CrossRef]
- Ye, X.; Li, F.-R.; Pan, Q.; Li, Z.; Yu, G.-Q.; Liu, H.; Liu, J.; Huai, P.-C.; Zhang, F.-R. Prevalence and Associated Factors of Sexually Transmitted Infections among Methamphetamine Users in Eastern China: A Cross-Sectional Study. BMC Infect. Dis. 2022, 22, 7. [Google Scholar] [CrossRef]
- Masiá, M.; Fernández-González, M.; García, J.A.; Padilla, S.; García-Payá, E.; Gutiérrez, A.; Tabla, V.O.D.L.; García-Abellán, J.; Agulló, V.; Gutiérrez, F. Infection with Chlamydia Trachomatis Increases the Risk of High-Grade Anal Intraepithelial Neoplasia in People Living With Human Immunodeficiency Virus. Clin. Infect. Dis. 2020, 70, 2161–2167. [Google Scholar] [CrossRef]
- Wang, K.; Muñoz, K.J.; Tan, M.; Sütterlin, C. Chlamydia and HPV Induce Centrosome Amplification in the Host Cell through Additive Mechanisms. Cell. Microbiol. 2021, 23, e13397. [Google Scholar] [CrossRef]
- Porras, C.; Tsang, S.H.; Herrero, R.; Guillén, D.; Darragh, T.M.; Stoler, M.H.; Hildesheim, A.; Wagner, S.; Boland, J.; Lowy, D.R.; et al. Efficacy of the Bivalent HPV Vaccine against HPV 16/18-Associated Precancer: Long-Term Follow-up Results from the Costa Rica Vaccine Trial. Lancet Oncol. 2020, 21, 1643–1652. [Google Scholar] [CrossRef]
- Hu, S.; Xu, X.; Zhu, F.; Hong, Y.; Hu, Y.; Zhang, X.; Pan, Q.; Zhang, W.; Zhang, C.; Yang, X.; et al. Efficacy of the AS04-Adjuvanted HPV-16/18 Vaccine in Young Chinese Women with Oncogenic HPV Infection at Baseline: Post-Hoc Analysis of a Randomized Controlled Trial. Hum. Vaccines Immunother. 2021, 17, 955–964. [Google Scholar] [CrossRef]
- Mariz, F.C.; Gray, P.; Bender, N.; Eriksson, T.; Kann, H.; Apter, D.; Paavonen, J.; Pajunen, E.; Prager, K.M.; Sehr, P.; et al. Sustainability of Neutralising Antibodies Induced by Bivalent or Quadrivalent HPV Vaccines and Correlation with Efficacy: A Combined Follow-up Analysis of Data from Two Randomised, Double-Blind, Multicentre, Phase 3 Trials. Lancet Infect. Dis. 2021, 21, 1458–1468. [Google Scholar] [CrossRef]
- Joura, E.A.; Ulied, A.; Vandermeulen, C.; Figueroa, M.R.; Seppä, I.; Aguado, J.J.H.; Ahonen, A.; Reich, O.; Virta, M.; Perino, A.; et al. Immunogenicity and Safety of a Nine-Valent Human Papillomavirus Vaccine in Women 27–45 years of Age Compared to Women 16–26 years of Age: An Open-Label Phase 3 Study. Vaccine 2021, 39, 2800–2809. [Google Scholar] [CrossRef]
- Pinto, L.A.; Wilkin, T.J.; Kemp, T.J.; Abrahamsen, M.; Isaacs-Soriano, K.; Pan, Y.; Webster-Cyriaque, J.; Palefsky, J.M.; Giuliano, A.R. Oral and Systemic HPV Antibody Kinetics Post-Vaccination among HIV-Positive and HIV-Negative Men. Vaccine 2019, 37, 2502–2510. [Google Scholar] [CrossRef]
- Wilkin, T.; Lee, J.Y.; Lensing, S.Y.; Stier, E.A.; Goldstone, S.E.; Berry, J.M.; Jay, N.; Aboulafia, D.; Cohn, D.L.; Einstein, M.H.; et al. Safety and Immunogenicity of the Quadrivalent Human Papillomavirus Vaccine in HIV-1–Infected Men. J. Infect. Dis. 2010, 202, 1246–1253. [Google Scholar] [CrossRef]
- Denny, L.; Hendricks, B.; Gordon, C.; Thomas, F.; Hezareh, M.; Dobbelaere, K.; Durand, C.; Hervé, C.; Descamps, D. Safety and Immunogenicity of the HPV-16/18 AS04-Adjuvanted Vaccine in HIV-Positive Women in South Africa: A Partially-Blind Randomised Placebo-Controlled Study. Vaccine 2013, 31, 5745–5753. [Google Scholar] [CrossRef]
- Munk-Madsen, M.Z.; Toft, L.; Kube, T.; Richter, R.; Ostergaard, L.; Søgaard, O.S.; Tolstrup, M.; Kaufmann, A.M. Cellular Immunogenicity of Human Papillomavirus Vaccines Cervarix and Gardasil in Adults with HIV Infection. Hum. Vaccines Immunother. 2018, 14, 909–916. [Google Scholar] [CrossRef]
- Toft, L.; Storgaard, M.; Müller, M.; Sehr, P.; Bonde, J.; Tolstrup, M.; Østergaard, L.; Søgaard, O.S. Comparison of the Immunogenicity and Reactogenicity of Cervarix and Gardasil Human Papillomavirus Vaccines in HIV-Infected Adults: A Randomized, Double-Blind Clinical Trial. J. Infect. Dis. 2014, 209, 1165–1173. [Google Scholar] [CrossRef]
- Chow, E.P.F.; Fairley, C.K.; Zou, H.; Wigan, R.; Garland, S.M.; Cornall, A.M.; Atchison, S.; Tabrizi, S.N.; Chen, M.Y. Human Papillomavirus Antibody Levels Following Vaccination or Natural Infection among Young Men Who Have Sex with Men. Clin. Infect. Dis. 2021, ciab1052. [Google Scholar] [CrossRef]
- Falcaro, M.; Castañon, A.; Ndlela, B.; Checchi, M.; Soldan, K.; Lopez-Bernal, J.; Elliss-Brookes, L.; Sasieni, P. The Effects of the National HPV Vaccination Programme in England, UK, on Cervical Cancer and Grade 3 Cervical Intraepithelial Neoplasia Incidence: A Register-Based Observational Study. Lancet 2021, 398, 2084–2092. [Google Scholar] [CrossRef]
- Donato, V.D.; Caruso, G.; Petrillo, M.; Kontopantelis, E.; Palaia, I.; Perniola, G.; Plotti, F.; Angioli, R.; Muzii, L.; Panici, P.B.; et al. Adjuvant Hpv Vaccination to Prevent Recurrent Cervical Dysplasia after Surgical Treatment: A Meta-Analysis. Vaccines 2021, 9, 410. [Google Scholar] [CrossRef]
- Lichter, K.; Krause, D.; Xu, J.; Tsai, S.H.L.; Hage, C.; Weston, E.; Eke, A.; Levinson, K. Adjuvant Human Papillomavirus Vaccine to Reduce Recurrent Cervical Dysplasia in Unvaccinated Women: A Systematic Review and Meta-Analysis. Obstet. Gynecol. 2020, 135, 1070–1083. [Google Scholar] [CrossRef]
- Firnhaber, C.; Swarts, A.; Jezile, V.; Mulongo, M.; Goeieman, B.; Williams, S.; Faesen, M.; Michelow, P.; Wilkin, T. Human Papillomavirus Vaccination Prior to Loop Electroexcision Procedure Does Not Prevent Recurrent Cervical High-Grade Squamous Intraepithelial Lesions in Women Living with Human Immunodeficiency Virus: A Randomized, Double-Blind, Placebo-Controlled Tria. Clin. Infect. Dis. 2021, 73, e2211–e2216. [Google Scholar] [CrossRef]
- Gosens, K.C.M.; Zee, R.P.V.D.; Heukelom, M.L.S.V.; Jongen, V.W.; Cairo, I.; Eeden, A.V.; Noesel, C.J.M.V.; Quint, W.G.V.; Pasmans, H.; Dijkgraaf, M.G.W.; et al. HPV Vaccination to Prevent Recurrence of Anal Intraepithelial Neoplasia in HIV+ MSM. AIDS 2021, 35, 1753–1764. [Google Scholar] [CrossRef]
- Hidalgo-Tenorio, C.; Pasquau, J.; Omar-Mohamed, M.; Sampedro, A.; López-Ruz, M.A.; López Hidalgo, J.; Ramírez-Taboada, J. Effectiveness of the Quadrivalent HPV Vaccine in Preventing Anal ≥HSILs in a Spanish Population of HIV+ MSM Aged >26 Years. Viruses 2021, 13, 144. [Google Scholar] [CrossRef]
- Wendland, E.M.; Kops, N.L.; Bessel, M.; Comerlato, J.; Maranhão, A.G.K.; Souza, F.M.A.; Villa, L.L.; Pereira, G.F.M. Effectiveness of a Universal Vaccination Program with an HPV Quadrivalent Vaccine in Young Brazilian Women. Vaccine 2021, 39, 1840–1845. [Google Scholar] [CrossRef]
- Clark, M.; Jembere, N.; Kupets, R. The Impact of a Universal Human Papilloma Virus (HPV) Vaccination Program on Lower Genital Tract Dysplasia and Genital Warts. Prev. Med. 2021, 150, 106641. [Google Scholar] [CrossRef]
- Chow, E.P.F.; Tabrizi, S.N.; Fairley, C.K.; Wigan, R.; Machalek, D.A.; Regan, D.G.; Hocking, J.S.; Garland, S.M.; Cornall, A.M.; Atchison, S.; et al. Prevalence of Human Papillomavirus in Teenage Heterosexual Males Following the Implementation of Female and Male School-Based Vaccination in Australia: 2014–2017. Vaccine 2019, 37, 6907–6914. [Google Scholar] [CrossRef]
- Díez-Domingo, J.; Sánchez-Alonso, V.; Villanueva, R.-J.; Acedo, L.; Tuells, J. Impact of a Gender-Neutral HPV Vaccination Program in Men Who Have Sex with Men (MSM). Int. J. Environ. Res. Public Health 2021, 18, 963. [Google Scholar] [CrossRef]
- Chow, E.P.F.; Carter, A.; Vickers, T.; Fairley, C.K.; McNulty, A.; Guy, R.J.; Regan, D.G.; Grulich, A.E.; Callander, D.; Khawar, L.; et al. Effect on Genital Warts in Australian Female and Heterosexual Male Individuals after Introduction of the National Human Papillomavirus Gender-Neutral Vaccination Programme: An Analysis of National Sentinel Surveillance Data from 2004–18. Lancet Infect. Dis. 2021, 21, 1747–1756. [Google Scholar] [CrossRef]
- García, F.J.Á.; Ortega, M.J.C.; Aldeán, J.Á.; Garcés-Sánchez, M.; Sánchez, N.G.; Llanos, E.G.; Merino, Á.H.; Arce, A.I.D.; Moína, M.M.; Melián, A.M.; et al. Immunisation Schedule of the Spanish Association of Paediatrics: 2020 Recommendations. An. Pediatr. 2020, 92, e1–e10. [Google Scholar] [CrossRef]





| Classification | HPV Types |
|---|---|
| High risk (group 1) | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 |
| Probably high risk (group 2A) | 68 |
| Possibly carcinogenic to humans (group 2B) | 26, 53, 66, 67, 70, 73, 82 30, 34, 69, 85, 97 |
| Unclassifiable as to carcinogenicity in humans (group 3) | 6, 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-González, A.; Cachay, E.; Ocampo, A.; Poveda, E. Update on the Epidemiological Features and Clinical Implications of Human Papillomavirus Infection (HPV) and Human Immunodeficiency Virus (HIV) Coinfection. Microorganisms 2022, 10, 1047. https://doi.org/10.3390/microorganisms10051047
Pérez-González A, Cachay E, Ocampo A, Poveda E. Update on the Epidemiological Features and Clinical Implications of Human Papillomavirus Infection (HPV) and Human Immunodeficiency Virus (HIV) Coinfection. Microorganisms. 2022; 10(5):1047. https://doi.org/10.3390/microorganisms10051047
Chicago/Turabian StylePérez-González, Alexandre, Edward Cachay, Antonio Ocampo, and Eva Poveda. 2022. "Update on the Epidemiological Features and Clinical Implications of Human Papillomavirus Infection (HPV) and Human Immunodeficiency Virus (HIV) Coinfection" Microorganisms 10, no. 5: 1047. https://doi.org/10.3390/microorganisms10051047
APA StylePérez-González, A., Cachay, E., Ocampo, A., & Poveda, E. (2022). Update on the Epidemiological Features and Clinical Implications of Human Papillomavirus Infection (HPV) and Human Immunodeficiency Virus (HIV) Coinfection. Microorganisms, 10(5), 1047. https://doi.org/10.3390/microorganisms10051047







