Infections Caused by Moellerella wisconsensis: A Case Report and a Systematic Review of the Literature
Abstract
:1. Introduction
Case Presentation
2. Materials and Methods
2.1. Study Design and Aims
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Assessment Risk of Bias
3. Results
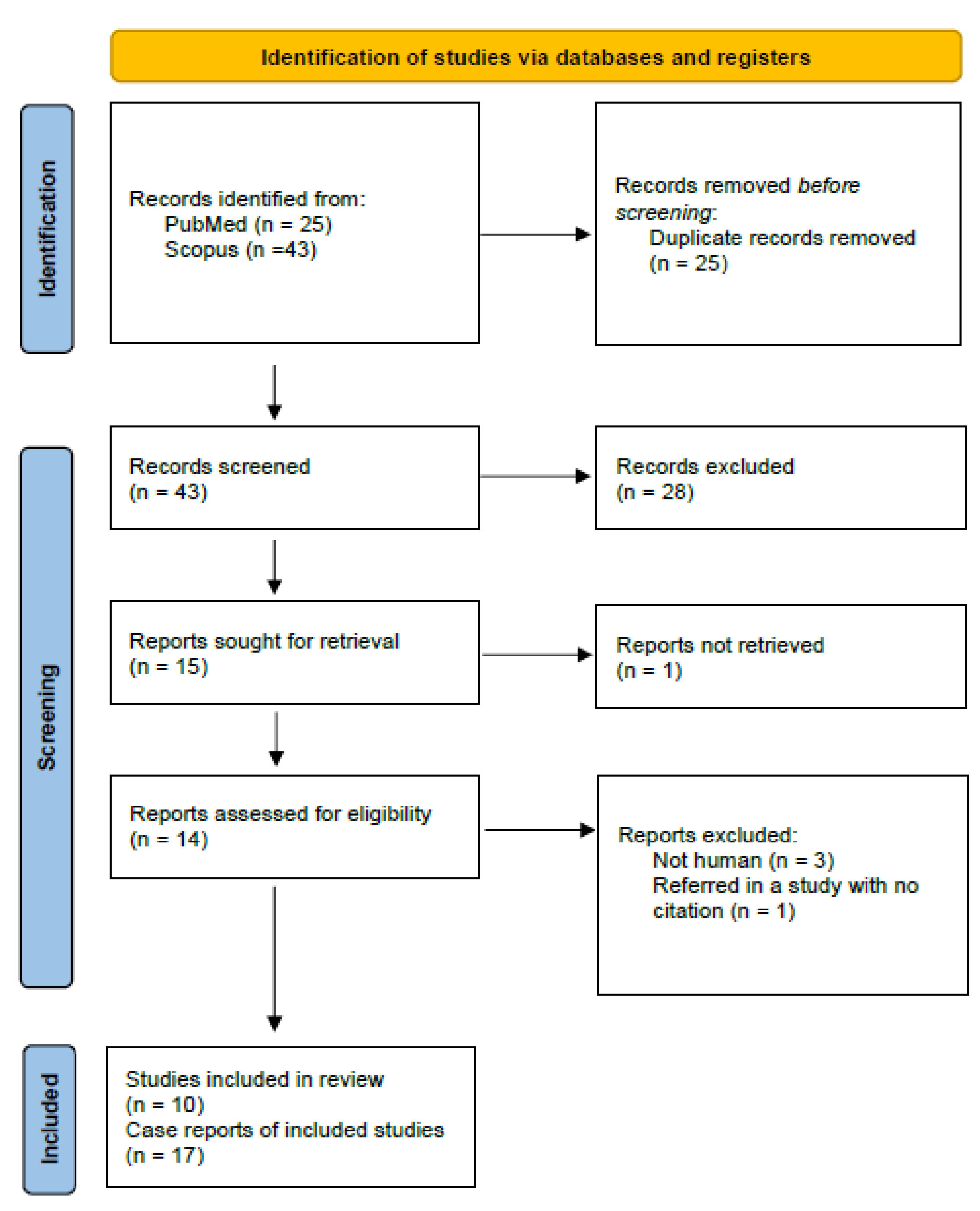
3.1. Study Selection
3.2. Study Characteristics
3.3. Origin of the Infection
3.4. Quality Appraisal
| Author | Year | Study Design | Country | Patient Age/Sex | Clinical Presentation | Sample |
|---|---|---|---|---|---|---|
| Hickman-Brenner [1] | 1984 | Case Series | Wisconsin, USA | 5 y/o ♀ | Diarrhea | Feces |
| Hickman-Brenner [1] | 1984 | Case Series | Wisconsin, USA | 29 y/o ♂ | Diarrhea | Feces |
| Hickman-Brenner [1] | 1984 | Case Series | Wisconsin, USA | 40 y/o ♂ | Bloody diarrhea | Feces |
| Hickman-Brenner [1] | 1984 | Case Series | Wisconsin, USA | 62 y/o ♂ | Gastroenteritis | Feces |
| Hickman-Brenner [1] | 1984 | Case Series | Wisconsin, USA | 16 y/o ♀ | Not reported | Feces |
| Hickman-Brenner [1] | 1984 | Case Series | Wisconsin, USA | 38 y/o ♀ | Not reported | Feces |
| Hickman-Brenner [1] | 1984 | Case Series | Virginia, USA | NA | Diarrhea | Feces |
| Hickman-Brenner [1] | 1984 | Case Series | New York, USA | NA | Not reported | Feces |
| Wittke [11] | 1985 | Case Report | Hamburg, Germany | 71 y/o ♂ | Acute cholecystitis | Bile |
| Ohanessian [12] | 1987 | Case Report | France | 77 y/o ♀ | Acute cholecystitis | Bile |
| Kubiniek [13] | 1995 | Case Report | France | 67 y/o ♀ | Small bowel perforation with peritonitis | Peritoneal fluid |
| Cardentey-Reyes [14] | 2009 | Case Report | Belgium | 46 y/o ♂ | Acute cholecystitis with peritonitis and secondary bacteremia | Bile, blood |
| Wallet [15] | 1994 | Case Report | Czech Republic | 20 y/o ♀ | Inhalation pneumonia, deep coma | Bronchial aspirate |
| Aller [16] | 2009 | Case Report | Spain | 80 y/o ♂ | Acute cholecystitis with secondary bacteremia | Bile, blood |
| Seyman [17] | 2013 | Case Report | Turkey | 53 y/o ♀ | Central venous catheter-related bloodstream | Pus from hemodialysis catheter insertion site, blood |
| Leroy [18] | 2016 | Case Report | Nantes, France | 64 y/o ♂ | Small bowel perforation with peritonitis | Peritoneal fluid |
| Ahmad [19] | 2020 | Case Report | India | 14 d/o ♀ | Diarrhea | Feces |
| Germanou | 2022 | Case Report | Limassol, Cyprus | 76 y/o ♂ | Urinary tract infection with secondary bacteremia | Pigtail end, urine, blood |
| Author | Year | Were Patient’s Demographic Characteristics Clearly Described? | Was the Patient’s History Clearly Described and Presented as a Timeline? | Was the Current Clinical Condition of the Patient on Presentation Clearly Described? | Were Diagnostic Tests or Assessment Methods and the Results Clearly Described? | Was the Intervention(s) or Treatment Procedure(s) Clearly Described? | Was the Post-Intervention Clinical Condition Clearly Described? | Were Adverse Events (Harms) or Unanticipated Events Identified and Described? | Does the Case Report Provide Takeaway Lessons? | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Ahmad [19] | 2020 | yes | no | no | yes | yes | yes | no | yes | Low |
| Leroy [18] | 2016 | yes | no | yes | yes | yes | yes | no | yes | Low |
| Seyman [17] | 2013 | yes | yes | yes | yes | yes | yes | no | yes | Low |
| Aller [16] | 2009 | yes | yes | yes | yes | yes | yes | no | yes | Low |
| Cardentey-Reyes [14] | 2009 | yes | yes | yes | yes | yes | yes | no | yes | Low |
| Kubiniek [13] | 1995 | yes | yes | yes | yes | yes | yes | no | yes | Low |
| Wallet [15] | 1994 | yes | no | yes | yes | no | yes | no | yes | Low |
| Ohanessian [12] | 1987 | yes | yes | yes | yes | yes | yes | no | yes | Low |
| Wittke [11] | 1985 | yes | no | yes | yes | yes | yes | yes | yes | Low |
| Hickman-Brenner [1] | 1984 | yes | no | yes | no | no | no | no | yes | High |
| yes | no | no | no | no | no | no | yes | |||
| yes | no | no | no | no | no | no | yes | |||
| no | no | no | no | no | no | no | yes | |||
| yes | no | no | no | no | no | no | yes | |||
| no | no | no | no | no | no | no | yes | |||
| no | no | no | no | no | no | no | yes | |||
| no | no | no | no | no | no | no | yes |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hickman-Brenner, F.W.; Huntley-Carter, G.P.; Saitoh, Y.; Steigerwalt, A.G.; Farmer, J.J., 3rd; Brenner, D.J. Moellerella wisconsensis, a new genus and species of Enterobacteriaceae found in human stool specimens. J. Clin. Microbiol. 1984, 19, 460–463. [Google Scholar] [CrossRef] [Green Version]
- Sandfort, R.F.; Murray, W.; Janda, J.M. Moellerella wisconsensis isolated from the oral cavity of a wild raccoon (Procyon lotor). Vector-Borne Zoonotic Dis. 2002, 2, 197–199. [Google Scholar] [CrossRef]
- Casalinuovo, F.; Musarella, R. Isolation of Moellerella wisconsensis from the lung of a goat. Vet. Microbiol. 2009, 138, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Bangert, R.L.; Ward, A.C.; Stauber, E.H.; Cho, B.R.; Widders, P.R. A survey of the aerobic bacteria in the feces of captive raptors. Avian. Dis. 1988, 32, 53–62. [Google Scholar] [CrossRef]
- Interaminense, J.A.; Nascimento, D.C.; Ventura, R.F.; Batista, J.E.; Souza, M.M.; Hazin, F.H.; Pontes-Filho, N.T.; Lima-Filho, J.V. Recovery and screening for antibiotic susceptibility of potential bacterial pathogens from the oral cavity of shark species involved in attacks on humans in Recife, Brazil. J. Med. Microbiol. 2010, 59, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Macé, S.; Joffraud, J.J.; Cardinal, M.; Malcheva, M.; Cornet, J.; Lalanne, V.; Chevalier, F.; Sérot, T.; Pilet, M.F.; Dousset, X. Evaluation of the spoilage potential of bacteria isolated from spoiled raw salmon (Salmo salar) fillets stored under modified atmosphere packaging. Int. J. Food Microbiol. 2013, 160, 227–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coton, M.; Joffraud, J.J.; Mekhtiche, L.; Leroi, F.; Coton, E. Biodiversity and dynamics of the bacterial community of packaged king scallop (Pecten maximus) meat during cold storage. Food Microbiol. 2013, 35, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiktorczyk-Kapischke, N.; Skowron, K.; Kwiecińska-Piróg, J.; Białucha, A.; Wałecka-Zacharska, E.; Grudlewska-Buda, K.; Kraszewska, Z.; Gospodarek-Komkowska, E. Flies as a potential vector of selected alert pathogens in a hospital environment. Int. J. Environ. Health Res. 2021, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.M.Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; Mu, P. Chapter 7: Systematic reviews of etiology and risk. In Joanna Briggs Institute Reviewer’s Manual; Aromataris, E., Munn, Z., Eds.; The Joanna Briggs Institute: Adelaide, Australia, 2017. [Google Scholar]
- Wittke, J.W.; Aleksic, S.; Wuthe, H.H. Isolation of Moellerella wisconsensis from an infected human gallbladder. Eur. J. Clin. Microbiol. 1985, 4, 351–352. [Google Scholar] [CrossRef]
- Ohanessian, J.H.; Fourcade, N.; Priolet, B.; Richard, C.; Bashour, G.; Dugelay, M. A propos d’une infection vesiculaire par Moellerella wisconsensis. Méd. Malpract. Infect. 1987, 17, 414–416. [Google Scholar] [CrossRef]
- Kubiniek, V.; Dahman, M.; Sicard, D.; Fosse, T. Peritoneal infection of Moellerella wisconsensis. Med. Malpract. Infect. 1995, 25, 760–761. [Google Scholar] [CrossRef]
- Cardentey-Reyes, A.; Jacobs, F.; Struelens, M.J.; Rodriguez-Villalobos, H. First case of bacteremia caused by Moellerella wisconsensis: Case report and a review of the literature. Infection 2009, 37, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Wallet, F.; Fruchart, A.; Bouvet, P.J.; Courcol, R.J. Isolation of Moellerella wisconsensis from bronchial aspirate. Eur. J. Clin. Microbiol. Infect. Dis. 1994, 13, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Aller, A.I.; Castro, C.; Medina, M.J.; González, M.T.; Sevilla, P.; Morilla, M.D.; Corzo, J.E.; Martín-Mazuelos, E. Isolation of Moellerella wisconsensis from blood culture from a patient with acute cholecystitis. Clin. Microbiol. Infect. 2009, 15, 1193–1194. [Google Scholar] [CrossRef] [Green Version]
- Seyman, D.; Sepin-Özen, N.; Berk, H.; Kızılateş, F.; Günay, V.; Öztoprak, N. First case of primary bacteremia caused by Moellerella wisconsensis: A case report and literature review. Klimik Dergisi 2013, 26, 119–121. [Google Scholar] [CrossRef]
- Leroy, A.G.; Malandain, D.; Duchalais, E.; Meurette, G.; Corvec, S. Accurate MALDI-TOF mass spectrometry identification of a colistin-resistant Moellerella wisconsensis strain. Med. Malpract. Infect. 2016, 46, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ali, S.M.; Khan, A.U. Co-existence of blaNDM-1 and blaVIM-1 producing Moellerella wisconsensis in NICU of North Indian Hospital. J. Infect. Dev. Ctries. 2020, 14, 228–231. [Google Scholar] [CrossRef]
- Stock, I.; Falsen, E.; Wiedemann, B. Moellerella wisconsensis: Identification, natural antibiotic susceptibility and its dependency on the medium applied. Diagn. Microbiol. Infect. Dis. 2003, 45, 1–11. [Google Scholar] [CrossRef]
- Marshall, A.R.; Al-Jumaili, I.J.; Bint, A.J. The isolation of Moellerella wisconsensis from stool samples in the U.K. J. Infect. 1986, 12, 31–33. [Google Scholar] [CrossRef]
- Zambarbieri, J.; Grilli, G.; Vitiello, T.; Scarpa, P. Urinary tract infection by atypical uropathogens in dogs. Vet. Ital. 2021, 57, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Cabadajova, D.; Kudrna, L. Moellerella wisconsensis—The first isolation and identification of a new genus and species of the family Enterobacteriaceae in Czechoslovakia. Cesk. Epidemiol. Mikrobiol. Imunol. 1988, 37, 45–48. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Germanou, D.; Spernovasilis, N.; Papadopoulos, A.; Christodoulou, S.; Agouridis, A.P. Infections Caused by Moellerella wisconsensis: A Case Report and a Systematic Review of the Literature. Microorganisms 2022, 10, 892. https://doi.org/10.3390/microorganisms10050892
Germanou D, Spernovasilis N, Papadopoulos A, Christodoulou S, Agouridis AP. Infections Caused by Moellerella wisconsensis: A Case Report and a Systematic Review of the Literature. Microorganisms. 2022; 10(5):892. https://doi.org/10.3390/microorganisms10050892
Chicago/Turabian StyleGermanou, Daphnie, Nikolaos Spernovasilis, Anastasios Papadopoulos, Sofia Christodoulou, and Aris P. Agouridis. 2022. "Infections Caused by Moellerella wisconsensis: A Case Report and a Systematic Review of the Literature" Microorganisms 10, no. 5: 892. https://doi.org/10.3390/microorganisms10050892
APA StyleGermanou, D., Spernovasilis, N., Papadopoulos, A., Christodoulou, S., & Agouridis, A. P. (2022). Infections Caused by Moellerella wisconsensis: A Case Report and a Systematic Review of the Literature. Microorganisms, 10(5), 892. https://doi.org/10.3390/microorganisms10050892







