Process Development for Benzyl Alcohol Production by Whole-Cell Biocatalysis in Stirred and Packed Bed Reactors
Abstract
1. Introduction
2. Materials and Methods
2.1. Biocatalyst Isolation and Identification
2.2. Biocatalyst Preparation
2.3. Single Aqueous Phase Systems
2.3.1. Aldehyde and Ketone Screening
2.3.2. Effect of Cosolvent and Substrate Concentrations
2.4. Two-Phase Systems
2.5. Bioreactions with Immobilized Cells
2.6. Scale-Up of the Reaction System
2.6.1. Stirred Reactor
2.6.2. Plug Flow Reactor
2.7. Analytical Methods
2.8. Statistical Analysis
3. Results and Discussion
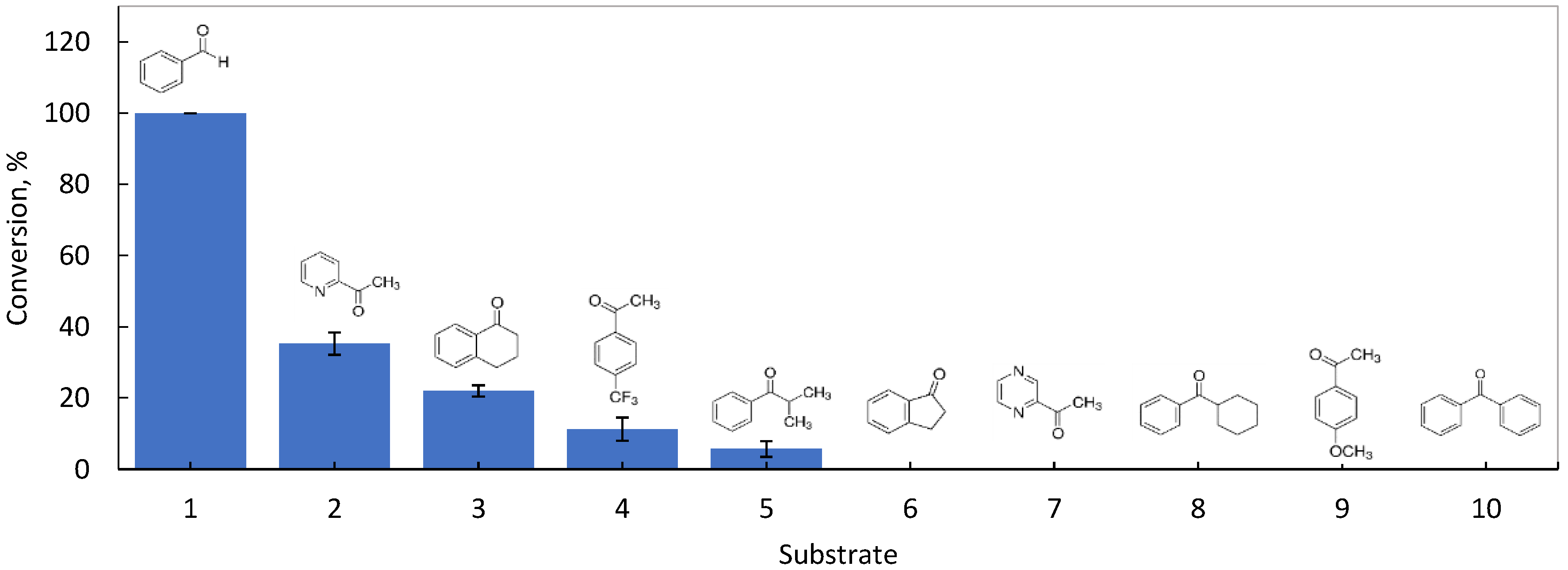
3.1. Screening for Activity with Aromatic Ketones
3.2. Bioprocess Development
3.2.1. One-Phase System
3.2.2. Two-Phase System
3.2.3. Immobilized vs. Free Cells
3.3. Scaling up of the Reaction System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stoye, D.; Freitag, W. Solvents. In Paints, Coatings and Solvents; Stoye, D., Freitag, W., Eds.; Wiley-VCH: Weinheim, Germany, 1998; pp. 277–373. [Google Scholar]
- Scognamiglio, J.; Jones, L.; Vitale, D.; Letizia, C.S.; Api, A.M. Fragrance material review on benzyl alcohol. Food Chem. Toxicol. 2012, 50, S140–S160. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panelon Food Additives Flavourings; Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Fürst, P.; Gürtler, R.; Gundert-Remy, U.; Husøy, T.; et al. Re-evaluation of benzyl alcohol (E1519) as food additive. EFSA J. 2019, 17, e05876. [Google Scholar] [PubMed]
- Corcoran, G.B.; Ray, S.D. Benzyl Alcohol. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 429–432. [Google Scholar]
- Perdomo, I.C.; Gianolio, S.; Pinto, A.; Romano, D.; Contente, M.L.; Paradisi, F.; Molinari, F. Efficient enzymatic preparation of flavor esters in water. J. Agric. Food Chem. 2019, 67, 6517–6522. [Google Scholar] [CrossRef] [PubMed]
- Shintre, M.S.; Ghadge, R.S.; Sawant, S.B. Lipolase catalyzed synthesis of benzyl esters of fatty acids. Biochem. Eng. J. 2002, 12, 131–141. [Google Scholar] [CrossRef]
- Behie, S.W.; Bonet, B.; Zacharia, V.M.; McClung, D.J.; Traxler, M.F. Molecules to ecosystems: Actinomycete natural products in situ. Front. Microbiol. 2017, 7, 2149. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.; Winnicki, T.; Majewska, K. Continuous transformation of benzaldehyde to benzyl alcohol by Rhodotorula mucilaginosa immobilized in an ultrafiltration cell. Biotechnol. Bioeng. 1983, 25, 1441–1452. [Google Scholar] [CrossRef]
- Pugh, S.; McKenna, R.; Halloum, I.; Nielsen, D.R. Engineering Escherichia coli for renewable benzyl alcohol production. Metab. Eng. Commun. 2015, 2, 39–45. [Google Scholar] [CrossRef]
- Solís Oba, A.; Martínez Pérez, R.M.; Cervantes Domínguez, F.; Pérez Méndez, H.I.; Solís Oba, M.; Manjarrez Álvarez, N. Biocatalytic reduction of benzaldehyde using vegetable wastes as enzyme sources. Acta Univ. 2017, 27, 13–18. [Google Scholar] [CrossRef]
- Martin, V.; Giorello, F.; Fariña, L.; Minteguiaga, M.; Salzman, V.; Boido, E.; Aguilar, P.S.; Gaggero, C.; Dellacassa, E.; Mas, A.; et al. De novo synthesis of benzenoid compounds by the yeast Hanseniaspora vineae increases the flavor diversity of wines. J. Agric. Food Chem. 2016, 64, 4574–4583. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, Y.; Chen, Y.; Chen, H.; Fan, C.; Mo, Q.; Yuan, J. One-pot cascade biotransformation for efficient synthesis of benzyl alcohol and its analogs. Chem. Asian J. 2020, 15, 1018–1021. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, M.; Alalawy, A.I.; Almutairi, F.M.; Al-Duais, M.A.; Wang, J.; Salama, E.-S. Identification and characterization of marine seaweeds for biocompounds production. Environ. Technol. Innov. 2021, 24, 101848. [Google Scholar] [CrossRef]
- Straathof, A.J.J.; Panke, S.; Schmid, A. The production of fine chemicals by biotransformations. Curr. Opin. Biotechnol. 2002, 13, 548–556. [Google Scholar] [CrossRef]
- Choi, J.-M.; Han, S.-S.; Kim, H.-S. Industrial applications of enzyme biocatalysis: Current status and future aspects. Biotechnol. Adv. 2015, 33, 1443–1454. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R. Whole cell biocatalysts: Essential workers from Nature to the industry. Microb. Biotechnol. 2017, 10, 250–263. [Google Scholar] [CrossRef]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic synthesis for industrial applications. Angew. Chem. Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef]
- Hauer, B. Embracing Nature’s catalysts: A viewpoint on the future of biocatalysis. ACS Catal. 2020, 10, 8418–8427. [Google Scholar] [CrossRef]
- Ferrer, M.; Méndez-García, C.; Bargiela, R.; Chow, J.; Alonso, S.; García-Moyano, A.; Bjerga, G.E.K.; Steen, I.H.; Schwabe, T.; Blom, C.; et al. Decoding the ocean’s microbiological secrets for marine enzyme biodiscovery. FEMS Microbiol. Lett. 2018, 366, fny285. [Google Scholar] [CrossRef]
- Rodrigues, C.J.C.; Pereira, R.F.S.; Fernandes, P.; Cabral, J.M.S.; de Carvalho, C.C.C.R. Cultivation-based strategies to find efficient marine biocatalysts. Biotechnol. J. 2017, 12, 1700036. [Google Scholar] [CrossRef]
- Matsuda, T.; Yamanaka, R.; Nakamura, K. Biocatalytic asymmetric reduction of C=O and activated C=C bonds in stereoselective synthesis. In Stereoselective Synthesis of Drugs and Natural Products; Andrushko, V., Andrushko, N., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2013; pp. 1–28. [Google Scholar]
- Rodrigues, C.J.C.; Sanches, J.M.; de Carvalho, C.C.C.R. Determining transaminase activity in bacterial libraries by time-lapse imaging. Chem. Commun. 2019, 55, 13538–13541. [Google Scholar] [CrossRef]
- Haby, B.; Hans, S.; Anane, E.; Sawatzki, A.; Krausch, N.; Neubauer, P.; Cruz Bournazou, M.N. Integrated robotic mini bioreactor platform for automated, parallel microbial cultivation with online data handling and process control. SLAS Technol. 2019, 24, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Wiltschi, B.; Cernava, T.; Dennig, A.; Galindo Casas, M.; Geier, M.; Gruber, S.; Haberbauer, M.; Heidinger, P.; Herrero Acero, E.; Kratzer, R.; et al. Enzymes revolutionize the bioproduction of value-added compounds: From enzyme discovery to special applications. Biotechnol. Adv. 2020, 40, 107520. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, C.C.C.R.; da Fonseca, M.M.R. 2.40-Biotransformations. In Comprehensive Biotechnology, 3rd ed.; Moo-Young, M., Ed.; Pergamon: Oxford, UK, 2019; pp. 574–585. [Google Scholar]
- Tufvesson, P.; Lima-Ramos, J.; Nordblad, M.; Woodley, J.M. Guidelines and cost analysis for catalyst production in biocatalytic processes. Org. Process Res. Dev. 2011, 15, 266–274. [Google Scholar] [CrossRef]
- Duetz, W.A.; van Beilen, J.B.; Witholt, B. Using proteins in their natural environment: Potential and limitations of microbial whole-cell hydroxylations in applied biocatalysis. Curr. Opin. Biotechnol. 2001, 12, 419–425. [Google Scholar] [CrossRef]
- Leon, R.; Fernandes, P.; Pinheiro, H.M.; Cabral, J.M.S. Whole-cell biocatalysis in organic media. Enzym. Microb. Technol. 1998, 23, 483–500. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R. Enzymatic and whole cell catalysis: Finding new strategies for old processes. Biotechnol. Adv. 2011, 29, 75–83. [Google Scholar] [CrossRef]
- Wachtmeister, J.; Rother, D. Recent advances in whole cell biocatalysis techniques bridging from investigative to industrial scale. Curr. Opin. Biotechnol. 2016, 42, 169–177. [Google Scholar] [CrossRef]
- Marques, M.P.C.; Carvalho, F.; de Carvalho, C.C.C.R.; Cabral, J.M.S.; Fernandes, P. Steroid bioconversion: Towards green processes. Food Bioprod. Process. 2010, 88, 12–20. [Google Scholar] [CrossRef]
- Gröger, H.; Hummel, W.; Borchert, S.; Kraußer, M. Reduction of ketones and aldehydes to alcohols. In Enzyme Catalysis in Organic Synthesis; Drauz, K., Gröger, H., May, O., Eds.; Wiley-VCH: Weinheim, Germany, 2012; pp. 1035–1110. [Google Scholar]
- Shortall, K.; Djeghader, A.; Magner, E.; Soulimane, T. Insights into aldehyde dehydrogenase enzymes: A structural perspective. Front. Mol. Biosci. 2021, 8, 659550. [Google Scholar] [CrossRef]
- Ellis, E.M. Microbial aldo-keto reductases. FEMS Microbiol. Lett. 2002, 216, 123–131. [Google Scholar] [CrossRef]
- Cheetham, P.S.J.; Blunt, K.W.; Bocke, C. Physical studies on cell immobilization using calcium alginate gels. Biotechnol. Bioeng. 1979, 21, 2155–2168. [Google Scholar] [CrossRef]
- Lee, B.-B.; Ravindra, P.; Chan, E.-S. Size and shape of calcium alginate beads produced by extrusion dripping. Chem. Eng. Technol. 2013, 36, 1627–1642. [Google Scholar] [CrossRef]
- Cao, D.; Xia, S.; Pan, P.; Zeng, H.; Li, C.-J.; Peng, Y. Light-driven MPV-type reduction of aryl ketones/aldehydes to alcohols with isopropanol under mild conditions. Green Chem. 2021, 23, 7539–7543. [Google Scholar] [CrossRef]
- Magano, J.; Dunetz, J.R. Large-scale carbonyl reductions in the pharmaceutical industry. Org. Process Res. Dev. 2012, 16, 1156–1184. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, A.S.; Walsh, A.M.; Sheehan, J.J.; Cotter, P.D.; Crispie, F.; McSweeney, P.L.H.; Kilcawley, K.N.; Rea, M.C. Omics-based insights into flavor development and microbial succession within surface-ripened cheese. mSystems 2018, 3, e00211–e00217. [Google Scholar] [CrossRef]
- Monnet, C.; Loux, V.; Gibrat, J.-F.; Spinnler, E.; Barbe, V.; Vacherie, B.; Gavory, F.; Gourbeyre, E.; Siguier, P.; Chandler, M.; et al. The Arthrobacter arilaitensis Re117 genome sequence reveals its genetic adaptation to the surface of cheese. PLoS ONE 2010, 5, e15489. [Google Scholar] [CrossRef]
- Jensen, P.R.; Mincer, T.J.; Williams, P.G.; Fenical, W. Marine actinomycete diversity and natural product discovery. Antonie Van Leeuwenhoek 2005, 87, 43–48. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, M.S. Antibacterial benzaldehydes produced by seaweed-derived Streptomyces atrovirens PK288-21. Fish. Sci. 2012, 78, 1065–1073. [Google Scholar] [CrossRef]
- Cao, X.; Tarr, M.A. Aldehyde and ketone photoproducts from solar-irradiated crude oil-seawater systems determined by Electrospray Ionization–Tandem Mass Spectrometry. Environ. Sci. Technol. 2017, 51, 11858–11866. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Y. Marine volatile organic compounds and their impacts on marine aerosol—A review. Sci. Total Environ. 2021, 768, 145054. [Google Scholar] [CrossRef] [PubMed]
- Świzdor, A.; Janeczko, T.; Dmochowska-Gładysz, J. Didymosphaeria igniaria: A new microorganism useful for the enantioselective reduction of aryl-aliphatic ketones. J. Ind. Microbiol. Biotechnol. 2010, 37, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Miyahara, Y.; Morii, N.; Okano, T.; Kubota, H.; Besser, T.E. Pentanol and benzyl alcohol attack bacterial surface structures differently. Appl. Environ. Microbiol. 2016, 82, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Konopásek, I.; Strzalka, K.; Svobodová, J. Cold shock in Bacillus subtilis: Different effects of benzyl alcohol and ethanol on the membrane organisation and cell adaptation. Biochim. Biophys. Acta 2000, 1464, 18–26. [Google Scholar] [CrossRef]
- Lozano, P.; Nieto, S.; Serrano, L.J.; Perez, J.; Sanchez-Gomez, G.; García-Verdugo, E.; Luis, V.S. Flow biocatalytic processes in ionic liquids and supercritical fluids. Mini Rev. Org. Chem. 2017, 14, 65–74. [Google Scholar] [CrossRef]
- Van Schie, M.M.C.H.; Spöring, J.-D.; Bocola, M.; Domínguez de María, P.; Rother, D. Applied biocatalysis beyond just buffers–From aqueous to unconventional media. Options Guidel. Green Chem. 2021, 23, 3191–3206. [Google Scholar] [CrossRef]
- Rodrigues, C.J.C.; Ferrer, M.; de Carvalho, C.C.C.R. ω-Transaminase-mediated asymmetric synthesis of (S)-1-(4-trifluoromethylphenyl)ethylamine. Catalysts 2021, 11, 307. [Google Scholar] [CrossRef]
- Bar, R. Effect of interphase mixing on a water–organic solvent two-liquid phase microbial system: Ethanol fermentation. J. Chem. Technol. Biotechnol. 1988, 43, 49–62. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R.; da Fonseca, M.M.R. Solvent toxicity in organic-aqueous systems analysed by multivariate analysis. Bioprocess Biosyst. Eng. 2004, 26, 361–375. [Google Scholar] [CrossRef]
- Notman, R.; Noro, M.; O’Malley, B.; Anwar, J. Molecular basis for dimethylsulfoxide (DMSO) action on lipid membranes. J. Am. Chem. Soc. 2006, 128, 13982–13983. [Google Scholar] [CrossRef]
- Lipinski, C. Drug solubility in water and dimethylsulfoxide. In Molecular Drug Properties; Mannhold, R., Ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 255–282. [Google Scholar]
- Ullah, I.; Khan, A.L.; Ali, L.; Khan, A.R.; Waqas, M.; Hussain, J.; Lee, I.-J.; Shin, J.-H. Benzaldehyde as an insecticidal, antimicrobial, and antioxidant compound produced by Photorhabdus temperata M1021. J. Microbiol. 2015, 53, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Jakoblinnert, A.; Rother, D. A two-step biocatalytic cascade in micro-aqueous medium: Using whole cells to obtain high concentrations of a vicinal diol. Green Chem. 2014, 16, 3472–3482. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R.; da Fonseca, M.M.R. Towards the bio-production of trans-carveol and carvone from limonene: Induction after cell growth on limonene and toluene. Tetrahedron Asymmetry 2003, 14, 3925–3931. [Google Scholar] [CrossRef]
- Grundtvig, I.P.R.; Heintz, S.; Krühne, U.; Gernaey, K.V.; Adlercreutz, P.; Hayler, J.D.; Wells, A.S.; Woodley, J.M. Screening of organic solvents for bioprocesses using aqueous-organic two-phase systems. Biotechnol. Adv. 2018, 36, 1801–1814. [Google Scholar] [CrossRef]
- Ashcroft, C.P.; Dunn, P.J.; Hayler, J.D.; Wells, A.S. Survey of solvent usage in papers published in organic process research & development 1997–2012. Org. Process Res. Dev. 2015, 19, 740–747. [Google Scholar]
- Constable, D.J.C.; Jimenez-Gonzalez, C.; Henderson, R.K. Perspective on Solvent Use in the Pharmaceutical Industry. Org. Process Res. Dev. 2007, 11, 133–137. [Google Scholar] [CrossRef]
- Baldascini, H.; Janssen, D.B. Interfacial inactivation of epoxide hydrolase in a two-liquid-phase system. Enzym. Microb. Technol. 2005, 36, 285–293. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R.; Wick, L.Y.; Heipieper, H.J. Cell wall adaptations of planktonic and biofilm Rhodococcus erythropolis cells to growth on C5 to C16 n-alkane hydrocarbons. Appl. Microbiol. Biotechnol. 2009, 82, 311–320. [Google Scholar] [CrossRef]
- Thomas, S.M.; DiCosimo, R.; Nagarajan, V. Biocatalysis: Applications and potentials for the chemical industry. Trends Biotechnol. 2002, 20, 238–242. [Google Scholar] [CrossRef]
- Polakovič, M.; Švitel, J.; Bučko, M.; Filip, J.; Neděla, V.; Ansorge-Schumacher, M.B.; Gemeiner, P. Progress in biocatalysis with immobilized viable whole cells: Systems development, reaction engineering and applications. Biotechnol. Lett. 2017, 39, 667–683. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Gotovtsev, P.M.; Yuzbasheva, E.Y.; Gorin, K.V.; Butylin, V.V.; Badranova, G.U.; Perkovskaya, N.I.; Mostova, E.B.; Namsaraev, Z.B.; Rudneva, N.I.; Komova, A.V.; et al. Immobilization of microbial cells for biotechnological production: Modern solutions and promising technologies. Appl. Biochem. Microbiol. 2015, 51, 792–803. [Google Scholar] [CrossRef]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From food industry to biomedical applications and management of metabolic disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Lindeque, R.M.; Woodley, J.M. Reactor selection for effective continuous biocatalytic production of pharmaceuticals. Catalysts 2019, 9, 262. [Google Scholar] [CrossRef]
- de Carvalho, C.C.R.; van Keulen, F.; Manuela, M.; da Fonseca, R. Production and recovery of limonene-1,2-diol and simultaneous resolution of a diastereomeric mixture of limonene-1,2-epoxide with whole cells of Rhodococcus erythropolis DCL14. Biocatal. Biotransform. 2000, 18, 223–235. [Google Scholar] [CrossRef]
- Britton, J.; Majumdar, S.; Weiss, G.A. Continuous flow biocatalysis. Chem. Soc. Rev. 2018, 47, 5891–5918. [Google Scholar] [CrossRef] [PubMed]
- Tufvesson, P.; Fu, W.; Jensen, J.S.; Woodley, J.M. Process considerations for the scale-up and implementation of biocatalysis. Food Bioprod. Process. 2010, 88, 3–11. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, C.J.C.; de Carvalho, C.C.C.R. Process Development for Benzyl Alcohol Production by Whole-Cell Biocatalysis in Stirred and Packed Bed Reactors. Microorganisms 2022, 10, 966. https://doi.org/10.3390/microorganisms10050966
Rodrigues CJC, de Carvalho CCCR. Process Development for Benzyl Alcohol Production by Whole-Cell Biocatalysis in Stirred and Packed Bed Reactors. Microorganisms. 2022; 10(5):966. https://doi.org/10.3390/microorganisms10050966
Chicago/Turabian StyleRodrigues, Carlos J. C., and Carla C. C. R. de Carvalho. 2022. "Process Development for Benzyl Alcohol Production by Whole-Cell Biocatalysis in Stirred and Packed Bed Reactors" Microorganisms 10, no. 5: 966. https://doi.org/10.3390/microorganisms10050966
APA StyleRodrigues, C. J. C., & de Carvalho, C. C. C. R. (2022). Process Development for Benzyl Alcohol Production by Whole-Cell Biocatalysis in Stirred and Packed Bed Reactors. Microorganisms, 10(5), 966. https://doi.org/10.3390/microorganisms10050966






