Abstract
Pantoea ananatis, a gram-negative bacterium belonging to the Erwiniaceae family, is a well-known phytopathogen isolated from many ecological niches and plant hosts. However, this bacterium also provides us with various beneficial characteristics, such as the growth promotion of their host plants and increased crop yield. Some isolated non-pathogenic strains are promising for the microbial production of useful substances. P. ananatis AJ13355 was isolated as an acidophilic bacterium and was used as an excellent host to produce L-glutamic acid under acidic conditions. The genome sequence of P. ananatis AJ13355 was determined, and specific genome-engineering technologies were developed. As a result, P. ananatis was successfully used to construct a bacterial strain that produces cysteine, a sulfur-containing amino acid that has been difficult to produce through fermentation because of complex regulation. Furthermore, by heterologous expression including plant-derived genes, construction of a strain that produces isoprenoids such as isoprene and linalool as secondary metabolites was achieved. P. ananatis is shown to be a useful host for the production of secondary metabolites, as well as amino acids, and is expected to be used as a platform for microbial production of bioactive substances, aromatic substances, and other high-value-added substances of plant origin in the future.
1. Introduction
Pantoea ananatis is a gram-negative, rod-shaped, aerobic, or facultatively anaerobic bacteria belonging to the class Gammaproteobacteria and was recently reclassified into the family Erwiniaceae from Enterobacteriaceae [1,2]. P. ananatis was first described as Erwinia ananas by Serrano [3]. Some strains of Enterobacter agglomerans, Erwinia herbicola, and Erwinia milletiae that form part of the E. herbicola–E. agglomerans complex was assigned to the genus Pantoea [4]. Later, Pantoea uredovora, a pathogen of Puccinia graminis, was shown to have a high level of genomic relatedness to P. ananas, and the two species were synonymized [5]. P. ananas proposed by Mergaert et al. [5] was corrected to P. ananatis by Trüper and De’Clari [6]. Due to this phylogenetic history, P. ananatis contains various kinds of plant pathogenic strains [1]. At the same time, strains that promote plant growth that are applicable to bioremediation, or have lignocellulose degradation capacity, have been found in recent years [7].
In the field of microbial biomanufacturing, Escherichia coli, a model organism and a member of the family Enterobacteriaceae, which is a gram-negative facultative anaerobic rod, has been used industrially for the production of various substances because of its high growth rate and sugar consumption activity in the neutral pH range [8,9]. Corynebacterium glutamicum, a gram-positive, rod-shaped bacterium, is capable of L-glutamate fermentation [10] and has been used for the industrial production of many substances by taking advantage of its characteristic cell surface [11,12]. To date, bacterial fermentation production has been realized by mainly using E. coli and C. glutamicum, and many tools for genetic engineering have been developed for both strains [9]. However, it was clear that if both strains had the trait of robustness to pH, they would be more desirable industrial substance-producing bacteria. P. ananatis AJ13355 was isolated as an acidophilic bacterium [13] and has been used as an excellent host organism in previous studies to produce amino acids such as L-glutamate [14], L-cysteine [15], and isoprenoids [16,17]. The emergence of strains closely related to E. coli and advantageous for industrial production is important in terms of increasing the potential for substance production. The purpose of this review is to overview what is known about these P. ananatis, to verify their potential for the production of useful substances, and to provide perspectives on their future. We describe the potential of this strain as a new platform for bacterial bioproduction through its discovery, the development of genome engineering methods, and the production of useful metabolites based not only on academic reports but also on relevant patent information.
2. Characteristic of Pantoea ananatis
P. ananatis has been isolated from various environments and hosts and is well-known for its phytopathogenicity. P. ananatis causes disease symptoms in many economically important agronomic crops and forest tree species worldwide [1]. However, several strains have been known to improve the growth of plants [7], such as papaya [18], red pepper [19], sugarcane [20,21], poplar [22], and rice [23,24]. Kim et al. [19,25] determined the genome sequence of P. ananatis B1–9 isolated from the rhizosphere of the green onion and reported that enhanced red pepper crop yield by approximately three times and showed phosphate solubilization, sulfur oxidation, nitrogen fixation, and indole-3-acetic acid (IAA) production activities. P. ananatis AMG521, isolated as an endophyte from rice paddies, showed the capacity to synthesize siderophores, cellulose, and IAA, and the capacity to solubilize phosphate and increase rice yield [23]. P. ananatis strain 1.38, isolated from the rhizosphere of rice (Oryza sativa L.), has been reported to have phosphate solubilization activity, siderophore and auxin production, and cellulose, lipase, and pectinase activities [24]. It has long been known that P. ananatis produces carotenoids, and it has been reported that introducing the carotenoid biosynthesis genes of P. ananatis into a microorganism that does not produce carotenoids leads to the production of lycopene, astaxanthin, and β-carotene [26,27]. Ten complete genomes of P. ananatis have been determined and registered. Four were pathogenic and four had useful traits (Table 1).

Table 1.
Complete genome of Pantoea ananatis.
3. Isolation of P. ananatis AJ 13355 and Genome-Editing Tools
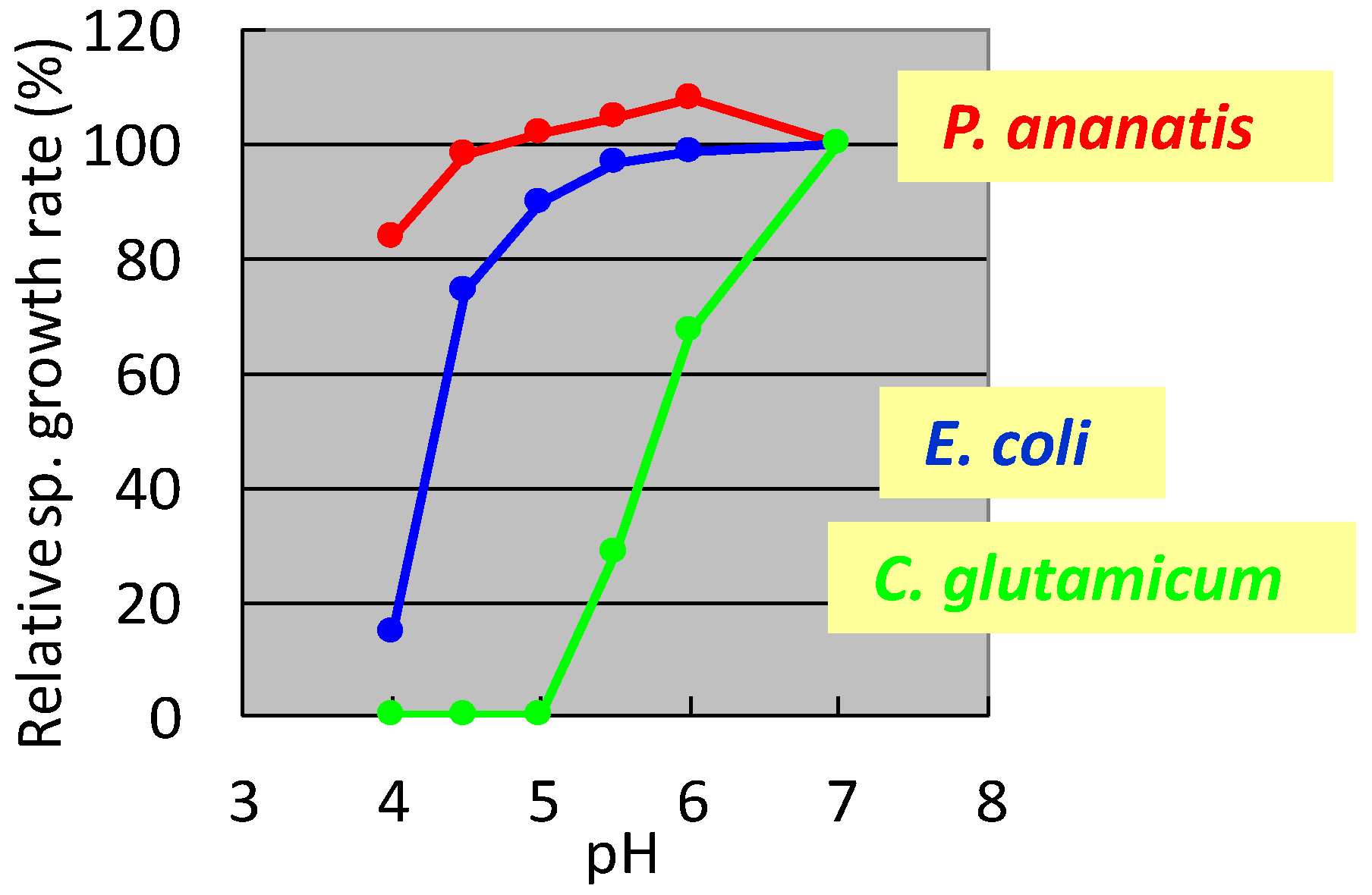
In the 1990s, researchers at Ajinomoto Co., Inc. developed L-glutamate fermentation under acidic conditions. The host needed to grow at low pH and resist high L-glutamate concentrations. In the mid-1990s, specialists from Ajinomoto Co., Inc. (Kawasaki, Japan) collected a gram-negative acidophilic bacterium from the soil of a tea plantation in Iwata City (Shizuoka, Japan), which was designated as strain AJ13355. After screening various strains for these properties, P. ananatis strain AJ13355 was selected as the host strain for glutamate fermentation under acidic conditions. This bacterium has proven capable of growing on various sugars and organic acids at acidic and neutral pH values and is resistant to high concentrations of L-glutamic acid [35]. The effects of pH on the specific growth rates of C. glutamicum, E. coli, and P. ananatis are shown in Figure 1.
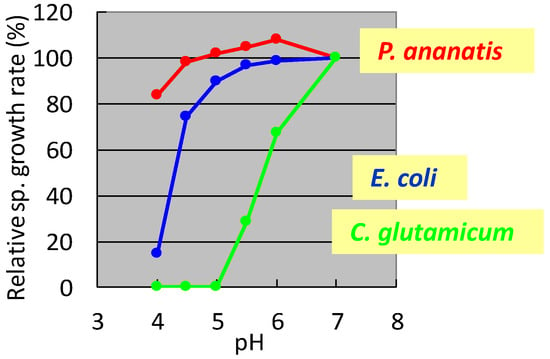
Figure 1.
Effect of pH on the specific growth rate of Corynebacterium glutamicum, Escherichia coli, and Pantoea ananatis. The growth rate of each strain at pH 7 was 100%. C. glutamicum: green, E. coli; blue, P. ananatis: red. The data are typical results from three or more independent experiments.
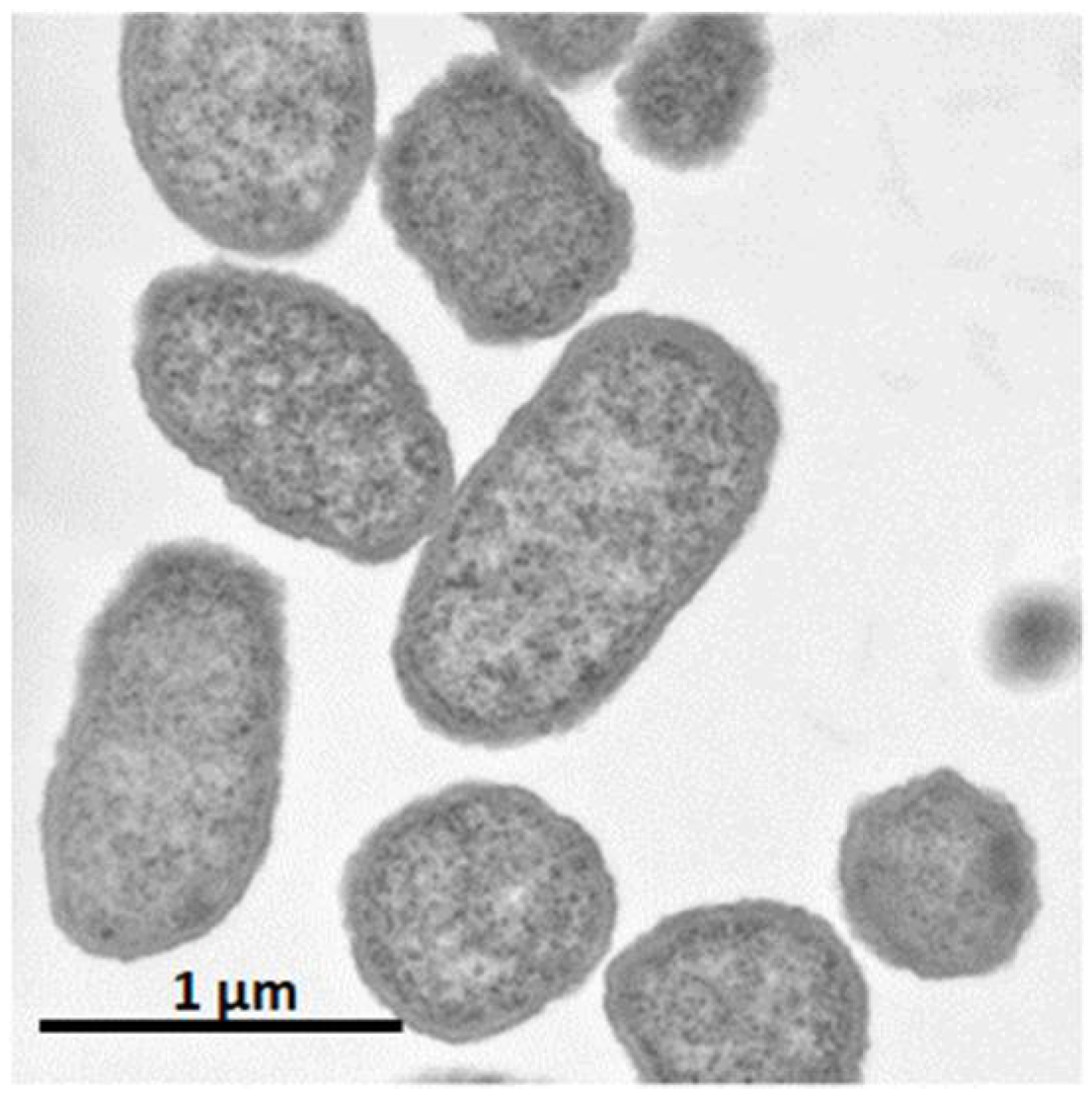
It was shown that C. glutamicum showed good growth only around neutral pH, while P. ananatis showed better growth than E. coli in acidic pH. According to standard microbiological tests on its bacteriological properties and nucleotide sequencing of its 16S rRNA [36], this strain was identified as P. ananatis [37]. The electron micrograph of P. ananatis AJ13355 shows the features of gram-negative and rod-shaped bacteria (Figure 2).
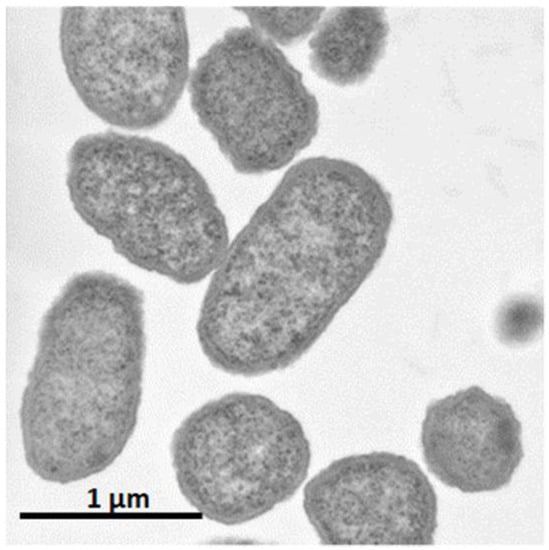
Figure 2.
Electron micrograph of Pantoea ananatis AJ13355 at a magnification of ×30,200.
The P. ananatis AJ13355 strain has passed all tests required for industrialization and has been recognized as a biosafety level 1 strain. The complete genomic sequence of P. ananatis AJ13355 was determined and found to consist of a single circular chromosome consisting of 4,555,536 bp (DDBJ: AP012032) and a circular plasmid (pEA320) with 321,744 bp (DDBJ: AP012033). After automated annotation, 4071 protein-coding sequences were identified in the P. ananatis AJ13355 genome [13]. The P. ananatis AJ13355 genome was compared with that of Escherichia coli, a model organism belonging to the Enterobacteriaceae family to which P. ananatis once belonged and which was utilized in the industrial production of various substances. Short colinear regions, which are identical to the DNA sequences in the E. coli MG1655 chromosome, were widely dispersed along the P. ananatis AJ13355 genome. Conjugal gene transfer from E. coli to P. ananatis, mediated by homologous recombination between short identical sequences, has also been experimentally demonstrated [13].
To develop a general genetic tool that can be used in P. ananatis AJ13355, Katashkina et al. [38] constructed novel non-mobilizable derivatives of RSF1010, a well-studied broad-host-range plasmid lacking all known DNA sequences involved in the mobilization process because of the exploitation of λ Red-driven recombination between the plasmid and an in vitro-constructed linear DNA fragment. Mobilization of the obtained RSFmob plasmid was not detected in standard tests, and high stability was demonstrated in E. coli and P. ananatis, which satisfies the biosafety requirements of genetically modified organisms used in scaled-up production [38].
To develop highly active producer strains with metabolically engineered pathways, it is necessary to manipulate many genes and express them individually at different levels or under separate regulatory controls. The construction of plasmid-less marker-less strains using sequential chromosome modifications, including deletions and integration of genetic material, has many advantages for further practical exploitation of these bacteria in industry. The λ Red-recombineering technique previously developed in E. coli is a high-performance tool for the rapid construction of precise genome modifications, such as deletion or insertion of genetic material, nucleotide changes, modification of regulatory regions, and construction of unmarked mutations. Although the expression of λ Red genes in P. ananatis was highly toxic, a mutant strain, SC17(0), grew well under simultaneous expression of λ gam, bet, and exo genes. Using this strain, site-specific and homologous recombination of phage λ, together with the possible transfer of marked mutations by electroporation of the chromosomal DNA, were adapted to genetically engineer P. ananatis AJ13355 and its derivatives [39]. Minaeva et al. [40] developed a new method of foreign DNA insertion for the step-by-step construction of plasmid-less marker-less recombinant E. coli strains with a chromosome structure designed in advance. The dual-in/out strategy is based on the initial Red-driven insertion of artificial φ80-attB sites into the desired points of the chromosome followed by two site-specific recombination processes: first, the φ80 system is used for integration of the recombinant DNA based on selective marker-carrier conditionally replicated plasmid with φ80-attP-site; and, second, the λ system is used for excision of the inserted vector part, including the plasmid ori-replication and the marker, flanked by λ-attL/R-sites [40]. Andreeva et al. [41] adopted a dual-in/out recombineering-driven strategy to integrate DNA fragments into targeted points on the E. coli chromosome for application in P. ananatis. P. ananatis pqqABCDEF was cloned in vivo and integrated into the chromosomes of P. ananatis using the dual-in/out strategy. The introduction of a second copy of pqqABCDEF to P. ananatis SC17(0) doubled the accumulation of PQQ [41]. This new approach has facilitated the design of recombinant marker-less and plasmid-less strains. It allows for the inclusion of large artificial inserts that are difficult to introduce using commonly used PCR-based recombination procedures. Katashkina et al. [42] further improved the dual-in/out system to a method that simultaneously incorporates a few DNA fragments into specific loci on the genome. They divided the mevalonic acid pathway into acetyl-CoA to mevalonic acid (MVA), MVA to phosphomevalonate, and the remaining pathway (see Isoprenoid production). Through the improved dual-in/out system and electroporation efficiency in P. ananatis, they were able to handle plasmids for all three parts simultaneously. They showed that their experimental design was sufficient to remove all selection markers [42]. Consequently, it is now possible to evaluate the production of genetically engineered strains that retain the desired multiple genetic traits, resulting in an increase in breeding speed.
4. L-Cysteine Production
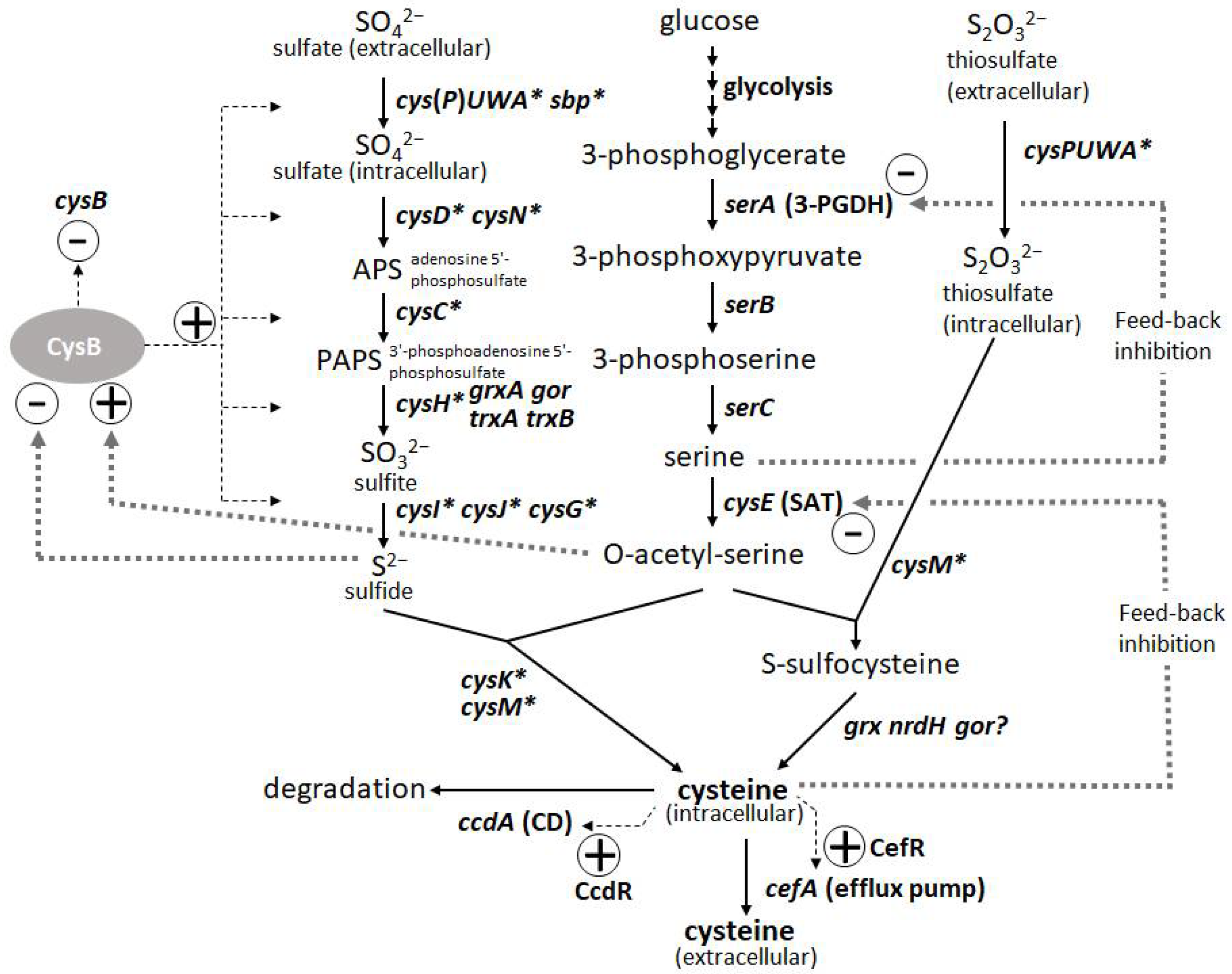
L-Cysteine is an important amino acid with many applications in various industries, including pharmaceuticals, food, and cosmetics. Bacterial fermentation is well-established for many major L-amino acids in commercial production; however, L-cysteine is one of the few exceptions, for which fermentative production methods have been demonstrated only in recent years and are still under development [43,44]. Because of the cytotoxicity of L-cysteine, bacterial cells are equipped with several modes of stringent metabolic regulation that allow them to strictly control the intracellular levels of L-cysteine, making overproduction of this compound more challenging. Dissecting these multifaceted and complicated intracellular regulations and freeing them from negative feedback controls is essential to achieve efficient overproduction. These regulatory mechanisms include feedback inhibition of two key enzymes, serine acetyltransferase (SAT) and 3-phosphoglycerate dehydrogenase (3-PGDH) by L-cysteine and L-serine, respectively, along with the L-cysteine biosynthetic pathway. CysB, a master regulator of sulfur assimilation and L-cysteine metabolism, controls the expression of most of the biosynthetic and metabolic genes associated with L-cysteine at the transcriptional level (Figure 3).
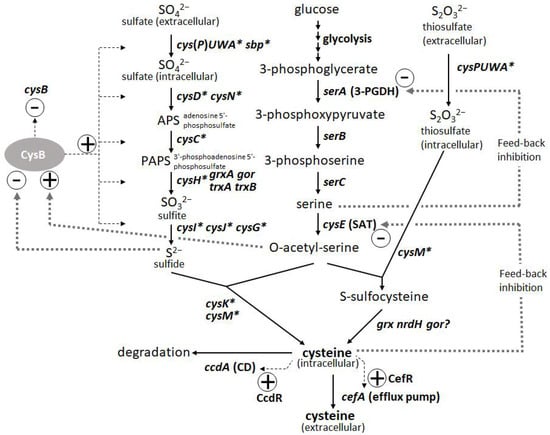
Figure 3.
Biosynthetic pathway and regulation of L-cysteine in Pantoea ananatis AJ13355 [45]. Thin and thick dotted lines indicate transcription and protein regulation, respectively. Asterisk indicates CysB regulon.
The degradation of L-cysteine provides another mode of regulation wherein cysteine desulfhydrases (CDs) play a central role in protecting bacterial cells from intracellular over-accumulation. The efflux systems of L-cysteine by specific exporters add an additional layer of regulation, in which they work as a safety valve to cope with the rapid increase in its intracellular levels. Thus, L-cysteine levels are regulated by its biosynthesis, degradation, and efflux [45]. To achieve overproduction, negative regulations, namely SAT and 3-PGDH, must be deregulated, and positive regulations, namely CysB regulons of biosynthesis, must be enhanced. CDs have to be removed, but they must be combined with the enhancement of efflux transporters because the transporters contribute to recovering damaged cells by accumulating L-cysteine under deficient conditions of CDs and promoting extracellular production of L-cysteine as its oxidized form L-cystine (L-cysteine can be easily oxidized extracellularly during aerobic cultivation). Once these core factors specific to L-cysteine production are regulated, general approaches such as enhancing bottlenecks and their biosynthetic pathways and shutting down the pathways to byproducts become more effective [15]. To date, among many amino-acid-producing microbes, P. ananatis and E. coli are advanced bacterial hosts for L-cysteine production [15,46].
The introduction of mutated key biosynthetic enzymes that are free from feedback inhibition is the first step in the development of amino-acid-producing microbes. There are two key enzymes in the L-cysteine biosynthetic pathway in P. ananatis: SAT and 3-PGDH encoded by cysE and serA, respectively. Mutations that remove the feedback inhibition by L-cysteine have been well-studied and applied to L-cysteine production in E. coli (Figure 3). Effective mutations identified for 3-PGDH in E. coli [47] could apply to the corresponding position of this enzyme in P. ananatis [48]. Kai et al. [49] identified many substantial effective mutations in the SAT of E. coli and P. ananatis that abolished feedback inhibition by comparing the crystal structures of SAT with and without the allosteric inhibitor. The basic preliminary L-cysteine-producing strains can be constructed using these mutated enzymes, which typically produce traces of L-cysteine in productive media.
The second major step in genetic engineering is identifying and removing the genes involved in L-cysteine degradation. In E. coli, at least five CDs (TnaA, MetC, MalY, CysK, and CysM [50,51]) and one cysteine desulfidase (YhaM [52,53]) have been identified and demonstrated to exert positive effects on L-cysteine production through gene deletion. While E. coli has developed rather complicated degradation mechanisms involving multiple degradation enzymes, P. ananatis possesses the only CD encoded by ccdA. CcdA in P. ananatis is the major and strongest CD that is induced intensively by L-cysteine using CcdR encoded adjacent to ccdA in the corresponding locus; therefore, this CD is proposed to have a more distinctive function in L-cysteine decomposition to cope with the sudden increase in both intracellular and extracellular L-cysteine levels [54]. Deleting ccdA in P. ananatis is very effective for producing L-cysteine, as it removes detectable levels of CD activity in cells [15,45]. P. ananatis may have advantages over E. coli in managing degradation activity. E. coli strains with multiple gene knockouts could still exhibit significant CD activity [51], indicating that there were additional unidentified CDs that presumably negatively affected L-cysteine production. Moreover, many of the CDs in E. coli have significant metabolic functions (e.g., TnaA is a tryptophanase, and CysM and CysK are cysteine synthases), whose deletion may affect essential physiological functions, including growth and biosynthesis of L-cysteine.
The third step was to identify and utilize the efflux system of L-cysteine. Because of the deletion of CD gene(s), the cells exhibited poor growth that was due to the increased intracellular levels of L-cysteine. The introduction of efflux pumps increases the production of L-cysteine and recovers the damaged cells from poor growth caused by accumulated L-cysteine. YdeD and YfiK are functional efflux pumps in E. coli for L-cysteine [54,55]. However, since they were identified while searching for factors effective for overproducing L-cysteine, their substrates and physiological function in L-cysteine metabolism and regulation are unclear. However, P. ananatis has developed more specific efflux pumps of L-cysteine, associated with its metabolism and regulation. CefA and CefB of P. ananatis were discovered by screening for factors that confer resistance to high concentrations of L-cysteine (Figure 3). CefA was found to be inducible by L-cysteine using its counterpart regulator CefR, encoded adjacent to cefA in the corresponding locus; therefore, it has been proposed that it functions as a specific safety valve of L-cysteine [45]. Both CefA and CefB were demonstrated to be involved in L-cysteine overproduction in P. ananatis, where they significantly contributed to high-level production and recovery from L-cysteine toxicity caused by the absence of its decomposer ccdA [15]. P. ananatis may have an advantage over E. coli in exporting L-cysteine because it possesses more specific and efficient efflux systems that may allow the export of L-cysteine specifically without leaking out some essential metabolites required for cell viability.
If core regulatory systems that negatively control intracellular L-cysteine levels are deregulated (i.e., 3-PGDH and SAT) or turned off (i.e., CcdA) and positive systems are turned on (i.e., CefA and CefB), bottlenecks can be enhanced and unnecessary waste pathways can be attenuated. In P. ananatis, many biosynthetic genes have been shown to have positive effects on the overproduction of L-cysteine, including cysM and cysPUWA [15,48]. The inactivation of adverse genes such as ydjN and fliY, which encode an importer of cystine [56,57,58,59] and yciW, which are involved in methionine metabolism [60], was also demonstrated to have positive effects on L-cysteine production [61,62,63]. Modulation of constitutively active CysB, a master cysteine regulator that controls most of the cys genes, is reported to be effective in E. coli. This could be potentially applicable to P. ananatis, as this system is common in both.
Consequently, P. ananatis serves as an advantageous microbial host for the fermentative production of L-cysteine. It has many common features with E. coli in sulfur and l-cysteine metabolism. Several useful genetic tools and databases developed for E. coli can be applied to P. ananatis. Moreover, P. ananatis may be advantageous over E. coli in the modulation and management of degradation and efflux systems because it has developed native systems that are more specific and efficient for handling L-cysteine. The current best-reported demonstration of L-cysteine production by P. ananatis achieved 2.2 g/L levels in test tube fermentation [15], which was comparable to a recent demonstration in E. coli (0.6 g/L in shake flask, 5.1 g/L in fed-batch jar fermenter [64]; 1.4 g/L in shake flask, 7.5 g/L in fed-batch jar fermenter [46]). Further tuning the critical genetic elements by developing a fully optimized jar fermenter process can elevate the titer tens of times.
5. Isoprenoid Production
Isoprenoids are a very diverse and large group of natural products with important properties in various industries, such as pharmaceuticals, flavors, fragrances, fuels, and fuel additives; however, because they are secondary metabolites, their production in vivo is minimal despite their important physiological roles. Microbial production of many isoprenoids is desirable and has been attempted mainly in E. coli and Saccharomyces cerevisiae [65]. The biosynthesis pathway of isoprenoids is divided into two parts: formation of building blocks, isopentenyl pyrophosphate (IPP)/dimethylallyl pyrophosphate (DMAPP) of all isoprenoids, and isoprenoid synthases, which are usually derived from plants or bacteria. The biosynthesis pathways of isoprenoid precursors are categorized mainly into the mevalonic acid (MVA) and methylerythritol phosphate (MEP) pathways [66]. The theoretical yields of isoprenoids have been analyzed by using the MEP and MVA pathways and the effect of the starting molecules [67]. Yang et al. [68] reported a study of isoprene fermentation using both pathways in E. coli. However, many other studies have not confirmed a successful combination of both pathways. One of the reasons for this is that the metabolic behavior of the MEP pathway has not yet been fully elucidated [69]. The MVA pathway is distributed in eukaryotes and archaea, and it is known that some pathways involve different enzymatic reactions depending on the species. In some prokaryotes, the presence of the MVA pathway has been suggested by genome sequences [70]. The existence of the MVA pathway was inferred based on the genome sequence information of the Corynebacterium variable. Mihara et al. [71] expressed a gene corresponding to the mevalonate kinase of C. variable in E. coli and successfully measured its activity as a mevalonate kinase.
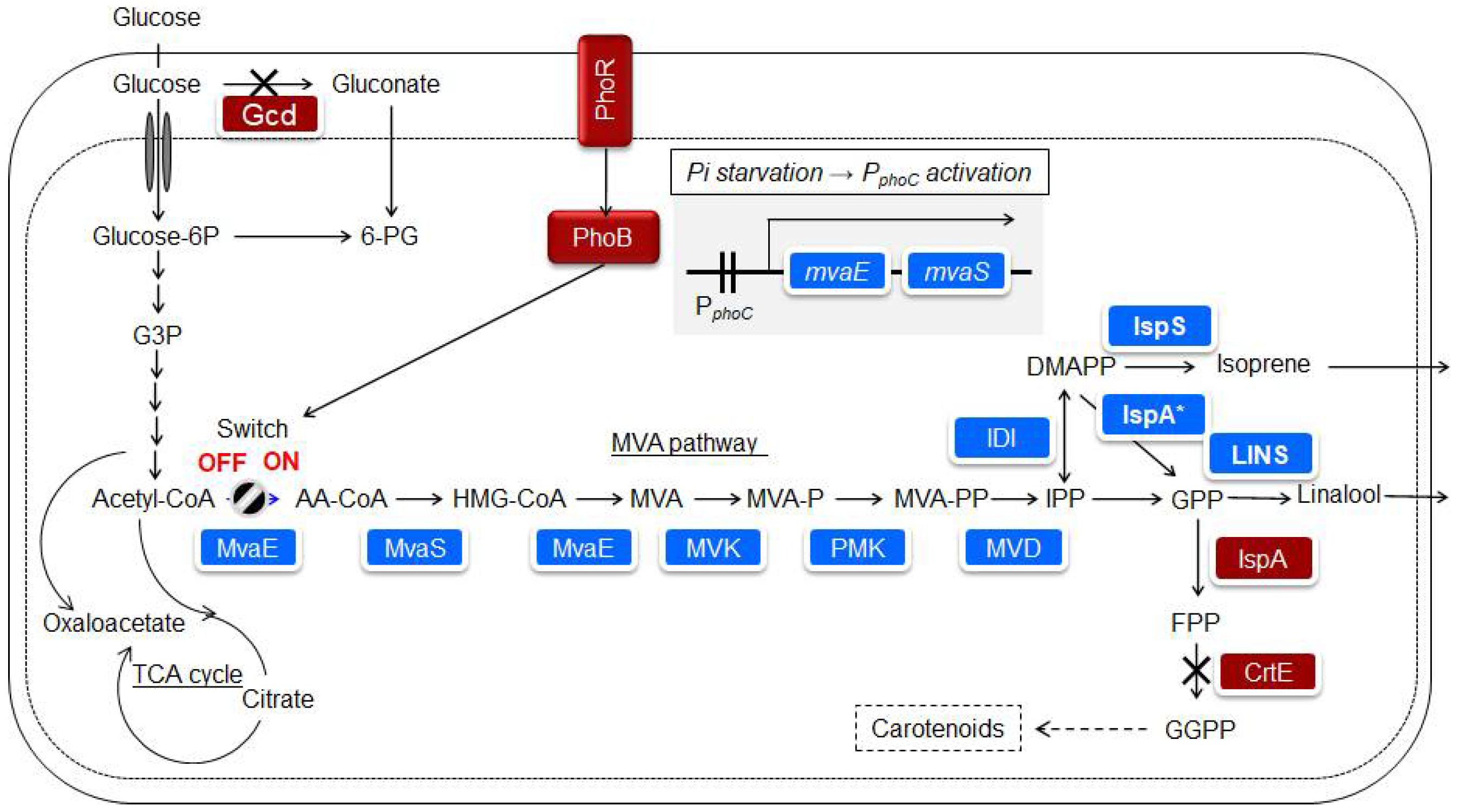
Isoprene is mainly produced from petroleum, is widely used in chemical industries, and is one of the desired compounds for microbial production. However, isoprene can quickly evaporate in the atmosphere because of its high vapor pressure. Bacillus subtilis and E. coli are known bacterial hosts [72], and it has been reported that 1.8 g/l of isoprene was produced in E. coli by isopropyl-β-D-thiogalactopyranoside (IPTG) induction of a heterologous MVA pathway [73]. The IPTG, or arabinose induction system, is widely known; however, it is not a panacea in terms of the responsiveness and cost of the induction system. P. ananatis is also known to possess the MEP pathway and produce carotenoids. Nitta et al. [16] succeeded in developing a control system for the heterologous MVA pathway, in which isoprene production was initiated when the phosphate source in the medium was consumed by P. ananatis. An overview of the isoprenoid biosynthetic pathway and strain construction is shown in Figure 4 [17]. In E. coli, the PhoB/PhoR two-component regulatory system responded to phosphate deficiency [74]. The promoters of phoC and pstS, which were assumed to be under the control of the PhoB/PhoR two-component regulatory system of P. ananatis, were used for MVA production. The mvaE and mvaS from Enterococcus faecalis, which encode enzymes in the pathway from acetyl-CoA to MVA, are concatenated to form an artificial operon linked to the promoters of the phoC or pstS genes in P. ananatis. A genetically modified strain was constructed by introducing one copy of this unit into the P. ananatis genome. In MVA fermentation using this strain, the accumulation of MVA was low under rich phosphate source conditions in the medium, while a significant amount of MVA was observed under a limited phosphate source [16]. This result suggests that the promoters of phoC and pstS can be used to produce substances that respond to phosphate concentration. Katashkina et al. [42] studied isoprene production via the MVA pathway in P. ananatis.
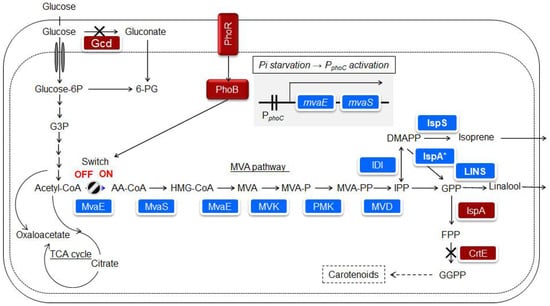
Figure 4.
Biosynthetic pathway introduced into Pantoea ananatis AJ13355 to produce isoprenoids. The introduced foreign genes or enzymes are shown on a blue background. Host-derived enzymes are shown on a brown background [17].
The mvaE and mvaS genes from E. faecalis were introduced under the control of a phosphate responsive promoter. Three genes encoding phosphomevalonate kinase, diphosphomevalonate decarboxylase, and isopentenyl pyrophosphate isomerase from S. cerevisiae were artificially constructed into operons and linked to the tac promoter, a constitutive promoter [42]. It has been reported that eukaryotic mevalonate kinase is inhibited by phosphate compounds in the isoprenoid biosynthesis pathway. Archaea synthesize IPPs using MVA as an intermediate, but the details of the biosynthetic pathway are known to be different from those of the MVA pathway that exists widely in eukaryotes. In terms of metabolic engineering, studies are being conducted to obtain mutants that release feedback inhibition of mevalonate kinase and search for mevalonate kinases that do not have feedback inhibition [75]. Kazieva et al. [76] purified His-tagged recombinant mevalonate kinases from the methanogens Methanosaeta concilii and Methanocella paludicola using E. coli and characterized them for activity and feedback inhibition. These enzymes had higher catalytic efficiencies (kcat/Km) than enzymes from Methanosarcina mazei, which were previously reported as mevalonate kinases that were not subject to feedback inhibition. These enzymes or the enzyme from M. mazei were also not subjected to feedback inhibition [76]. The expression of the gene encoding mevalonate kinase from M. paludicola was implemented by introducing an expression unit using the tac promoter into a locus separate from the artificial operon. As a result, all genes of the MVA pathway were integrated into the genome of P. ananatis, and all enzymes were functional during isoprene production in P. ananatis [42].
Isoprene is finally produced by isoprene synthase using DMAPP as a substrate (Figure 4). Isoprene synthase has been mostly derived from plants [77]. Expression plasmids for Mucuna, Kudzu, and Populus isoprene synthases were constructed and heterologously expressed in E. coli. Crude protein extracts were prepared from these strains. A comparison of the isoprene production capacity per crude protein extract suggested that the enzyme from Mucuna exhibited the highest apparent activity [78]. Previous studies on poplar-derived isoprene synthase expressed in E. coli reported that an apparent Michaelis constant for DMAPP was 2.45 mM [79]. This suggests significant room for improvement in the use of isoprene synthase as an enzyme in material production. Nakata et al. [80] designed and evaluated isoprene synthase mutants from Mucuna based on 3D structure prediction. After conducting a simple purification, the substrate and enzyme were mixed, and the amount of isoprene generated was measured for each mutant after 18 h of incubation. A C521E mutant, which showed a 9.4-fold increase in isoprene production capacity compared to the wild-type enzyme, was successfully obtained. Isoprene synthase from Mucuna was expressed using a high-copy number vector [42]. Nitta et al. [16] analyzed the change in isoprene production over time using strains that retained these genetic traits in fed-batch cultures.
Isoprene production started when the inorganic phosphorus concentration in the culture medium was depleted, and the isoprene concentration in the off-gas of the fermenter reached 450 ppm. This indicated that the MVA pathway and isoprene synthase were successfully introduced into P. ananatis to synthesize isoprene [16]. In general, in the production of useful substances, a higher concentration or accumulation of the target substance has an advantage in terms of production cost. Isoprene has been reported to have an explosive range, with a lower explosive limit of 1.5 vol% and an upper explosive limit of 8.9% [81]. Because isoprene is a highly volatile substance, it requires a combination of technologies not manageable using conventional fermentation technologies, such as gas recovery technology and hazardous material handling in fermentation facilities. From the sustainability viewpoint, it is desirable to study the manufacturing method after considering the starting materials, economic rationality, and safety design.
Linalool is a monoterpenoid compound that is widely used for flavor. In the biosynthetic pathway, geranyl pyrophosphate (GPP) is a substrate of linalool synthase (Figure 4). Previous studies have shown that E. coli IspA produces farnesyl pyrophosphate more than GPP, and a mutant form of IspA (IspA * S80F) is effective for studying the biosynthesis of monoterpenoids [82]. Linalools also have an intramolecular chiral center; therefore, S and R forms exist. A set of strains with almost the same genetic traits as isoprene-producing strains, but with linalool synthase and IspA * instead of isoprene synthase, were constructed [83]. In addition, unlike isoprene, linalool remains in the solution and continues to adhere to the microorganisms, which may cause harmful effects such as inhibition of respiratory activity. As a result, the two-phase culture method was adopted, with isopropyl myristate added as a solvent. Thirteen candidate linalool synthases were introduced into P. ananatis, and their ability to produce linalool and optical activity was confirmed. The results showed that 100% S linalool synthesis was observed when the enzymes from Malus domestica and Actinidia arguta were used, and 100% R linalool synthesis was observed when the enzymes from Ocimum basilicum and Streptomyces clavuligerus were used [83]. Nitta et al. [17] heterologously expressed S-linalool synthase from A. arguta in P. ananatis, prepared a crude enzyme extract, examined the expression levels in the soluble and insoluble fractions, and found that most of the S-linalool synthase was present in the insoluble fraction. For substantial production, it is desirable to identify S-linalool synthase in the soluble fraction. The expression level in the soluble fraction was increased by adding solubilizing tags and examining the frequency of codon usage [17]. The structural features of class I terpene synthases distributed in plants, including isoprene synthase and S-linalool synthase, are relatively similar [84]. In the expression of plant-derived Class I terpene synthases in microbial hosts, from the viewpoint of enhancing the substance production capacity, it is suggested that various devices, such as the design of gene expression units, improvement of enzyme kinetics, and enhancement of solubilization rate, are necessary. Nitta et al. [17] studied the culture conditions and expression method of the enzyme for (S)-linalool production. The results showed that (S)-linalool accumulation had a production capacity of > 10 g/L. By implementing several strategies, high production capacity was achieved. The following methods were used to achieve high production capacity: (1) introduction of a tag to increase the solubility of linalool synthase, (2) increase in the ability to synthesize GPP as a precursor, which increases the activity of the enzyme in the cell, and (3) adoption of a culture method that separates the growth of the strains from the production of (S)-linalool, including a two-phase culture and phosphate-responsible promoter [17].
In an earlier study, Streptomyces and E. coli were widely used as hosts for microbial production of isoprenoids. Hoshino et al. [83] introduced genes for linalool synthase and IspA * into wild-type strains of E. coli and P. ananatis, respectively, and found that linalool accumulation was more than doubled in the wild strain of P. ananatis. The P. ananatis AJ13355 strain was also shown to be a suitable platform for producing terpene compounds. With further strain improvement and process development, the microbial production technology of monoterpenoid compounds by P. ananatis strain AJ13355 is expected to reach a commercial level.
6. Summary of P. ananatis Features
Among the P. ananatis strains identified to date, including those that promote plant growth from phytopathogens, much knowledge is accumulating on strain AJ 13355 as a strain with industrial production advantages. The characteristics of P. ananatis strain AJ 13355 useful for industrial production are summarized in Table 2. The pH growth range in an acidic region is broader than E. coli and C. glutamicum (Figure 1). Optimal growth temperature is 34 °C lower than E. coli (37 °C) and higher than C. glutamicum (31.5 °C) [11]. In the properties related to recombinant DNA, broad-host-range plasmids are available [38]. Genome engineering technologies have been developed to a level comparable to E. coli [39,40,41]. Gene regulation is generally similar to E. coli, but partly different, which was described in the regulation of L-cysteine [11,15]. P. ananatis can express actinomycetes and plant-derived genes [16,17,83], which can be advantageous for secondary metabolite production. Organic solvent tolerance is generally equivalent to E. coli; however, wild-strain-based production of isoprenoids was better in P. ananatis than in E. coli [83].

Table 2.
Characteristics of Pantoea ananatis AJ13355.
7. Conclusions
Among the isolated non-pathogenic P. ananatis, the P. ananatis strain AJ13355 was a suitable platform for producing useful substances. Genetic tools are now available for P. ananatis AJ13355, and it is becoming possible to breed it with the speed and accuracy of E. coli. It is also expected that P. ananatis AJ13355 has a wider acidic pH range than other common fermentation bacteria and is more resistant to stress. P. ananatis has different metabolisms and regulations from those of model industrial organisms, and breeding strategies can be developed to take advantage of its characteristics. It is possible to introduce heterologous biosynthetic pathways into P. ananatis, and it has been shown that it can be used to produce isoprenoids that are volatile and harmful to bacterial cells in two-phase cultivation processes using organic solvents.
Currently, the technology to produce value-added substances of plant origin, such as bioactive and aromatic substances, is one of the expected directions of bioproduction in the future. From the perspective of the sustainable development goals (SDGs), there is also a need for technology to produce many types of petrochemicals with plant-derived sugars produced using microbial fermentation. For this purpose, a high throughput and rapid development of a production platform using P. ananatis AJ13355 combined with further synthetic biology methods is required. In addition, the production system must be optimized for industrialization. We hope that these efforts will make P. ananatis AJ13355 a more suitable strain for the industrial production of useful substances and contribute to fermentation production.
Author Contributions
All authors contributed to the conceptualization, writing—original draft preparation, and writing—review and editing of this article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors thank Oonuki, A., Tajima, Y., and Katashkina, J. I. for carefully reading and providing critical comments on this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coutinho, T.A.; Venter, S.N. Pantoea ananatis: An Unconventional Plant Pathogen. Mol. Plant Pathol. 2009, 10, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Adeolu, M.; Alnajar, S.; Naushad, S.; Gupta, R.S. Genome-Based Phylogeny and Taxonomy of the ‘Enterobacteriales’: Proposal for Enterobacterales ord. nov. Divided into the Families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5575–5599. [Google Scholar] [CrossRef] [PubMed]
- Serrano, F.B. Bacterial Fruitlet Brown-Rot of Pineapple in the Philippines. Philipp. J. Sci. 1928, 36, 271–324. [Google Scholar]
- Gavini, F.; Mergaert, J.; Beji, A.; Mielcarek, C.; Izard, D.; Kersters, K.; De Ley, J. Transfer of Enterobacter agglomerans (Beijerinck 1888) Ewing and Fife 1972 to Pantoea gen. nov. as Pantoea agglomerans comb. nov. and Description of Pantoea dispersa sp. nov. Int. J. Syst. Bacteriol. 1989, 39, 337–345. [Google Scholar] [CrossRef]
- Mergaert, J.; Verdonck, L.; Kersters, K. Transfer of Erwinia ananas (Synonym, Erwinia uredovora) and Erwinia stewartii to the Genus Pantoea emend. as Pantoea ananas (Serrano 1928) comb. nov. and Pantoea stewartii (Smith 1898) comb. nov., Respectively, and Description of Pantoea stewartii subsp. indologenes subsp. nov. Int. J. Syst. Bacteriol. 1993, 43, 162–173. [Google Scholar] [CrossRef]
- Trüper, H.G.; De’Clari, L. Taxonomic Note: Necessary Correction of Specific Epithets Formed as Substantives (Nouns) “in Apposition”. Int. J. Syst. Evol. 1997, 47, 908–909. [Google Scholar] [CrossRef] [Green Version]
- Weller-Stuart, T.; De Maayer, P.; Coutinho, T. Pantoea ananatis: Genomic Insights into a Versatile Pathogen. Mol. Plant Pathol. 2017, 18, 1191–1198. [Google Scholar] [CrossRef]
- Becker, J.; Wittmann, C. Advanced Biotechnology: Metabolically Engineered Cells for the Bio-Based Production of Chemicals and Fuels, Materials, and Health-Care Products. Angew. Chem. Int. Ed. Engl. 2015, 54, 3328–3350. [Google Scholar] [CrossRef]
- Pontrelli, S.; Chiu, T.Y.; Lan, E.I.; Chen, F.Y.; Chang, P.; Liao, J.C. Escherichia coli as a Host for Metabolic Engineering. Metab. Eng. 2018, 50, 16–46. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, S.; Udaka, S.; Shimono, M. Studies on The Amino Acid Fermentation. J. Gen. Appl. Microbiol. 1957, 3, 193–205. [Google Scholar] [CrossRef]
- Becker, J.; Wittmann, C. Bio-Based Production of Chemicals, Materials and Fuels—Corynebacterium glutamicum as Versatile Cell Factory. Curr. Opin. Biotechnol. 2012, 23, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, Y.; Matsuzawa, H. Recent Progress in Production of Amino Acid-Derived Chemicals Using Corynebacterium glutamicum. World J. Microbiol. Biotechnol. 2021, 37, 49. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Kadotani, N.; Izui, H.; Katashkina, J.I.; Kuvaeva, T.M.; Andreeva, I.G.; Golubeva, L.I.; Malko, D.B.; Makeev, V.J.; Mashko, S.V.; et al. The Complete Genome Sequence of Pantoea ananatis AJ13355, an Organism with Great Biotechnological Potential. Appl. Microbiol. Biotechnol. 2012, 93, 331–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usuda, Y.; Hara, Y.; Kojima, H. Toward Sustainable Amino Acid Production. In Advances in Biochemical Engineering/Biotechnology; Yokota, A., Ikeda, M., Eds.; Springer: Tokyo, Japan, 2017; Volume 159, pp. 289–304. [Google Scholar] [CrossRef]
- Takumi, K.; Ziyatdinov, M.K.; Samsonov, V.; Nonaka, G. Fermentative Production of Cysteine by Pantoea ananatis. Appl. Environ. Microbiol. 2017, 83, e02502-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitta, N.; Tajima, Y.; Katashkina, J.I.; Yamamoto, Y.; Onuki, A.; Rachi, H.; Kazieva, E.; Nishio, Y. Application of Inorganic Phosphate Limitation to Efficient Isoprene Production in Pantoea ananatis. J. Appl. Microbiol. 2020, 128, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Tajima, Y.; Yamamoto, Y.; Moriya, M.; Matsudaira, A.; Hoshino, Y.; Nishio, Y.; Usuda, Y. Fermentative Production of Enantiopure (S)-Linalool Using a Metabolically Engineered Pantoea ananatis. Microb. Cell Fact. 2021, 20, 54. [Google Scholar] [CrossRef]
- Thomas, P.; Kumari, S.; Swarna, G.K.; Gowda, T.K.S. Papaya Shoot Tip Associated Endophytic Bacteria Isolated from In Vitro Cultures and Host–Endophyte Interaction In Vitro and In Vivo. Can. J. Microbiol. 2007, 53, 380–390. [Google Scholar] [CrossRef]
- Kim, S.-N.; Cho, W.K.; Kim, W.-I.; Jee, H.J.; Park, C.-S. Growth Promotion of Pepper Plants by Pantoea ananatis B1-9 and Its Efficient Endophytic Colonization Capacity in Plant Tissues. Plant Pathol. J. 2012, 28, 270–281. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.-Y.; Zeng, Q.; Nong, Z.M.; Ye, X.-L.; Cen, Z.-L.; Li, Y.-R.; Hu, C.-J. Identification of an Endophytic Nitrogen-Fixing Bacterium NN08200 from Sugarcane and Its Growth Promotion of Sugarcane. Microbiol. China. 2019, 46, 1336–1345. [Google Scholar] [CrossRef]
- Zeng, Q.; Shi, G.; Nong, Z.; Ye, X.; Hu, C. Complete Genome Sequence of Pantoea ananatis strain NN08200, an Endophytic Bacterium Isolated from Sugarcane. Curr. Microbiol. 2020, 77, 1864–1870. [Google Scholar] [CrossRef] [Green Version]
- Gkorezis, P.; Van Hamme, J.D.; Bottos, E.M.; Thijs, S.; Balseiro-Romero, M.; Monterroso, C.; Kidd, P.S.; Rineau, F.; Weyens, N.; Vangronsveld, J. Draft Genome Sequence of Pantoea ananatis GB1, a Plant-Growth-Promoting Hydrocarbonoclastic Root Endophyte, Isolated at a Diesel Fuel Phytoremediation Site Planted with Populus. Genome Announc. 2016, 4, e00028-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megías, E.; Megías, M.; Ollero, F.J.; Hungria, M. Draft Genome Sequence of Pantoea ananatis strain AMG521, a Rice Plant Growth-Promoting Bacterial Endophyte Isolated from the Guadalquivir Marshes in Southern Spain. Genome Announc. 2016, 4, e01681-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megías, E.; Dos Reis Junior, F.B.; Ribeiro, R.A.; Ollero, F.J.; Megías, M.; Hungria, M. Draft Genome Sequence of Pantoea ananatis strain 1.38, a Bacterium Isolated from the Rhizosphere of Oryza sativa var. Puntal That Shows Biotechnological Potential as an Inoculant. Genome Announc. 2018, 6, e01547-17. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.H.; Kang, B.R.; Rong, X.; McSpadden Gardener, B.B.; Ji, H.J.; Park, C.S.; Kim, Y.C. Draft Genome Sequence of Pantoea ananatis B1-9, a Nonpathogenic Plant Growth-Promoting Bacterium. J. Bacteriol. 2012, 194, 729. [Google Scholar] [CrossRef] [Green Version]
- Misawa, N.; Shimada, H. Metabolic Engineering for the Production of Carotenoids in non-Carotenogenic Bacteria and Yeasts. J. Biotechnol. 1997, 59, 169–181. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, J.E.; Lee, S.H.; Park, H.M.; Choi, M.S.; Kim, J.Y.; Lee, S.H.; Shin, Y.C.; Keasling, J.D.; Kim, S.W. Engineering the Lycopene Synthetic Pathway in E. coli by Comparison of the Carotenoid Genes of Pantoea agglomerans and Pantoea ananatis. Appl. Microbiol. Biotechnol. 2007, 74, 131–139. [Google Scholar] [CrossRef]
- De Maayer, P.; Chan, W.Y.; Venter, S.N.; Toth, I.K.; Birch, P.R.; Joubert, F.; Coutinho, T.A. Genome Sequence of Pantoea ananatis LMG20103, the Causative Agent of Eucalyptus Blight and Dieback. J. Bacteriol. 2010, 192, 2936–2937. [Google Scholar] [CrossRef] [Green Version]
- Choi, O.; Lim, J.Y.; Seo, Y.S.; Hwang, I.; Kim, J. Complete Genome Sequence of the Rice Pathogen Pantoea ananatis strain PA13. J. Bacteriol. 2012, 194, 531. [Google Scholar] [CrossRef] [Green Version]
- De Maayer, P.; Chan, W.Y.; Rezzonico, F.; Bühlmann, A.; Venter, S.N.; Blom, J.; Goesmann, A.; Frey, J.E.; Smits, T.H.; Duffy, B.; et al. Complete Genome Sequence of Clinical Isolate Pantoea ananatis LMG 5342. J. Bacteriol. 2012, 194, 1615–1616. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Liu, R.; Niu, Y.; Lin, H.; Ye, W.; Guo, L.; Hu, X. Whole Genome Sequence of Pantoea ananatis R100, an Antagonistic Bacterium Isolated from Rice Seed. J. Biotechnol. 2016, 225, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Yu, J.; Jia, M.; Zheng, L.; Feng, Y. Indole Enhances the Survival of Pantoea ananatis YJ76 in Face of Starvation Conditions. J. Basic Microbiol. 2017, 57, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Stice, S.P.; Stumpf, S.D.; Gitaitis, R.D.; Kvitko, B.H.; Dutta, B. Pantoea ananatis Genetic Diversity Analysis Reveals Limited Genomic Diversity as Well as Accessory Genes Correlated with Onion Pathogenicity. Front. Microbiol. 2018, 9, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Yang, C.; Ji, Z.; Zeng, Y.; Liang, Y.; Hou, Y. First Report of New Bacterial Leaf Blight of Rice Caused by Pantoea ananatis in Southeast China. Plant Dis. 2021, 106, PDIS-05. [Google Scholar] [CrossRef] [PubMed]
- Moriya, M.; Izui, H.; Ono, E.; Matsui, K.; Ito, H.; Hara, Y. L-Glutamic Acid-Producing Bacterium and Method for Producing L-Glutamic Acid. U.S. Patent 6,331,419, 18 December 2001. [Google Scholar]
- Kwon, S.W.; Go, S.J.; Kang, H.W.; Ryu, J.C.; Jo, J.K. Phylogenetic Analysis of Erwinia species Based on 16S RRNA Gene Sequences. Int. J. Syst. Bacteriol. 1997, 47, 1061–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, Y.; Tateyama, Y.; Sato, M. Process for Producing L-Glutamic Acid. U.S. Patent US7,354,744, 8 April 2008. [Google Scholar]
- Katashkina, J.I.; Kuvaeva, T.M.; Andreeva, I.G.; Skorokhodova, A.Y.; Biryukova, I.V.; Tokmakova, I.L.; Golubeva, L.I.; Mashko, S.V. Construction of Stably Maintained Non-Mobilizable Derivatives of RSF1010 Lacking All Known Elements Essential for Mobilization. BMC Biotechnol. 2007, 7, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katashkina, J.I.; Hara, Y.; Golubeva, L.I.; Andreeva, I.G.; Kuvaeva, T.M.; Mashko, S.V. Use of the λ Red-Recombineering Method for Genetic Engineering of Pantoea ananatis. BMC Mol. Biol. 2009, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Minaeva, N.I.; Gak, E.R.; Zimenkov, D.V.; Skorokhodova, A.Y.; Biryukova, I.V.; Mashko, S.V. Dual-In/Out Strategy for Genes Integration into Bacterial Chromosome: A Novel Approach to Step-by-Step Construction of Plasmid-Less Marker-Less Recombinant E. coli Strains with Predesigned Genome Structure. BMC Biotechnol. 2008, 8, 63. [Google Scholar] [CrossRef] [Green Version]
- Andreeva, I.G.; Golubeva, L.I.; Kuvaeva, T.M.; Gak, E.R.; Katashkina, J.I.; Mashko, S.V. Identification of Pantoea ananatis Gene Encoding Membrane Pyrroloquinoline Quinone (PQQ)-Dependent Glucose Dehydrogenase and pqqABCDEF Operon Essential for PQQ Biosynthesis. FEMS Microbiol. Lett. 2011, 318, 55–60. [Google Scholar] [CrossRef]
- Katashkina, J.I.; Kazieva, E.D.; Tajima, Y.; Mashko, S.V. Increased Isoprene Production by the Recombinant Pantoea ananatis strain due to the Balanced Amplification of Mevalonate Pathway Genes. Appl. Biochem. Microbiol. 2019, 55, 850–860. [Google Scholar] [CrossRef]
- Wada, M.; Takagi, H. Metabolic Pathways and Biotechnological Production of L-Cysteine. Appl. Microbiol. Biotechnol. 2006, 73, 48–54. [Google Scholar] [CrossRef]
- Takagi, H.; Ohtsu, I. L-Cysteine Metabolism and Fermentation in Microorganisms. Adv. Biochem. Eng. Biotechnol. 2017, 159, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Takumi, K.; Nonaka, G. Bacterial Cysteine-Inducible Cysteine Resistance Systems. J. Bacteriol. 2016, 198, 1384–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Hou, Y.; Wang, Y.; Li, Z. Enhancement of Sulfur Conversion Rate in the Production of L-Cysteine by Engineered Escherichia coli. J. Agric. Food Chem. 2020, 68, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Al-Rabiee, R.; Zhang, Y.; Grant, G.A. The Mechanism of Velocity Modulated Allosteric Regulation in D-3-phosphoglycerate Dehydrogenase. Site-Directed Mutagenesis of Effector Binding Site Residues. J. Biol. Chem. 1996, 271, 23235–23238. [Google Scholar] [CrossRef] [Green Version]
- Takumi, K.; Nonaka, G. An L-Amino Acid-Producing Bacterium and a Method for Producing an L-Amino Acid. European Patent EP2,218,729, 9 July 2014. [Google Scholar]
- Kai, Y.; Kashiwagi, T.; Ishikawa, K.; Ziyatdinov, M.K.; Redkina, E.I.; Kiriukhin, M.Y.; Gusyatiner, M.M.; Kobayashi, S.; Takagi, H.; Suzuki, E.E. Engineering of Escherichia coli L-Serine O-acetyltransferase on the Basis of Crystal Structure: Desensitization to Feedback Inhibition by L-Cysteine. Protein Eng. Des. Sel. 2006, 19, 163–167. [Google Scholar] [CrossRef]
- Awano, N.; Wada, M.; Kohdoh, A.; Oikawa, T.; Takagi, H.; Nakamori, S. Effect of Cysteine Desulfhydrase Gene Disruption on L-Cysteine Overproduction in Escherichia coli. Appl. Microbiol. Biotechnol. 2003, 62, 239–243. [Google Scholar] [CrossRef]
- Awano, N.; Wada, M.; Mori, H.; Nakamori, S.; Takagi, H. Identification and Functional Analysis of Escherichia coli Cysteine Desulfhydrases. Appl. Environ. Microbiol. 2005, 71, 4149–4152. [Google Scholar] [CrossRef] [Green Version]
- Shimada, T.; Tanaka, K.; Ishihama, A. Transcription Factor DecR (YbaO) Controls Detoxification of L-Cysteine in Escherichia coli. Microbiology 2016, 162, 1698–1707. [Google Scholar] [CrossRef]
- Nonaka, G.; Takumi, K. Cysteine Degradation Gene yhaM, Encoding Cysteine Desulfidase, Serves as a Genetic Engineering Target to Improve Cysteine Production in Escherichia coli. AMB Express 2017, 7, 90. [Google Scholar] [CrossRef] [Green Version]
- Dassler, T.; Maier, T.; Winterhalter, C.; Böck, A. Identification of a Major Facilitator Protein from Escherichia coli Involved in Efflux of Metabolites of the Cysteine Pathway. Mol. Microbiol. 2000, 36, 1101–1112. [Google Scholar] [CrossRef]
- Franke, I.; Resch, A.; Dassler, T.; Maier, T.; Böck, A. YfiK from Escherichia coli Promotes Export of O-Acetylserine and Cysteine. J. Bacteriol. 2003, 185, 1161–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtsu, I.; Wiriyathanawudhiwong, N.; Morigasaki, S.; Nakatani, T.; Kadokura, H.; Takagi, H. The L-Cysteine/L-Cystine Shuttle System Provides Reducing Equivalents to the Periplasm in Escherichia coli. J. Biol. Chem. 2010, 285, 17479–17487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtsu, I.; Kawano, Y.; Suzuki, M.; Morigasaki, S.; Saiki, K.; Yamazaki, S.; Nonaka, G.; Takagi, H. Uptake of L-Cystine via an ABC Transporter Contributes Defense of Oxidative Stress in the L-Cystine Export-Dependent Manner in Escherichia coli. PLoS ONE 2015, 10, e0120619. [Google Scholar] [CrossRef] [Green Version]
- Chonoles Imlay, K.R.; Korshunov, S.; Imlay, J.A. Physiological Roles and Adverse Effects of the Two Cystine Importers of Escherichia Coli. J. Bacteriol. 2015, 197, 3629–3644. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, S.; Takei, K.; Nonaka, G. ydjN Encodes an S-Sulfocysteine Transporter Required by Escherichia coli for Growth on S-Sulfocysteine as a Sulfur Source. FEMS Microbiol. Lett. 2016, 363, fnw185. [Google Scholar] [CrossRef] [Green Version]
- Kawano, Y.; Ohtsu, I.; Tamakoshi, A.; Shiroyama, M.; Tsuruoka, A.; Saiki, K.; Takumi, K.; Nonaka, G.; Nakanishi, T.; Hishiki, T.; et al. Involvement of the yciW Gene in L-Cysteine and L-Methionine Metabolism in Escherichia coli. J. Biosci. Bioeng. 2015, 119, 310–313. [Google Scholar] [CrossRef]
- Nonaka, G. L-Cysteine-Producing Bacterium and a Method for Producing L-Cysteine. U.S. Patent US8,647,847, 11 February 2014. [Google Scholar]
- Nonaka, G. L-Cysteine-Producing Bacterium and a Method for Producing L-Cysteine. U.S. Patent US20,100,233,765, 16 September 2010. [Google Scholar]
- Kawano, Y.; Ohtsu, I.; Takumi, K.; Tamakoshi, A.; Nonaka, G.; Funahashi, E.; Ihara, M.; Takagi, H. Enhancement of L-Cysteine Production by Disruption of yciW in Escherichia coli. J. Biosci. Bioeng. 2015, 119, 176–179. [Google Scholar] [CrossRef]
- Liu, H.; Fang, G.; Wu, H.; Li, Z.; Ye, Q. L-Cysteine Production in Escherichia coli Based on Rational Metabolic Engineering and Modular Strategy. Biotechnol. J. 2018, 13, 1700695. [Google Scholar] [CrossRef]
- Wang, C.; Liwei, M.; Park, J.B.; Jeong, S.H.; Wei, G.; Wang, Y.; Kim, S.W. Microbial Platform for Terpenoid Production: Escherichia coli and Yeast. Front. Microbiol. 2018, 9, 2460. [Google Scholar] [CrossRef]
- Chang, M.C.Y.; Keasling, J.D. Production of Isoprenoid Pharmaceuticals by Engineered Microbes. Nat. Chem. Biol. 2006, 2, 674–681. [Google Scholar] [CrossRef]
- Gruchattka, E.; Hädicke, O.; Klamt, S.; Schütz, V.; Kayser, O. In Silico Profiling of Escherichia coli and Saccharomyces cerevisiae as Terpenoid Factories. Microb. Cell Fact. 2013, 12, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Gao, X.; Jiang, Y.; Sun, B.; Gao, F.; Yang, S. Synergy Between Methylerythritol Phosphate Pathway and Mevalonate Pathway for Isoprene Production in Escherichia coli. Metab. Eng. 2016, 37, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Volke, D.C.; Rohwer, J.; Fischer, R.; Jennewein, S. Investigation of the Methylerythritol 4-Phosphate Pathway for Microbial Terpenoid Production Through Metabolic Control Analysis. Microb. Cell Fact. 2019, 18, 192. [Google Scholar] [CrossRef] [Green Version]
- Lombard, J.; Moreira, D. Origins and Early Evolution of the Mevalonate Pathway of Isoprenoid Biosynthesis in the Three Domains of Life. Mol. Biol. Evol. 2011, 28, 87–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihara, Y.; Rachi, H.; Nishio, Y.; Katashkina, Y.J.; Kazieva, D.E.; Andreeva, G.I. Method of Producing Isoprene Monomer. U.S. Patent US10,184,137, 22 January 2019. [Google Scholar]
- Ye, L.; Lv, X.; Yu, H. Engineering Microbes for Isoprene Production. Metab. Eng. 2016, 38, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Wang, C.; Jang, H.J.; Cha, M.S.; Park, J.E.; Jo, S.Y.; Choi, E.S.; Kim, S.W. Isoprene Production by Escherichia coli Through the Exogenous Mevalonate Pathway with Reduced Formation of Fermentation Byproducts. Microb. Cell Fact. 2016, 15, 214. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, Y.J.; Wanner, B.L. Global Regulation by the Seven-Component Pi Signaling System. Curr. Opin. Microbiol. 2010, 13, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Primak, Y.A.; Du, M.; Miller, M.C.; Wells, D.H.; Nielsen, A.T.; Weyler, W.; Beck, Z.Q. Characterization of a Feedback-Resistant Mevalonate Kinase from the Archaeon Methanosarcina mazei. Appl. Environ. Microbiol. 2011, 77, 7772–7778. [Google Scholar] [CrossRef] [Green Version]
- Kazieva, E.; Yamamoto, Y.; Tajima, Y.; Yokoyama, K.; Katashkina, J.; Nishio, Y. Characterization of Feedback-Resistant Mevalonate Kinases from the Methanogenic Archaeons Methanosaeta concilii and Methanocella paludicola. Microbiology 2017, 163, 1283–1291. [Google Scholar] [CrossRef]
- Murrell, J.C.; McGenity, T.J.; Crombie, A.T. Microbial Metabolism of Isoprene: A Much-Neglected Climate-Active Gas. Microbiology 2020, 166, 600–613. [Google Scholar] [CrossRef]
- Hayashi, Y.; Harada, M.; Takaoka, S.; Fukushima, Y.; Yokoyama, K.; Nishio, Y.; Tajima, Y.; Mihara, Y.; Nakata, K. Isoprene Synthase and Gene Encoding the Same, and Method for Producing Isoprene Monomer. Japanese Patent JP6,254,728, 27 December 2017. [Google Scholar]
- Schnitzler, J.P.; Zimmer, I.; Bachl, A.; Arend, M.; Fromm, J.; Fischbach, R.J. Biochemical Properties of Isoprene Synthase in Poplar (Populus × canescens). Planta 2005, 222, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Tajima, Y.; Tagami, U.; Oku, T.; Kashima, Y. Modified Isoprene Synthase. U.S. Patent US9,890,373, 13 February 2018. [Google Scholar]
- Sigma-Aldrich. Material Safe Data Sheet of Isoprene from. Available online: https://www.sigmaaldrich.com/JP/en/sds/aldrich/464953 (accessed on 5 December 2021).
- Reiling, K.K.; Yoshikuni, Y.; Martin, V.J.J.; Newman, J.; Bohlmann, J.; Keasling, J.D. Mono and Diterpene Production in Escherichia coli. Biotechnol. Bioeng. 2004, 87, 200–212. [Google Scholar] [CrossRef]
- Hoshino, Y.; Moriya, M.; Matsudaira, A.; Katashkina, J.I.; Nitta, N.; Nishio, Y.; Usuda, Y. Stereospecific Linalool Production Utilizing Two-Phase Cultivation System in Pantoea ananatis. J. Biotechnol. 2020, 324, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Honzatko, R.B.; Peters, R.J. Terpenoid Synthase Structures: A so Far Incomplete View of Complex Catalysis. Nat. Prod. Rep. 2012, 29, 1153–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).