A Journey into Animal Models of Human Osteomyelitis: A Review
Abstract
:1. Background
2. Materials and Methods
3. Bacterial Biofilm
3.1. Device-Related and Non-Device-Related Biofilm Infections
3.2. Microbiology of Bacterial Osteomyelitis
4. Immune Response
5. Elements of Bone Anatomy
5.1. Histopathological Generalities on Osteomyelitis
5.2. Periprosthetic Joint Infection (PJI)
5.3. Post-Traumatic Osteomyelitis
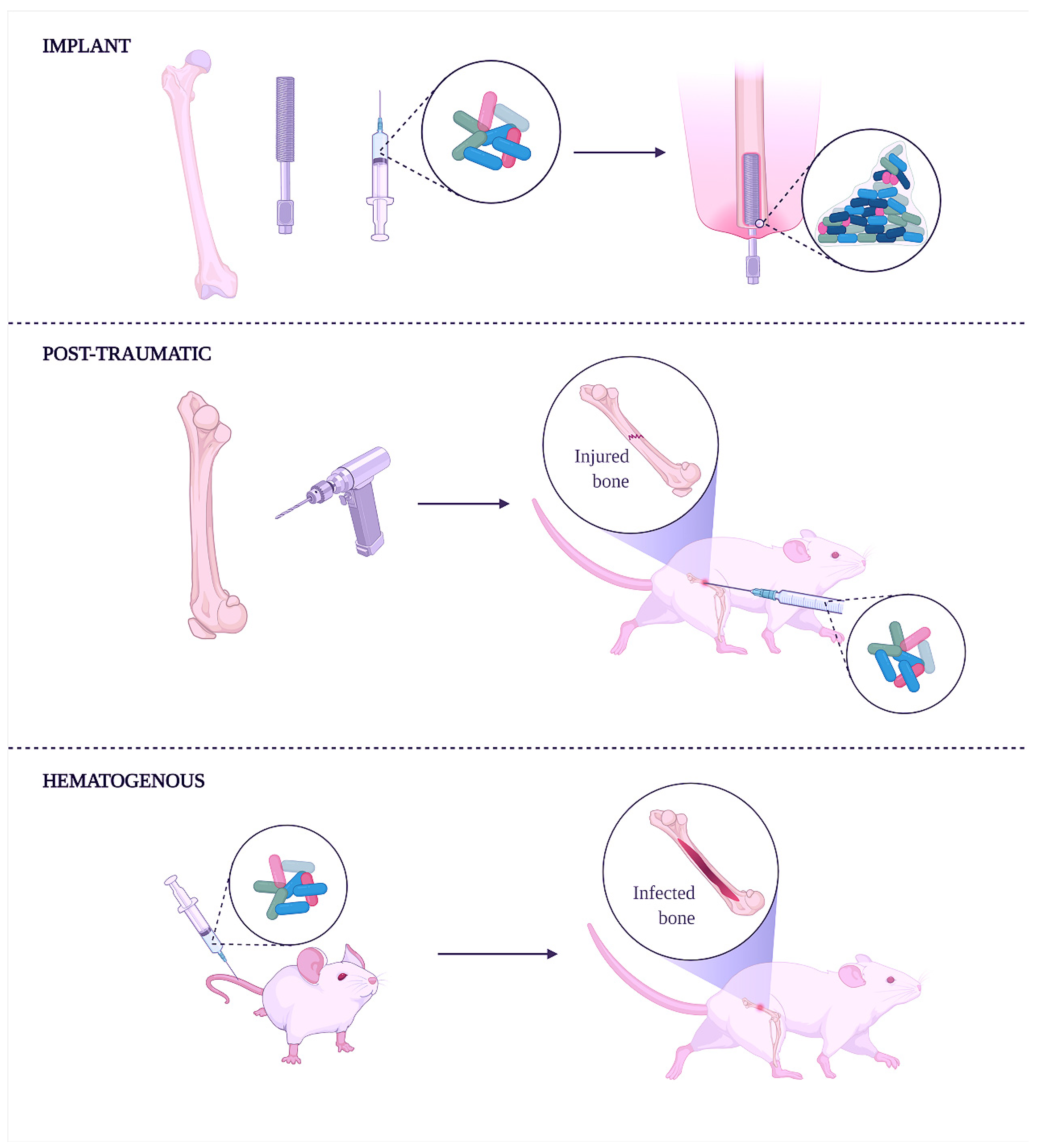
6. Animal Models of Osteomyelitis Overview
6.1. The History of Animals as Models of Osteomyelitis
6.2. What Should Be Considered When Designing Animal Models of Osteomyelitis?
6.3. Which Is the Most Appropriate Animal Model for the Experimental Procedure?
6.4. Age of the Animals and Route of Inoculation
6.5. Bacterial Species Used to Induce Experimental Osteomyelitis
7. Specific Animal Models
7.1. Rat Models
7.2. Mouse Models
7.3. Rabbit Models
7.4. Poultry Models
7.5. Large Animal Models
7.5.1. Canine Models
7.5.2. Small Ruminants’ Models (Sheep and Goat)
7.5.3. Porcine Models
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fox, J.G.; Anderson, L.C.; Loew, F.M.; Quimby, F.W. Laboratory Animal Medicine, 3rd ed.; Anderson, L., Otto, G., Pritchett-Corning, K., Whary, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; ISBN 9780124166134. [Google Scholar]
- Sellick, J.; Lowther, J. Enhancing the Protection of Animals Used for Scientific Purposes. Environ. Law Manag. 2011, 23, 75–82. [Google Scholar]
- Moreau, A. The next Personalized Medicine Evolution in Orthopedics: How Diagnosing and Treating Scoliosis Are about to Change. Pers. Med. 2017, 14, 89–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirschmann, M.T.; Friederich, N.F.; Becker, R.; Karlsson, J. Personalised Medicine in Knee Arthroplasty: We Need More Science! Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1357–1358. [Google Scholar] [CrossRef] [Green Version]
- McKinley, T.O.; Lisboa, F.A.; Horan, A.D.; Gaski, G.E.; Mehta, S. Precision Medicine Applications to Manage Multiply Injured Patients With Orthopaedic Trauma. J. Orthop. Trauma 2019, 33, S25–S29. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Mirzaei, R.; Montanaro, L.; Arciola, C.R. Hijacking of Immune Defences by Biofilms: A Multifront Strategy. Biofouling 2019, 35, 1055–1074. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial Biofilm and Associated Infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Mirzaei, R.; Mohammadzadeh, R.; Alikhani, M.Y.; Shokri Moghadam, M.; Karampoor, S.; Kazemi, S.; Barfipoursalar, A.; Yousefimashouf, R. The Biofilm-Associated Bacterial Infections Unrelated to Indwelling Devices. IUBMB Life 2020, 72, 1271–1285. [Google Scholar] [CrossRef]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as Complex Differentiated Communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef] [Green Version]
- Costerton, J.W.; Geesey, G.G.; Cheng, K.J. A Citation Classic Commentrary on “How Bacteria Stick”. Sci. Am. 1978, 238, 86–95. [Google Scholar]
- Kalishwaralal, K.; BarathManiKanth, S.; Pandian, S.R.K.; Deepak, V.; Gurunathan, S. Silver Nanoparticles Impede the Biofilm Formation by Pseudomonas Aeruginosa and Staphylococcus Epidermidis. Colloids Surf. B Biointerfaces 2010, 79, 340–344. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Microbial Life on Surfaces. Emerg Infect Diseases. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Lembre, P.; Lorentz, C.; Di, P. Exopolysaccharides of the Biofilm Matrix: A Complex Biophysical World. In The Complex World of Polysaccharides; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.; Pang, S.; Borsje, C.; Sleutels, T.; Hamelers, B.; ter Heijne, A. Real-Time Monitoring of Biofilm Thickness Allows for Determination of Acetate Limitations in Bio-Anodes. Bioresour. Technol. Rep. 2022, 18, 101028. [Google Scholar] [CrossRef]
- Panda, S.; Singh, D.V. Biofilm Formation by Ica-Negative Ocular Isolates of Staphylococcus Haemolyticus. Front. Microbiol. 2018, 9, 2687. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm Formation in Staphylococcus Implant Infections. A Review of Molecular Mechanisms and Implications for Biofilm-Resistant Materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef] [PubMed]
- Wilking, J.N.; Zaburdaev, V.; De Volder, M.; Losick, R.; Brenner, M.P.; Weitz, D.A. Liquid Transport Facilitated by Channels in Bacillus Subtilis Biofilms. Proc. Natl. Acad. Sci. USA 2013, 110, 848–852. [Google Scholar] [CrossRef] [Green Version]
- Stewart, P.S. Diffusion in Biofilms WHY IS DIFFUSION AN IMPORTANT PROCESS. J. Bacteriol. 2003, 185, 1485–1491. [Google Scholar] [CrossRef] [Green Version]
- Kong, K.F.; Vuong, C.; Otto, M. Staphylococcus Quorum Sensing in Biofilm Formation and Infection. Int. J. Med. Microbiol. 2006, 296, 133–139. [Google Scholar] [CrossRef]
- Vuong, C.; Kocianova, S.; Yao, Y.; Carmody, A.B.; Otto, M. Increased Colonization of Indwelling Medical Devices by Quorum-Sensing Mutants of Staphylococcus Epidermidis In Vivo. J. Infect. Dis. 2004, 190, 1498–1505. [Google Scholar] [CrossRef] [Green Version]
- Di Cagno, R.; De Angelis, M.; Calasso, M.; Gobbetti, M. Proteomics of the Bacterial Cross-Talk by Quorum Sensing. J. Proteom. 2011, 74, 19–34. [Google Scholar] [CrossRef]
- Turan, N.B.; Chormey, D.S.; Büyükpınar, Ç.; Engin, G.O.; Bakirdere, S. Quorum Sensing: Little Talks for an Effective Bacterial Coordination. TrAC-Trends Anal. Chem. 2017, 91, 1–11. [Google Scholar] [CrossRef]
- George, E.A.; Muir, T.W. Molecular Mechanisms of Agr Quorum Sensing in Virulent Staphylococci. ChemBioChem 2007, 8, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Resch, A.; Rosenstein, R.; Nerz, C.; Götz, F. Differential Gene Expression Profiling of Staphylococcus Aureus Cultivated under Biofilm and Planktonic Conditions. Appl. Environ. Microbiol. 2005, 71, 2663–2676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, E.C.; Singh, A.; Gibson, T.W.G.; Scott Weese, J. Biofilm-Associated Gene Expression in Staphylococcus Pseudintermedius on a Variety of Implant Materials. Vet. Surg. 2016, 45, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, E.A.L.; Picech, F.; Renna, M.S.; Baravalle, C.; Andreotti, C.S.; Russi, R.; Calvinho, L.F.; Diez, C.; Dallard, B.E. Detection of Staphylococcus Aureus Adhesion and Biofilm-Producing Genes and Their Expression during Internalization in Bovine Mammary Epithelial Cells. Vet. Microbiol. 2016, 183, 69–77. [Google Scholar] [CrossRef]
- Koenig, R.L.; Ray, J.L.; Maleki, S.J.; Smeltzer, M.S.; Hurlburt, B.K. Staphylococcus Aureus AgrA Binding to the RNAIII-Agr Regulatory Region. J. Bacteriol. 2004, 186, 7549–7555. [Google Scholar] [CrossRef] [Green Version]
- Karatan, E.; Watnick, P. Signals, Regulatory Networks, and Materials That Build and Break Bacterial Biofilms. Microbiol. Mol. Biol. Rev. 2009, 73, 310–347. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Gowardman, J.; Morrison, M.; Krause, L.; Playford, E.G.; Rickard, C.M. Molecular Investigation of Bacterial Communities on Intravascular Catheters: No Longer Just Staphylococcus. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1189–1198. [Google Scholar] [CrossRef]
- Cue, D.; Lei, M.G.; Lee, C.Y. Genetic Regulation of the Intercellular Adhesion Locus in Staphylococci. Front. Cell. Infect. Microbiol. 2012, 2, 38. [Google Scholar] [CrossRef] [Green Version]
- Boudjemaa, R.; Steenkeste, K.; Canette, A.; Briandet, R.; Marlière, C. Direct Observation of the Cell-Wall Remodeling in Adhering Staphylococcus Aureus 27217_ An AFM Study Supported by SEM and TEM. Cell Surf. 2019, 5, 100018. [Google Scholar] [CrossRef]
- Araújo, G.R.D.S.; Viana, N.B.; Gómez, F.; Pontes, B.; Frases, S. The Mechanical Properties of Microbial Surfaces and Biofilms. Cell Surf. 2019, 5, 100028. [Google Scholar] [CrossRef]
- Elbourne, A.; Chapman, J.; Gelmi, A.; Cozzolino, D.; Crawford, R.J.; Khanh, V. Bacterial-Nanostructure Interactions: The Role of Cell Elasticity and Adhesion Forces. J. Colloid Interface Sci. 2019, 546, 192–210. [Google Scholar] [CrossRef] [PubMed]
- Ammar, Y.; Swailes, D.; Bridgens, B.; Chen, J. Surface & Coatings Technology In Fl Uence of Surface Roughness on the Initial Formation of Bio Fi Lm. Surf. Coat. Technol. J. 2015, 284, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Logan, B.E. Bacterial Adhesion to Glass and Metal-Oxide Surfaces. Colloids Surf. B Biointerfaces 2004, 36, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Larsen, L.H.; Lorenzen, J.A.N.; Hall-stoodley, L.; Kikhney, J.; Moter, A.; Thomsen, T.R. Microbiological Diagnosis of Device-Related Biofilm Infections. Acta Pharmacol. Microbiol. Immunol. Scandinava 2017, 125, 289–303. [Google Scholar] [CrossRef] [Green Version]
- Maki, D.G.; Kluger, D.M.; Crnich, C.J. The Risk of Bloodstream Infection in Adults with Different Intravascular Devices: A Systematic Review of 200 Published Prospective Studies. Mayo Clin. Proc. 2006, 81, 1159–1171. [Google Scholar] [CrossRef] [Green Version]
- Dym, H.; Zeidan, J. Microbiology of Acute and Chronic Osteomyelitis and Antibiotic Treatment. Dent. Clin. N. A. 2017, 61, 271–282. [Google Scholar] [CrossRef]
- Baur, D.A.; Altay, M.A.; Flores-hidalgo, A.; Ort, Y.; Quereshy, F.A. Chronic Osteomyelitis of the Mandible: Diagnosis and Management—An Institution’s Experience Over 7 Years. J. Oral Maxillofac. Surg. 2015, 73, 655–665. [Google Scholar] [CrossRef]
- Lazar, V.; Ditu, L.; Alina, L.; Popa, M.; Chifiriuc, C. Impact of Dental Plaque Biofilms in Periodontal Disease: Management and Future in Therapy Impact of Dental Plaque Biofilms Periodontal Disease: Management and Future Therapy. In Periodontal Disease: Management and Future Therapy, Periodontitis—A Useful Reference; InTech Open: London, UK, 2017. [Google Scholar]
- Marshall, T.G.; Marshall, F.E. Sarcoidosis Succumbs to Antibiotics—Implications for Autoimmune Disease. Autoimmun. Rev. 2004, 3, 295–300. [Google Scholar] [CrossRef]
- Brady, R.A.; Leid, J.G.; Calhoun, J.H.; Costerton, J.W.; Shirtliff, M.E. Osteomyelitis and the Role of Biofilms in Chronic Infection. FEMS Immunol. Med. Microbiol. 2008, 52, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Thakolkaran, N.; Shetty, A. Acute Hematogenous Osteomyelitis in Children. Ochsner J. 2019, 19, 116–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, R.A.; Leid, J.G.; Costerton, J.W.; Shirtliff, M.E. Osteomyelitis: Clinical Overview and Mechanisms of Infection Persistence. Clin. Microbiol. Newsl. 2006, 28, 65–72. [Google Scholar] [CrossRef]
- Castellazzi, L.; Mantero, M.; Esposito, S. Update on the Management of Pediatric Acute Osteomyelitis and Septic Arthritis. Int. J. Mol. Sci. 2016, 17, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckles, V.L.L.; Jones, H.W.; Harrison, W.J. Chronic Haematogenous Osteomyelitis in Children. J. Bone Jt. Surg. 2010, 92, 1138–1143. [Google Scholar] [CrossRef] [Green Version]
- Zimmerli, W.; Sendi, P. Orthopaedic Biofilm Infections. Apmis 2017, 125, 353–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsagris, V.; Vliora, C.; Mihelarakis, I.; Syridou, G.; Pasparakis, D.; Lebessi, E.; Tsolia, M. Salmonella Osteomyelitis in Previously Healthy Children. Pediatr. Infect. Dis. J. 2016, 35, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Du, J.; Chen, L. Salmonella Osteomyelitis in a Previously Healthy Neonate: A Case Report and Review of the Literature. Ital. J. Pediatr. 2018, 44, 8–10. [Google Scholar] [CrossRef] [Green Version]
- Weiss-Salz, I.; Yagupsky, P. Kingella Kingae Infections in Children: An Update. In Hot Topics in Infection and Immunity in Children VIII; Springer: New York, NY, USA, 2011; ISBN 9781461402046. [Google Scholar]
- Dodwell, E.R. Osteomyelitis and Septic Arthritis in Children: Current Concepts. Curr. Opin. Pediatr. 2013, 25, 58–63. [Google Scholar] [CrossRef]
- Martin, A.C.; Anderson, D.; Lucey, J.; Guttinger, R.; Burgner, D.P.; Blyth, C.C. Predictors of Outcome in Pediatric Osteomyelitis Five Years Experience in a Single Tertiary Center. Pediatr. Infect. Dis. 2016, 35, 387–391. [Google Scholar] [CrossRef]
- Arnold, J.C.; Bradley, J.S. Osteoarticular Infections in Children. Infect. Dis. Clin. 2015, 29, 557–574. [Google Scholar] [CrossRef]
- Ratnayake, K.; Davis, A.J.; Brown, L.; Young, T.P. American Journal of Emergency Medicine Pediatric Acute Osteomyelitis in the Postvaccine, Methicillin-Resistant Staphylococcus Aureus Era. Am. J. Emerg. Med. 2015, 33, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef]
- Aytaç, S.; Schnetzke, M.; Swartman, B.; Herrmann, P.; Woelfl, C.; Heppert, V.; Alfred, P.; Thorsten, G. Posttraumatic and Postoperative Osteomyelitis: Surgical Revision Strategy with Persisting Fistula. Arch. Orthop. Trauma Surg. 2014, 134, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.M.; McDonald, J.R. Osteomyelitis: Approach to Diagnosis and Treatment. Phys. Sportsmed. 2008, 36, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Bryers, J.D. Medical Biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [CrossRef]
- Darisipudi, M.N.; Nordengrün, M.; Bröker, B.M.; Péton, V. Messing with the Sentinels-The Interaction of Staphylococcus Aureus with Dendritic Cells. Microorganisms 2018, 6, 87. [Google Scholar] [CrossRef] [Green Version]
- Friedl, P.; Weigelin, B. Interstitial Leukocyte Migration and Immune Function. Nat. Immunol. 2008, 9, 960–969. [Google Scholar] [CrossRef]
- Wong, C.H.Y.; Heit, B.; Kubes, P. Molecular Regulators of Leucocyte Chemotaxis during Inflammation. Cardiovasc. Res. 2010, 86, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An Overview of the Role of Neutrophils in Innate Immunity, Inflammation and Host-Biomaterial Integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef]
- Günther, F.; Wabnitz, G.H.; Stroh, P.; Prior, B.; Obst, U.; Samstag, Y.; Wagner, C.; Hänsch, G.M. Host Defence against Staphylococcus Aureus Biofilms Infection: Phagocytosis of Biofilms by Polymorphonuclear Neutrophils (PMN). Mol. Immunol. 2009, 46, 1805–1813. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2013, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stroh, P.; Günther, F.; Meyle, E.; Prior, B.; Wagner, C.; Hänsch, G.M. Host Defence against Staphylococcus Aureus Biofilms by Polymorphonuclear Neutrophils: Oxygen Radical Production but Not Phagocytosis Depends on Opsonisation with Immunoglobulin G. Immunobiology 2011, 216, 351–357. [Google Scholar] [CrossRef]
- Scherr, T.D.; Hanke, M.L.; Huang, O.; James, D.B.A.; Horswill, A.R.; Bayles, K.W.; Fey, P.D.; Torres, V.J.; Kielian, T. Staphylococcus Aureus Biofilms Induce Macrophage Dysfunction Through Leukocidin AB and Alpha-Toxin. mBio 2015, 6, e01021-15. [Google Scholar] [CrossRef] [Green Version]
- González, J.F.; Hahn, M.M.; Gunn, J.S. Chronic Biofilm-Based Infections: Skewing of the Immune Response. Pathog. Dis. 2018, 76, fty023. [Google Scholar] [CrossRef] [PubMed]
- Meyle, E.; Stroh, P.; GüNther, F.; Hoppy-Tichy, T.; Wagner, C.; HäNsch, G.M. Destruction of Bacterial Biofilms by Polymorphonuclear Neutrophils: Relative Contribution of Phagocytosis, DNA Release, and Degranulation. Int. J. Artif. Organs 2010, 33, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Ammons, M.C.; Copié, V. Mini-Review: Lactoferrin: A Bioinspired, Anti-Biofilm Therapeutic. Biofouling 2013, 29, 443–455. [Google Scholar] [CrossRef] [Green Version]
- Wagner, C.; Obst, U.; Hänsch, G.M. Implant-Associated Posttraumatic Osteomyelitis: Collateral Damage by Local Host Defense? Int. J. Artif. Organs 2005, 28, 1172–1180. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Gamberini, S.; Donati, M.E.; Pirini, V.; Visai, L.; Speziale, P.; Montanaro, L. Antibiotic Resistance in Exopolysaccharide-Forming Staphylococcus Epidermidis Clinical Isolates from Orthopaedic Implant Infections. Biomaterials 2005, 26, 6530–6535. [Google Scholar] [CrossRef]
- Meyle, E.; Brenner-Weiss, G.; Obst, U.; Prior, B.; Hänsch, G.M. Immune Defense against S. Epidermidis Biofilms: Components of the Extracellular Polymeric Substance Activate Distinct Bactericidal Mechanisms of Phagocytic Cells. Int. J. Artif. Organs 2012, 35, 700–712. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Zhou, R.; Ng, S.C.; Li, J.; Huang, M.; Zhou, F.; Wang, X.; Shen, B.; Kamm, M.A.; et al. Characteristics of Fecal and Mucosa-Associated Microbiota in Chinese Patients with Inflammatory Bowel Disease. Medicine 2014, 93, e51. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.; Yuan, L.; Wang, J.; Wang, F.; Yang, X.-K.; Zhang, F.; Song, J.; Ma, X.; Cheng, Q.; Song, G. Lipopolysaccharide (LPS) Induces the Apoptosis and Inhibits Osteoblast Differentiation through JNK Pathway in MC3T3-E1 Cells. Inflammation 2014, 37, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.J., Jr.; Ward, C.L.; Romano, D.R.; Hurtgen, B.J.; Hardy, S.K.; Woodbury, R.L.; Trevino, A.V.; Rathbone, C.R.; Wenke, J.C. Staphylococcus Aureus Biofilms Decrease Osteoblast Viability, Inhibits Osteogenic Differentiation, and Increases Bone Resorption in Vitro. BMC Musculoskelet. Disord. 2013, 14, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, G.; O’Rourke, K.; Chinnaiyan, A.M.; Gentz, R.; Ebner, R.; Ni, J.; Dixit, V.M. The Receptor for the Cytotoxic Ligand TRAIL. Science 1997, 276, 111–113. [Google Scholar] [CrossRef]
- Schneider, P.; Thome, M.; Burns, K.; Bodmer, J.L.; Hofmann, K.; Kataoka, T.; Holler, N.; Tschopp, J. TRAIL Receptors 1 (DR4) and 2 (DR5) Signal FADD-Dependent Apoptosis and Activate NF-KappaB. Immunity 1997, 7, 831–836. [Google Scholar] [CrossRef] [Green Version]
- Emery, J.G.; McDonnell, P.; Burke, M.B.; Deen, K.C.; Lyn, S.; Silverman, C.; Dul, E.; Appelbaum, E.R.; Eichman, C.; DiPrinzio, R.; et al. Osteoprotegerin Is a Receptor for the Cytotoxic Ligand TRAIL. J. Biol. Chem. 1998, 273, 14363–14367. [Google Scholar] [CrossRef] [Green Version]
- Gallo, R.L.; Huttner, K.M. Antimicrobial Peptides: An Emerging Concept in Cutaneous Biology. J. Investig. Dermatol. 1998, 111, 739–743. [Google Scholar] [CrossRef] [Green Version]
- Dunsche, A.; Açil, Y.; Siebert, R.; Harder, J.; Schröder, J.M.; Jepsen, S. Expression Profile of Human Defensins and Antimicrobial Proteins in Oral Tissues. J. Oral Pathol. Med. 2001, 30, 154–158. [Google Scholar] [CrossRef]
- Warnke, P.H.; Springer, I.N.; Russo, P.A.J.; Wiltfang, J.; Essig, H.; Kosmahl, M.; Sherry, E.; Acil, Y. Innate Immunity in Human Bone. Bone 2006, 38, 400–408. [Google Scholar] [CrossRef]
- Varoga, D.; Pufe, T.; Mentlein, R.; Kohrs, S.; Grohmann, S.; Tillmann, B.; Hassenpflug, J.; Paulsen, F. Expression and Regulation of Antimicrobial Peptides in Articular Joints. Ann. Anat.-Anat. Anz. 2005, 187, 499–508. [Google Scholar] [CrossRef]
- Varoga, D.; Tohidnezhad, M.; Paulsen, F.; Wruck, C.J.; Brandenburg, L.; Mentlein, R.; Lippross, S.; Hassenpflug, J.; Besch, L.; Müller, M.; et al. The Role of Human Beta-Defensin-2 in Bone. J. Anat. 2008, 213, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Baltensperger, M.; Grätz, K.; Bruder, E.; Lebeda, R.; Makek, M.; Eyrich, G. Is Primary Chronic Osteomyelitis a Uniform Disease? Proposal of a Classification Based on a Retrospective Analysis of Patients Treated in the Past 30 Years. J. Cranio-Maxillofac. Surg. 2004, 32, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shavit, Z. Taking a Toll on the Bones: Regulation of Bone Metabolism by Innate Immune Regulators. Autoimmunity 2008, 41, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Zwerina, J. Positive Regulators of Osteoclastogenesis and Bone Resorption in Rheumatoid Arthritis. Arthritis Res. Ther. 2011, 13, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redlich, K.; Smolen, J.S. Inflammatory Bone Loss: Pathogenesis and Therapeutic Intervention. Nat. Rev. Drug Discov. 2012, 11, 234–250. [Google Scholar] [CrossRef]
- Lebowitz, D.; Wolter, L.; Zenklusen, C.; Chouiter, A.; Malinverni, R. TB Determined: Tuberculous Osteomyelitis. Am. J. Med. 2014, 127, 198–201. [Google Scholar] [CrossRef]
- Miller, L.S.; O’Connell, R.M.; Gutierrez, M.A.; Pietras, E.M.; Shahangian, A.; Gross, C.E.; Thirumala, A.; Cheung, A.L.; Cheng, G.; Modlin, R.L. MyD88 Mediates Neutrophil Recruitment Initiated by IL-1R but Not TLR2 Activation in Immunity against Staphylococcus Aureus. Immunity 2006, 24, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.S.; Pietras, E.M.; Uricchio, L.H.; Hirano, K.; Rao, S.; Lin, H.; O’Connell, R.M.; Iwakura, Y.; Cheung, A.L.; Cheng, G.; et al. Inflammasome-Mediated Production of IL-1beta Is Required for Neutrophil Recruitment against Staphylococcus Aureus in Vivo. J. Immunol. 2007, 179, 6933–6942. [Google Scholar] [CrossRef] [Green Version]
- Stork, L.C.; Peterson, V.M.; Rundus, C.H.; Robinson, W.A. Interleukin-1 Enhances Murine Granulopoiesis in Vivo. Exp. Hematol. 1988, 16, 163–167. [Google Scholar]
- Nakai, S.; Aihara, K.; Hirai, Y. Interleukin-1 Potentiates Granulopoiesis and Thrombopoiesis by Producing Hematopoietic Factors in Vivo. Life Sci. 1989, 45, 585–591. [Google Scholar] [CrossRef]
- Putnam, N.E.; Fulbright, L.E.; Curry, J.M.; Ford, C.A.; Petronglo, J.R.; Hendrix, A.S.; Cassat, J.E. MyD88 and IL-1R Signaling Drive Antibacterial Immunity and Osteoclast-Driven Bone Loss during Staphylococcus Aureus Osteomyelitis. PLoS Pathog. 2019, 15, e1007744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boskey, A.L.; Posner, A.S. Bone Structure, Composition, and Mineralization. Orthop. Clin. N. A. 1984, 15, 597–612. [Google Scholar] [CrossRef]
- Cowan, P.T.; Kahai, P. Anatomy, Bones. In In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Unal, M.; Cingoz, F.; Bagcioglu, C.; Sozer, Y.; Akkus, O. Interrelationships between Electrical, Mechanical and Hydration Properties of Cortical Bone. J. Mech. Behav. Biomed. Mater. 2018, 77, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Conrad, D.A. Acute Hematogenous Osteomyelitis. Pediatr. Rev. 2010, 31, 464–471. [Google Scholar] [CrossRef]
- Rosenberg, A.E. Bones, Joints and Soft Tissue Tumors. In Robbins and Cotran-Pathologic Basis of Disease; WB Saunders: Philadelphia, PA, USA, 2010; pp. 1237–1240. [Google Scholar]
- Birt, M.C.; Anderson, D.W.; Bruce Toby, E.; Wang, J. Osteomyelitis: Recent Advances in Pathophysiology and Therapeutic Strategies. J. Orthop. 2017, 14, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Trampuz, A.; Zimmerli, W. Antimicrobial Agents in Orthopaedic Surgery: Prophylaxis and Treatment. Drugs 2006, 66, 1089–1105. [Google Scholar] [CrossRef] [PubMed]
- Benito, N.; Franco, M.; Coll, P.; Gálvez, M.L.; Jordán, M.; López-Contreras, J.; Pomar, V.; Monllau, J.C.; Mirelis, B.; Gurguí, M. Etiology of Surgical Site Infections after Primary Total Joint Arthroplasties. J. Orthop. Res. 2014, 32, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; Townsend, R. Pharmacological Agents for Soft Tissue and Bone Infected with MRSA: Which Agent and for How Long? Injury 2011, 42 (Suppl. S5), S7–S10. [Google Scholar] [CrossRef]
- van de Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Infection of Orthopedic Implants and the Use of Antibiotic-Loaded Bone Cements. A Review. Acta Orthop. Scand. 2001, 72, 557–571. [Google Scholar] [CrossRef]
- Williams, R.J.; Henderson, B.; Nair, S.P. Staphylococcus Aureus Fibronectin Binding Proteins A and B Possess a Second Fibronectin Binding Region That May Have Biological Relevance to Bone Tissues. Calcif. Tissue Int. 2002, 70, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Kalita, S.J.; Verma, S. Nanocrystalline Hydroxyapatite Bioceramic Using Microwave Radiation: Synthesis and Characterization. Mater. Sci. Eng. C 2010, 30, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of Orthopedic Implants with Emphasis on Bacterial Adhesion Process and Techniques Used in Studying Bacterial-Material Interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garzoni, C.; Kelley, W.L. Return of the Trojan Horse: Intracellular Phenotype Switching and Immune Evasion by Staphylococcus Aureus. EMBO Mol. Med. 2011, 3, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Sendi, P.; Proctor, R.A. Staphylococcus Aureus as an Intracellular Pathogen: The Role of Small Colony Variants. Trends Microbiol. 2009, 17, 54–58. [Google Scholar] [CrossRef]
- Proctor, R.A.; von Eiff, C.; Kahl, B.C.; Becker, K.; McNamara, P.; Herrmann, M.; Peters, G. Small Colony Variants: A Pathogenic Form of Bacteria That Facilitates Persistent and Recurrent Infections. Nat. Rev. Microbiol. 2006, 4, 295–305. [Google Scholar] [CrossRef]
- Bose, D.; Kugan, R.; Stubbs, D.; McNally, M. Management of Infected Nonunion of the Long Bones by a Multidisciplinary Team. Bone Jt. J. 2015, 97, 814–817. [Google Scholar] [CrossRef]
- Lovati, A.B.; Luca, C.; Bottagisio, M.; Monti, L.; De Vecchi, E.; Previdi, S.; Accetta, R.; Drago, L. Modeling Staphylococcus Epidermidis—Induced Non-Unions: Subclinical and Clinical Evidence in Rats. PLoS ONE 2016, 11, e0147447. [Google Scholar] [CrossRef] [Green Version]
- Kruse Johansen, L.; Jensen, H.E. Animal Models of Hematogenous Staphylococcus Aureus Osteomyelitis in Long Bones: A Review. Orthop. Res. Rev. 2013, 5, 51–64. [Google Scholar] [CrossRef] [Green Version]
- An, Y.H.; Kang, Q.K.; Arciola, C.R. Animal Models of Osteomyelitis. Int. J. Artif. Organs 2006, 29, 407–420. [Google Scholar] [CrossRef]
- Lexer, E. Zur Experimentellen Erzeugung Osteomyelitischer Herde. Arch. Klin. Chir. 1894, 48, 181–200. [Google Scholar]
- Rodet, A. Physiologie Pathologique—Étude Expérimentale Sur l’ostéomyelite Infectieuse. Comptes Rendus Acad. Sci. 1885, 99, 569–571. [Google Scholar]
- Thompson, R.H.; Dubos, R.J. Production of Experimental Osteomyelitis in Rabbits by Intravenous Injection of Staphylococcus Aureus. J. Exp. Med. 1938, 68, 191–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haldeman, K.O. No TitleAcute Osteomyelitis. A Clinical and Experimental Study. Surg. Gynecol. Obstet. 1934, 59, 25–31. [Google Scholar]
- Starr, C.L. Acute Hematogenous Osteomyelitis. Clarence L. Starr, M.D. (1868–1928). 1922. Clin. Orthop. 2002, 403, 4–7. [Google Scholar]
- Scheman, L.; Janota, M.; Lewin, P. The production of experimental osteomyelitis: Preliminary report. J. Am. Med. Assoc. 1941, 117, 1525–1529. [Google Scholar] [CrossRef]
- Zak, O.; Zak, F.; Rich, R.; Tosch, W.; Kradolfer, F.; WM, S. Experimental Staphylococcal Osteomyelitis in Rats: Therapy with Rifampin and Cloxacillin, Alone or in Combination. In Current Chemotherapy and Immunotherapy; American Society for Microbiology: Washington, DC, USA, 1982; pp. 973–974. [Google Scholar]
- Hamblen, D.L. Hyperbaric Oxygenation. Its Effect on Experimental Staphylococcal Osteomyelitis in Rats. J. Bone Jt. Surg. Am. 1968, 50, 1129–1141. [Google Scholar] [CrossRef]
- Ueno, H. A Study on Experimental Pyogenic Osteomyelitis. Especially the Production of Experimental Osteomyelitis in Mice. J. Jpn. Orthop. Assoc. 1974, 48, 291–301. [Google Scholar]
- Fitzgerald, R.H.J. Experimental Osteomyelitis: Description of a Canine Model and the Role of Depot Administration of Antibiotics in the Prevention and Treatment of Sepsis. J. Bone Jt. Surg. Am. 1983, 65, 371–380. [Google Scholar] [CrossRef]
- Deysine, M.; Rosario, E.; Isenberg, H.D. Acute Hematogenous Osteomyelitis: An Experimental Model. Surgery 1976, 79, 97–99. [Google Scholar]
- Passl, R.; Weber, P.; Egkher, E.; Kovac, W. Acute and chronic experimental posttraumatic osteomyelitis in guinea pigs. Helv. Chir. Acta 1979, 46, 163–165. [Google Scholar] [PubMed]
- Emslie, K.R.; Nade, S. Acute Hematogenous Staphylococcal Osteomyelitis. A Description of the Natural History in an Avian Model. Am. J. Pathol. 1983, 110, 333–345. [Google Scholar] [PubMed]
- Emslie, K.R.; Ozanne, N.R.; Nade, S.M. Acute Haematogenous Osteomyelitis: An Experimental Model. J. Pathol. 1983, 141, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.L.; Galloway, R.H.; Baumgartner, J.C.; Barsoum, I.S. Development of Chronic Mandibular Osteomyelitis in a Miniswine Model. J. Oral Maxillofac. Surg. 1993, 51, 1358–1362. [Google Scholar] [CrossRef]
- Kaarsemaker, S.; Walenkamp, G.H.; vd Bogaard, A.E. New Model for Chronic Osteomyelitis with Staphylococcus Aureus in Sheep. Clin. Orthop. 1997, 339, 246–252. [Google Scholar] [CrossRef]
- Curtis, M.J.; Brown, P.R.; Dick, J.D.; Jinnah, R.H. Contaminated Fractures of the Tibia: A Comparison of Treatment Modalities in an Animal Model. J. Orthop. Res. 1995, 13, 286–295. [Google Scholar] [CrossRef]
- Waldvogel, F.A.; Medoff, G.; Swartz, M.N. Osteomyelitis: A Review of Clinical Features, Therapeutic Considerations and Unusual Aspects. 3. Osteomyelitis Associated with Vascular Insufficiency. N. Engl. J. Med. 1970, 282, 316–322. [Google Scholar] [CrossRef]
- Arens, D.; Wilke, M.; Calabro, L.; Hackl, S.; Zeiter, S.; Zderic, I.; Richards, R.G.; Moriarty, T.F. A Rabbit Humerus Model of Plating and Nailing Osteosynthesis with and without Staphylococcus Aureus Osteomyelitis. Eur. Cells Mater. 2015, 30, 148–162. [Google Scholar] [CrossRef]
- Mandal, R.K.; Jiang, T.; Al-Rubaye, A.A.; Rhoads, D.D.; Wideman, R.F.; Zhao, J.; Pevzner, I.; Kwon, Y.M. An Investigation into Blood Microbiota and Its Potential Association with Bacterial Chondronecrosis with Osteomyelitis (BCO) in Broilers. Sci. Rep. 2016, 6, 25882. [Google Scholar] [CrossRef] [Green Version]
- Robinson, D.A.; Bechtold, J.E.; Carlson, C.S.; Evans, R.B.; Conzemius, M.G. Development of a Fracture Osteomyelitis Model in the Rat Femur. J. Orthop. Res. 2011, 29, 131–137. [Google Scholar] [CrossRef]
- Kobayakawa, H. A study on experimental pyogenic osteomyelitis. 1. The preferential site of hematogenous osteomyelitis. 2. The role of foreign body in hematogenous infection (author’s transl). Nihon Seikeigeka Gakkai Zasshi 1979, 53, 761–775. [Google Scholar] [PubMed]
- Pesanti, E.L.; Lorenzo, J.A. Osteoclasts and Effects of Interleukin 4 in Development of Chronic Osteomyelitis. Clin. Orthop. 1998, 355, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Aerssens, J.; Boonen, S.; Lowet, G.; Dequeker, J. Interspecies Differences in Bone Composition, Density, and Quality: Potential Implications for in Vivo Bone Research. Endocrinology 1998, 139, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Swindle, M.M. Comparative Anatomy and Physiology of the Pig. Scand. J. Lab. Anim. Sci. 1998, 25, 11–21. [Google Scholar]
- Jensen, H.E.; Nielsen, O.L.E.L.; Agerholm, J.S.; Iburg, T.; Johansen, L.K.; Johannesson, E.; Møller, M.; Jahn, L.; Munk, L.; Aalbæk, B.; et al. A Non-Traumatic Staphylococcus Aureus Osteomyelitis Model in Pigs. In Vivo 2010, 264, 257–264. [Google Scholar]
- Mader, J.T. Animal Models of Osteomyelitis. Am. J. Med. 1985, 78, 213–217. [Google Scholar] [CrossRef]
- Weaver, J.; Tayler, M. Experimental Staphylococcaemia and Hematogenous Osteomyelitis. J. Bone Jt. Surg. Am. 1943, 25, 791–802. [Google Scholar]
- Chadha, H.S.; Fitzgerald, R.H.J.; Wiater, P.; Sud, S.; Nasser, S.; Wooley, P.H. Experimental Acute Hematogenous Osteomyelitis in Mice. I. Histopathological and Immunological Findings. J. Orthop. Res. 1999, 17, 376–381. [Google Scholar] [CrossRef]
- Yoon, K.S.; Fitzgerald, R.H.J.; Sud, S.; Song, Z.; Wooley, P.H. Experimental Acute Hematogenous Osteomyelitis in Mice. II. Influence of Staphylococcus Aureus Infection on T-Cell Immunity. J. Orthop. Res. 1999, 17, 382–391. [Google Scholar] [CrossRef]
- Blevins, J.S.; Elasri, M.O.; Allmendinger, S.D.; Beenken, K.E.; Skinner, R.A.; Thomas, J.R.; Smeltzer, M.S. Role of SarA in the Pathogenesis of Staphylococcus Aureus Musculoskeletal Infection. Infect. Immun. 2003, 71, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Cheng, L.I.; Helfer, D.R.; Ashbaugh, A.G.; Miller, R.J.; Tzomides, A.J.; Thompson, J.M.; Ortines, R.V.; Tsai, A.S.; Liu, H.; et al. Mouse Model of Hematogenous Implant-Related Staphylococcus Aureus Biofilm Infection Reveals Therapeutic Targets. Proc. Natl. Acad. Sci. USA 2017, 114, E5094–E5102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jødal, L.; Nielsen, O.L.; Afzelius, P.; Alstrup, A.K.O.; Hansen, S.B. Blood Perfusion in Osteomyelitis Studied with [(15)O]Water PET in a Juvenile Porcine Model. EJNMMI Res. 2017, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansen, L.K.; Svalastoga, E.L.; Frees, D.; Aalbæk, B.; Koch, J.; Iburg, T.M.; Nielsen, O.L.; Leifsson, P.S.; Jensen, H.E. A New Technique for Modeling of Hematogenous Osteomyelitis in Pigs: Inoculation into Femoral Artery. J. Investig. Surg. 2013, 26, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, J.H.; Manring, M.M. Adult Osteomyelitis. Infect. Dis. Clin. N. A. 2005, 19, 765–786. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, S. An Experimental Study on Pyogenic Osteomyelitis with Special Reference to Polymicrobial Infections. Nihon Seikeigeka Gakkai Zasshi 1985, 59, 429–441. [Google Scholar] [PubMed]
- Sakaeda, H. Experimental Polymicrobial Osteomyelitis Produced by Both Aerobic and Anaerobic Opportunistic Pathogens. Nihon Seikeigeka Gakkai Zasshi 1988, 62, 791–802. [Google Scholar] [PubMed]
- Wong, R.M.Y.; Li, T.K.; Li, J.; Ho, W.T.; Chow, S.K.H.; Leung, S.S.Y.; Cheung, W.H.; Ip, M. A Systematic Review on Current Osteosynthesis-Associated Infection Animal Fracture Models. J. Orthop. Transl. 2020, 23, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Helbig, L.; Guehring, T.; Rosenberger, S.; Ivanova, A.; Kaeppler, K.; Fischer, C.A.; Moghaddam, A.; Schmidmaier, G. A New Animal Model for Delayed Osseous Union Secondary to Osteitis. BMC Musculoskelet. Disord. 2015, 16, 362. [Google Scholar] [CrossRef] [Green Version]
- Schaer, T.P.; Stewart, S.; Hsu, B.B.; Klibanov, A.M. Hydrophobic Polycationic Coatings That Inhibit Biofilms and Support Bone Healing during Infection. Biomaterials 2012, 33, 1245–1254. [Google Scholar] [CrossRef]
- Bilgili, F.; Balci, H.I.; Karaytug, K.; Sariyilmaz, K.; Atalar, A.C.; Bozdag, E.; Tuna, M.; Bilgic, B.; Gurler, N. Can Normal Fracture Healing Be Achieved When the Implant Is Retained on the Basis of Infection? An Experimental Animal Model. Clin. Orthop. 2015, 473, 3190–3196. [Google Scholar] [CrossRef] [Green Version]
- Norden, C.W. Experimental Osteomyelitis. I. A Description of the Model. J. Infect. Dis. 1970, 122, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Rissing, J.P.; Buxton, T.B.; Fisher, J.; Harris, R.; Shockley, R.K. Arachidonic Acid Facilitates Experimental Chronic Osteomyelitis in Rats. Infect. Immun. 1985, 49, 141–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottagisio, M.; Coman, C.; Lovati, A.B. Animal Models of Orthopaedic Infections. A Review of Rabbit Models Used to Induce Long Bone Bacterial Infections. J. Med. Microbiol. 2019, 68, 506–537. [Google Scholar] [CrossRef]
- Chen, M.-F.; Chang, C.-H.; Chiang-Ni, C.; Hsieh, P.-H.; Shih, H.-N.; Ueng, S.W.N.; Chang, Y. Rapid Analysis of Bacterial Composition in Prosthetic Joint Infection by 16S RRNA Metagenomic Sequencing. Infection 2019, 8, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Ivy, M.I.; Thoendel, M.J.; Jeraldo, P.R.; Greenwood-Quaintance, K.E.; Hassen, A.D.; Abdel, M.P.; Chia, N.; Yao, J.Z.; Tande, A.J.; Mandrekar, J.N.; et al. Direct Detection and Identification of Prosthetic Joint Infection Pathogens in Synovial Fluid by Metagenomic Shotgun Sequencing. J. Clin. Microbiol. 2018, 56, e00402-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pineda, C.; Espinosa, R.; Pena, A. Radiographic Imaging in Osteomyelitis: The Role of Plain Radiography, Computed Tomography, Ultrasonography, Magnetic Resonance Imaging, and Scintigraphy. Semin. Plast. Surg. 2009, 1, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.; Rojavin, Y.; Jamali, A.; Wasielewski, S.; Salgado, C. Animal Models for the Study of Osteomyelitis. Semin. Plast. Surg. 2009, 23, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Reizner, W.; Hunter, J.G.; O’Malley, N.T.; Southgate, R.D.; Schwarz, E.M.; Kates, S.L. A Systematic Review of Animal Models for Staphylococcus Aureus Osteomyelitis. Eur. Cells Mater. 2015, 27, 196–212. [Google Scholar] [CrossRef]
- Lucke, M.; Schmidmaier, G.; Sadoni, S.; Wildemann, B.; Schiller, R.; Haas, N.P.; Raschke, M. Gentamicin Coating of Metallic Implants Reduces Implant-Related Osteomyelitis in Rats. Bone 2003, 32, 521–531. [Google Scholar] [CrossRef]
- Bisland, S.K.; Chien, C.; Wilson, B.C.; Burch, S. Pre-Clinical in Vitro and in Vivo Studies to Examine the Potential Use of Photodynamic Therapy in the Treatment of Osteomyelitis. Photochem. Photobiol. Sci. 2006, 5, 31–38. [Google Scholar] [CrossRef]
- Akiyama, T.; Miyamoto, H.; Yonekura, Y.; Tsukamoto, M.; Ando, Y.; Noda, I.; Sonohata, M.; Mawatari, M. Silver Oxide-Containing Hydroxyapatite Coating Has in Vivo Antibacterial Activity in the Rat Tibia. J. Orthop. Res. 2013, 31, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Ozturan, K.E.; Yucel, I.; Kocoglu, E.; Cakici, H.; Guven, M. Efficacy of Moxifloxacin Compared to Teicoplanin in the Treatment of Implant-Related Chronic Osteomyelitis in Rats. J. Orthop. Res. 2010, 28, 1368–1372. [Google Scholar] [CrossRef]
- Solov’ev, M.M.; Alekhova, T.M.; Mel’tsova, G.M.; Solov’eva, A.M.; Murzin, B.A. The modelling of mandibular osteomyelitis. Stomatologiia 1992, 1, 6–8. [Google Scholar]
- Hienz, S.A.; Sakamoto, H.; Flock, J.I.; Mörner, A.C.; Reinholt, F.P.; Heimdahl, A.; Nord, C.E. Development and Characterization of a New Model of Hematogenous Osteomyelitis in the Rat. J. Infect. Dis. 1995, 171, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Kalteis, T.; Beckmann, J.; Schröder, H.-J.; Schaumburger, J.; Linde, H.-J.; Lerch, K.; Lehn, N. Treatment of Implant-Associated Infections with Moxifloxacin: An Animal Study. Int. J. Antimicrob. Agents 2006, 27, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Harrasser, N.; Gorkotte, J.; Obermeier, A.; Feihl, S.; Straub, M.; Slotta-Huspenina, J.; von Eisenhart-Rothe, R.; Moser, W.; Gruner, P.; de Wild, M.; et al. A New Model of Implant-Related Osteomyelitis in the Metaphysis of Rat Tibiae. BMC Musculoskelet. Disord. 2016, 17, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haenle, M.; Zietz, C.; Lindner, T.; Arndt, K.; Vetter, A.; Mittelmeier, W.; Podbielski, A.; Bader, R. A Model of Implant-Associated Infection in the Tibial Metaphysis of Rats. Sci. World J. 2013, 2013, 481975. [Google Scholar] [CrossRef]
- Fan, Y.; Xiao, Y.; Sabuhi, W.A.; Leape, C.P.; Gil, D.; Grindy, S.; Muratoglu, O.K.; Bedair, H.; Collins, J.E.; Randolph, M.; et al. Longitudinal Model of Periprosthetic Joint Infection in the Rat. J. Orthop. Res. 2020, 38, 1101–1112. [Google Scholar] [CrossRef]
- Nelson, D.R.; Buxton, T.B.; Luu, Q.N.; Rissing, J.P. An Antibiotic Resistant Experimental Model of Pseudomonas Osteomyelitis. Infection 1990, 18, 246–248. [Google Scholar] [CrossRef]
- Hendricks, K.J.; Burd, T.A.; Anglen, J.O.; Simpson, A.W.; Christensen, G.D.; Gainor, B.J. Synergy Between Staphylococcus Aureus and Pseudomonas Aeruginosa in a Rat Model of Complex Orthopaedic Wounds. JBJS 2001, 83, 855–861. [Google Scholar] [CrossRef]
- Mecikoglu, M.; Saygi, B.; Yildirim, Y.; Karadag-Saygi, E.; Ramadan, S.S.; Esemenli, T. The Effect of Proteolytic Enzyme Serratiopeptidase in the Treatment of Experimental Implant-Related Infection. J. Bone Jt. Surg. Am. 2006, 88, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Thormann, U.; Sommer, U.; Stötzel, S.; Mohamed, W.; Schnettler, R.; Domann, E.; Chakraborty, T.; Alt, V. Impact of Prophylactic CpG Oligodeoxynucleotide Application on Implant-Associated Staphylococcus Aureus Bone Infection. Bone 2015, 78, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Lovati, A.B.; Romanò, C.L.; Monti, L.; Vassena, C.; Previdi, S.; Drago, L. Does PGE1 Vasodilator Prevent Orthopaedic Implant-Related Infection in Diabetes? Preliminary Results in a Mouse Model. PLoS ONE 2014, 9, e94758. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Gromov, K.; Søballe, K.; Puzas, J.E.; O’Keefe, R.J.; Awad, H.; Drissi, H.; Schwarz, E.M. Quantitative Mouse Model of Implant-Associated Osteomyelitis and the Kinetics of Microbial Growth, Osteolysis, and Humoral Immunity. J. Orthop. Res. 2008, 26, 96–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernthal, N.M.; Stavrakis, A.I.; Billi, F.; Cho, J.S.; Kremen, T.J.; Simon, S.I.; Cheung, A.L.; Finerman, G.A.; Lieberman, J.R.; Adams, J.S.; et al. A Mouse Model of Post-Arthroplasty Staphylococcus Aureus Joint Infection to Evaluate in Vivo the Efficacy of Antimicrobial Implant Coatings. PLoS ONE 2010, 5, e12580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funao, H.; Ishii, K.; Nagai, S.; Sasaki, A.; Hoshikawa, T.; Aizawa, M.; Okada, Y.; Chiba, K.; Koyasu, S.; Toyama, Y.; et al. Establishment of a Real-Time, Quantitative, and Reproducible Mouse Model of Staphylococcus Osteomyelitis Using Bioluminescence Imaging. Infect. Immun. 2012, 80, 733–741. [Google Scholar] [CrossRef] [Green Version]
- Pribaz, J.R.; Bernthal, N.M.; Billi, F.; Cho, J.S.; Ramos, R.I.; Guo, Y.; Cheung, A.L.; Francis, K.P.; Miller, L.S. Mouse Model of Chronic Post-Arthroplasty Infection: Noninvasive in Vivo Bioluminescence Imaging to Monitor Bacterial Burden for Long-Term Study. J. Orthop. Res. 2012, 30, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Yoshii, T.; Magara, S.; Miyai, D.; Nishimura, H.; Kuroki, E.; Furudoi, S.; Komori, T.; Ohbayashi, C. Local Levels of Interleukin-1β, -4, -6, and Tumor Necrosis Factor α in an Experimental Model of Murine Osteomyelitis Due to Staphylococcus Aureus. Cytokine 2002, 19, 59–65. [Google Scholar] [CrossRef]
- Heim, C.E.; Vidlak, D.; Scherr, T.D.; Kozel, J.A.; Holzapfel, M.; Muirhead, D.E.; Kielian, T. Myeloid-Derived Suppressor Cells Contribute to Staphylococcus aureus Orthopedic Biofilm Infection. J. Immunol. 2014, 192, 3778–3792. [Google Scholar] [CrossRef] [Green Version]
- Heim, C.E.; Vidlak, D.; Scherr, T.D.; Hartman, C.W.; Garvin, K.L.; Kielian, T. IL-12 Promotes Myeloid-Derived Suppressor Cell Recruitment and Bacterial Persistence during Staphylococcus aureus Orthopedic Implant Infection. J. Immunol. 2015, 194, 3861–3872. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lin, Y.; Cheng, C.; Chen, P.; Zhang, P.; Wu, H.; Li, K.; Deng, Y.; Qian, J.; Zhang, X.; et al. NF-ΚB/TWIST1 Mediates Migration and Phagocytosis of Macrophages in the Mice Model of Implant-Associated Staphylococcus Aureus Osteomyelitis. Front. Microbiol. 2020, 11, 1301. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, G.; Wallimann, A.; Rangel-Moreno, J.; de Mesy Bentley, K.L.; Hildebrand, M.; Mys, K.; Kenney, H.M.; Sumrall, E.T.; Daiss, J.L.; Zeiter, S.; et al. Humanized Mice Exhibit Exacerbated Abscess Formation and Osteolysis During the Establishment of Implant-Associated Staphylococcus Aureus Osteomyelitis. Front. Immunol. 2021, 12, 651515. [Google Scholar] [CrossRef]
- Andriole, V.T.; Nagel, D.A.; Southwick, W.O. A Paradigm for Human Chronic Osteomyelitis. J. Bone Jt. Surg. Am. 1973, 55, 1511–1515. [Google Scholar] [CrossRef]
- Arens, S.; Kraft, C.; Schlegel, U.; Printzen, G.; Perren, S.M.; Hansis, M. Susceptibility to Local Infection in Biological Internal Fixation. Experimental Study of Open vs Minimally Invasive Plate Osteosynthesis in Rabbits. Arch. Orthop. Trauma Surg. 1999, 119, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, T.F.; Campoccia, D.; Nees, S.K.; Boure, L.P.; Richards, R.G. In Vivo Evaluation of the Effect of Intramedullary Nail Microtopography on the Development of Local Infection in Rabbits. Int. J. Artif. Organs 2010, 33, 667–675. [Google Scholar] [CrossRef]
- Ashhurst, D.E.; Hogg, J.; Perren, S.M. A Method for Making Reproducible Experimental Fractures of the Rabbit Tibia. Injury 1982, 14, 236–242. [Google Scholar] [CrossRef]
- Worlock, P.; Slack, R.; Harvey, L.; Mawhinney, R. An Experimental Model of Post-Traumatic Osteomyelitis in Rabbits. Br. J. Exp. Pathol. 1988, 69, 235–244. [Google Scholar]
- Kraft, C.N.; Schlegel, U.; Pfluger, D.; Eijer, H.; Textor, J.; Hansis, M.; Arens, S. Radiological Signs of Osteitis around Extramedullary Metal Implants. A Radiographic-Microbiological Correlative Analysis in Rabbit Tibiae after Local Inoculation of Staphylococcus Aureus. Arch. Orthop. Trauma Surg. 2001, 121, 338–342. [Google Scholar] [CrossRef]
- Johansson, A.; Svensson, O.; Blomgren, G.; Eliasson, G.; Nord, C.E. Anaerobic Osteomyelitis. A New Experimental Rabbit Model. Clin. Orthop. 1991, 265, 297–301. [Google Scholar] [CrossRef]
- Schulz, S.; Steinhart, H.; Mutters, R. Chronic Osteomyelitis in a New Rabbit Model. J. Investig. Surg. 2001, 14, 121–131. [Google Scholar] [CrossRef]
- Belmatoug, N.; Crémieux, A.C.; Bleton, R.; Volk, A.; Saleh-Mghir, A.; Grossin, M.; Garry, L.; Carbon, C. A New Model of Experimental Prosthetic Joint Infection Due to Methicillin-Resistant Staphylococcus Aureus: A Microbiologic, Histopathologic, and Magnetic Resonance Imaging Characterization. J. Infect. Dis. 1996, 174, 414–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, M.R.; Poelstra, K.A.; Sherrell, J.C.; Kwon, M.S.; Belzile, E.L.; Brown, T.E. A Novel Total Knee Arthroplasty Infection Model in Rabbits. J. Orthop. Res. 2005, 23, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Morrissy, R.T.; Haynes, D.W. Acute Hematogenous Osteomyelitis: A Model with Trauma as an Etiology. J. Pediatr. Orthop. 1989, 9, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Whalen, J.L.; Fitzgerald, R.H.J.; Morrissy, R.T. A Histological Study of Acute Hematogenous Osteomyelitis Following Physeal Injuries in Rabbits. J. Bone Jt. Surg. Am. 1988, 70, 1383–1392. [Google Scholar] [CrossRef]
- Johansson, A.; Lindgren, J.U.; Nord, C.E.; Svensson, O. Material and Design in Haematogenous Implant-Associated Infections in a Rabbit Model. Injury 1999, 30, 651–657. [Google Scholar] [CrossRef]
- Sharun, K.; Pawde, A.M.; Banu, S.A.; Manjusha, K.M.; Kalaiselvan, E.; Kumar, R.; Kinjavdekar, P. Amarpal Development of a Novel Atrophic Non-Union Model in Rabbits: A Preliminary Study. Ann. Med. Surg. 2021, 68, 102558. [Google Scholar] [CrossRef]
- Daum, R.S.; Davis, W.H.; Farris, K.B.; Campeau, R.J.; Mulvihill, D.M.; Shane, S.M. A Model of Staphylococcus Aureus Bacteremia, Septic Arthritis, and Osteomyelitis in Chickens. J. Orthop. Res. 1990, 8, 804–813. [Google Scholar] [CrossRef]
- Griffith, G.L.; Hopkinson, W.I.; Lloyd, J. Staphylococcal Necrosis of the Head of the Femur in Broiler Chickens. Aust. Vet. J. 1984, 61, 293. [Google Scholar] [CrossRef]
- Carnaghan, R.B.A. Spinal Cord Compression in Fowls Due to Spondylitis Caused by Staphylococcus Pyogenes. J. Comp. Pathol. 1966, 76, 9–14. [Google Scholar] [CrossRef]
- Wideman, R.F.; Prisby, R.D. Bone Circulatory Disturbances in the Development of Spontaneous Bacterial Chondronecrosis with Osteomyelitis: A Translational Model for the Pathogenesis of Femoral Head Necrosis. Front. Endocrinol. 2013, 3, 183. [Google Scholar] [CrossRef] [Green Version]
- Greene, E.; Flees, J.; Dhamad, A.; Alrubaye, A.; Hennigan, S.; Pleimann, J.; Smeltzer, M.; Murray, S.; Kugel, J.; Goodrich, J.; et al. Double-Stranded RNA Is a Novel Molecular Target in Osteomyelitis Pathogenesis: A Translational Avian Model for Human Bacterial Chondronecrosis with Osteomyelitis. Am. J. Pathol. 2019, 189, 2077–2089. [Google Scholar] [CrossRef] [PubMed]
- Petty, W.; Spanier, S.; Shuster, J.J.; Silverthorne, C. The Influence of Skeletal Implants on Incidence of Infection. Experiments in a Canine Model. J. Bone Jt. Surg.-Ser. A 1985, 67, 1236–1244. [Google Scholar] [CrossRef]
- Garvin, K.L.; Miyano, J.A.; Robinson, D.; Giger, D.; Novak, J.; Radio, S. Polylactide/Polyglycolide Antibiotic Implants in the Treatment of Osteomyelitis. A Canine Model. J. Bone Jt. Surg.-Ser. A 1994, 76, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Kieser, D.C.; Kanade, S.; Waddell, N.J.; Kieser, J.A.; Theis, J.C.; Swain, M.V. The Deer Femur-A Morphological and Biomechanical Animal Model of the Human Femur. Biomed. Mater. Eng. 2014, 24, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.F.; Clasper, J.C.; Parker, S.J.; Watkins, P.E. Early Intramedullary Nailing in an Animal Model of a Heavily Contaminated Fracture of the Tibia. J. Orthop. Res. 2002, 20, 648–653. [Google Scholar] [CrossRef] [Green Version]
- Salgado, C.J.; Jamali, A.A.; Mardini, S.; Buchanan, K.; Veit, B. A Model for Chronic Osteomyelitis Using Staphylococcus Aureus in Goats. Clin. Orthop. 2005, 246–250. [Google Scholar] [CrossRef]
- Beardmore, A.A.; Brooks, D.E.; Wenke, J.C.; Thomas, D.B. Effectiveness of Local Antibiotic Delivery with an Osteoinductive and Osteoconductive Bone-Graft Substitute. J. Bone Jt. Surg.-Ser. A 2005, 87, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Tran, N.; Tran, P.A.; Jarrell, J.D.; Engiles, J.B.; Thomas, N.P.; Young, M.D.; Hayda, R.A.; Born, C.T. In Vivo Caprine Model for Osteomyelitis and Evaluation of Biofilm-Resistant Intramedullary Nails. BioMed Res. Int. 2013, 2013, 674378. [Google Scholar] [CrossRef] [Green Version]
- van der Borden, A.J.; Maathuis, P.G.M.; Engels, E.; Rakhorst, G.; van der Mei, H.C.; Busscher, H.J.; Sharma, P.K. Prevention of Pin Tract Infection in External Stainless Steel Fixator Frames Using Electric Current in a Goat Model. Biomaterials 2007, 28, 2122–2126. [Google Scholar] [CrossRef]
- Stewart, S.; Barr, S.; Engiles, J.; Hickok, N.J.; Shapiro, I.M.; Richardson, D.W.; Parvizi, J.; Schaer, T.P. Vancomycin-Modified Implant Surface Inhibits Biofilm Formation and Supports Bone-Healing in an Infected Osteotomy Model in Sheep. J. Bone Jt. Surg. Am. 2012, 94, 1406–1415. [Google Scholar] [CrossRef] [Green Version]
- Dehring, D.J.; Crocker, S.H.; Wismar, B.L.; Steinberg, S.M.; Lowery, B.D.; Cloutier, C.T. Comparison of Live Bacteria Infusions in a Porcine Model of Acute Respiratory Failure. J. Surg. Res. 1983, 34, 151–158. [Google Scholar] [CrossRef]
- Jensen, L.K.; Koch, J.; Dich-Jorgensen, K.; Aalbæk, B.; Petersen, A.; Fuursted, K.; Bjarnsholt, T.; Kragh, K.N.; Tøtterup, M.; Bue, M.; et al. Novel Porcine Model of Implant-Associated Osteomyelitis: A Comprehensive Analysis of Local, Regional, and Systemic Response. J. Orthop. Res. 2017, 35, 2211–2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, P.F.; Watkins, P.E. The Prevention of Experimental Osteomyelitis in a Model of Gunshot Fracture in the Pig. Eur. J. Orthop. Surg. Traumatol. 2001, 11, 237–241. [Google Scholar] [CrossRef]
- Jensen, L.K.; Johansen, A.S.B.; Jensen, H.E. Porcine Models of Biofilm Infections with Focus on Pathomorphology. Front. Microbiol. 2017, 8, 1961. [Google Scholar] [CrossRef] [PubMed]
- Koschmieder, R.; Ritzerfeld, W.; Homeyer, L. Gentamycinzusatz Zum Polymethylmethacrylat Zur Behandlung von Knocheninfektionen. Z. Orthop. 1975, 113, 147–149. [Google Scholar] [PubMed]
- Rink, A.; Santschi, E.M.; Beattie, C.W. Normalized CDNA Libraries from a Porcine Model of Orthopedic Implant-Associated Infection. Mamm. Genome 2002, 13, 198–205. [Google Scholar] [CrossRef]
- Johansen, L.K.; Frees, D.; Aalbæk, B.; Koch, J.; Iburg, T.; Nielsen, O.L.; Leifsson, P.S.; Jensen, H.E. A Porcine Model of Acute, Haematogenous, Localized Osteomyelitis Due to Staphylococcus Aureus: A Pathomorphological Study. Apmis 2011, 119, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Johansen, L.K.; Koch, J.; Frees, D.; Aalbæk, B.; Nielsen, O.L.; Leifsson, P.S.; Iburg, T.M.; Svalastoga, E.; Buelund, L.E.; Bjarnsholt, T.; et al. Pathology and Biofilm Formation in a Porcine Model of Staphylococcal Osteomyelitis. J. Comp. Pathol. 2012, 147, 343–353. [Google Scholar] [CrossRef]
- Johansen, L.K.; Koch, J.; Kirketerp-Møller, K.; Wamsler, O.J.; Nielsen, O.L.; Leifsson, P.S.; Frees, D.; AalbæK, B.; Jensen, H.E. Therapy of Haematogenous Osteomyelitis—A Comparative Study in a Porcine Model and Angolan Children. In Vivo 2013, 27, 305–312. [Google Scholar]
- Tøttrup, M.; Bue, M.; Koch, J.; Jensen, L.K.; Hanberg, P.; Aalbæk, B.; Fuursted, K.; Jensen, H.E.; Søballe, K. Effects of Implant-Associated Osteomyelitis on Cefuroxime Bone Pharmacokinetics. J Bone Jt. Surg. Am. 2016, 98, 363–369. [Google Scholar] [CrossRef]
- Jensen, L.K. Implant-Associated Osteomyelitis: Development, Characterisation, and Application of a Porcine Model. Apmis 2021, 129, 1–44. [Google Scholar] [CrossRef] [PubMed]
| Species | Pros [1,2,3,134,135,136,137,138,139] | Cons [1,2,3,134,135,136,137,138,139] |
|---|---|---|
| Small models | Evaluation of pathophysiology and novel treatment strategies | Failure in systemic antibiotic treatment evaluation studies due to the physiology of the gastrointestinal tract |
| Adaptable to pathological conditions (easy manipulation) | Very small joints–in situ examination is impossible | |
| Development of well-characterized mouse strains (knock-out or transgenic models) | Limitations associated with existing surgical approaches | |
| Use of specific and well-known antibodies | Limited or rapid cortical remodeling | |
| Bone turnover is similar to human | Cortical bone composition (e.g., hydroxyproline and protein content) differs from that of humans | |
| Biohazard risk related to handling infected animals (infected bites) | ||
| Growth plates never close in mice and rats | ||
| Ethical concerns | ||
| Large models | Higher life span | Ethical concerns |
| Larger skeletal surfaces allow mimicking internal and external fixation techniques and implants commonly used in humans | High cost (breeding, manipulation) | |
| Rate of osteogenesis (sheep and goat) | Higher rate of bone growth than humans (porcine) | |
| High similarity to human bones in density and mineral composition (dog and porcine) | Bones are denser and present fewer Harversian canals (sheep) |
| Model/Strain | Gender | Age/Weight | Microorganism/ Concentration | Disease Model | Site of Inoculum | Osteomyelitis Induction | Timepoint | Aim of the Study | Results | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Inbred cr/rar and outbred Sprague–Dawley albino | M | Inbred: 200–400 g; Sprague–Dawley albino rats: 400–500 g | S. aureus phage type 52/52A/80. 3.0 × 106 CFU | CO | Tibia (bone marrow) | Sodium morrhuate 5% and arachidonic acid | 35 days | To verify if arachidonic acid could facilitate experimental osteomyelitis | Arachidonic acid was a strong facilitator of osteomyelitis. | [159] |
| Sprague–Dawley albino | M | 300–400 g | P. aeruginosa (field strain isolated from a patient with osteomyelitis). 2.4, 3.3, 4.8, 6.4, and 7.1 log CFU were used to determine ID50. Log 5.8 CFU was used for experimental inoculation. | CH | Tibial metaphysis | 21 days (for ID50 determination) and 63 days for the experiment | To create a rat model of chronic P. aeruginosa osteomyelitis that did not require promoting agents | The ID50 was log 4.0 CFU with an ID100 of log 6.4. In the rat model, the establishment of P. aeruginosa osteomyelitis does not require promoting agents. | [176] | |
| White | na | na | S. aureus | Mandibular osteomyelitis | Drilled cavity in mandibular cavity | na | na | To develop a reliable model of mandibular osteomyelitis | Preclinical study of new drugs and physiotherapeutic methods can be obtained after the used of this model | [170] |
| Wistar | F | 180–220 g | S. aureus Phillips (field strains from osteomyelitis). 5 × 104, 5 × 106, 5 × 107, or 5 × 108 CFU (surgically exposed mandible). 5 × 106, 5 × 107, or 5 × 108 CFU (surgically exposed tibia). 5 × 106 and 5 × 107 CFU (control B and control C). 108 CFU (control D). | HO | Intramedullary injection of sodium morrhuate in mandible and tibia. Bacteria were injected into the femoral vein. | Sodium morrhuate 5% | 14 days | To establish and evaluate a new rat model of haematogenous osteomyelitis | No pathologic changes were produced in animals undergoing only surgery but receiving sodium morrhuate (control). In the treated group, osteomyelitis was successfully established. | [171] |
| Sprague–Dawley | M | 300 g | S. aureus ATCC 29213 or P. aeruginosa ATCC 27853. 103 CFU in one group and 106 CFU in the other for S. aureus and ascending concentrations for P. aeruginosa. 103 CFU of S. aureus, 103 CFU of P. aeruginosa, or 103 CFU of both S. aureus and P. aeruginosa. | Complex orthopaedic wounds | Lumbar spinous process | na | 14 days | To determine whether synergy exists between S. aureus and P. aeruginosa in a rat model of complex orthopaedic wounds | When low levels of each organism were present in the wound, synergy existed. The ability of S. aureus to cause infections qis enhanced by low concentrations of P. aeruginosa. | [177] |
| Sprague–Dawley | F | 5 months | S. aureus ATCC 49230. 103 CFU | Implant–related osteomyelitis | Proximal tibia metaphysis (medullary cavity) | Poly (D,L–lactide)–coated Kirschner wire | 42 days | To test the efficacy of a new biodegradable, gentamicin–loaded poly(D,L–lactide) coating | The implant–related infection was significantly reduced by PDLLA + 10% gentamicin. | [166] |
| Sprague–Dawley | F | 250–300 g | S. aureus Xen29 (genetically engineered using Gram–positive lux transposon plasmid pAUL–Atn 4001 luxABCDE kmr). 106 CFU/mL. Biofilm–coated K–wire (0.5 cm long). | Implant–related osteomyelitis (biofilm model) | Proximal anterior margin of the tibial epicondyle | Arachidonic acid (50 µg/mL in 0.9% NaCl solution) | 10 days | To investigate photodynamic therapy (PDT) as alternative treatment for osteomyelitis using bioluminescence | 1 mM of 5–aminolevulinic acid and methylene blue 0.1 mM can mediate the sensitivity of S. aureus at 5 J cm−2 light dose with ≥4log10 cell kill | [167] |
| Sprague–Dawley | na | 417 g | S. epidermidis ATCC 35984. 105 CFU/cavity | Implant–related infection | Cortex of the intercondylar notch of the femur | na | 14 days (control group), 56 days (treated groups) | To evaluate the effect of serratiopeptidase in the eradication of periarticular hardware | Bacterial growth was reduced in the treated group by serratiopeptidase and antibiotic together compared to animals inoculated with antibiotics alone | [178] |
| Wistar | M | 12–14 weeks, 423–481 g | S. aureus ATCC 29213. 107 CFU | Implant–associated infection | Femoral medullary cavity | na | 21 days | To assess the antibiotic efficacy of moxifloxacin in implant–associated infections | Animal mortality 0%. The efficacy of moxifloxacin was significantly greater (p < 0.01) than that of vancomycin. | [172] |
| Wistar | na | 300–350 g | S. aureus ATCC 29213. 107 CFU | Implant–related chronic osteomyelitis | Medullary cavity of femur | na | 28 days | To test moxifloxacin compared to teicoplanin in chronic implant–related osteomyelitis | For moxifloxacin–group compared to teicoplanin–group the decrease of bacterial counts was more prominent (p = 0.001). | [169] |
| Sprague–Dawley | M | 250–300 g | S. aureus (field strain isolated from a patient with an infected total hip arthroplasty). 104 CFU | Femur fracture model | Medullary cavity of femur | na | 21 days | To develop a model of induced implant–associated osteomyelitis following fracture repair | Between the control and S. aureus group, by one week after surgery/inoculation, significant differences in the radiographic score for osteomyelitis were detected. | [137] |
| Sprague–Dawley | M | 10–w, 283–401 g | Methicillin–resistant S. aureus (field strain isolated from a patient with septicemia). 1.0 × 102 CFU | Implant–related osteomyelitis | Tibial medullary cavity | na | 28 days | To develop an antibacterial coating with Ag–containing hydroxyapatite (Ag–HA) | Antibacterial activity of Ag–HA coating was shown against MRSA. Serum Ag ion concentrations reached a peak at about 48 h | [168] |
| Sprague–Dawley | F | na | S. aureus ATCC 25923. 106, 105, 104 and 103 CFU | Implant–associated infection | Medial proximal tibial metaphysis | na | 42 days | To evaluate a novel animal model for the generation of implant–associated infections in the tibial metaphysis of rats | A higher viable count was observed in peri–implant bone samples from animals inoculated with 106 CFU. However, there could be no correlation between initial load and concentration after sacrifice. | [174] |
| Sprague–Dawley | M | 12 w | S. aureus DSM 28763 (field strain isolated from wound infection; genome sequenced, biofilm producer). 103 CFU | Implant–related infection | Tibia | na | 42 days | To determine if the prophylactic administration of TLR9 ligand CpG ODN type B would affect a model of implant–related chronic infection | Results indicated that the bacterial load in the infected tibia was reduced at the beginning of infection but failed to prevent the development of chronic infection. | [179] |
| Wistar | M | 12 weeks, 300–350 g | Methicillin–resistant S. epidermidis strain GOI1153754–03–14 (field strain from infected knee prosthesis). 1 × 103, 1 × 105 and 1 × 108 CFU/rat | Fracture model | Non–critical midshaft full–thickness defect in femur | na | 56 days | To understand the role of subclinical bacterial contaminations in the non–union development | Bone healing was prevented in low–grade S. epidermidis contamination. Bacterial inoculum and non–union rate followed a dose–dependent relation | [114] |
| Wistar | M | 5 months, 353–401 g | S. aureus ATCC25923. 102 CFU (Group I–IIA), 103 CFU (Group I–IIB) | Implant–related osteomyelitis | Proximal lateral tibial metaphysis | na | 42 days | To evaluate a low bacterial inocula animal model of tibial metaphysis and investigate osseointegration of the implants coated with hydroxyapatite (HA) and low–dosed HA–silver (HA–Ag) | No systemic infection registered. Infection was induced, independently whether bacterial load used and implant inserted. | [173] |
| Sprague–Dawley | M | 350–400 g | S. aureus Xen 29 ATCC 12600. 2 × 107 CFU (injected in 8 mm length–hole), 8 × 107 CFU (injected into the joints) | Periprosthetic joint infection | Lateral femoral condyle | na | 28 days | To develop a joint replacement model with ultrahigh molecular weight polyethylene (UHMWPE) and titanium components | Clinical infection indicators such as osteolysis, loosening of the implants were observed for 4 weeks | [175] |
| Model/Strain | Gender | Age/Weight | Microorganism/Concentration | Disease Model | Site of Inoculum | Timepoint | Aim of the Study | Results | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| CD1 | na | >6 months | S. aureus ATTC 49230. 2 to 3 mm length of 4–0 suture seeded with S. aureus. | Chronic osteomyelitis | Proximal tibia | 2 h–28 days | To investigate the role of interleukin 4 in osteoclast activation and development of chronic osteomyelitis | IL4 may help to block the osteoclast reaction, which leads to more bone loss. | [139] |
| C3H/HeJ | na | 8–10 weeks; 20–25 g | S. aureus LS-1. 5 × 107 UFC (injected into the tail vein). | Acute hematogenous osteomyelitis | Tibia (incomplete cartilaginous fracture) | 7 days | To study the immunological responses to S. aureus bone infection | An increase of splenic B lymphocytes and in lymph–node CD4+ T lymphocytes was observed. | [145] |
| ICR | F | 5–weeks; 25 g | S. aureus E-31461. 4.6 × 105 CFU/suture | Tibial osteomyelitis | Tibia | 28 days | To evaluate local levels of IL-1 β, IL–4, IL-6, and TNF–α, in a model of murine osteomyelitis due to S. aureus | Levels of IL-1β and IL-6 in infected bone were elevated in the early post–infection period and then decreased. TNF levels remained elevated 3 to 28 days post–infection, while IL–4 levels were elevated late in the infection. | [185] |
| C57BL/6 | F | 6–8 weeks | S. aureus UAMS-1 ATCC 49230 and S. aureus Xen29 (derived from ATCC 12600). 9.5 ± 3.7 × 105 CFU/pin of UAMS-1 and 4.2 ± 0.5 × 105 CFU/pin of Xen29. | Implant–associated osteomyelitis | Tibial metaphysis | 18 days | To develop a novel murine model of implant–associated osteomyelitis using steel pin coated with S. aureus | Histology confirmed all the characteristics of the associated implant. After one week, the mice produced IgM, which converted to IgG 11 days after implantation. | [181] |
| C57BL/6 wildtype and LysEGFP | M | 12–weeks | S. aureus SH1000 strain, ALC2906 (contains the shuttle plasmid pSK236 with the penicillin–binding protein 2 promoter fused to the luxABCDE reporter cassette). 5 × 102, 5 × 103 and 5 × 104 CFU/mouse | Post–arthroplasty infections | Knee joint | 7 and 14 days | To develop a model of post–arthroplasty Infection combining the use of bioluminescent bacteria and genetically engineered mice that possess fluorescent neutrophils (LysEGFP mice) | Chronic osteomyelitis was developed in mice infected with a low bacterial load, while acute osteomyelitis was developed in those who received 103 and 104. In vivo bioluminescence EGFP–neutrophil signals and fluorescence of LysEGFP mice are highly correlated with Ex vivo bacterial counts. | [182] |
| BALB/c | M | 12 weeks; 20–25 g | S. aureus Xen-29. 1.0 × 108 CFU | Osteomyelitis | Femur | Not necessary | To establish a real–time quantitative mouse model of osteomyelitis using bioluminescence imaging | In infected mice, serum levels of interleukin–6, interleukin–1β and C–reactive protein were significantly higher. | [183] |
| C57BL/6 and LysEGFP | M | 12–weeks | S. aureus ALC2906 S. aureus Xen29 (derived from the pleural fluid isolate ATCC 12600 with an antibiotic marker), S. aureus Xen40 (derived from the osteomyelitis isolate UAMS–1, inside chromosome) and S. aureus Xen36 (derived from the bacteremia isolate ATCC 49525, integrated into a stable plasmid). 1 × 102, 1 × 103 and 1 × 104 CFU. | Post–arthroplasty infections | Femur | 42 days | To study the pathogenesis of post–arthroplasty infections with the use of bioimaging and non–invasive technology | A chronic post–arthroplasty infection model was developed. Up until day 10 ALC2906 had an increase in bioluminescent signals. On day 42, biofilms were detected on the implants inoculated with ALC2906. These results suggest that the construct was lost during in vivo replication. | [184] |
| NOD/ShiLtJ | F | 23.3 ± 1.3 g | S. aureus. 103 CFU/mouse | Implant related infection | Femoral canal | 28 days | To investigate the effect of a PGE1 vasodilator on the incidence of surgical infections in diabetic mice | Limited signs of infection were identified in mice treated with the combination of a PGE1 and an antibiotic using micro–CT and histological analysis. | [180] |
| C57BL/6 | M | 8 weeks | S. aureus USA300 LAC. 103 CFU | Orthopaedic biofilm infection | Femur | 28 days | To examine the functional role of Myeloid–derived suppressor cells in shaping the anti–inflammatory milieu during S. aureus orthopedic biofilm infection | Increased expression of Arg–1, iNOS and IL-10. Bacterial clearance was improved due to the targeted depletion of MDSC and neutrophils using mAb 1A8 (antiLy6G). | [186] |
| C57BL/6NCr | M | 8 weeks | S. aureus isolate USA300 LAC. 103 CFU | Orthopaedic implant infection | Femur | 28 days | To study the pro–inflammatory ability of IL-12 in myeloid–derived suppressor cell recruitment and bacterial persistence | Several cytokines (IL-12p40, IL-1β, TNF–α, and G–CSF) and chemokines (CXCL2, CCL5) were significantly elevated. In both p40 and p35 KO Mice MDSC recruitment was significantly reduced. | [187] |
| C57BL/6 | M | 8 weeks | S. aureus. 2 × 103 CFU | Implant–associated osteomyelitis | Mid–diaphysis of the femur | 3 days | To develop a model of implant–associated S. aureus osteomyelitis to study the expression of osteomyelitis associated genes (ERBB2, TWIST1, and NANOG) | Around the infected implant, an upregulation of TWIST1 in macrophages and an accumulation of macrophages was observed. In addition, the expression of TWIST1, MMP9, and MMP13, together with the migration and phagocytosis function of 264.7 cells were increased. | [188] |
| (NOD)–scid IL2Rγnull (NSG) mice | F | na | S. aureus. 5 × 105 CFU/mL | Orthopaedic implant infection | Tibia | 14 days | To study the response of human immune cells during chronic S. aureus bone infections engrafting mice with hematopoietic stem cells (huNSG) | Compared to the control group, huNSG mice have increased weight loss, osteolysis, and bacterial spread to internal organs. Moreover, through flow cytometry and immunohistochemistry, more human T cells are present in infected huNGS mice than in uninfected ones. | [189] |
| Model/Strain | Gender | Age/Weight | Microorganism/Concentration | Disease Model | Site of Inoculum | Osteomyelitis Induction | Timepoint | Aim of the Study | Results | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Rabbits | na | 2–2.25 lb | S. aureus | HO and direct inoculation | Metaphysis of the tibia | 5% sodium morrhuate | 8 weeks | Preliminary report on new method of producing experimental osteomyelitis in rabbits | The authors were able to keep the animals alive for an indefinite period (at least 8 weeks). | [122] |
| New Zealand white rabbits | na | 4–4.5 lb | S. aureus phage type 52/52A/80 (field strain isolated from a child with osteomyelitis), S. aureus 209 P and P. mirabilis (field strain from a urinary–tract infection). 3 × 106 CFU (S. aureus phage type 52/52A/80 only). | Chronic osteomyelitis | Tibial medullary cavity | 5% sodium morrhuate | 60–180 days | To establish a model of chronic osteomyelitis. | The injection of S. aureus together with sodium morrhuate could induce osteomyelitis. Animal inoculated with P. mirabilis showed the same radiologic lesions observed from those infected with S. aureus | [158] |
| New Zealand white rabbits | F | 3–6 kg | S. aureus. 2 × 104, 2 × 105, 2 × 106, 2 × 107, 2 × 108, 2 × 109 CFU | Implant–related osteomyelitis | Tibial marrow cavity | na | 180 days | A new model of chronic staphylococcal osteomyelitis | The results of this study indicate that chronic staphylococcal osteomyelitis can be produced in the rabbit tibia in the presence of a metallic implant. | [190] |
| New Zealand white rabbits | M | >3.5 kg | S. aureus (phage type 29). 105, 106, 107 CFU | Implant–related osteomyelitis | Tibial medullary cavity | na | 8 weeks | To develop a model of induced implant–associated osteomyelitis following fracture repair | The study was successful in developing a model that could be used for other studies in osteomyelitis | [194] |
| New Zealand white rabbits | na | na | Bacteroides fragilis. 107 CFU | Single strain osteomyelitis infection | Medullary cavity of the proximal tibial metaphysis | na | 18 weeks | To test a new anaerobic osteomyelitis model | This method gave a high infection rate with reproducible immunologic, roentgenographic, and histologic reactions | [196] |
| Chinchilla–Bastard rabbits | F | 3.25–4.79 kg | S. aureus. 3 × 105 CFU | Implant–related osteomyelitis | Proximal end of the femur | 0.1 mL 5% sodium morrhuate | 6–8 weeks | To find a rabbit model to perform more local therapeutic strategies on the infected bone | The new technique did not influence the motion of the hind limb and mimicked well the intramedullary pinning of long fractured bones, but it did pose some risks for postoperative infections | [197] |
| New Zealand White rabbits | M | ±4.2 kg | MRSA. 0 CFU in one knee and 104 CFU in the contralateral knee (Group A) 102 CFU in one knee and 103 CFU in the contralateral knee (Group B). | Implant–related osteomyelitis | Knee | na | 7 days | To design and evaluate a novel small animal model for the investigation of biomaterial centered infection in total joint arthroplasty | This model closely simulates the biologics, and not the mechanics, of human prosthetic knee replacement and is a valuable tool to develop new systemic and local anti–infective strategies | [199] |
| New Zealand White rabbits | M and F | 7–8 months, 2.04 ± 0.09 kg | na | Segmental bone defect in the radial diaphysis | Radius bone | na | 30 days | Development of a novel atrophic non–union model in rabbits | The radiographic signs of healing were completely absent in all the rabbits on 30th postoperative day, indicating inert bone ends. | [203] |
| Model/Strain | Gender | Age/Weight | Microorganism/Concentration | Disease Model | Site of Inoculum | Timepoint | Aim of the Study | Results | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Fowl Rhode Island Red | na | 4–6 weeks | S. pyogenes; 3 strains: Strain 1641; Strain 8217 phage type 81/+; strain 8272 type 29/7/42E/42D/81. 0–108 CFU | Spondylitis | Intravenous | 15–18 days | To describe outbreaks caused by S. pyogenes and report the disease’s experimental reproduction. | The condition was not verified on individual birds but on a flock basis; furthermore, did not appear to be related to particular poultry breeds. | [206] |
| Broiler | M | 29–days | S. aureus phage type 6/42E/53/77/83A/84. 1 × 104 to 108 CFU/kg | Acute hematogenous osteomyelitis | Wing vein | 6, 12, 24, 48, 96, and 192 h | To describe a highly reproducible experimental model of acute hematogenous osteomyelitis in chickens closely mimics the human disease. | Osteomyelitis was produced quite easily. Within the periosteum adjacent to the metaphysis lesions were observed, while in the lungs, liver and kidneys, no bacterial lesions were observed. | [129] |
| Broiler | M | 35–days | S. aureus. 105 CFU in one group and 107 CFU in the other. | Acute osteomyelitis | Intravenous | 14 days | To record a flock outbreak of femoral head necrosis in broiler chickens due to infection with S. aureus. | It has been observed that. S. aureus is a pathogen with a tropism for bone growth. | [205] |
| Broiler | M | 30–days | S. aureus strain Duntravis, capsular type 8 isolate. 105, 106, or 107 CFU | Osteomyelitis and septic arthritis | Intravenous | 14 days | To study the occurrence, magnitude, and kinetics of bacteremia and the resultant osteomyelitis and septic arthritis | From 1 to 23 h after inoculation, osteomyelitis remained uniform in continuously bacteremic animals | [204] |
| Model/Strain | Gender | Age/Weight | Microorganism/ Concentration | Disease Model | Site of Inoculum | Timepoint | Aim of the Study | Results | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Goats | M/F | 1–4 years, 25–45 kg | S. aureus ATCC 700260. 4 × 109 CFU | Chronic osteomyelitis | Tibia | 12–16 weeks | To develop a model of tibial osteomyelitis | 96% of the animal have radiographic evidence of osteomyelitis. Local osteomyelitis was developed. | [213] |
| Suffolk–cross breed | F | 55–80 kg | S. aureus ATCC 29213. 3 × 108 CFU | Fracture | Tibia | 3 weeks | To study the outcome of a heavily contaminated fracture | The entire length of the implant induced infection in animals. intramedullary nailing should not be used as a first treatment for heavily contaminated fractures. | [212] |
| Texel crossbreed sheep | na | 3–5 years, 47–64 kg | S. aureus PS 8386. 8 × 108 CFU | Chronic osteomyelitis | Tibia (3% tetradecylsodiumsulphate solution used as sclerosing agent) | 12 weeks | To develop a large animal model for chronic osteomyelitis | Localized soft tissue swelling, pain during the acute phase, and limping in all sheep were considered clinical signs of infection. | [132] |
| Spanish goats | na | 37–50 kg | S. aureus ATCC 29213. 9.42 × 104 CFU | Open fracture | Proximal tibial metaphysis | 21 days | To evaluate the prophylactic treatment of bone–graft substitute using locally delivered antimicrobial | The use of tobramycin–impregnated calcium sulfate pellets and demineralized bone matrix prevented intramedullary dissemination of S. aureus | [214] |
| Goat | F | 40–50 kg | S. aureus ATCC 25923. 2 × 104 CFU | Open fracture | Tibia (intramedullary nails) | 5 weeks | To develop a large animal model to study antimicrobial coated bone implants | Intramedullary nails allowed to treat fracture. At 5 weeks, the treated goats lost 7% of initial body weight but was able to ambulate. The control animals were not able to deambulate and lost 8.4% of initial body weight. | [215] |
| Saanen goats | F | na | S. epidermidis HBH276 (field strain isolated from neonate). 3 × 105 CFU | Orthopaedic infection | Tibia | 3 weeks | To study the impact of an electric percutaneous current in preventing implant associated infection. | The low amperage electric current prevents infections of percutaneous pins implanted in Tibia | [216] |
| Dorset–cross ewes | na | 2.5–3.5-years | S. aureus. 2.5 × 106 CFU | Implant–associated infection | Tibia | 3 months | To develop a surface modification of titanium fracture hardware with vancomycin to prevent bacterial colonization in a large animal model | The modified titanium plates treated with antibiotic–derived compounds inhibited the colonization of the implant. Moreover, treated groups showed bone–healing. | [217] |
| Model/Strain | Gender | Age/Weight | Microorganism/Concentration | Disease Model | Site of Inoculum | Timepoint | Aim of the Study | Results | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Domestic Landrace | na | 12 weeks | S. aureus (haemolyticus). 2 × 108 CFU | Infectious bone diseases | Femur | 16 days | To study the effect of gentamicin embedded in palacos bone cement. | The number of germ populations is reduced significantly by the antibiotic released in a microbiologically active concentration. The number of germs in control group remained at a high level | [222] |
| Yucatan mini swine | F | 2–5 years, 68–95 kg | S. aureus ATCC strains 6538P, 25923, and 29213. 108–109 CFU | Chronic Mandibular Osteomyelitis | Mandible (5% sodium morrhuate as inducing agent) | 8 weeks | To develop chronic mandibular osteomyelitis in miniature swine | Clinical evidence of mandibular osteomyelitis was developed in all mini swine by eight weeks post-infection. At this time, S. aureus was recovered from all six mini swine where bone wax had been used to seal the trephine hole, but not from the two PMMA animals | [131] |
| Large White | F | 45.4 kg | S. aureus ATCC 29213. 107 CFU | Experimental osteomyelitis in a model of gunshot fracture | Tibia | 14 days | To create a model of ballistic wounding in the proximal tibia of pigs | The incidence of osteomyelitis was significantly reduced thanks to treatment with antibiotics. The histological examination confirmed the diagnosis of osteomyelitis based on the presence of purulent material inside associated bone and osteonecrosis. | [220] |
| Large White × Pietrain male castrated | na | 50–65 kg | S. aureus (field strain isolated from a human orthopaedic infection). 1.2 × 103 CFU | Orthopaedic implant–associated infection | Tibia | 28 days | To create cDNA libraries that reflected changes in immune cell function after exposure to infection with Staph. aureus | 7620 ESTs were clustered into 1029 clusters with an average of 3.6 sequences and 3846 singletons. | [223] |
| Yorkshire–Landrace crossbred | F | 8 weeks, 20–25 kg | S. aureus strain S54F9 (clinical strain isolated from a chronic embolic porcine lung abscess). 2 × 109–2.5 × 109 CFU once (group 1 and 2) or twice (group 3 and 4) at 0 h and 12 h. | Non–traumatic osteomyelitis | Ear vein | 6–12–24–48 h | To evaluate the pig as a model for the development of osteomyelitis following haematogenous spread of S. aureus | Disseminated micro abscesses within the lungs by 6 h were developed (but disappeared at 48 h). Within bones, lesions were localized in separate foci. | [142] |
| Yorkshire–Landrace crossbred | F | 8–9 weeks, 15 kg; | S. aureus strain S54F9 (field strain isolated from a chronic embolic porcine lung abscess). 75, 7.5 × 102, 7.5 × 103, 7.5 × 104, 7.5 × 105 CFU | Acute haematogenous localized osteomyelitis | Brachial artery | 5–15 days | To develop a porcine model for haematogenous localized osteomyelitis | Any lesion was not developed in low dose infection models. Pigs inoculated with 5000 and 50,000 CFU ⁄ kg BW only developed micro abscesses in bones of the infected legs. In trabecular osteonecrosis, bone lesions were evident. | [224] |
| Yorkshiree Landrace–cross pigs | F | 12-weeks, 30 kg | S. aureus strains UAMS–1 (isolated from a case of human osteomyelitis), NCTC–8325–4 (isolated from a case of human sepsis) and S54F9 (isolated from a chronic embolic pulmonary abscess in a Danish slaughter pig). 3 × 105 CFU | Haematogenous osteomyelitis | Ear vein | 11 or 15 days | To compare the infection potential of the porcine strain (S54F9) with two S. aureus strains of human origin (UAMS–1 and NCTC–8325–4) in this model and to examine the development of HO with a focus on pathology and the localization and microenvironment of S. aureus | In three, one, and none of the recipients of porcine and human strains, respectively, bone lesions were present. On the CT scans, the vascularized bone tissue was seen as foci of increased opacity. | [225] |
| Yorkshire–Landrace crossbred pigs | F | 12 weeks, 30 kg | S. aureus S54F9. 3 × 105 CFU | Haematogenous osteomyelitis | Femoral artery | 11–15 days | To describe a new intra–arterial inoculation technique in a porcine model of juvenile haematogenous osteomyelitis | Percutaneous catheterization is not an option due to the depth of the artery’s position. This model provides a reliable method for detecting lesions that discriminates the naturally occurring HO in long bones. | [150] |
| Specific pathogen–free | na | 12 weeks, 30 kg | S. aureus S54F9. 1.5 × 107 CFU (pig no.1) 1.5 × 108 CFU (pig no.2) | Haematogenous osteomyelitis | Femoral condyle | 6–8 days | To examine the histological bone changes of experimentally induced osteomyelitis in the porcine model | Lesions found in animals resemble those found in children suffering from haematogenous osteomyelitis | [226] |
| Danish Landrace | F | 67–77 kg | S. aureus strain S54F9, (spa type t1333). 104 CFU | Implant–associated osteomyelitis | Tibia | 5 days | To investigate cefuroxime penetration during implant–associated osteomyelitis | In the implant cavities, lesions referable to bone destruction were found; no alteration in adjacent areas was noted. Cefuroxime penetration into infected bone was incomplete. | [227] |
| Danish SPF Landrace | F | 3–8 months | S. aureus strain S54F9 (spa–type t1333). Low dose (102 and 103 CFU) High dose (104 CFU) | Implant associated osteomyelitis | Tibia | 5 days | To describe a novel porcine implant associated osteomyelitis model. | A significantly higher volume of bone lesion, number of neutrophils, concentration of acute–phase proteins in serum and enlargement of regional lymph nodes were induced by a high inoculum. Therefore, a threshold of 40 neutrophils for 10 high power fields was considered for the histopathological diagnosis of high–grade IAO. | [219] |
| Landrace SPF | F | 35 kg | S. aureus S45F9. 104 CFU | Implant–associated osteomyelitis | Tibia | 2–4–6 days | To elucidate how deep implant–associated osteomyelitis can go into the peri–implanted bone tissue within a week | On implants and from 25 µm to 6 mm into pathological bone area S. aureus bacteria were identified. | [228] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meroni, G.; Tsikopoulos, A.; Tsikopoulos, K.; Allemanno, F.; Martino, P.A.; Soares Filipe, J.F. A Journey into Animal Models of Human Osteomyelitis: A Review. Microorganisms 2022, 10, 1135. https://doi.org/10.3390/microorganisms10061135
Meroni G, Tsikopoulos A, Tsikopoulos K, Allemanno F, Martino PA, Soares Filipe JF. A Journey into Animal Models of Human Osteomyelitis: A Review. Microorganisms. 2022; 10(6):1135. https://doi.org/10.3390/microorganisms10061135
Chicago/Turabian StyleMeroni, Gabriele, Alexios Tsikopoulos, Konstantinos Tsikopoulos, Francesca Allemanno, Piera Anna Martino, and Joel Fernando Soares Filipe. 2022. "A Journey into Animal Models of Human Osteomyelitis: A Review" Microorganisms 10, no. 6: 1135. https://doi.org/10.3390/microorganisms10061135
APA StyleMeroni, G., Tsikopoulos, A., Tsikopoulos, K., Allemanno, F., Martino, P. A., & Soares Filipe, J. F. (2022). A Journey into Animal Models of Human Osteomyelitis: A Review. Microorganisms, 10(6), 1135. https://doi.org/10.3390/microorganisms10061135








