Transcriptome Analysis of Intracellular Amastigotes of Clinical Leishmania infantum Lines from Therapeutic Failure Patients after Infection of Human Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Compounds
2.2. Culture of Leishmania infantum Lines and THP-1 Cells
2.3. In Vitro Macrophage Infection
2.4. RNA Isolation and cDNA Library Preparation
2.5. RNA-seq Data Generation, Pre-Processing
2.6. Data Analysis
2.7. Differential Expression Analysis
2.8. Enrichment Analysis
2.9. Protein–Protein Interaction Network Analysis
3. Results and Discussion
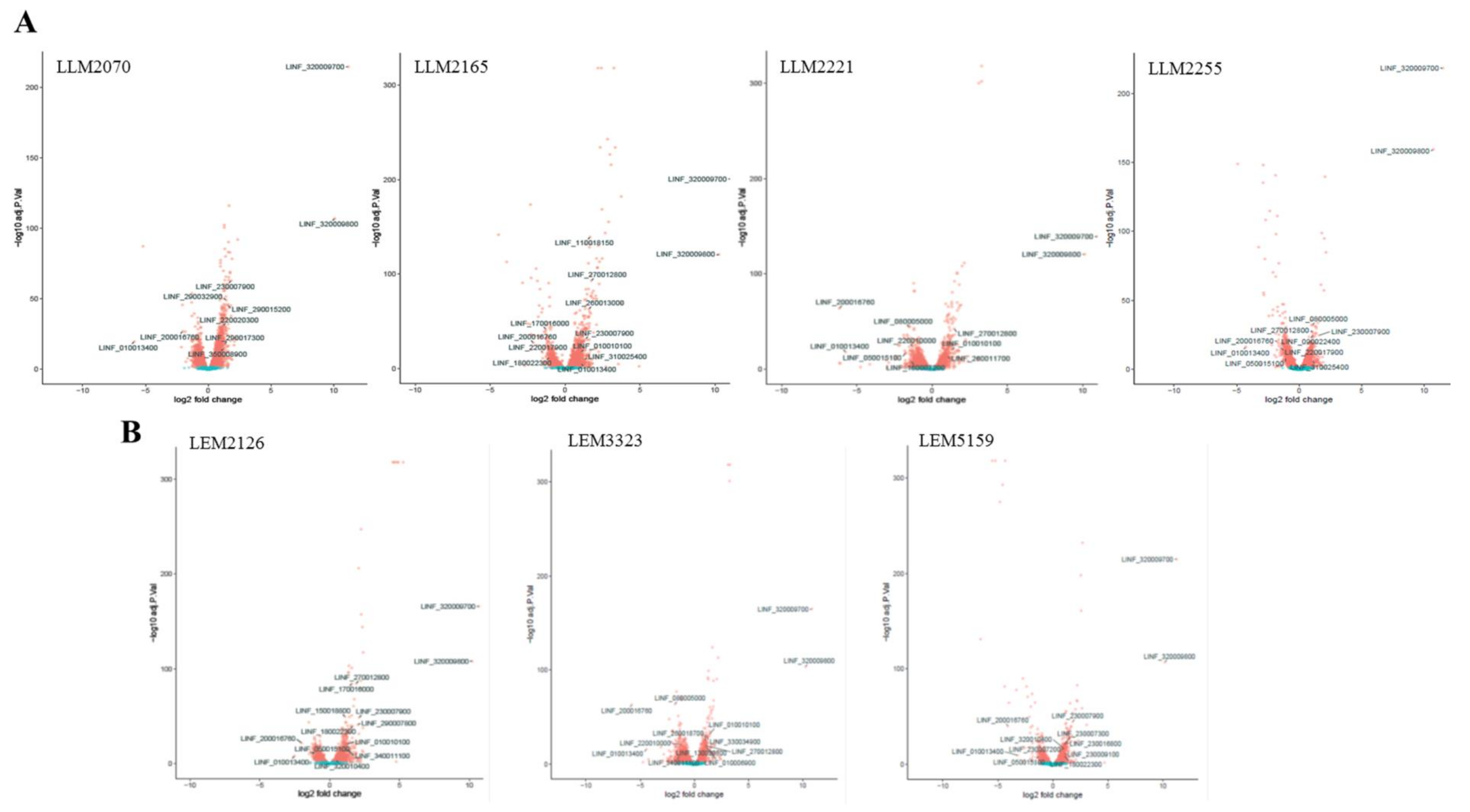
3.1. Transcriptomic Profile of L. infantum Lines after Infection of THP-1 Cells
3.2. Gene Ontology (GO) Enrichment Analysis of the DEGs
3.3. Most Relevant DEGs of L. infantum Lines after Late-Timepoint THP-1 Cell Infection
3.4. Functional and Physical Protein Associations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef]
- Vanaerschot, M.; Dumetz, F.; Roy, S.; Ponte-Sucre, A.; Arevalo, J.; Dujardin, J.C. Treatment failure in leishmaniasis: Drug-resistance or another (epi-) phenotype? Expert Rev. Anti-Infect. Ther. 2014, 12, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Gourbal, B.; Sonuc, N.; Bhattacharjee, H.; Legare, D.; Sundar, S.; Ouellette, M.; Rosen, B.P.; Mukhopadhyay, R. Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J. Biol. Chem. 2004, 279, 31010–31017. [Google Scholar] [CrossRef] [PubMed]
- Marquis, N.; Gourbal, B.; Rosen, B.P.; Mukhopadhyay, R.; Ouellette, M. Modulation in aquaglyceroporin AQP1 gene transcript levels in drug-resistant Leishmania. Mol. Microbiol. 2005, 57, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.M.; Baba, E.H.; Machado-de-Avila, R.A.; Chavez-Olortegui, C.; Demicheli, C.P.; Frézard, F.; Monte-Neto, R.L.; Murta, S.M. Silver and Nitrate Oppositely Modulate Antimony Susceptibility through Aquaglyceroporin 1 in Leishmania (Viannia) Species. Antimicrob. Agents Chemother. 2016, 60, 4482–4489. [Google Scholar] [CrossRef]
- Jhingran, A.; Chawla, B.; Saxena, S.; Barrett, M.P.; Madhubala, R. Paromomycin: Uptake and resistance in Leishmania donovani. Mol. Biochem. Parasitol. 2009, 164, 111–117. [Google Scholar] [CrossRef]
- Seifert, K.; Pérez-Victoria, F.J.; Stettler, M.; Sánchez-Cañete, M.P.; Castanys, S.; Gamarro, F.; Croft, S.L. Inactivation of the miltefosine transporter, LdMT, causes miltefosine resistance that is conferred to the amastigote stage of Leishmania donovani and persists in vivo. Int. J. Antimicrob. Agents 2007, 30, 229–235. [Google Scholar] [CrossRef]
- Pérez-Victoria, F.J.; Sánchez-Cañete, M.P.; Seifert, K.; Croft, S.L.; Sundar, S.; Castanys, S.; Gamarro, F. Mechanisms of experimental resistance of Leishmania to miltefosine: Implications for clinical use. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer. Chemother. 2006, 9, 26–39. [Google Scholar] [CrossRef]
- Barrera, M.C.; Rojas, L.J.; Weiss, A.; Fernandez, O.; McMahon-Pratt, D.; Saravia, N.G.; Gomez, M.A. Profiling gene expression of antimony response genes in Leishmania (Viannia) panamensis and infected macrophages and its relationship with drug susceptibility. Acta Trop. 2017, 176, 355–363. [Google Scholar] [CrossRef]
- Haimeur, A.; Brochu, C.; Genest, P.; Papadopoulou, B.; Ouellette, M. Amplification of the ABC transporter gene PGPA and increased trypanothione levels in potassium antimonyl tartrate (SbIII) resistant Leishmania tarentolae. Mol. Biochem. Parasitol. 2000, 108, 131–135. [Google Scholar] [CrossRef]
- Légaré, D.; Richard, D.; Mukhopadhyay, R.; Stierhof, Y.D.; Rosen, B.P.; Haimeur, A.; Papadopoulou, B.; Ouellette, M. The Leishmania ATP-binding cassette protein PGPA is an intracellular metal-thiol transporter ATPase. J. Biol. Chem. 2001, 276, 26301–26307. [Google Scholar] [CrossRef] [PubMed]
- Perea, A.; Manzano, J.I.; Castanys, S.; Gamarro, F. The LABCG2 Transporter from the Protozoan Parasite Leishmania Is Involved in Antimony Resistance. Antimicrob. Agents Chemother. 2016, 60, 3489–3496. [Google Scholar] [CrossRef] [PubMed]
- Castanys-Muñoz, E.; Pérez-Victoria, J.M.; Gamarro, F.; Castanys, S. Characterization of an ABCG-like transporter from the protozoan parasite Leishmania with a role in drug resistance and transbilayer lipid movement. Antimicrob. Agents Chemother. 2008, 52, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, M.; Légaré, D.; Haimeur, A.; Grondin, K.; Roy, G.; Brochu, C.; Papadopoulou, B. ABC transporters in Leishmania and their role in drug resistance. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer. Chemother. 1998, 1, 43–48. [Google Scholar] [CrossRef]
- Coelho, A.C.; Messier, N.; Ouellette, M.; Cotrim, P.C. Role of the ABC transporter PRP1 (ABCC7) in pentamidine resistance in Leishmania amastigotes. Antimicrob. Agents Chemother. 2007, 51, 3030–3032. [Google Scholar] [CrossRef]
- Carnielli, J.B.T.; Crouch, K.; Forrester, S.; Silva, V.C.; Carvalho, S.F.G.; Damasceno, J.D.; Brown, E.; Dickens, N.J.; Costa, D.L.; Costa, C.H.N.; et al. A Leishmania infantum genetic marker associated with miltefosine treatment failure for visceral leishmaniasis. EBioMedicine 2018, 36, 83–91. [Google Scholar] [CrossRef]
- Adaui, V.; Lye, L.F.; Akopyants, N.S.; Zimic, M.; Llanos-Cuentas, A.; Garcia, L.; Maes, I.; De Doncker, S.; Dobson, D.E.; Arevalo, J.; et al. Association of the Endobiont Double-Stranded RNA Virus LRV1 With Treatment Failure for Human Leishmaniasis Caused by Leishmania braziliensis in Peru and Bolivia. J. Infect. Dis. 2016, 213, 112–121. [Google Scholar] [CrossRef]
- Bourreau, E.; Ginouves, M.; Prévot, G.; Hartley, M.A.; Gangneux, J.P.; Robert-Gangneux, F.; Dufour, J.; Sainte-Marie, D.; Bertolotti, A.; Pratlong, F.; et al. Presence of Leishmania RNA Virus 1 in Leishmania guyanensis Increases the Risk of First-Line Treatment Failure and Symptomatic Relapse. J. Infect. Dis. 2016, 213, 105–111. [Google Scholar] [CrossRef]
- Singh, N. Drug resistance mechanisms in clinical isolates of Leishmania donovani. Indian J. Med. Res. 2006, 123, 411–422. [Google Scholar]
- Ghosh, S.; Biswas, S.; Mukherjee, S.; Pal, A.; Saxena, A.; Sundar, S.; Dujardin, J.C.; Das, S.; Roy, S.; Mukhopadhyay, R.; et al. A Novel Bioimpedance-Based Detection of Miltefosine Susceptibility Among Clinical Leishmania donovani Isolates of the Indian Subcontinent Exhibiting Resistance to Multiple Drugs. Front. Cell. Infect. Microbiol. 2021, 11, 768830. [Google Scholar] [CrossRef]
- Purkait, B.; Kumar, A.; Nandi, N.; Sardar, A.H.; Das, S.; Kumar, S.; Pandey, K.; Ravidas, V.; Kumar, M.; De, T.; et al. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob. Agents Chemother. 2012, 56, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Do Monte-Neto, R.L.; Coelho, A.C.; Raymond, F.; Légaré, D.; Corbeil, J.; Melo, M.N.; Frézard, F.; Ouellette, M. Gene expression profiling and molecular characterization of antimony resistance in Leishmania amazonensis. PLoS Negl. Trop. Dis. 2011, 5, e1167. [Google Scholar] [CrossRef]
- Matrangolo, F.S.; Liarte, D.B.; Andrade, L.C.; de Melo, M.F.; Andrade, J.M.; Ferreira, R.F.; Santiago, A.S.; Pirovani, C.P.; Silva-Pereira, R.A.; Murta, S.M. Comparative proteomic analysis of antimony-resistant and -susceptible Leishmania braziliensis and Leishmania infantum chagasi lines. Mol. Biochem. Parasitol. 2013, 190, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Rastrojo, A.; García-Hernández, R.; Vargas, P.; Camacho, E.; Corvo, L.; Imamura, H.; Dujardin, J.C.; Castanys, S.; Aguado, B.; Gamarro, F.; et al. Genomic and transcriptomic alterations in Leishmania donovani lines experimentally resistant to antileishmanial drugs. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 246–264. [Google Scholar] [CrossRef] [PubMed]
- Patino, L.H.; Imamura, H.; Cruz-Saavedra, L.; Pavia, P.; Muskus, C.; Méndez, C.; Dujardin, J.C.; Ramírez, J.D. Major changes in chromosomal somy, gene expression and gene dosage driven by Sb(III) in Leishmania braziliensis and Leishmania panamensis. Sci. Rep. 2019, 9, 9485. [Google Scholar] [CrossRef] [PubMed]
- Patino, L.H.; Muskus, C.; Ramírez, J.D. Transcriptional responses of Leishmania (Leishmania) amazonensis in the presence of trivalent sodium stibogluconate. Parasites Vectors 2019, 12, 348. [Google Scholar] [CrossRef]
- Andrade, J.M.; Gonçalves, L.O.; Liarte, D.B.; Lima, D.A.; Guimarães, F.G.; de Melo Resende, D.; Santi, A.M.M.; de Oliveira, L.M.; Velloso, J.P.L.; Delfino, R.G.; et al. Comparative transcriptomic analysis of antimony resistant and susceptible Leishmania infantum lines. Parasites Vectors 2020, 13, 600. [Google Scholar] [CrossRef]
- Perea-Martínez, A.; García-Hernández, R.; Manzano, J.I.; Gamarro, F. Transcriptomic Analysis in Human Macrophages Infected with Therapeutic Failure Clinical Isolates of Leishmania infantum. ACS Infect. Dis. 2022, 8, 800–810. [Google Scholar] [CrossRef]
- García-Hernández, R.; Manzano, J.I.; Perea-Martínez, A.; Gamarro, F. New Insights on Drug-Resistant Clinical Isolates of Leishmania infantum-Infected Human Macrophages as Determined by Comparative Transcriptome Analyses. Omics J. Integr. Biol. 2022, 26, 165–177. [Google Scholar] [CrossRef]
- Zhang, S.; Kim, C.C.; Batra, S.; McKerrow, J.H.; Loke, P. Delineation of diverse macrophage activation programs in response to intracellular parasites and cytokines. PLoS Negl. Trop. Dis. 2010, 4, e648. [Google Scholar] [CrossRef]
- Chaussabel, D.; Semnani, R.T.; McDowell, M.A.; Sacks, D.; Sher, A.; Nutman, T.B. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood 2003, 102, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, C.; Díaz-Toro, Y.; Tellez, J.; Castilho, T.M.; Rojas, R.; Ettinger, N.A.; Tikhonova, I.; Alexander, N.D.; Valderrama, L.; Hager, J.; et al. Human macrophage response to L. (Viannia) panamensis: Microarray evidence for an early inflammatory response. PLoS Negl. Trop. Dis. 2012, 6, e1866. [Google Scholar] [CrossRef] [PubMed]
- Rastrojo, A.; Carrasco-Ramiro, F.; Martín, D.; Crespillo, A.; Reguera, R.M.; Aguado, B.; Requena, J.M. The transcriptome of Leishmania major in the axenic promastigote stage: Transcript annotation and relative expression levels by RNA-seq. BMC Genom. 2013, 14, 223. [Google Scholar] [CrossRef] [PubMed]
- Rastrojo, A.; Corvo, L.; Lombraña, R.; Solana, J.C.; Aguado, B.; Requena, J.M. Analysis by RNA-seq of transcriptomic changes elicited by heat shock in Leishmania major. Sci. Rep. 2019, 9, 6919. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.I.; Muxel, S.M.; Zampieri, R.A.; Müller, K.E.; Nerland, A.H.; Floeter-Winter, L.M. Differential immune response modulation in early Leishmania amazonensis infection of BALB/c and C57BL/6 macrophages based on transcriptome profiles. Sci. Rep. 2019, 9, 19841. [Google Scholar] [CrossRef]
- Shadab, M.; Das, S.; Banerjee, A.; Sinha, R.; Asad, M.; Kamran, M.; Maji, M.; Jha, B.; Deepthi, M.; Kumar, M.; et al. RNA-Seq Revealed Expression of Many Novel Genes Associated With Leishmania donovani Persistence and Clearance in the Host Macrophage. Front. Cell. Infect. Microbiol. 2019, 9, 17. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Dillon, L.A.; Belew, A.T.; Bravo, H.C.; Mosser, D.M.; El-Sayed, N.M. Dual Transcriptome Profiling of Leishmania-Infected Human Macrophages Reveals Distinct Reprogramming Signatures. mBio 2016, 7, e00027-16. [Google Scholar] [CrossRef]
- Dillon, L.A.; Suresh, R.; Okrah, K.; Corrada Bravo, H.; Mosser, D.M.; El-Sayed, N.M. Simultaneous transcriptional profiling of Leishmania major and its murine macrophage host cell reveals insights into host-pathogen interactions. BMC Genom. 2015, 16, 1108. [Google Scholar] [CrossRef]
- Inbar, E.; Hughitt, V.K.; Dillon, L.A.; Ghosh, K.; El-Sayed, N.M.; Sacks, D.L. The Transcriptome of Leishmania major Developmental Stages in Their Natural Sand Fly Vector. mBio 2017, 8, e00029-17. [Google Scholar] [CrossRef]
- Verma, A.; Bhandari, V.; Deep, D.K.; Sundar, S.; Dujardin, J.C.; Singh, R.; Salotra, P. Transcriptome profiling identifies genes/pathways associated with experimental resistance to paromomycin in Leishmania donovani. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Dumetz, F.; Imamura, H.; Sanders, M.; Seblova, V.; Myskova, J.; Pescher, P.; Vanaerschot, M.; Meehan, C.J.; Cuypers, B.; De Muylder, G.; et al. Modulation of Aneuploidy in Leishmania donovani during Adaptation to Different In Vitro and In Vivo Environments and Its Impact on Gene Expression. mBio 2017, 8, e00599-17. [Google Scholar] [CrossRef] [PubMed]
- Fiebig, M.; Kelly, S.; Gluenz, E. Comparative Life Cycle Transcriptomics Revises Leishmania mexicana Genome Annotation and Links a Chromosome Duplication with Parasitism of Vertebrates. PLoS Pathog. 2015, 11, e1005186. [Google Scholar] [CrossRef]
- González-de la Fuente, S.; Peiró-Pastor, R.; Rastrojo, A.; Moreno, J.; Carrasco-Ramiro, F.; Requena, J.M.; Aguado, B. Resequencing of the Leishmania infantum (strain JPCM5) genome and de novo assembly into 36 contigs. Sci. Rep. 2017, 7, 18050. [Google Scholar] [CrossRef]
- Lachaud, L.; Bourgeois, N.; Plourde, M.; Leprohon, P.; Bastien, P.; Ouellette, M. Parasite susceptibility to amphotericin B in failures of treatment for visceral leishmaniasis in patients coinfected with HIV type 1 and Leishmania infantum. Clin. Infect. Dis. 2009, 48, e16–e22. [Google Scholar] [CrossRef] [PubMed]
- Enríquez, R.; Sirvent, A.E.; Padilla, S.; Toro, P.; Sánchez, M.; Millán, I. Membranoproliferative glomerulonephritis due to visceral leishmaniasis in an HIV patient. Am. J. Case Rep. 2015, 16, 8–11. [Google Scholar] [CrossRef]
- Sánchez-Fernández, E.M.; García-Moreno, M.I.; Arroba, A.I.; Aguilar-Diosdado, M.; Padrón, J.M.; García-Hernández, R.; Gamarro, F.; Fustero, S.; Sánchez-Aparicio, J.E.; Masgrau, L.; et al. Synthesis of polyfluoroalkyl sp(2)-iminosugar glycolipids and evaluation of their immunomodulatory properties towards anti-tumor, anti-leishmanial and anti-inflammatory therapies. Eur. J. Med. Chem. 2019, 182, 111604. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef]
- Hendrickx, S.; Van Bockstal, L.; Caljon, G.; Maes, L. In-depth comparison of cell-based methodological approaches to determine drug susceptibility of visceral Leishmania isolates. PLoS Negl. Trop. Dis. 2019, 13, e0007885. [Google Scholar] [CrossRef]
- Andrés-León, E.; Núñez-Torres, R.; Rojas, A.M. miARma-Seq: A comprehensive tool for miRNA, mRNA and circRNA analysis. Sci. Rep. 2016, 6, 25749. [Google Scholar] [CrossRef]
- Andrés-León, E.; Rojas, A.M. miARma-Seq, a comprehensive pipeline for the simultaneous study and integration of miRNA and mRNA expression data. Methods 2019, 152, 31–40. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A quality control tool for high throughput sequence data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 1 May 2022).
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Nikolayeva, O.; Robinson, M.D. edgeR for differential RNA-seq and ChIP-seq analysis: An application to stem cell biology. Methods Mol. Biol. 2014, 1150, 45–79. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [PubMed]
- Reeb, P.D.; Bramardi, S.J.; Steibel, J.P. Assessing Dissimilarity Measures for Sample-Based Hierarchical Clustering of RNA Sequencing Data Using Plasmode Datasets. PLoS ONE 2015, 10, e0132310. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Alves-Ferreira, E.V.C.; Ferreira, T.R.; Walrad, P.; Kaye, P.M.; Cruz, A.K. Leishmania braziliensis prostaglandin F(2α) synthase impacts host infection. Parasites Vectors 2020, 13, 9. [Google Scholar] [CrossRef]
- Monné, M.; Miniero, D.V.; Daddabbo, L.; Palmieri, L.; Porcelli, V.; Palmieri, F. Mitochondrial transporters for ornithine and related amino acids: A review. Amino Acids 2015, 47, 1763–1777. [Google Scholar] [CrossRef]
- Pérez-Victoria, F.J.; Sánchez-Cañete, M.P.; Castanys, S.; Gamarro, F. Phospholipid translocation and miltefosine potency require both L. donovani miltefosine transporter and the new protein LdRos3 in Leishmania parasites. J. Biol. Chem. 2006, 281, 23766–23775. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef]
- Figarella, K.; Uzcategui, N.L.; Zhou, Y.; LeFurgey, A.; Ouellette, M.; Bhattacharjee, H.; Mukhopadhyay, R. Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: Possible role in volume regulation and osmotaxis. Mol. Microbiol. 2007, 65, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.F. ABC transporters: From microorganisms to man. Annu. Rev. Cell Biol. 1992, 8, 67–113. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.S.; Monte Neto, R.L.; Andrade, J.M.; Santi, A.M.; Reis, P.G.; Frézard, F.; Murta, S.M. Molecular characterization of the MRPA transporter and antimony uptake in four New World Leishmania spp. susceptible and resistant to antimony. Int. J. Parasitol. Drugs Drug Resist. 2013, 3, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Santos, J.M.; Parodi-Talice, A.; Castanys, S.; Gamarro, F. The overexpression of an intracellular ABCA-like transporter alters phospholipid trafficking in Leishmania. Biochem. Biophys. Res. Commun. 2005, 330, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Engel, K.; Höhne, W.; Häberle, J. Mutations and polymorphisms in the human argininosuccinate synthetase (ASS1) gene. Hum. Mutat. 2009, 30, 300–307. [Google Scholar] [CrossRef]
- Xie, L.; Gross, S.S. Argininosuccinate synthetase overexpression in vascular smooth muscle cells potentiates immunostimulant-induced NO production. J. Biol. Chem. 1997, 272, 16624–16630. [Google Scholar] [CrossRef]
- El Fadili, K.; Drummelsmith, J.; Roy, G.; Jardim, A.; Ouellette, M. Down regulation of KMP-11 in Leishmania infantum axenic antimony resistant amastigotes as revealed by a proteomic screen. Exp. Parasitol. 2009, 123, 51–57. [Google Scholar] [CrossRef]
- Lakhal-Naouar, I.; Jardim, A.; Strasser, R.; Luo, S.; Kozakai, Y.; Nakhasi, H.L.; Duncan, R.C. Leishmania donovani argininosuccinate synthase is an active enzyme associated with parasite pathogenesis. PLoS Negl. Trop. Dis. 2012, 6, e1849. [Google Scholar] [CrossRef]
- Kaur, J.; Tiwari, R.; Kumar, A.; Singh, N. Bioinformatic Analysis of Leishmania donovani Long-Chain Fatty Acid-CoA Ligase as a Novel Drug Target. Mol. Biol. Int. 2011, 2011, 278051. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luque-Ortega, J.R.; Rivas, L. Miltefosine (hexadecylphosphocholine) inhibits cytochrome c oxidase in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 2007, 51, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- López, C.; Chevalier, N.; Hannaert, V.; Rigden, D.J.; Michels, P.A.; Ramirez, J.L. Leishmania donovani phosphofructokinase. Gene characterization, biochemical properties and structure-modeling studies. Eur. J. Biochem. 2002, 269, 3978–3989. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.M.; Kinkead, J.; McNae, I.W.; Vásquez-Valdivieso, M.; Wear, M.A.; Michels, P.A.M.; Walkinshaw, M.D. Kinetic and structural studies of Trypanosoma and Leishmania phosphofructokinases show evolutionary divergence and identify AMP as a switch regulating glycolysis versus gluconeogenesis. FEBS J. 2020, 287, 2847–2861. [Google Scholar] [CrossRef] [PubMed]
- García-Huertas, P.; Mejía-Jaramillo, A.M.; Machado, C.R.; Guimarães, A.C.; Triana-Chávez, O. Prostaglandin F2α synthase in Trypanosoma cruzi plays critical roles in oxidative stress and susceptibility to benznidazole. R. Soc. Open Sci. 2017, 4, 170773. [Google Scholar] [CrossRef]
- Piñeyro, M.D.; Arias, D.; Parodi-Talice, A.; Guerrero, S.; Robello, C. Trypanothione Metabolism as Drug Target for Trypanosomatids. Curr. Pharm. Des. 2021, 27, 1834–1846. [Google Scholar] [CrossRef]
- Hillebrand, H.; Schmidt, A.; Krauth-Siegel, R.L. A second class of peroxidases linked to the trypanothione metabolism. J. Biol. Chem. 2003, 278, 6809–6815. [Google Scholar] [CrossRef]
- Currier, R.B.; Ulrich, K.; Leroux, A.E.; Dirdjaja, N.; Deambrosi, M.; Bonilla, M.; Ahmed, Y.L.; Adrian, L.; Antelmann, H.; Jakob, U.; et al. An essential thioredoxin-type protein of Trypanosoma brucei acts as redox-regulated mitochondrial chaperone. PLoS Pathog. 2019, 15, e1008065. [Google Scholar] [CrossRef]
- Mukherjee, A.; Roy, G.; Guimond, C.; Ouellette, M. The gamma-glutamylcysteine synthetase gene of Leishmania is essential and involved in response to oxidants. Mol. Microbiol. 2009, 74, 914–927. [Google Scholar] [CrossRef]
- Das, S.; Shah, P.; Tandon, R.; Yadav, N.K.; Sahasrabuddhe, A.A.; Sundar, S.; Siddiqi, M.I.; Dube, A. Over-Expression of Cysteine Leucine Rich Protein Is Related to SAG Resistance in Clinical Isolates of Leishmania donovani. PLoS Negl. Trop. Dis. 2015, 9, e0003992. [Google Scholar] [CrossRef][Green Version]
- Alves-Ferreira, E.V.; Toledo, J.S.; De Oliveira, A.H.; Ferreira, T.R.; Ruy, P.C.; Pinzan, C.F.; Santos, R.F.; Boaventura, V.; Rojo, D.; López-Gonzálvez, Á.; et al. Differential Gene Expression and Infection Profiles of Cutaneous and Mucosal Leishmania braziliensis Isolates from the Same Patient. PLoS Negl. Trop. Dis. 2015, 9, e0004018. [Google Scholar] [CrossRef] [PubMed]
- Moen, S.O.; Fairman, J.W.; Barnes, S.R.; Sullivan, A.; Nakazawa-Hewitt, S.; Van Voorhis, W.C.; Staker, B.L.; Lorimer, D.D.; Myler, P.J.; Edwards, T.E. Structures of prostaglandin F synthase from the protozoa Leishmania major and Trypanosoma cruzi with NADP. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2015, 71, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Guimond, C.; Trudel, N.; Brochu, C.; Marquis, N.; El Fadili, A.; Peytavi, R.; Briand, G.; Richard, D.; Messier, N.; Papadopoulou, B.; et al. Modulation of gene expression in Leishmania drug resistant mutants as determined by targeted DNA microarrays. Nucleic Acids Res. 2003, 31, 5886–5896. [Google Scholar] [CrossRef]
- Mukherjee, A.; Padmanabhan, P.K.; Singh, S.; Roy, G.; Girard, I.; Chatterjee, M.; Ouellette, M.; Madhubala, R. Role of ABC transporter MRPA, gamma-glutamylcysteine synthetase and ornithine decarboxylase in natural antimony-resistant isolates of Leishmania donovani. J. Antimicrob. Chemother. 2007, 59, 204–211. [Google Scholar] [CrossRef]
- Kumar, A.; Sisodia, B.; Misra, P.; Sundar, S.; Shasany, A.K.; Dube, A. Proteome mapping of overexpressed membrane-enriched and cytosolic proteins in sodium antimony gluconate (SAG) resistant clinical isolate of Leishmania donovani. Br. J. Clin. Pharmacol. 2010, 70, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, M.C.; Bourassa, S.; Légaré, D.; Poirier, G.G.; Droit, A.; Ouellette, M. Quantitative proteomic analysis of amphotericin B resistance in Leishmania infantum. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.C.; Silva, W.R.; Azevedo, A.F.; Santos, P.L.; Teixeira, S.A.; Muscará, M.N.; Thomazzi, S.M.; Almeida, R.P.; Fernandes, R.P.; Scher, R. Expression of glyceraldehyde 3-phosphate dehydrogenase is enhanced in Leishmania spp naturally resistant to nitric oxide. Genet. Mol. Res. GMR 2015, 14, 7113–7121. [Google Scholar] [CrossRef]
- Zufferey, R.; Ben Mamoun, C. Leishmania major expresses a single dihydroxyacetone phosphate acyltransferase localized in the glycosome, important for rapid growth and survival at high cell density and essential for virulence. J. Biol. Chem. 2006, 281, 7952–7959. [Google Scholar] [CrossRef]



| GO Term | GO ID | Lines | DEGs | ||
|---|---|---|---|---|---|
| Up | Down | Hypothetical | |||
| Cellular protein modification process | GO:0006464 | LEM2126 | 23 | 0 | 0 |
| LEM3323 | 7 | 36 | 1 | ||
| LLM2221 | 4 | 17 | 1 | ||
| LLM2070 | 26 | 3 | 0 | ||
| Protein modification process | GO:0036211 | LEM2126 | 23 | 0 | 0 |
| LEM3323 | 7 | 36 | 1 | ||
| LLM2221 | 4 | 17 | 1 | ||
| LLM2070 | 28 | 4 | 0 | ||
| Macromolecular modification | GO:0043412 | LEM2126 | 24 | 0 | 0 |
| LEM3323 | 9 | 38 | 2 | ||
| LLM2221 | 7 | 21 | 2 | ||
| LLM2070 | 26 | 3 | 0 | ||
| Protein phosphorylation | GO:0006468 | LEM2126 | 21 | 0 | 0 |
| LEM3323 | 2 | 28 | 0 | ||
| Phosphorylation | GO:0016310 | LEM2126 | 21 | 0 | 0 |
| LEM3323 | 2 | 28 | 0 | ||
| Phosphate-containing compound metabolic process | GO:0006796 | LEM2126 | 24 | 1 | 0 |
| LEM3323 | 2 | 33 | 0 | ||
| Phosphorus metabolic process | GO:0006793 | LEM2126 | 24 | 1 | 0 |
| LEM3323 | 2 | 33 | 0 | ||
| Gene ID | Description | Leishmania Line | Log2 FC | FDR |
|---|---|---|---|---|
| 1. Transport, uptake, and efflux across cellular membranes | ||||
| LINF_160007200 | Mitochondrial ornithine transporter 1-like protein | LLM2221 | −1.38 | 4.61 × 10−9 |
| LINF_130020800 | Phospholipid-transporting ATPase 1-like protein | LEM3323 | −1.13 | 4.33 × 10−16 |
| LINF_320010400 | LEM3 (ligand-effect modulator 3) family/CDC50 family, putative | LEM5159 | 1.01 | 3.13 × 10−17 |
| LEM2126 | 2.34 | 0.000207 | ||
| LINF_220020300 | Aquaporin, putative | LLM2070 | 1.20 | 1.66 × 10−31 |
| LINF_230007200 | ATP-binding cassette protein subfamily C, member 1, putative | LEM5159 | 1.04 | 3.13 × 10−17 |
| LINF_230007300 | ATP-binding cassette protein subfamily C, member 2, putative | LEM5159 | 1.01 | 4.16 × 10−21 |
| LINF_110018150 | ATP-binding cassette protein subfamily A, member 2, putative | LLM2165 | 1.67 | 4.87 × 10−140 |
| 2. Energy metabolism | ||||
| LINF_230007900 | Argininosuccinate synthase, putative | LEM5159 | 1.79 | 2.69 × 10−45 |
| LEM2126 | 2.12 | 1.61 × 10−49 | ||
| LLM2070 | 1.83 | 1.38 × 10−63 | ||
| LLM2255 | 1.60 | 2.23 × 10−25 | ||
| LLM2165 | 1.37 | 3.22 × 10−32 | ||
| LINF_010010100 | Fatty-acyl-CoA synthetase 2, putative | LEM3323 | 1.43 | 4.06 × 10−33 |
| LEM2126 | 1.26 | 1.59 × 10−21 | ||
| LLM2221 | 1.32 | 4.60 × 10−21 | ||
| LLM2165 | 1.07 | 2.85 × 10−18 | ||
| LINF_230009100 | Cytochrome c oxidase subunit 10, putative | LEM5159 | 1.02 | 4.96 × 10−12 |
| LINF_290032900 | ATP-dependent phosphofructokinase | LLM2070 | 1.44 | 1.14 × 10−49 |
| 3. Redox metabolism and detoxification | ||||
| LINF_270012800 | Amino acid transporter, putative | LEM3323 | 1.00 | 1.55 × 10−20 |
| LEM2126 | 1.90 | 2.50 × 10−85 | ||
| LLM2221 | 1.46 | 3.52 × 10−43 | ||
| LLM2255 | 1.10 | 1.93 × 10−27 | ||
| LLM2165 | 1.75 | 8.01 × 10−94 | ||
| LINF_290017300 | Tryparedoxin-like protein | LLM2070 | 1.30 | 1.48 × 10−18 |
| LINF_150018800 | Tryparedoxin peroxidase | LEM2126 | 1.10 | 2.51 × 10−50 |
| LINF_260013000 | Type II (glutathione peroxidase-like) tryparedoxin peroxidase | LLM2165 | 1.58 | 1.00 × 10−63 |
| LINF_350008900 | Thioredoxin, putative | LLM2070 | 1.21 | 1.94 × 10−15 |
| LINF_260011700 | Thioredoxin, putative | LLM2221 | 1.01 | 6.25 × 10−14 |
| LINF_010006900 | Low-molecular-weight phosphotyrosine protein phosphatase, putative | LEM3323 | 1.10 | 9.28 × 10−19 |
| LINF_180022300 | Gamma-glutamylcysteine synthetase, putative | LEM5159 | 1.03 | 4.98 × 10−10 |
| LEM2126 | 1.65 | 1.81 × 10−40 | ||
| LLM2165 | −1.04 | 1.04 × 10−12 | ||
| LINF_340011100 | Leucine-rich repeat protein, putative | LEM3323 | −1.36 | 3.02 × 10−9 |
| LEM2126 | 1.22 | 3.05 × 10−16 | ||
| LINF_320009800 | Leucine-rich repeat, putative | LEM3323 | 10.25 | 2.69 × 10−104 |
| LEM5159 | 10.18 | 9.34 × 10−108 | ||
| LEM2126 | 10.21 | 8.44 × 10−109 | ||
| LLM2221 | 10.14 | 2.12 × 10−121 | ||
| LLM2070 | 10.08 | 7.91 × 10−108 | ||
| LLM2255 | 10.74 | 4.15 × 10−160 | ||
| LLM2165 | 10.26 | 1.49 × 10−121 | ||
| LINF_050015100 | 3-mercaptopyruvate sulfurtransferase | LEM5159 | −1.25 | 1.82 × 10−9 |
| LEM2126 | −1.24 | 2.38 × 10−10 | ||
| LLM2221 | −1.76 | 9.80 × 10−18 | ||
| LLM2255 | −1.06 | 6.27 × 10−9 | ||
| LINF_220010000 | Acetyltransferase (GNAT) family, putative | LEM3323 | −1.21 | 7.79 × 10−22 |
| LLM2221 | −1.28 | 2.13 × 10−24 | ||
| LINF_330034900 | Metallopeptidase, clan MF, family M17 | LEM3323 | 1.18 | 9.33 × 10−31 |
| LINF_080005000 | Adaptor complex protein (AP) 3 delta subunit 1, putative | LEM3323 | −1.79 | 1.25 × 10−64 |
| LLM2221 | −1.52 | 5.91 × 10−45 | ||
| LLM2255 | 1.07 | 6.32 × 10−33 | ||
| LINF_170016000 | META domain containing protein, putative | LEM2126 | 1.59 | 2.73 × 10−85 |
| LLM2165 | −1.28 | 1.61 × 10−42 | ||
| LINF_230016800 | Alcohol dehydrogenase-zinc-containing-like protein | LEM5159 | 1.21 | 1.63 × 10−22 |
| LINF_290015200 | 5-histidylcysteine sulfoxide synthase, putative | LLM2070 | 1.57 | 6.10 × 10−46 |
| LINF_290007800 | D-lactate dehydrogenase-like protein | LEM2126 | 2.02 | 6.78 × 10−42 |
| LINF_310025400 | Acetylornithine deacetylase-like protein | LLM2255 | 1.06 | 1.11 × 10−6 |
| LLM2165 | 1.29 | 5.34 × 10−10 | ||
| LINF_220017900 | ChaC-like protein, putative | LLM2255 | −1.46 | 1.59 × 10−16 |
| LLM2165 | −1.50 | 6.53 × 10−18 | ||
| LINF_320009700 | Prostaglandin f synthase, putative | LEM3323 | 10.85 | 9.59 × 10−166 |
| LEM5159 | 11.25 | 1.29 × 10−215 | ||
| LEM2126 | 10.69 | 8.55 × 10−167 | ||
| LLM2221 | 10.95 | 6.08 × 10−140 | ||
| LLM2070 | 11.17 | 1.74 × 10−215 | ||
| LLM2255 | 11.50 | 4.43 × 10−219 | ||
| LLM2165 | 11.24 | 1.72 × 10−201 | ||
| 4. Hypothetical | ||||
| LINF_200016760 | Unspecified product | LEM3323 | −5.77 | 6.91 × 10−64 |
| LEM5159 | −4.08 | 6.84 × 10−41 | ||
| LEM2126 | −1.99 | 1.30 × 10−22 | ||
| LLM2221 | −6.16 | 1.47 × 10−64 | ||
| LLM2070 | −2.02 | 1.38 × 10−27 | ||
| LLM2255 | −1.73 | 9.19 × 10−21 | ||
| LLM2165 | −2.11 | 4.98 × 10−28 | ||
| Protein Code | Description | Relationship Score |
|---|---|---|
| XP_001464336.1 | Putative glutathione-S-transferase/glutaredoxin | 0.919 |
| XP_001467729.1 | Putative prostaglandin f synthase (280 aa) | 0.803 |
| XP_001468134.1 | Putative d-xylulose reductase | 0.727 |
| ENOL | Enolase | 0.685 |
| GPD | Glycerol-3-phosphate dehydrogenase [NAD+], glycosomal | 0.661 |
| XP_001463690.1 | Dihydroxyacetone kinase 1-like protein | 0.650 |
| ALDH2 | Aldehyde dehydrogenase, mitochondrial precursor | 0.632 |
| GSH1 | Gamma-glutamylcysteine synthetase | 0.625 |
| XP_001467057.1 | Putative alcohol dehydrogenase | 0.575 |
| XP_001466975.1 | Putative pyridoxal kinase | 0.566 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Hernández, R.; Perea-Martínez, A.; Manzano, J.I.; Terrón-Camero, L.C.; Andrés-León, E.; Gamarro, F. Transcriptome Analysis of Intracellular Amastigotes of Clinical Leishmania infantum Lines from Therapeutic Failure Patients after Infection of Human Macrophages. Microorganisms 2022, 10, 1304. https://doi.org/10.3390/microorganisms10071304
García-Hernández R, Perea-Martínez A, Manzano JI, Terrón-Camero LC, Andrés-León E, Gamarro F. Transcriptome Analysis of Intracellular Amastigotes of Clinical Leishmania infantum Lines from Therapeutic Failure Patients after Infection of Human Macrophages. Microorganisms. 2022; 10(7):1304. https://doi.org/10.3390/microorganisms10071304
Chicago/Turabian StyleGarcía-Hernández, Raquel, Ana Perea-Martínez, José Ignacio Manzano, Laura C. Terrón-Camero, Eduardo Andrés-León, and Francisco Gamarro. 2022. "Transcriptome Analysis of Intracellular Amastigotes of Clinical Leishmania infantum Lines from Therapeutic Failure Patients after Infection of Human Macrophages" Microorganisms 10, no. 7: 1304. https://doi.org/10.3390/microorganisms10071304
APA StyleGarcía-Hernández, R., Perea-Martínez, A., Manzano, J. I., Terrón-Camero, L. C., Andrés-León, E., & Gamarro, F. (2022). Transcriptome Analysis of Intracellular Amastigotes of Clinical Leishmania infantum Lines from Therapeutic Failure Patients after Infection of Human Macrophages. Microorganisms, 10(7), 1304. https://doi.org/10.3390/microorganisms10071304








