Phage Products for Fighting Antimicrobial Resistance
Abstract
:1. Introduction
2. Phage Biology
3. Phages Products in Plant Health
4. Phage Products in Animal Health
5. Phages Products in Food Health
6. Phage Products in Human Health
7. Advantages and Disadvantages of Phage Therapy
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lobanovska, M.; Pilla, G. Penicillin’s Discovery and Antibiotic Resistance: Lessons for the Future? Yale J. Biol. Med. 2017, 90, 135–145. [Google Scholar]
- Oechslin, F. Resistance Development to Bacteriophages Occurring during Bacteriophage Therapy. Viruses 2018, 10, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell Infect. Microbiol. 2021, 11, 771510. [Google Scholar] [CrossRef]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.W.; Cha, C.J. Antibiotic resistome from the One-Health perspective: Understanding and controlling antimicrobial resistance transmission. Exp. Mol. Med. 2021, 53, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.P.; Costa, A.R.; Pinto, G.; Meneses, L.; Azeredo, J. Current challenges and future opportunities of phage therapy. FEMS Microbiol. Rev. 2020, 44, 684–700. [Google Scholar] [CrossRef]
- Group The Bacteriophage Ecology. Phage Companies. Available online: http://companies.phage.org/ (accessed on 29 April 2022).
- Bacteriophage.news. Bacteriophage Products. Available online: https://www.bacteriophage.news/phage-products/ (accessed on 29 April 2022).
- Squires, R.A. Bacteriophage therapy for challenging bacterial infections: Achievements, limitations and prospects for future clinical use by veterinary dermatologists. Vet. Dermatol. 2021, 32, 587-e158. [Google Scholar] [CrossRef] [PubMed]
- Abd-Allah, I.M.; El-Housseiny, G.S.; Yahia, I.S.; Aboshanab, K.M.; Hassouna, N.A. Rekindling of a Masterful Precedent; Bacteriophage: Reappraisal and Future Pursuits. Front. Cell Infect. Microbiol. 2021, 11, 635597. [Google Scholar] [CrossRef]
- Gordillo Altamirano, F.L.; Barr, J.J. Phage Therapy in the Postantibiotic Era. Clin. Microbiol. Rev. 2019, 32, e00066-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moelling, K.; Broecker, F.; Willy, C. A Wake-Up Call: We Need Phage Therapy Now. Viruses 2018, 10, 688. [Google Scholar] [CrossRef] [Green Version]
- Dion, M.B.; Oechslin, F.; Moineau, S. Phage diversity, genomics and phylogeny. Nat. Rev. Microbiol. 2020, 18, 125–138. [Google Scholar] [CrossRef]
- Salmond, G.P.; Fineran, P.C. A century of the phage: Past, present and future. Nat. Rev. Microbiol. 2015, 13, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Olszak, T.; Latka, A.; Roszniowski, B.; Valvano, M.A.; Drulis-Kawa, Z. Phage Life Cycles Behind Bacterial Biodiversity. Curr. Med. Chem. 2017, 24, 3987–4001. [Google Scholar] [CrossRef] [Green Version]
- Cahill, J.; Young, R. Phage Lysis: Multiple Genes for Multiple Barriers. Adv. Virus Res. 2019, 103, 33–70. [Google Scholar]
- Sunderland, K.S.; Yang, M.; Mao, C. Phage-Enabled Nanomedicine: From Probes to Therapeutics in Precision Medicine. Angew. Chem. Int. Ed. Engl. 2017, 56, 1964–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciejewska, B.; Olszak, T.; Drulis-Kawa, Z. Applications of bacteriophages versus phage enzymes to combat and cure bacterial infections: An ambitious and also a realistic application? Appl. Microbiol. Biotechnol. 2018, 102, 2563–2581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argov, T.; Azulay, G.; Pasechnek, A.; Stadnyuk, O.; Ran-Sapir, S.; Borovok, I.; Sigal, N.; Herskovits, A.A. Temperate bacteriophages as regulators of host behavior. Curr. Opin. Microbiol. 2017, 38, 81–87. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Węgrzyn, G.; Jończyk-Matysiak, E.; Borysowski, J.; Weber-Dąbrowska, B. Phage therapy: Current status and perspectives. Med. Res. Rev. 2020, 40, 459–463. [Google Scholar] [CrossRef]
- Monteiro, R.; Pires, D.P.; Costa, A.R.; Azeredo, J. Phage Therapy: Going Temperate? Trends Microbiol. 2019, 27, 368–378. [Google Scholar] [CrossRef] [Green Version]
- Bhargava, K.; Nath, G.; Bhargava, A.; Aseri, G.K.; Jain, N. Phage therapeutics: From promises to practices and prospectives. Appl. Microbiol. Biotechnol. 2021, 105, 9047–9067. [Google Scholar] [CrossRef]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front. Pharmacol. 2019, 10, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luong, T.; Salabarria, A.C.; Roach, D.R. Phage Therapy in the Resistance Era: Where Do We Stand and Where Are We Going? Clin. Ther. 2020, 42, 1659–1680. [Google Scholar] [CrossRef] [PubMed]
- Farooq, T.; Hussain, M.D.; Shakeel, M.T.; Tariqjaveed, M.; Aslam, M.N.; Naqvi SA, H.; Amjad, R.; Tang, Y.; She, X.; He, Z. Deploying Viruses against Phytobacteria: Potential Use of Phage Cocktails as a Multifaceted Approach to Combat Resistant Bacterial Plant Pathogens. Viruses 2022, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Sundin, G.W.; Wang, N. Antibiotic Resistance in Plant-Pathogenic Bacteria. Annu. Rev. Phytopathol. 2018, 56, 161–180. [Google Scholar] [CrossRef]
- Svircev, A.; Roach, D.; Castle, A. Framing the Future with Bacteriophages in Agriculture. Viruses 2018, 10, 218. [Google Scholar] [CrossRef] [Green Version]
- Buttimer, C.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and Bacterial Plant Diseases. Front. Microbiol. 2017, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Xing, S.; Liu, J.; Tang, X.; Ruan, L.; Sun, M.; Tong, Y.; Peng, D. Isolation and characterization of a novel phage Xoo-sp2 that infects Xanthomonas oryzae pv. oryzae. J. Gen. Virol. 2018, 99, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Born, Y.; Fieseler, L.; Thöny, V.; Leimer, N.; Duffy, B.; Loessner, M.J. Engineering of Bacteriophages Y2::dpoL1-C and Y2::luxAB for Efficient Control and Rapid Detection of the Fire Blight Pathogen, Erwinia amylovora. Appl. Environ. Microbiol. 2017, 83, e00341-17. [Google Scholar] [CrossRef] [Green Version]
- Kering, K.K.; Kibii, B.J.; Wei, H. Biocontrol of phytobacteria with bacteriophage cocktails. Pest. Manag. Sci. 2019, 75, 1775–1781. [Google Scholar] [CrossRef]
- Clavijo-Coppens, F.; Ginet, N.; Cesbron, S.; Briand, M.; Jacques, M.A.; Ansaldi, M. Novel Virulent Bacteriophages Infecting Mediterranean Isolates of the Plant Pest Xylella fastidiosa and Xanthomonas albilineans. Viruses 2021, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Ahern, S.J.; Das, M.; Bhowmick, T.S.; Young, R.; Gonzalez, C.F. Characterization of novel virulent broad-host-range phages of Xylella fastidiosa and Xanthomonas. J. Bacteriol. 2014, 196, 459–471. [Google Scholar] [CrossRef] [PubMed]
- de Melo, A.G.; Levesque, S.; Moineau, S. Phages as friends and enemies in food processing. Curr. Opin. Biotechnol. 2018, 49, 185–190. [Google Scholar] [CrossRef]
- Sommer, J.; Trautner, C.; Witte, A.K.; Fister, S.; Schoder, D.; Rossmanith, P.; Mester, P.J. Don’t Shut the Stable Door after the Phage Has Bolted-The Importance of Bacteriophage Inactivation in Food Environments. Viruses 2019, 11, 468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Połaska, M.; Sokołowska, B. Bacteriophages-a new hope or a huge problem in the food industry. AIMS Microbiol. 2019, 5, 324–346. [Google Scholar] [CrossRef] [PubMed]
- PLUS Erwiphage. Erwiphage PLUS. 2022. Available online: https://www.erwiphage.com/ (accessed on 29 April 2022).
- Phage Fixed. Applications. 2022. Available online: https://www.fixed-phage.com/ (accessed on 29 April 2022).
- Protection World Animal. EU Bans the Routine Use of Antibiotics in Farmed Animals. 2022. Available online: https://www.worldanimalprotection.org/european-union-bans-antibiotic-overuse-farmed-animals-animal-welfare (accessed on 29 April 2022).
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Gigante, A.; Atterbury, R.J. Veterinary use of bacteriophage therapy in intensively-reared livestock. Virol. J. 2019, 16, 155. [Google Scholar] [CrossRef] [Green Version]
- Loponte, R.; Pagnini, U.; Iovane, G.; Pisanelli, G. Phage Therapy in Veterinary Medicine. Antibiotics 2021, 10, 421. [Google Scholar] [CrossRef]
- Hawkins, C.; Harper, D.; Burch, D.; Anggård, E.; Soothill, J. Topical treatment of Pseudomonas aeruginosa otitis of dogs with a bacteriophage mixture: A before/after clinical trial. Vet. Microbiol. 2010, 146, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Dec, M.; Wernicki, A.; Urban-Chmiel, R. Efficacy of experimental phage therapies in livestock. Anim. Health Res. Rev. 2020, 21, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Han, G.; Li, Z.; Cun, S.; Hao, B.; Zhang, J.; Liu, X. Bacteriophage therapy in aquaculture: Current status and future challenges. Folia Microbiol. 2022, 1–18. [Google Scholar] [CrossRef]
- Islam, M.R.; Martinez-Soto, C.E.; Lin, J.T.; Khursigara, C.M.; Barbut, S.; Anany, H. A systematic review from basics to omics on bacteriophage applications in poultry production and processing. Crit. Rev. Food Sci. Nutr. 2021, 1–33. [Google Scholar] [CrossRef]
- Laboratories Delmont. What is Staphage Lysate (SPL)? 2022. Available online: https://delmontlabs.com/ (accessed on 29 April 2022).
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage Applications for Food Production and Processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef] [Green Version]
- Pharmaceuticals Proteon. Products. 2022. Available online: https://www.proteonpharma.com/ (accessed on 29 April 2022).
- Phagelab. Development. 2022. Available online: https://phage-lab.com/development/ (accessed on 29 April 2022).
- Heyse, S.; Hanna, L.F.; Woolston, J.; Sulakvelidze, A.; Charbonneau, D. Bacteriophage cocktail for biocontrol of Salmonella in dried pet food. J. Food Prot. 2015, 78, 97–103. [Google Scholar] [CrossRef]
- Soffer, N.; Abuladze, T.; Woolston, J.; Li, M.; Hanna, L.F.; Heyse, S.; Charbonneau, D.; Sulakvelidze, A. Bacteriophages safely reduce Salmonella contamination in pet food and raw pet food ingredients. Bacteriophage 2016, 6, e1220347. [Google Scholar] [CrossRef] [Green Version]
- Alomari, M.M.M.; Dec, M.; Urban-Chmiel, R. Bacteriophages as an Alternative Method for Control of Zoonotic and Foodborne Pathogens. Viruses 2021, 13, 2348. [Google Scholar] [CrossRef]
- Wójcik, E.A.; Stańczyk, M.; Wojtasik, A.; Kowalska, J.D.; Nowakowska, M.; Łukasiak, M.; Bartnicka, M.; Kazimierczak, J.; Dastych, J. Comprehensive Evaluation of the Safety and Efficacy of BAFASAL(®) Bacteriophage Preparation for the Reduction of Salmonella in the Food Chain. Viruses 2020, 12, 742. [Google Scholar] [CrossRef]
- UniFAHS. Products. 2022. Available online: https://www.unifahs.com/# (accessed on 29 April 2022).
- Żbikowska, K.; Michalczuk, M.; Dolka, B. The Use of Bacteriophages in the Poultry Industry. Animals 2020, 10, 872. [Google Scholar] [CrossRef]
- Clavijo, V.; Baquero, D.; Hernandez, S.; Farfan, J.C.; Arias, J.; Arévalo, A.; Donado-Godoy, P.; Vives-Flores, M. Phage cocktail SalmoFREE® reduces Salmonella on a commercial broiler farm. Poult. Sci. 2019, 98, 5054–5063. [Google Scholar] [CrossRef]
- Phageseeker. Products. 2022. Available online: http://www.phageseeker.com/ (accessed on 29 April 2022).
- Miller, R.W.; Skinner, E.J.; Sulakvelidze, A.; Mathis, G.F.; Hofacre, C.L. Bacteriophage therapy for control of necrotic enteritis of broiler chickens experimentally infected with Clostridium perfringens. Avian Dis. 2010, 54, 33–40. [Google Scholar] [CrossRef]
- Pharma ACD. CUSTUS®YRS: Bacteriophages against Yersiniosis. 2022. Available online: https://acdpharma.com/custusyrs-eng/ (accessed on 29 April 2022).
- SciPhage. Products. 2022. Available online: http://sciphage.com/products/ (accessed on 29 April 2022).
- Schulz, P.; Pajdak-Czaus, J.; Robak, S.; Dastych, J.; Siwicki, A.K. Bacteriophage-based cocktail modulates selected immunological parameters and post-challenge survival of rainbow trout (Oncorhynchus mykiss). J. Fish Dis. 2019, 42, 1151–1160. [Google Scholar] [CrossRef]
- Runda Biotechnology Pharmaceutical. News. 2022. Available online: http://www.rundabio.cn/news/1946.html (accessed on 29 April 2022).
- Cytophage. BACTERIOPHAGE. 2022. Available online: https://cytophage.com/ (accessed on 29 April 2022).
- Intermediates Pathway. Products. 2022. Available online: http://www.pathway-intermediates.com/ (accessed on 29 April 2022).
- Bacteriophage. Phage-Products. 2022. Available online: https://www.bacteriophage.news/phage-products/ (accessed on 1 May 2022).
- MicroMir. Micromir Drugs. 2022. Available online: https://micromir.bio/ (accessed on 29 April 2022).
- Gambino, M.; Brøndsted, L. Looking into the future of phage-based control of zoonotic pathogens in food and animal production. Curr. Opin. Biotechnol. 2021, 68, 96–103. [Google Scholar] [CrossRef]
- López-Cuevas, O.; Medrano-Félix, J.A.; Castro-Del Campo, N.; Chaidez, C. Bacteriophage applications for fresh produce food safety. Int. J. Environ. Health Res. 2021, 31, 687–702. [Google Scholar] [CrossRef]
- Li, M.; Lin, H.; Jing, Y.; Wang, J. Broad-host-range Salmonella bacteriophage STP4-a and its potential application evaluation in poultry industry. Poult. Sci. 2020, 99, 3643–3654. [Google Scholar] [CrossRef]
- Vikram, A.; Tokman, J.I.; Woolston, J.; Sulakvelidze, A. Phage Biocontrol Improves Food Safety by Significantly Reducing the Level and Prevalence of Escherichia coli O157:H7 in Various Foods. J. Food Prot. 2020, 83, 668–676. [Google Scholar] [CrossRef]
- Ushanov, L.; Lasareishvili, B.; Janashia, I.; Zautner, A.E. Application of Campylobacter jejuni Phages: Challenges and Perspectives. Animals 2020, 10, 279. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.; Ali, Z.; Khan, M.; Bostan, N.; Naseem, S. The dawn of phage therapy. Rev. Med. Virol. 2019, 29, e2041. [Google Scholar] [CrossRef]
- (FSN) Food Safety News. EU Project Uses Phages to Tackle Campylobacter in Poultry. 2020. Available online: https://www.foodsafetynews.com/2020/11/eu-project-uses-phages-to-tackle-campylobacter-in-poultry/ (accessed on 1 May 2022).
- Dissanayake, U.; Ukhanova, M.; Moye, Z.D.; Sulakvelidze, A.; Mai, V. Bacteriophages Reduce Pathogenic Escherichia coli Counts in Mice Without Distorting Gut Microbiota. Front. Microbiol. 2019, 10, 1984. [Google Scholar] [CrossRef]
- Phageguard. Phage Technology Which Food Pathogen Can We Help You Fight? 2022. Available online: https://phageguard.com/ (accessed on 29 April 2022).
- Zhang, X.; Niu, Y.D.; Nan, Y.; Stanford, K.; Holley, R.; McAllister, T.; Narváez-Bravo, C. SalmoFresh™ effectiveness in controlling Salmonella on romaine lettuce, mung bean sprouts and seeds. Int. J. Food Microbiol. 2019, 305, 108250. [Google Scholar] [CrossRef]
- Grant, A.; Parveen, S.; Schwarz, J.; Hashem, F.; Vimini, B. Reduction of Salmonella in ground chicken using a bacteriophage. Poult. Sci. 2017, 96, 2845–2852. [Google Scholar] [CrossRef]
- Production Arm & Hammer Animal and Food. Phage Technology Reduces Salmonella in Ground Turkey. 2020. Available online: https://ahfoodchain.com/en/segments/food-production/products/finalyse-sal (accessed on 29 April 2022).
- Diana, G.; Lorena, R.-R.; Lucía, F.; Beatriz, M.; Ana, R.; Pilar, G. Applicability of commercial phage-based products against Listeria monocytogenes for improvement of food safety in Spanish dry-cured ham and food contact surfaces. Food Control 2017, 73, 1474–1482. [Google Scholar]
- Chibeu, A.; Agius, L.; Gao, A.; Sabour, P.M.; Kropinski, A.M.; Balamurugan, S. Efficacy of bacteriophage LISTEX™P100 combined with chemical antimicrobials in reducing Listeria monocytogenes in cooked turkey and roast beef. Int. J. Food Microbiol. 2013, 167, 208–214. [Google Scholar] [CrossRef]
- Truchado, P.; Elsser-Gravesen, A.; Gil, M.I.; Allende, A. Post-process treatments are effective strategies to reduce Listeria monocytogenes on the surface of leafy greens: A pilot study. Int. J. Food Microbiol. 2020, 313, 108390. [Google Scholar] [CrossRef]
- Pennone, V.; Sanz-Gaitero, M.; O’Connor, P.; Coffey, A.; Jordan, K.; van Raaij, M.J.; McAuliffe, O. Inhibition of L. monocytogenes Biofilm Formation by the Amidase Domain of the Phage vB_LmoS_293 Endolysin. Viruses 2019, 11, 722. [Google Scholar] [CrossRef] [Green Version]
- Intralytix. Products. 2022. Available online: http://intralytix.com/index.php?page=prod (accessed on 29 April 2022).
- Soffer, N.; Woolston, J.; Li, M.; Das, C.; Sulakvelidze, A. Bacteriophage preparation lytic for Shigella significantly reduces Shigella sonnei contamination in various foods. PLoS ONE 2017, 12, e0175256. [Google Scholar] [CrossRef] [Green Version]
- Suh, G.A.; Lodise, T.P.; Tamma, P.D.; Knisely, J.M.; Alexander, J.; Aslam, S.; Barton, K.D.; Bizzell, E.; Totten KM, C.; Campbell, J.L.; et al. Considerations for the Use of Phage Therapy in Clinical Practice. Antimicrob. Agents Chemother. 2022, 66, e0207121. [Google Scholar] [CrossRef]
- Nikolich, M.P.; Filippov, A.A. Bacteriophage Therapy: Developments and Directions. Antibiotics 2020, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Reuter, M.; Kruger, D.H. Approaches to optimize therapeutic bacteriophage and bacteriophage-derived products to combat bacterial infections. Virus Genes 2020, 56, 136–149. [Google Scholar] [CrossRef]
- Rhoads, D.D.; Wolcott, R.D.; Kuskowski, M.A.; Wolcott, B.M.; Ward, L.S.; Sulakvelidze, A. Bacteriophage therapy of venous leg ulcers in humans: Results of a phase I safety trial. J. Wound Care 2009, 18, 237–238, 240–243. [Google Scholar] [CrossRef]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Kakasis, A.; Panitsa, G. Bacteriophage therapy as an alternative treatment for human infections. A comprehensive review. Int. J. Antimicrob. Agents 2019, 53, 16–21. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Łobocka, M.; Głowacka-Rutkowska, A.; Bednarek, A.; Borysowski, J.; Jończyk-Matysiak, E.; Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Bagińska, N.; et al. Phage Therapy: What Have We Learned? Viruses 2018, 10, 288. [Google Scholar] [CrossRef] [Green Version]
- Petrovic Fabijan, A.; Lin RC, Y.; Ho, J.; Maddocks, S.; Ben Zakour, N.L.; Iredell, J.R. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat. Microbiol. 2020, 5, 465–472. [Google Scholar] [CrossRef]
- Kifelew, L.G.; Warner, M.S.; Morales, S.; Vaughan, L.; Woodman, R.; Fitridge, R.; Mitchell, J.G.; Speck, P. Efficacy of phage cocktail AB-SA01 therapy in diabetic mouse wound infections caused by multidrug-resistant Staphylococcus aureus. BMC Microbiol. 2020, 20, 204. [Google Scholar] [CrossRef]
- Law, N.; Logan, C.; Yung, G.; Furr, C.L.; Lehman, S.M.; Morales, S.; Rosas, F.; Gaidamaka, A.; Bilinsky, I.; Grint, P.; et al. Successful adjunctive use of bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa infection in a cystic fibrosis patient. Infection 2019, 47, 665–668. [Google Scholar] [CrossRef]
- Terwilliger, A.; Clark, J.; Karris, M.; Hernandez-Santos, H.; Green, S.; Aslam, S.; Maresso, A. Phage Therapy Related Microbial Succession Associated with Successful Clinical Outcome for a Recurrent Urinary Tract Infection. Viruses 2021, 13, 2049. [Google Scholar] [CrossRef]
- Abedon, S.T.; Danis-Wlodarczyk, K.M.; Alves, D.R. Phage Therapy in the 21st Century: Is There Modern, Clinical Evidence of Phage-Mediated Efficacy? Pharmaceuticals 2021, 14, 1157. [Google Scholar] [CrossRef]
- Galtier, M.; De Sordi, L.; Sivignon, A.; de Vallée, A.; Maura, D.; Neut, C.; Rahmouni, O.; Wannerberger, K.; Darfeuille-Michaud, A.; Desreumaux, P.; et al. Bacteriophages Targeting Adherent Invasive Escherichia coli Strains as a Promising New Treatment for Crohn’s Disease. J. Crohns. Colitis 2017, 11, 840–847. [Google Scholar] [CrossRef] [Green Version]
- Pharma Pherecydes. Candidats. 2022. Available online: https://www.pherecydes-pharma.com/produits-candidats/ (accessed on 29 April 2022).
- Therapeutics Phico. Products. 2022. Available online: https://www.phicotx.co.uk/#products (accessed on 29 April 2022).
- Microgen. Products. 2022. Available online: https://www.microgen.ru/en/products/bakteriofagi/ (accessed on 29 April 2022).
- Biomx. Pipeline. 2022. Available online: https://www.biomx.com/ (accessed on 29 April 2022).
- Phagelux. Leading Products. 2022. Available online: http://www.phagelux.com/ (accessed on 29 April 2022).
- Czaplewski, L.; Bax, R.; Clokie, M.; Dawson, M.; Fairhead, H.; Fischetti, V.A.; Foster, S.; Gilmore, B.F.; Hancock, R.E.; Harper, D.; et al. Alternatives to antibiotics-a pipeline portfolio review. Lancet Infect. Dis. 2016, 16, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.H.; Park, W.B.; Cho, J.E.; Choi, Y.J.; Choi, S.J.; Jun, S.Y.; Kang, C.K.; Song, K.H.; Choe, P.G.; Bang, J.H.; et al. Effects of Phage Endolysin SAL200 Combined with Antibiotics on Staphylococcus aureus Infection. Antimicrob. Agents Chemother. 2018, 62, e00731-18. [Google Scholar] [CrossRef] [Green Version]
- Jun, S.Y.; Jang, I.J.; Yoon, S.; Jang, K.; Yu, K.S.; Cho, J.Y.; Seong, M.W.; Jung, G.M.; Yoon, S.J.; Kang, S.H. Pharmacokinetics and Tolerance of the Phage Endolysin-Based Candidate Drug SAL200 after a Single Intravenous Administration among Healthy Volunteers. Antimicrob. Agents Chemother. 2017, 61, e02629-16. [Google Scholar] [CrossRef] [Green Version]
- Cass, J.; Barnard, A.; Fairhead, H. Engineered Bacteriophage as a Delivery Vehicle for Antibacterial Protein, SASP. Pharmaceuticals 2021, 14, 1038. [Google Scholar] [CrossRef] [PubMed]
- BioPreparation Eliava. Products. 2022. Available online: https://eliava-institute.org/?lang=en (accessed on 29 April 2022).
- Mai, V.; Ukhanova, M.; Reinhard, M.K.; Li, M.; Sulakvelidze, A. Bacteriophage administration significantly reduces Shigella colonization and shedding by Shigella-challenged mice without deleterious side effects and distortions in the gut microbiota. Bacteriophage 2015, 5, e1088124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R&D BioNTech. Synthetic lysins. 2022. Available online: https://www.phagomed.com/overview (accessed on 29 April 2022).
- Zheng, D.W.; Dong, X.; Pan, P.; Chen, K.W.; Fan, J.X.; Cheng, S.X.; Zhang, X.Z. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat. Biomed. Eng. 2019, 3, 717–728. [Google Scholar] [CrossRef]
- Biochimpharm. Products. 2022. Available online: https://biochimpharm.ge/en/ (accessed on 29 April 2022).
- Ghosh, C.; Sarkar, P.; Issa, R.; Haldar, J. Alternatives to Conventional Antibiotics in the Era of Antimicrobial Resistance. Trends Microbiol. 2019, 27, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Immunopreparat Aziya. Production. 2022. Available online: https://www.aziyaimmunopreparat.uz/eng/ (accessed on 29 April 2022).
- Pharma, M.B. Products. 2022. Available online: https://www.mbph.cz/produkty (accessed on 29 April 2022).
- Phagex. Phage Products. 2022. Available online: https://bacteriophages.info/en/bacteriophage/ (accessed on 29 April 2022).
- Therapeutics Adaptive Phage. Development Pipeline. 2022. Available online: https://www.aphage.com/science/pipeline/ (accessed on 29 April 2022).
- Selle, K.; Fletcher, J.R.; Tuson, H.; Schmitt, D.S.; McMillan, L.; Vridhambal, G.S.; Rivera, A.J.; Montgomery, S.A.; Fortier, L.C.; Barrangou, R.; et al. In Vivo Targeting of Clostridioides difficile Using Phage-Delivered CRISPR-Cas3 Antimicrobials. mBio 2020, 11, e00019-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Science Ellis Day Skin. Serums. 2022. Available online: https://www.ellisdayskinscience.com/products/balancing-phage-serum (accessed on 29 April 2022).
- Phyla. Our Technology: Bacteriophages. 2022. Available online: https://phylabiotics.com/ (accessed on 29 April 2022).
- Domingo-Calap, P.; Delgado-Martínez, J. Bacteriophages: Protagonists of a Post-Antibiotic Era. Antibiotics 2018, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Pirnay, J.P.; Merabishvili, M.; Van Raemdonck, H.; De Vos, D.; Verbeken, G. Bacteriophage Production in Compliance with Regulatory Requirements. Methods Mol. Biol. 2018, 1693, 233–252. [Google Scholar]
- Oliveira, H.; São-José, C.; Azeredo, J. Phage-Derived Peptidoglycan Degrading Enzymes: Challenges and Future Prospects for In Vivo Therapy. Viruses 2018, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Gondil, V.S.; Harjai, K.; Chhibber, S. Endolysins as emerging alternative therapeutic agents to counter drug-resistant infections. Int. J. Antimicrob. Agents 2020, 55, 105844. [Google Scholar] [CrossRef]
- Totté, J.E.E.; van Doorn, M.B.; Sgma, P. Successful Treatment of Chronic Staphylococcus aureus-Related Dermatoses with the Topical Endolysin Staphefekt SA.100: A Report of 3 Cases. Case Rep. Dermatol. 2017, 9, 19–25. [Google Scholar] [CrossRef]
- Gerstmans, H.; Rodríguez-Rubio, L.; Lavigne, R.; Briers, Y. From endolysins to Artilysin®s: Novel enzyme-based approaches to kill drug-resistant bacteria. Biochem. Soc. Trans. 2016, 44, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, J.Y.; Jun, K.I.; Kang, C.K.; Song, K.H.; Choe, P.G.; Bang, J.H.; Kim, E.S.; Park, S.W.; Kim, H.B.; Kim, N.J.; et al. Efficacy of Intranasal Administration of the Recombinant Endolysin SAL200 in a Lethal Murine Staphylococcus aureus Pneumonia Model. Antimicrob. Agents Chemother. 2019, 63, e02009-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, V.G., Jr.; Das, A.F.; Lipka-Diamond, J.; Schuch, R.; Pomerantz, R.; Jáuregui-Peredo, L.; Bressler, A.; Evans, D.; Moran, G.J.; Rupp, M.E.; et al. Exebacase for patients with Staphylococcus aureus bloodstream infection and endocarditis. J. Clin. Investig. 2020, 130, 3750–3760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moorthy, G.S.; Greenberg, R.G.; Hornik, C.D.; Cassino, C.; Ghahramani, P.; Kumar, K.R.; Fowler, V.G.; Cohen-Wolkowiez, M. Safety and Pharmacokinetics of Exebacase in an Infant with Disseminated Staphylococcus aureus Infection. Clin. Infect. Dis. 2021, 1–12. [Google Scholar] [CrossRef]
- Abdelkader, K.; Gerstmans, H.; Saafan, A.; Dishisha, T.; Briers, Y. The Preclinical and Clinical Progress of Bacteriophages and Their Lytic Enzymes: The Parts are Easier than the Whole. Viruses 2019, 11, 96. [Google Scholar] [CrossRef] [Green Version]
- Borin, J.M.; Avrani, S.; Barrick, J.E.; Petrie, K.L.; Meyer, J.R. Coevolutionary phage training leads to greater bacterial suppression and delays the evolution of phage resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2104592118. [Google Scholar] [CrossRef]
- Hampton, H.G.; Watson, B.N.J.; Fineran, P.C. The arms race between bacteria and their phage foes. Nature 2020, 577, 327–336. [Google Scholar] [CrossRef]
- Barrangou, R.; Horvath, P. A decade of discovery: CRISPR functions and applications. Nat. Microbiol. 2017, 2, 17092. [Google Scholar] [CrossRef]
- Hussain, F.A.; Dubert, J.; Elsherbini, J.; Murphy, M.; VanInsberghe, D.; Arevalo, P.; Kauffman, K.; Rodino-Janeiro, B.K.; Gavin, H.; Gomez, A.; et al. Rapid evolutionary turnover of mobile genetic elements drives bacterial resistance to phages. Science 2021, 374, 488–492. [Google Scholar] [CrossRef]
- Barber, O.W.; Miramontes, I.M.; Jain, M.; Ozer, E.A.; Hartmann, E.M. The Future of Bacteriophage Therapy Will Promote Antimicrobial Susceptibility. mSystems 2021, 6, e0021821. [Google Scholar] [CrossRef]
- Burmeister, A.R.; Fortier, A.; Roush, C.; Lessing, A.J.; Bender, R.G.; Barahman, R.; Turner, P.E. Pleiotropy complicates a trade-off between phage resistance and antibiotic resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 11207–11216. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Van Belleghem, J.D.; de Vries, C.R.; Burgener, E.; Chen, Q.; Manasherob, R.; Aronson, J.R.; Amanatullah, D.F.; Tamma, P.D.; Suh, G.A. The Safety and Toxicity of Phage Therapy: A Review of Animal and Clinical Studies. Viruses 2021, 13, 1268. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Silk route to the acceptance and re-implementation of bacteriophage therapy. Biotechnol. J. 2016, 11, 595–600. [Google Scholar]
- Fauconnier, A. Phage Therapy Regulation: From Night to Dawn. Viruses 2019, 11, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Barceló, C. The disparate effects of bacteriophages on antibiotic-resistant bacteria. Emerg. Microbes Infect. 2018, 7, 168. [Google Scholar] [CrossRef]
- Bonilla, N.; Barr, J.J. Phage on Tap: A Quick and Efficient Protocol for the Preparation of Bacteriophage Laboratory Stocks. Methods Mol. Biol. 2018, 1838, 37–46. [Google Scholar]
- Luong, T.; Salabarria, A.C.; Edwards, R.A.; Roach, D.R. Standardized bacteriophage purification for personalized phage therapy. Nat. Protoc. 2020, 15, 2867–2890. [Google Scholar] [CrossRef]
- Sybesma, W.; Rohde, C.; Bardy, P.; Pirnay, J.P.; Cooper, I.; Caplin, J.; Chanishvili, N.; Coffey, A.; De Vos, D.; Scholz, A.H.; et al. Silk Route to the Acceptance and Re-Implementation of Bacteriophage Therapy-Part II. Antibiotics 2018, 7, 35. [Google Scholar]
- Bretaudeau, L.; Tremblais, K.; Aubrit, F.; Meichenin, M.; Arnaud, I. Good Manufacturing Practice (GMP) Compliance for Phage Therapy Medicinal Products. Front. Microbiol. 2020, 11, 1161. [Google Scholar] [CrossRef]

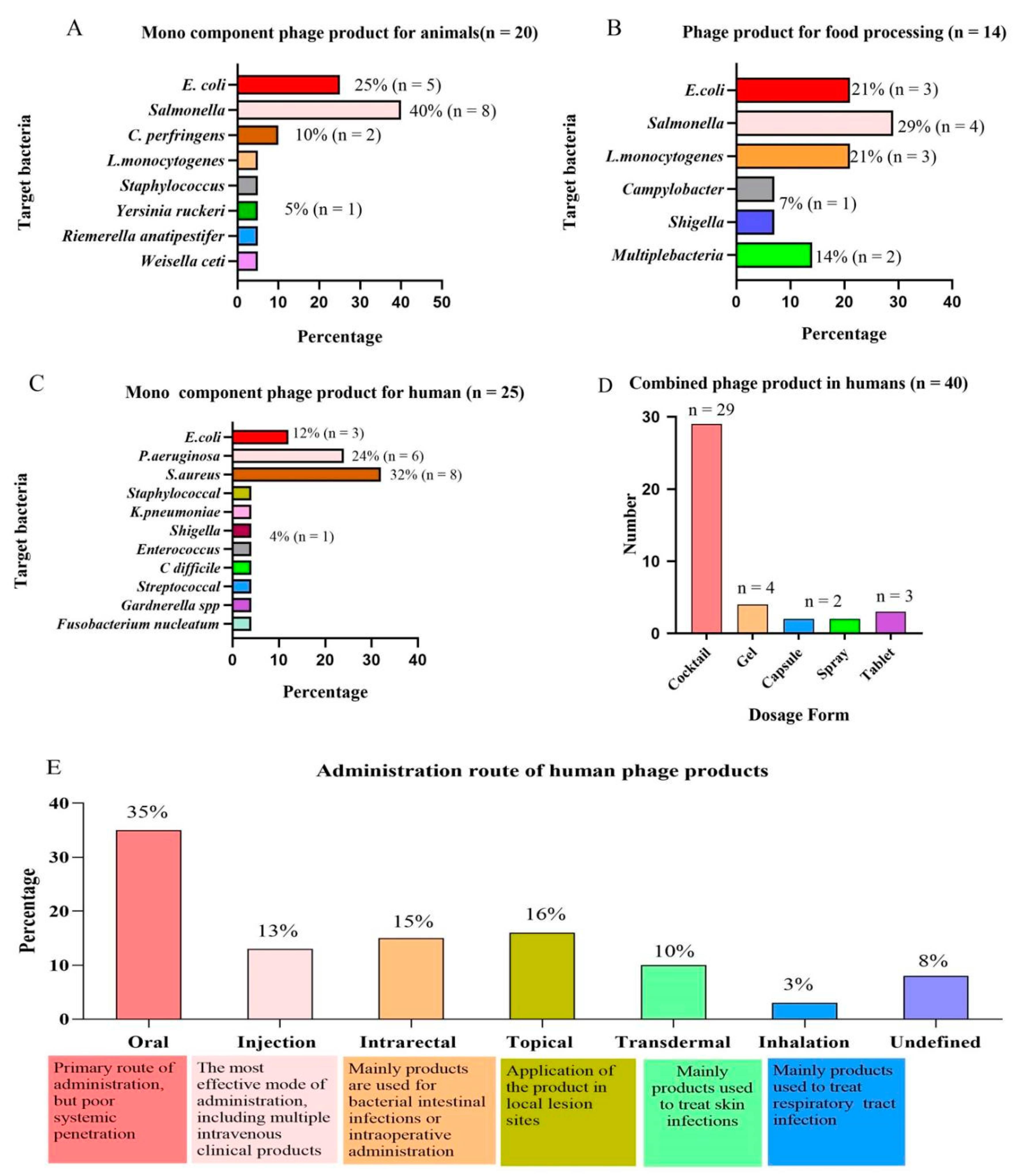
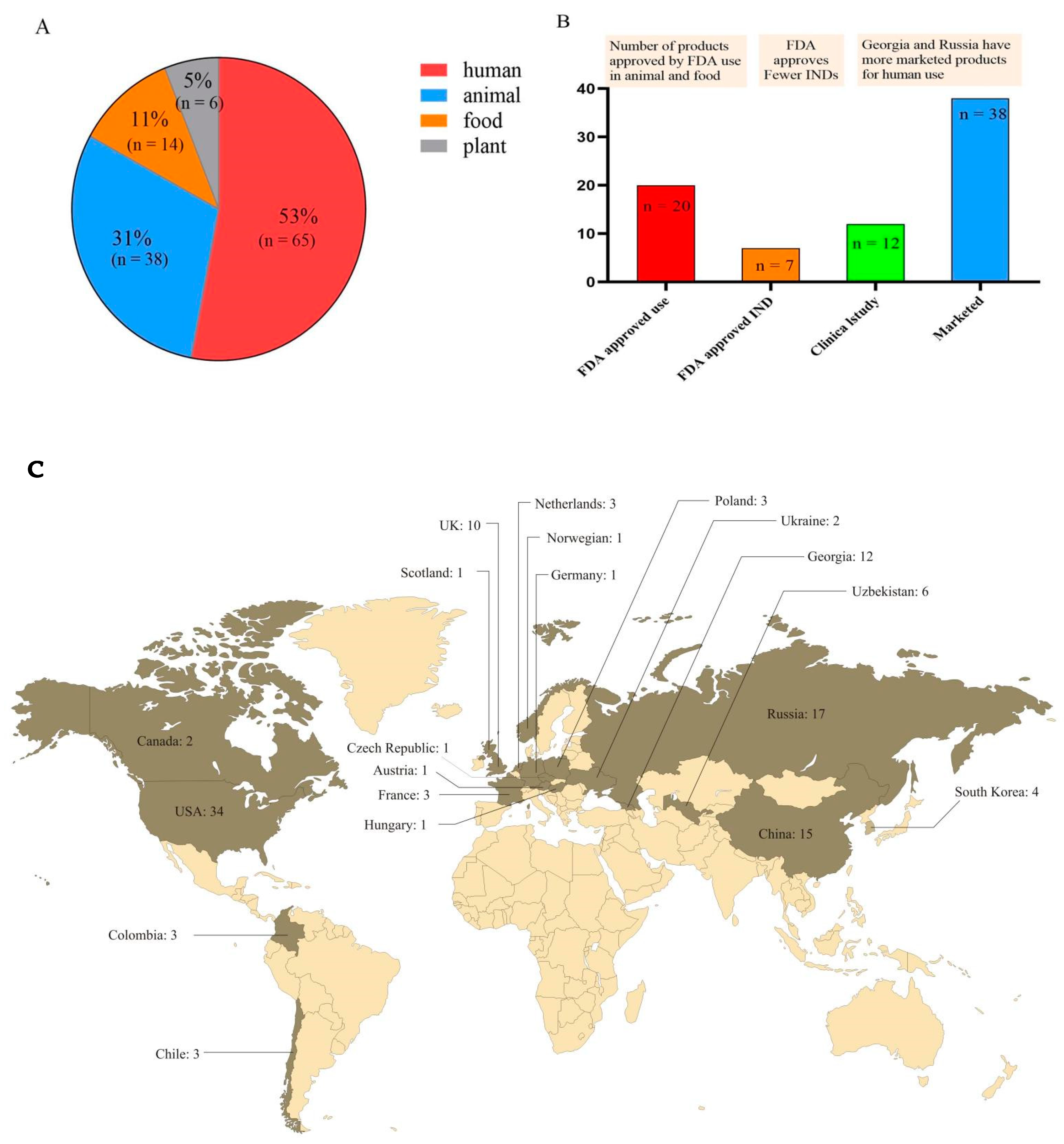
| Target Bacteria | Company | Products | Regulatory Approval | Certifications | Application | Ref. |
|---|---|---|---|---|---|---|
| Soft rot Enterobacteriacea | APS Biocontrol Ltd. (SCO) | Biolyse® BP | Approved | UK, European | Food processing assistants in the potato packaging industry | [34] |
| Clavibacter michiganensis | OmniLytics Inc. (USA) | AgriPhage CMM™ | EPA approved | USA, Canada | Tomato bacterial canker | [35,36] |
| Xanthomonas citri | AgriPhage™ Citrus Canker | Citrus canker | [27] | |||
| Erwinia amylovora | AgriPhage™ Fire Blight | Fire blight for apples and pears | ||||
| Erwiphage PLUS (HU) | Erwiphage | Undefined | Hungary | Fire blight caused by plants in the rose family | [37] | |
| Variety of bacteria | Fixed-Phage (UK) | agriPHIX™ | UK | Effective improvement of storage for a range of crops | [38] |
| Target Bacteria | Company | Products | Regulatory Approval | Certifications | Application | Ref. |
|---|---|---|---|---|---|---|
| E. coli | Intralytix (USA) | Ecolicide® | FDA | USA | For E. coli O157:H7 contamination in Pet Food | [48] |
| Ecolicide PX™ | For E. coli O157:H7 contamination on animal fur | |||||
| Arm and Hammer Animal & Food Production (USA) | Finalyse® | USDA, FSIS | USA | A preharvest antimicrobial hide wash used to reduce E. coli O157:H7 | [34] | |
| Proteon Pharmaceuticals (POL) | BAFACOL™ | EFSA | Poland | Feed additive to prevent pathogenic E. coli in poultry. | [49] | |
| Phagelab (CHI) | Swine product | Undefined | Chile | Liquid Food Additive Eliminates E. coli in Swine | [50] | |
| Salmonella | Intralytix (USA) | SalmoLyse® | FDA | USA | Salmonella Contamination in Pet Food | [51,52] |
| PLSV-1™ | Salmonella Contamination in Poultry | [44,53] | ||||
| Proteon Pharmaceuticals (POL) | BAFASAL+G® | EFSA | Poland | Feed additive to treat the digestive tract of poultry | [51,54] | |
| UniFAHS | SalmoGuard | FDA | Southeast Asian countries | Poultry feed additives | [52,55] | |
| OmniLytics Inc. (USA) | BacWash™ | USDA | USA | For Hides of livestock surface disinfection | [56] | |
| SciPhage (CO) | SalmoFree® | Undefined | Colombia | Feed additive for control of Salmonella infection in poultry | [57] | |
| PhagePharm (CHN) | NuoAnSha | Approved | China | Improve the breeding environment | [58] | |
| Phagelab (CHI) | Poultry product | Undefined | Chile | Liquid food additive to eliminate Salmonella in broilers. | [50] | |
| L. monocytogenes | Intralytix (USA) | ListPhage™ | FDA | USA | L. monocytogenes in pet food | [48] |
| C. perfringens | INT-401™ | FDA, FSIS | Against Poultry C. perfringens | [59] | ||
| PhagePharm (CHN) | NuoAnSuoQing | Approved | China | Necrotizing enteritis, diarrhea, intestinal bleeding caused by C. perfringens | [58] | |
| staphylococcus | Delmont Laboratories (USA) | Staphage Lysate(SPL)® | FDA | USA | Staphylococcal skin infections in dogs | [47] |
| Yersinia ruckeri | ACD Pharma (NOR) | CUSTUS®YRS | FOT | Norwegian | Various bacteria in aquaculture farms | [60] |
| R. anatipestifer | PhagePharm (CHN) | JiangYanQing | Approved | China | Decontamination of R.anatipestifer in aquaculture environments | [58] |
| Weisella ceti | SciPhage (CO) | Weissella Ceti Phages | Undefined | Colombia | control weissellosis in trout | [61] |
| Variety of bacteria | CJ CheilJedang Research Institute of Biotechnology (KOR) | Biotector®S | Undefined | South Korea | Feed additive for poultry and pigs against Salmonella, C.perfringens, E. coli. | [35] |
| Phagelab (CHI) | cattle product | Chile | Food additive prevent infectious diarrhea caused by E. coli and Salmonella. | [50] | ||
| Proteon Pharmaceuticals (POL) | BAFADOR® | EFSA | Poland | Fish feed additive against Aeromonas and Pseudomonas | [62] | |
| PhagePharm (CHN) | NuoAnQing | Approved | China | Improve the breeding environment | [58] | |
| YaLiNing | ||||||
| Varmsphage (CHN) | ChangShi | Infections caused by E. coli and Salmonella | [63] | |||
| Cytophage (CAN) | Poultry Feed Additives | Undefined | Canada | Prevents the common bacterial infections in chickens | [64] | |
| swine bacteriophage | Against the common bacterial infections in swine | |||||
| Fixed-Phage (UK) | aquaPHIX™ | Approved | UK | Added to the feed as a solvent | [37,49] | |
| farmPHIX™ | Feed additives | |||||
| petPHIX™ | Topical application of gels and creams | |||||
| Pathway Intermediates (KOR) | ProBe-Bac | FDA | South Korea | ProBe-Bac SE for pigs; ProBe-Bac PE for poulty | [65] | |
| Phagelux (CHN) | LUNIN | Approved | China | for poultry diseases | [66] | |
| LUZON | for swine disease | |||||
| LUMON | for cattle disease | |||||
| MicroMir (RUS) | Vetagin® | Approved | Russia | Prevention of bacterial endometritis, abscess and myositis in dairy cows | [67] | |
| Bronchophage | Control of common bacteria associated with lower respiratory tract disease | |||||
| Phagovet | Prevention of bacterial diseases in broilers |
| Target Bacteria | Company | Phage Products | Regulatory Approval | Certifications | Application | Ref. |
|---|---|---|---|---|---|---|
| E. coli | Intralytix (USA) | EcoShield PX™ | FDA | Canada; Israel; USA | Eliminate E. coli O157:H7 contamination prior to grinding or packaging | [71,75] |
| Micreos (NED) | PhageGuard E™ | USA | E. coli O157 on beef carcasses, primals, subs and trimmings. | [76] | ||
| FINK TEC GmbH (GER) | Secure Shield E1 | Used in beef products, turkey and other foods | [48] | |||
| Salmonella | Intralytix (USA) | SalmoFresh™ | USA, Canada, Israel | Food additives for poultry, fish, shellfish, fruits and vegetables. | [77] | |
| Micreos (NED) | PhageGuard S™ | Canada; Israel; Halal; OMRI; SKAL | In spray or dipping form for poultry, meat. | [78] | ||
| Phagelux (CHN) | SalmoPro® | Canada, China | As an antibacterial processing aid in food. | [56] | ||
| Arm and Hammer Animal & Food Production (USA) | Finalyse™SAL | Undefined | USA | For Salmonella in poultry products. | [79] | |
| L.monocytogenes | Intralytix (USA) | ListShield™ | FDA | USA | Food additives for poultry, fish, shellfish, fruits and vegetables. | [80] |
| Listex™ | [81] | |||||
| Micreos (NED) | PhageGuard Listex™ | Swiss; Israel; Halal; Canada; Kosher OMRI; SKAL | In spray or dipping form for poultry, meat. | [82,83] | ||
| Campylobacter | Intralytix (USA) | Compyshield™ | USA | Food additives for raw red meat | [84] | |
| Shigella | Intralytix (USA) | ShigaShield™ | Removal of Shigella from meat and vegetables | [53,85] | ||
| Variety of bacteria | Brimrose Technology Corporation (USA) | EnkoPhagum | Approved | Salmonella, Shigella, E. coli, Staphylococcus in meat products. | [53] | |
| Fixed-Phage (UK) | safePHIX™ | Undefined | UK | Against bacteria in the food cold chain | [38] |
| Mono Component | ||||||
|---|---|---|---|---|---|---|
| Target Bacteria | Company | Product | Regulatory Approval | Route of Administration | Application | Ref. |
| E. coli | Intralytix (USA) | EcoActive™ | FDA approved IND, Phase 1/2a | oral | Targeting adherent-invasive E. coli | [98] |
| Pherecydes Pharma (FRA) | PhagUTI | Phase I/II | Undefined | Treating E. coli Urinary Tract Infections | [99] | |
| Phico Therapeutics (UK) | SASPject PT5 | Uundefined | Intravenous injection | Fights diseases caused by E. coli | [100] | |
| P. aeruginosa | Microgen (RUS) | Bacteriophage P. aeruginosa | Russian Federation national standard certification | Oral intrarectal, or Intracavitary injection | Treatment and prevention of diseases caused by P. aeruginosa | [101] |
| Armata (USA) | AP-PA02; AP-PA03 | FDA approved IND, Phase 1b/2 | Inhalation | Treatment of respiratory tract infections caused by P. aeruginosa, especially in patients with CF | [95] | |
| BiomX (USA) | BX004 | Preclinical | Oral | [102] | ||
| Phagelux (CHN) | PGX0100 | FDA approved IND, preclinical | Transdermal | Spray and gel for burn care | [103] | |
| Phico Therapeutics (UK) | SASPject PT3 | undefined | Undefined | Against P. aeruginosa infection | [104] | |
| Pherecydes Pharma (FRA) | Pneumo Phage | Phase I/II clinical trials are expected to start in 2023 | Inhalation | Treatment of acute P. aeruginosa respiratory tract infection | [99] | |
| S. aureus | Microgen (RUS) | Staphylococcal bacteriophage | Russian Federation national standard certification | Inhalation | Treatment of Suppurative Inflammation and Intestinal Disorders Caused by Staphylococci | [101] |
| Armata (USA) | AP-SA01; AP-SA02 | FDA approved IND, Phase 1b/2 | Intravenous injection | Treatment of resistant and refractory Staphylococcus aureus bacteremia and diabetic foot ulcers | [93,94] | |
| BiomX (USA) | BX005 | Preclinical stage | Transdermal | Atopic dermatitis caused by S. aureus | [102] | |
| Phagelux (CHN) | PL-01-SZ | China NMPA IND submission expected in 2022 | S.aureus lyase, a hydrogel formulation for the treatment of eczema | [103] | ||
| PL-06-FC | P.acnes and S.aureus lyase, hydrogel for acne treatment | |||||
| iNtODEWorld (KOR) | N-Rephasin® SAL200 | Phase II | Intravenous injection | Effective against MRSA | [105,106] | |
| Pherecydes Pharma (FRA) | Phage Cocktail | Phase I/II | Undefined | Fights bone and joint infections (IOA) and diabetic foot ulcers (UPD) caused by S.aureus. | [99] | |
| Phico Therapeutics (UK) | SASPject PT1.2 | Phase I | Engineered phages deliver genes for antimicrobial proteins (SASPs) that rapidly kill S. aureus | [107] | ||
| Staphylococcal | Eliava Bio Preparation (GEO) | Staphylococcal Bacteriophage | Georgian Approval | Oral or intrarectal | Prevention and treatment of postoperative wound infections, Staphylococcal infections | [108] |
| K.pneumoniae | BiomX (USA) | BX003 | Phase I | Oral | Targeting K. pneumoniae bacterial strains present in the gut of IBD and PSC patients | [102] |
| Shigella | Intralytix (USA) | ShigActive™ | FDA approved IND,2021 | Oral | Prevention of human diseases caused by Shigella infection | [109] |
| Enterococcus | VRELysin™ | Undefined | Undefined | Colonization with antibiotic-resistant Enterococci and associated bacteremia | [84] | |
| C difficile | AmpliPhi (UK) | AmpliPhage-004 | Pre-phase 1 | Undefined | Against C. difficile (including highly virulent RT027) | [104] |
| Streptococcal | Microgen(RUS) | Streptococcal bacteriophage | Russian Federation national standard certification | Oral, topical and intrarectal | Treatment diseases caused by Streptococcus | [101] |
| Gardnerella spp | BioNTech R&D(AUT) | PM-477 | Preclinical | Undefined | Recurrent bacterial vaginosis, synthetic lysosomes | [110] |
| Fusobacterium nucleatum | BiomX (USA) | engineered phage | Preclinical | Intravenous injection | Targeting Fusobacterium nucleatum bacteria present in the tumor micro environment. | [111] |
| Combining targets against variety of bacteria | ||||||
| Dosage Form | Company | Product | Regulatory approval | Route of administration | Application | Ref |
| Phage spray | Biochimpharm (GEO) | Phagyo®spray | Georgian Approved | Topical | Treatment and prophylaxis of bacterial purulent–inflammatory infections (multiple microorganims) | [112] |
| Phage tablet | Septaphage®table | Oral | ||||
| Phage cocktail | Septaphage® | |||||
| Phagyo® | ||||||
| PhageStaph | ||||||
| Phage capsule | Travelphag™ | For bacterial infections, indigestion | ||||
| Phage cocktail | Microgen (RUS) | Salmonella groups A,B,C,D, bacteriophage | Russian Federation national standard certification | Oral, intrarectal | Treatment and Prevention of Diseases Caused by Salmonella | [101] |
| E.coli-Proteus bacteriophage | Oral, topical and intrarectal | Treatment and prevention of purulent inflammatory and enteric diseases, dysbacteriosis caused by bacteria Proteus and enterotoxigenic E.coli | ||||
| Klebsiella purified polyvalent bacteriophage | Specific lysis of K. pneumoniae, K. odorifera, K. rhinosclerosis. | |||||
| Intesti-bacteriophage | Treatment and prevention of bacillary dysentery | |||||
| Sextaphage ® Polyvalent Pyobacteriophage | Treatment and prevention of purulent inflammation and intestinal diseases | |||||
| Complex Pyobacteriophage | Specific lysis of Staphylococcus, Streptococcus, Enterococcus, Proteus, K.pneumoniae, P.aeruginosa and E. coli. | |||||
| Dysentery polyvalent bacteriophage | Oral and intrarectal | Specific lysis of the bacillary dysentery pathogen | ||||
| Phage cocktail | Eliava Bio Preparation (GEO) | Pyo-Phage | Georgian Approved | Oral, intrarectal, or intracavitary injection | Treatment and prevention of bacterial purulent inflammation and intestinal infections. | [113] |
| Fersisi-Phage | ||||||
| Intesti-Phage | Oral or intrarectal | |||||
| SES-phage | Rectal, or intracavitary injection | |||||
| ENKO-Phage | Oral | |||||
| Phage spray | Aziya Immunopreparat (UZ) | Bacteriophage Staphylococcus spray MediPhag | Marketed | Topical(spray) | A mix of sterile lysate phages against S. aureus | [114] |
| Phage cocktail | Bacteriophage Staphylococcus liquid MediPhag | Oral | ||||
| Bacteriophage Salmonella polyvalent MediPhag | Treatment and prevention of multiple serotypes of Salmonella | |||||
| Bacteriophage Dysenteric Polyvalent MediPhag | A mix of sterile lysate phages against Shigella | |||||
| GastroFag polyvalent MediPhag | Fight enteric diseases such as Salmonella, Proteus, S.aureus, P.aeruginosa, E.coli | |||||
| Phage capsule | Bacteriophage dysenteric polyvalent “MediPhag” | A white gelatin capsule containing lyophilizied dried bacteriophage capsules against Shigella | ||||
| phage tablet | MB Pharma (CZ) | LYZODOL® | Marketed | Oral | Against S.aureus, K.pneumoniae, Lelliottia amnigena, Propionibacterium acnes causing respiratory infections. | [115] |
| Phage gel | MicroMir (RUS) | Phagodent | Marketed | Topical | Contains 72 phage complexes to normalize oral microflora | [76] |
| Phagoderm | Skin gel containing 64 phages to prevent bacterial infection of the skin. | |||||
| Phagogyn | Gel containing 74 phages that prevent bacterial diseases of the reproductive system. | |||||
| Otophagus | Gel containing 69 phages that prevent bacterial and suppurative inflammation of the ear, nose and throat | |||||
| Phage cocktail | Phagex (UKR) | Pyofag® | Marketed | Oral and topical | Treatment of pathogenic factors in purulent inflammation and intestinal diseases caused by Streptococcus pyogenes, S.aureus, E.coli, P.aeruginosa, Proteus vulgaris, Proteus mirabilis | [116] |
| Intestifag® polyvalent bacteriophage | Fights intestinal diseases caused by Shigella, Salmonella, E. coli, P. aeruginosa, Enterococcus faecalis, S. aureus | |||||
| Phico Therapeutics (UK) | SASPject PT4 | Undefined | Intravenous injection | Treatment and prevention of diseases caused by K. pneumoniae and E.coli | [100] | |
| Phagelux (CHN) | BACTELIDE™ | FDA approved IND, preclinical | Transdermal | Patches and sprays for pressure ulcers | [103] | |
| Fixed-Phage (UK) | mediPHIX™ | Undefined | Undefined | Effective against a variety of bacteria | [38] | |
| Adaptive Phage Therapeutics (USA) | PhageBank | FDA approved IND, Phase 1/2 | Intravenous injection | Treat Diabetic Foot Osteomyelitis, Prosthetic Joint Infection, Chronic Recurrent UTI, Ophthalmic Infection, Cystic Fibrosis-related Lung Infection | [117] | |
| Locus Biosciences (KOR) | crPhage™ | Phase 1b | Injection | Combined with CRISPR-Cas3 to enhance bactericidal efficacy against various bacterial diseases such as IBD and UTI | [118] | |
| Ellis Day Skin Science (USA) | Balancing Phage Serum | Marketed | Transdermal | Eliminate bacteria associated with blemishes and acne to balance the skin microbiome | [119] | |
| Hydrating Phage Serum | ||||||
| PHYLA (USA) | Phortify Probiotic Serum | Marketed | A probiotic serum that targets and neutralizes acne-causing bacteria | [120] | ||
| SciPhage (CO) | AcneFree | Undefined | Fights acne-targeting bacteria | [61] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wang, W.; Zhang, Z.; Gu, Y.; Huang, A.; Wang, J.; Hao, H. Phage Products for Fighting Antimicrobial Resistance. Microorganisms 2022, 10, 1324. https://doi.org/10.3390/microorganisms10071324
Huang Y, Wang W, Zhang Z, Gu Y, Huang A, Wang J, Hao H. Phage Products for Fighting Antimicrobial Resistance. Microorganisms. 2022; 10(7):1324. https://doi.org/10.3390/microorganisms10071324
Chicago/Turabian StyleHuang, Yuanling, Wenhui Wang, Zhihao Zhang, Yufeng Gu, Anxiong Huang, Junhao Wang, and Haihong Hao. 2022. "Phage Products for Fighting Antimicrobial Resistance" Microorganisms 10, no. 7: 1324. https://doi.org/10.3390/microorganisms10071324
APA StyleHuang, Y., Wang, W., Zhang, Z., Gu, Y., Huang, A., Wang, J., & Hao, H. (2022). Phage Products for Fighting Antimicrobial Resistance. Microorganisms, 10(7), 1324. https://doi.org/10.3390/microorganisms10071324







