Mycelium vs. Fruiting Bodies of Edible Fungi—A Comparison of Metabolites
Abstract
1. Introduction
2. From Mycelium to Fruiting Body: Differential Gene Expression
3. From Genomics to Proteomics: Proteins, Enzymes and Amino Acids
4. Polysaccharides
5. Small Metabolites
6. Minerals
7. Effects of the Chemical Environment on the Concentration of Metabolites
8. Chemical Composition of Mycelia and Fruiting Bodies Differs Depending on Strain
9. Ecological Interactions Affect Fungal Growth
10. Toxins in Edible Fungi
11. The Potential of Fermenter Produced Fungal Mycelia as Future Meat Substitutes
12. Differences between Fermenter Produced Fungal Mycelia and Fruiting Bodies
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- United Nations. We Can End Poverty—Millenium Development Goals and Beyond. 2015. Available online: https://www.un.org/millenniumgoals/ (accessed on 8 June 2022).
- European Commission. Cluster 6: Food, Bioeconomy, Natural Resources, Agriculture and Environment. Available online: https://ec.europa.eu/info/research-and-innovation/funding/funding-opportunities/funding-programmes-and-open-calls/horizon-europe/cluster-6-food-bioeconomy-natural-resources-agriculture-and-environment_en (accessed on 8 June 2022).
- European Commission. Europäischer Grüner Deal. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_de (accessed on 8 June 2022).
- Xu, X.; Sharma, P.; Shu, S.; Lin, T.-S.; Ciais, P.; Tubiello, F.N.; Smith, P.; Campbell, N.; Jain, A.K. Global greenhouse gas emissions from animal-based foods are twice those of plant-based foods. Nat. Food 2021, 2, 724–732. [Google Scholar] [CrossRef]
- McCartney, P. Less Meat, Less Heat; Claudius Publishers: Munich, Germany, 2019. [Google Scholar]
- Palanisamy, M.; Franke, K.; Berger, R.G.; Heinz, V.; Topfl, S. High moisture extrusion of lupin protein: Influence of extrusion parameters on extruder responses and product properties. J. Sci. Food Agric. 2019, 99, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, R.H. Production of Mushroom Fruiting Bodies on the Surface of Submerged Cultures. Mycologia 1978, 70, 179–184. [Google Scholar] [CrossRef]
- Sailaja, B.; Radhika, B. A review on production of edible mushrooms and their applications. Int. J. Pharm. Biol. Sci. 2018, 8, 265–283. [Google Scholar]
- Burger, F.; Koch, M.; Fraatz, M.A.; Omarini, A.B.; Berger, R.G.; Zorn, H. Production of an Anise- and Woodruff-like Aroma by Monokaryotic Strains of Pleurotus sapidus Grown on Citrus Side Streams. Molecules 2022, 27, 651. [Google Scholar] [CrossRef]
- Beelman, R.B.; Royse, D.J.; Chikthimmah, N. Bioactive Components in Button Mushroom Agaricus bisporus (J. Lge) Imbach (Agaricomycetideae) of Nutritional, Medicinal, and Biological Importance. Int. J. Med. Mushrooms 2003, 5, 18. [Google Scholar] [CrossRef]
- Song, H.-Y.; Kim, D.-H.; Kim, J.-M. Comparative transcriptome analysis of dikaryotic mycelia and mature fruiting bodies in the edible mushroom Lentinula edodes. Sci. Rep. 2018, 8, 8983. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, X.; Liu, W. De novo transcriptomic analysis during Lentinula edodes fruiting body growth. Gene 2018, 641, 326–334. [Google Scholar] [CrossRef]
- Vetchinkina, E.; Fomin, A.; Navolokin, N.; Shirokov, A. Proteins and polysaccharides from vegetative mycelium of medicinal basidiomycete Lentinus edodes display cytotoxicity towards human and animal cancer cell lines. Int. J. Biol. Macromol. 2022, 195, 398–411. [Google Scholar] [CrossRef]
- Gao, S.; Wang, G.; Huang, Z.; Lei, X.; Bian, Y.; Liu, Y.; Huang, W. Selection of Reference Genes for qRT-PCR Analysis in Lentinula edodes after Hot-Air Drying. Molecules 2018, 24, 136. [Google Scholar] [CrossRef]
- Lu, Y.P.; Liao, J.H.; Guo, Z.J.; Cai, Z.X.; Chen, M.Y. Genome Survey and Transcriptome Analysis on Mycelia and Primordia of Agaricus blazei. Biomed. Res. Int. 2020, 2020, 1824183. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-Z.; Yao, F.; Yin, C.-M.; Sihi, D.-F.; Gao, H. Exogenous Induction of Ergosterol Synthesis in Agaricus blazei. Mod. Food Sci. Technol. 2021, 37, 65–72. [Google Scholar] [CrossRef]
- De Groot, P.W.; Schaap, P.J.; Sonnenberg, A.S.; Visser, J.; Van Griensven, L.J. The Agaricus bisporus hypA gene encodes a hydrophobin and specifically accumulates in peel tissue of mushroom caps during fruit body development. J. Mol. Biol. 1996, 257, 1008–1018. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Y.; Keerio, A.A.; Ma, A. Identification of hydrophobin genes and their physiological functions related to growth and development in Pleurotus ostreatus. Microbiol. Res. 2021, 247, 126723. [Google Scholar] [CrossRef]
- Qi, Y.; Sun, X.; Ma, L.; Wen, Q.; Qiu, L.; Shen, J. Identification of two Pleurotus ostreatus blue light receptor genes (PoWC-1 and PoWC-2) and in vivo confirmation of complex PoWC-12 formation through yeast two hybrid system. Fungal Biol. 2020, 124, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Du, F.; Zou, Y.; Hu, Q. Transcriptomics Analysis of Primordium Formation in Pleurotus eryngii. Genes 2021, 12, 1863. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Zajul, M.M. Biotechnologische Produktion von Basidiomyceten-Proteinen auf industriellen Nebenströmen zur Herstellung von Nahrungsmitteln. Ph.D. Thesis, Justus-Liebig-Universität Gießen, Gießen, Germany, 2017. [Google Scholar]
- Ahlborn, J.; Stephan, A.; Meckel, T.; Maheshwari, G.; Rühl, M.; Zorn, H. Upcycling of food industry side streams by basidiomycetes for production of a vegan protein source. Int. J. Recycl. Org. Waste Agric. 2019, 8, 447–455. [Google Scholar] [CrossRef]
- Onuoha, S.C.; Okoroh, P.N.; Tom-Quinn, R.A. Proximate Composition, Essential Heavy Metal Concentrations and Nutrient Density of the Mycelium and Fruiting Bodies of Organically Cultivated Pleurotus ostreatus. J. Appl. Life Sci. Int. 2021, 24, 44–52. [Google Scholar] [CrossRef]
- Vetter, J. [Mineral and amino acid contents of edible, cultivated shii-take mushrooms (Lentinus edodes)]. Z. Lebensm. Unters. Forsch. 1995, 201, 17–19. [Google Scholar] [CrossRef]
- Valenzuela-Cobos, J.D.; Grijalva-Endara, A.; Marcillo-Vellejo, R.; Garcés-Moncayo, M.F. Production and characterization of reconstituted strains of Pleurotus spp. cultivated on different agricultural wastes. Rev. Mex. De Ing. Química 2020, 19, 1493–1504. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, W.; Li, Z.; Huang, L.; Ban, L.; Peng, Y. Comparison and analysis of amino acid of fruit body and mycelium in Agaricus brunnescens Peck. Food Res. Dev. 2017, 38, 109–111. [Google Scholar]
- Finnigan, T.J.A.; Wall, B.T.; Wilde, P.J.; Stephens, F.B.; Taylor, S.L.; Freedman, M.R. Mycoprotein: The Future of Nutritious Nonmeat Protein, a Symposium Review. Curr. Dev. Nutr. 2019, 3, nzz021. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.A.; Goodenough, P.W. Fruiting of Agaricus bisporus. Arch. Microbiol. 1977, 114, 161–165. [Google Scholar] [CrossRef]
- Ding, Q.; Zhao, H.; Zhu, P.; Jiang, X.; Nie, F.; Li, G. Genome-wide identification and expression analyses of C2H2 zinc finger transcription factors in Pleurotus ostreatus. PeerJ 2022, 10, e12654. [Google Scholar] [CrossRef]
- Xu, D.; Lu, J.; Wang, Y.; Keerio, A.A.; Zheng, L.; Chen, L.; Ma, A. Identification and In Silico Analysis of Lectins in Gray Oyster Culinary-Medicinal Mushroom Pleurotus ostreatus (Agaricomycetes) Based on the Transcriptomes. Int. J. Med. Mushrooms 2019, 21, 1193–1205. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Watanabe, H.; Nagai, M.; Nakade, K.; Takahashi, M.; Sato, T. Lentinula edodes tlg1 encodes a thaumatin-like protein that is involved in lentinan degradation and fruiting body senescence. Plant Physiol. 2006, 141, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Manzi, P.; Gambelli, L.; Marconi, S.; Vivanti, V.; Pizzoferrato, L. Nutrients in edible mushrooms: An inter-species comparative study. Food Chem. 1999, 65, 477–482. [Google Scholar] [CrossRef]
- Zhang, Y.; Venkitasamy, C.; Pan, Z.; Liu, W.; Zhao, L. Novel Umami Ingredients: Umami Peptides and Their Taste. J. Food Sci. 2017, 82, 16–23. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yoshikawa, T.; Ikeda, S.; Ninomiya, T. Measurement of the relative taste intensity of some L-α-amino acids 5′-nucleotides. J. Food Sci. 1971, 36, 846–849. [Google Scholar] [CrossRef]
- Calonje, M.; García Mendoza, C.; Novaes-Ledieu, M. New contributions to the wall polysaccharide structure of vegetative mycelium and fruit body cell walls of Agaricus bisporus. Microbiologia 1996, 12, 599–606. [Google Scholar]
- Liu, Y.; Zheng, D.; Wang, D.; Su, L.; Wang, Q.; Li, Y. Immunomodulatory Activities of Polysaccharides from White Button Mushroom, Agaricus bisporus (Agaricomycetes), Fruiting Bodies and Cultured Mycelia in Healthy and Immunosuppressed Mice. Int. J. Med. Mushrooms 2019, 21, 13–27. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.-Z.; Zhang, L.-J.; Song, C.-Y.; Shang, X.-D.; Gu, Y.-F. Physico-chemical properties and immunocompetence in vitro of polysaccharides of Lentinula edodes fruiting bodies at different growth stages. Mycosystema 2020, 39, 1559–1567. [Google Scholar] [CrossRef]
- Sheng, K.; Wang, C.; Chen, B.; Kang, M.; Wang, M.; Liu, K.; Wang, M. Recent advances in polysaccharides from Lentinus edodes (Berk.): Isolation, structures and bioactivities. Food Chem. 2021, 358, 129883. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.S.P.; Behera, B.; Mishra, D.; Maiti, T.K. Immune augmentation and Dalton’s Lymphoma tumor inhibition by glucans/glycans isolated from the mycelia and fruit body of Pleurotus ostreatus. Int. Immunopharmacol. 2015, 25, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Sun, H.; Wu, L.; Kong, X.; Song, Q.; Zhu, Z. Structural characterization and inhibition on α-glucosidase of the polysaccharides from fruiting bodies and mycelia of Pleurotus eryngii. Int. J. Biol. Macromol. 2020, 156, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.V.C.; Fernandes, Â.; Oliveira, M.; Calhelha, R.C.; Barros, L.; Martins, A.; Ferreira, I. Development of nutraceutical formulations based on the mycelium of Pleurotus ostreatus and Agaricus bisporus. Food Funct. 2017, 8, 2155–2164. [Google Scholar] [CrossRef]
- Ghahremani-Majd, H.; Dashti, F. Chemical composition and antioxidant properties of cultivated button mushrooms (Agaricus bisporus). Hortic. Environ. Biotechnol. 2015, 56, 376–382. [Google Scholar] [CrossRef]
- Kała, K.; Kryczyk-Poprawa, A.; Rzewińska, A.; Muszyńska, B. Fruiting bodies of selected edible mushrooms as a potential source of lovastatin. Eur. Food Res. Technol. 2020, 246, 713–722. [Google Scholar] [CrossRef]
- Kabbaj, W.; Breheret, S.; Guimberteau, J.; Talou, T.; Olivier, J.M.; Bensoussan, M.; Sobal, M.; Roussos, S. Comparison of volatile compound production in fruit body and in mycelium of Pleurotus ostreatus identified by submerged and solid-state cultures. Appl. Biochem. Biotechnol. 2002, 102–103, 463–469. [Google Scholar] [CrossRef]
- Papaspyridi, L.; Sinanoglou, V.; Strati, I.; Katapodis, P.; Christakopoulos, P. Fatty acid profile of Pleurotus ostreatus and Ganoderma australe grown naturally and in a batch bioreactor. Acta Aliment. 2013, 42, 328–337. [Google Scholar] [CrossRef]
- Tan, Y.H.; Moore, D. High concentrations of mannitol in the shiitake mushroom Lentinula edodes. Microbios 1994, 79, 31–35. [Google Scholar] [PubMed]
- Grembecka, M. Sugar alcohols—their role in the modern world of sweeteners: A review. Eur. Food Res. Technol. 2015, 241, 377–383. [Google Scholar] [CrossRef]
- Kokkoris, V.; Massas, I.; Polemis, E.; Koutrotsios, G.; Zervakis, G.I. Accumulation of heavy metals by wild edible mushrooms with respect to soil substrates in the Athens metropolitan area (Greece). Sci. Total Environ. 2019, 685, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, B.; Krakowska, A.; Sułkowska-Ziaja, K.; Opoka, W.; Reczyński, W.; Baś, B. In vitro cultures and fruiting bodies of culinary-medicinal Agaricus bisporus (white button mushroom) as a source of selected biologically-active elements. J. Food Sci. Technol. 2015, 52, 7337–7344. [Google Scholar] [CrossRef]
- Zięba, P.; Kała, K.; Włodarczyk, A.; Szewczyk, A.; Kunicki, E.; Sękara, A.; Muszyńska, B. Selenium and Zinc Biofortification of Pleurotus eryngii Mycelium and Fruiting Bodies as a Tool for Controlling Their Biological Activity. Molecules 2020, 25, 889. [Google Scholar] [CrossRef]
- Muszyńska, B.; Kała, K.; Włodarczyk, A.; Krakowska, A.; Ostachowicz, B.; Gdula-Argasińska, J.; Suchocki, P. Lentinula edodes as a Source of Bioelements Released into Artificial Digestive Juices and Potential Anti-inflammatory Material. Biol. Trace Elem. Res. 2020, 194, 603–613. [Google Scholar] [CrossRef]
- Falandysz, J.; Fernandes, A.R.; Meloni, D. An overview of the lithium content and lithiation of the cultivable macrofungal species, Agaricus bisporus and Pleurotus spp. Trends Food Sci. Technol. 2022, 119, 338–347. [Google Scholar] [CrossRef]
- Rodríguez Pérez, S.; García Oduardo, N.; Bermúdez Savón, R.C.; Fernández Boizán, M.; Augur, C. Decolourisation of mushroom farm wastewater by Pleurotus ostreatus. Biodegradation 2008, 19, 519–526. [Google Scholar] [CrossRef]
- Wang, J.T.; Wang, Q.; Han, J.R. Yield, polysaccharides content and antioxidant properties of the mushroom Agaricus subrufescens produced on different substrates based on selected agricultural wastes. Sci. Hortic. 2013, 157, 84–89. [Google Scholar] [CrossRef]
- Xu, S.; Wang, F.; Fu, Y.; Li, D.; Sun, X.; Li, C.; Song, B.; Li, Y. Effects of mixed agro-residues (corn crop waste) on lignin-degrading enzyme activities, growth, and quality of Lentinula edodes. RSC Adv. 2020, 10, 9798–9807. [Google Scholar] [CrossRef]
- Vlasenko, E.; Kuznetsova, O. The Influence of Complex Additives on the Synthesis of Aroma Substances by Gray Oyster Culinary-Medicinal Mushroom, Pleurotus ostreatus (Agaricomycetes) during the Substrate Cultivation. Int. J. Med. Mushrooms 2020, 22, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, W.; Yang, Y.; Zhang, J.; Feng, J.; Yu, H.; Zhou, S.; Li, X.; Liu, Y. Effects of culture substrates on taste component content and taste quality of Lentinula edodes. Int. J. Food Sci. Technol. 2017, 52, 981–991. [Google Scholar] [CrossRef]
- Pinela, J.; Omarini, A.B.; Stojković, D.; Barros, L.; Postemsky, P.D.; Calhelha, R.C.; Breccia, J.; Fernández-Lahore, M.; Soković, M.; Ferreira, I. Biotransformation of rice and sunflower side-streams by dikaryotic and monokaryotic strains of Pleurotus sapidus: Impact on phenolic profiles and bioactive properties. Food Res. Int. 2020, 132, 109094. [Google Scholar] [CrossRef] [PubMed]
- Krahe, N.-K. Intraspezifische Variabilität Alkenspaltender Enzymaktivitäten in Monokaryotischen und Dikaryotischen Stämmen von Pleurotus sapidus. Ph.D. Thesis, Gottfried Wilhelm Leibniz Universität Hannover, Hannover, Germany, 2021. [Google Scholar]
- Mshandete, A.M.; Mgonja, J.R. Submerged liquid fermentation of some Tanzanian basidiomycetes for the production of mycelial biomass, exopolysaccharides and mycelium protein using wastes peels media. ARPN J. Agric. Biol. Sci. 2009, 4, 1–13. [Google Scholar]
- Papadaki, A.; Kachrimanidou, V.; Papanikolaou, S.; Philippoussis, A.; Diamantopoulou, P. Upgrading Grape Pomace through Pleurotus spp. Cultivation for the Production of Enzymes and Fruiting Bodies. Microorganisms 2019, 7, 207. [Google Scholar] [CrossRef]
- Ding, Z.; Chen, Y.; Xu, Z.; Peng, L.; Xu, G.; Gu, Z.; Zhang, L.; Shi, G.; Zhang, K. Production and characterization of laccase from Pleurotus ferulae in submerged fermentation. Ann. Microbiol. 2014, 64, 121–129. [Google Scholar] [CrossRef]
- Grosse, M.; Wu, S.; Krings, U.; Berger, R.G. Formation of Decatrienones with a Pineapple-like Aroma from 1-(13)C-Acetate by Cell Cultures of the Birch Polypore, Fomitopsis betulina. J. Agric. Food Chem. 2020, 68, 1678–1683. [Google Scholar] [CrossRef]
- Ramachandran, R.; Jebapriya, R.; Gnanadoss, J.J. Production of cellulase and laccase using Pleurotus sp. under submerged and solid-state fermentation. Int. J. Curr. Sci. 2013, 6, 7–13. [Google Scholar]
- Rebhun, M.; Hadar, Y. Use of Agro-Industrial Waste for Production of Laccase and Manganese Peroxidase from White-Rot Basidiomycetes. Int. J. Med. Mushrooms 2005, 7, 459–460. [Google Scholar] [CrossRef]
- Win, T.T.; Ohga, S. Study on the Cultivation of Agaricus blazei (Almond Mushroom) Grwon on Compost Mixed with Selected Agro-Residues. Adv. Microbiol. 2018, 8, 778–789. [Google Scholar] [CrossRef][Green Version]
- Wolters, N.; Schabronath, C.; Schembecker, G.; Merz, J. Efficient conversion of pretreated brewer’s spent grain and wheat bran by submerged cultivation of Hericium erinaceus. Bioresour. Technol. 2016, 222, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Krakowska, A.; Zięba, P.; Włodarczyk, A.; Kała, K.; Sułkowska-Ziaja, K.; Bernaś, E.; Sękara, A.; Ostachowicz, B.; Muszyńska, B. Selected edible medicinal mushrooms from Pleurotus genus as an answer for human civilization diseases. Food Chem. 2020, 327, 127084. [Google Scholar] [CrossRef] [PubMed]
- Malarczyk, E.; Zinko, E.; Nowak, G.; Ziaja, J.; Kochmanska-Rdest, J.; Leonowicz, A. The relation between the concentration of phenolic activity of manganese peroxidase in cultures of two species of Pleurotus during fruit body formation. In Proceedings of the TAPPI Biological Science Symposium, San Francisco, CA, USA, 19–23 October 1997; pp. 391–393. [Google Scholar]
- Siwulski, M.; Rzymski, P.; Budka, A.; Kalač, P.; Budzyńska, S.; Dawidowicz, L.; Hajduk, E.; Kozak, L.; Budzulak, J.; Sobieralski, K. The effect of different substrates on the growth of six cultivated mushroom species and composition of macro and trace elements in their fruiting bodies. Eur. Food Res. Technol. 2019, 245, 419–431. [Google Scholar] [CrossRef]
- Grosse, M.; Strauss, E.; Krings, U.; Berger, R.G. Response of the sesquiterpene synthesis in submerged cultures of the Basidiomycete Tyromyces floriformis to the medium composition. Mycologia 2019, 111, 885–894. [Google Scholar] [CrossRef]
- Krahe, N.-K.; Berger, R.G.; Witt, M.; Zorn, H.; Omarini, A.B.; Ersoy, F. Monokaryotic Pleurotus sapidus Strains with Intraspecific Variability of an Alkene Cleaving DyP-Type Peroxidase Activity as a Result of Gene Mutation and Differential Gene Expression. Int. J. Mol. Sci. 2021, 22, 1363. [Google Scholar] [CrossRef]
- Sarris, D.; Philippoussis, A.; Mallouchos, A.; Diamantopoulou, P. Valorization of low-cost, carbon-rich substrates by edible ascomycetes and basidiomycetes grown on liquid cultures. FEMS Microbiol. Lett. 2020, 367, fnaa168. [Google Scholar] [CrossRef]
- Leong, C.C.; Ho, W.Y.; Yeap, S.K.; Krishnen, G.; Chong, Z.X.; Ho, J.S.; Lim, P.T.; Ten, S.T. Assessment of phylogenetic, growth, and antioxidant capacity of Pleurotus spp. in Malaysia. J. Food Process. Preserv. 2021, 45, e15554. [Google Scholar] [CrossRef]
- Calabretti, A.; Mang, S.M.; Becce, A.; Castronuovo, D.; Cardone, L.; Candido, V.; Camele, I. Comparison of bioactive substances content between commercial and wild-type isolates of Pleurotus eryngii. Sustainability 2021, 13, 3777. [Google Scholar] [CrossRef]
- Zheng, W.-M.; Zhang, L.-J.; Li, Q.-Z.; Song, C.-Y.; Shang, X.-D.; Tan, Q. Genetic law of important agronomic traits of Lentinula edodes in F2 and F3 generations. Mycosystema 2021, 40, 3129–3142. [Google Scholar] [CrossRef]
- Sousa, M.; Costa, L.; Malantrucco, T.; Pereira, T.; Bastos, S.; Dias, E. Nutritional and enzymatic potential of the Lentinula edodes strains. Int. J. Curr. Res. 2016, 8, 34557–34561. [Google Scholar]
- Ryu, S.-R.; Bak, W.-C.; Koo, C.-D.; Lee, B.-H. Studies on breeding and cultivation characteristics of Lentinula edodes strains for sawdust cultivation. Korean J. Mycol. 2009, 37, 65–72. [Google Scholar] [CrossRef][Green Version]
- Omarini, A.B.; Plagemann, I.; Schimanski, S.; Krings, U.; Berger, R.G. Crosses between monokaryons of Pleurotus sapidus or Pleurotus florida show an improved biotransformation of (+)-valencene to (+)-nootkatone. Bioresour. Technol. 2014, 171, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Linke, D.; Omarini, A.B.; Takenberg, M.; Kelle, S.; Berger, R.G. Long-Term Monokaryotic Cultures of Pleurotus ostreatus var. florida Produce High and Stable Laccase Activity Capable to Degrade ss-Carotene. Appl. Biochem. Biotechnol. 2019, 187, 894–912. [Google Scholar] [CrossRef] [PubMed]
- Vreeburg, S.; Nygren, K.; Aanen, D.K. Unholy marriages and eternal triangles: How competition in the mushroom life cycle can lead to genomic conflict. Philos. Trans. R. Soc. B 2016, 371, 20150533. [Google Scholar] [CrossRef]
- Dobbs, E.; Deakin, G.; Bennett, J.; Fleming-Archibald, C.; Jones, I.; Grogan, H.; Burton, K. Viral interactions and pathogenesis during multiple viral infections in Agaricus bisporus. Mbio 2021, 12, e03470-20. [Google Scholar] [CrossRef]
- Kumar, B.; Kumari, C.; Kumar, M. Effect of bio-fertilizers on mycelial growth and physical properties of white button mushroom Agaricus bisporus (Lange) Imbach]. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 2216–2222. [Google Scholar] [CrossRef]
- Pandin, C.; Le Coq, D.; Deschamps, J.; Védie, R.; Rousseau, T.; Aymerich, S.; Briandet, R. Complete genome sequence of Bacillus velezensis QST713: A biocontrol agent that protects Agaricus bisporus crops against the green mould disease. J. Biotechnol. 2018, 278, 10–19. [Google Scholar] [CrossRef]
- Kertesz, M.A.; Thai, M. Compost bacteria and fungi that influence growth and development of Agaricus bisporus and other commercial mushrooms. Appl. Microbiol. Biotechnol. 2018, 102, 1639–1650. [Google Scholar] [CrossRef]
- Ghasemi, S.; Harighi, B.; Azizi, A.; Mojarrab, M. Reduction of brown blotch disease and tyrosinase activity in Agaricus bisporus infected by Pseudomonas tolaasii upon treatment with endofungal bacteria. Physiol. Mol. Plant Pathol. 2020, 110, 101474. [Google Scholar] [CrossRef]
- Lakkireddy, K.; Khonsuntia, W.; Kües, U. Mycoparasite Hypomyces odoratus infests Agaricus xanthodermus fruiting bodies in nature. AMB Express 2020, 10, 141. [Google Scholar] [CrossRef]
- Song, H.-Y.; Kim, N.; Kim, D.-H.; Kim, J.-M. The PoV mycovirus affects extracellular enzyme expression and fruiting body yield in the oyster mushroom, Pleurotus ostreatus. Sci. Rep. 2020, 10, 1094. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Naraian, R. Enhanced growth and yield of oyster mushroom by growth-promoting bacteria Glutamicibacter arilaitensis MRC119. J. Basic Microbiol. 2021, 61, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Bánfi, R.; Pohner, Z.; Szabó, A.; Herczeg, G.; Kovács, G.M.; Nagy, A.; Márialigeti, K.; Vajna, B. Succession and potential role of bacterial communities during Pleurotus ostreatus production. FEMS Microbiol. Ecol. 2021, 97, fiab125. [Google Scholar] [CrossRef] [PubMed]
- Liebenow, H.; Hahn, A.; Michalak, H. Risiko Pilze—Einschätzung und Hinweise; BfR-Pressestelle: Berlin, Germany, 2005. [Google Scholar]
- Speroni, J.J.; Beelman, R.B.; Schisler, L.C. Factors Influencing the Agaritine Content in Cultivated Mushrooms, Agaricus bisporus. J. Food Prot. 1983, 46, 506–509. [Google Scholar] [CrossRef]
- Shephard, S.E.; Schlatter, C. Covalent binding of agaritine to DNA in vivo. Food Chem. Toxicol. 1998, 36, 971–974. [Google Scholar] [CrossRef]
- Sepcić, K.; Berne, S.; Potrich, C.; Turk, T.; Macek, P.; Menestrina, G. Interaction of ostreolysin, a cytolytic protein from the edible mushroom Pleurotus ostreatus, with lipid membranes and modulation by lysophospholipids. Eur. J. Biochem. 2003, 270, 1199–1210. [Google Scholar] [CrossRef]
- Stephany, M.P.; Chung, S.; Handler, M.Z.; Handler, N.S.; Handler, G.A.; Schwartz, R.A. Shiitake Mushroom Dermatitis: A Review. Am. J. Clin. Dermatol. 2016, 17, 485–489. [Google Scholar] [CrossRef]
- Humpenöder, F.; Bodirsky, B.L.; Weindl, I.; Lotze-Campen, H.; Linder, T.; Popp, A. Projected environmental benefits of replacing beef with microbial protein. Nature 2022, 605, 90–96. [Google Scholar] [CrossRef]
- Krings, U.; Berger, R.G. Dynamics of sterols and fatty acids during UV-B treatment of oyster mushroom. Food Chem. 2014, 149, 10–14. [Google Scholar] [CrossRef]
- Singh, U.; Gautam, A.; Singha, T.K.; Tiwari, A.; Tiwari, P.; Sahai, V.; Sharma, S. Mass production of Pleurotus eryngii mycelia under submerged culture conditions with improved minerals and vitamin D2. LWT 2020, 131, 109665. [Google Scholar] [CrossRef]
- Bundesamt f. Strahlenschutz. Wildpilze—Bedenkenloser Genuss? Bundesamt für Strahlenschutz, Presse-und Öffentlichkeitsarbeit: Salzgitter, Germany, 2019. [Google Scholar]
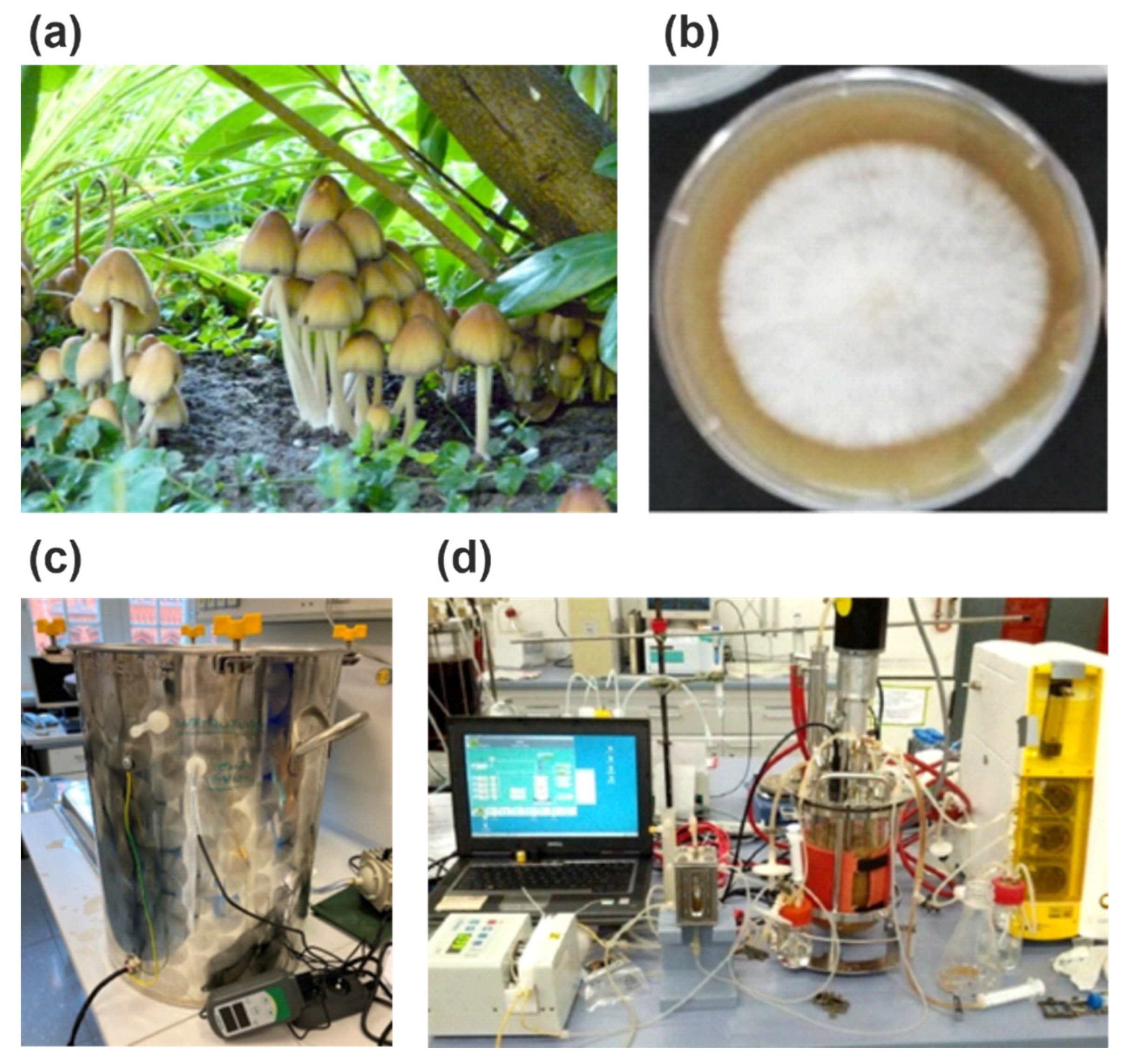
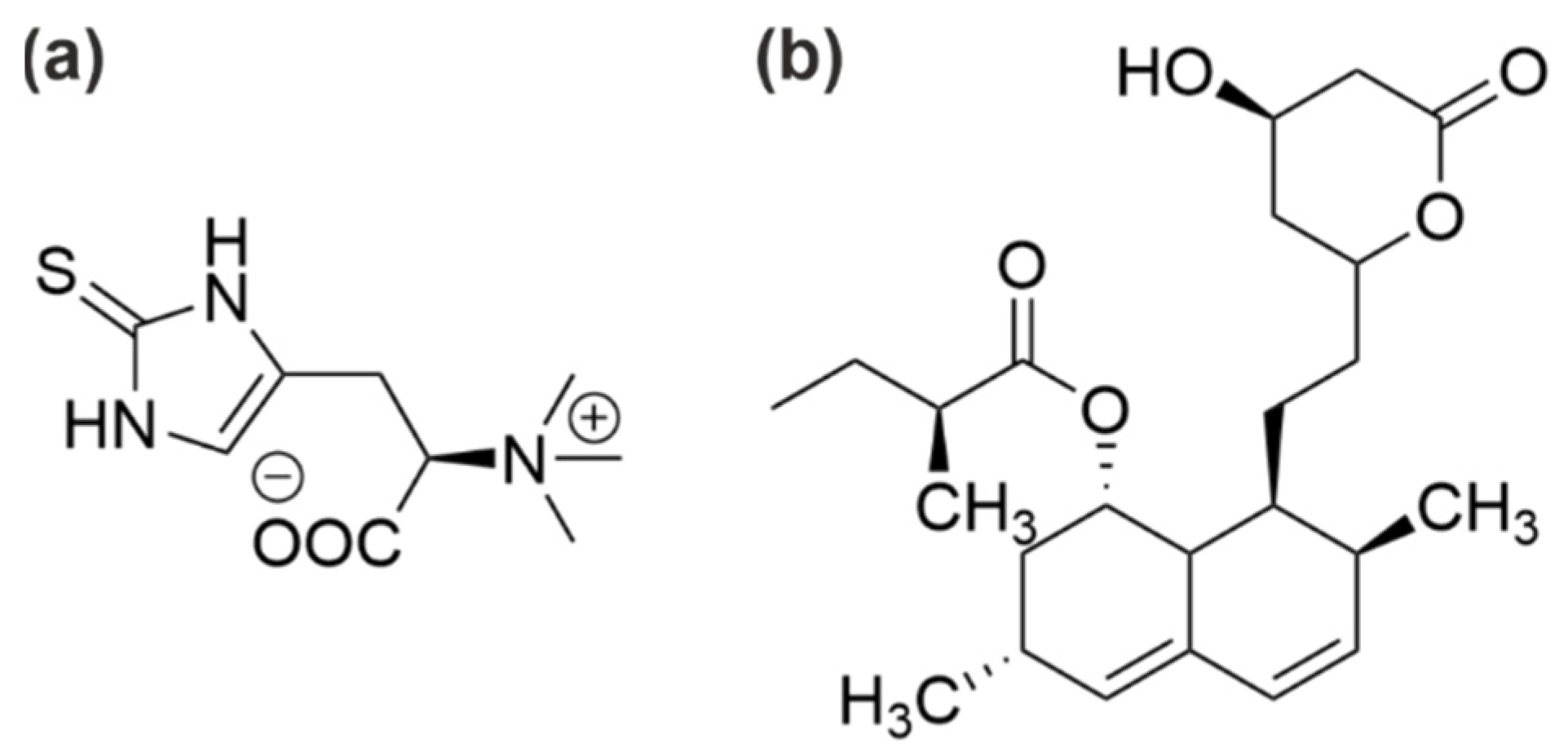
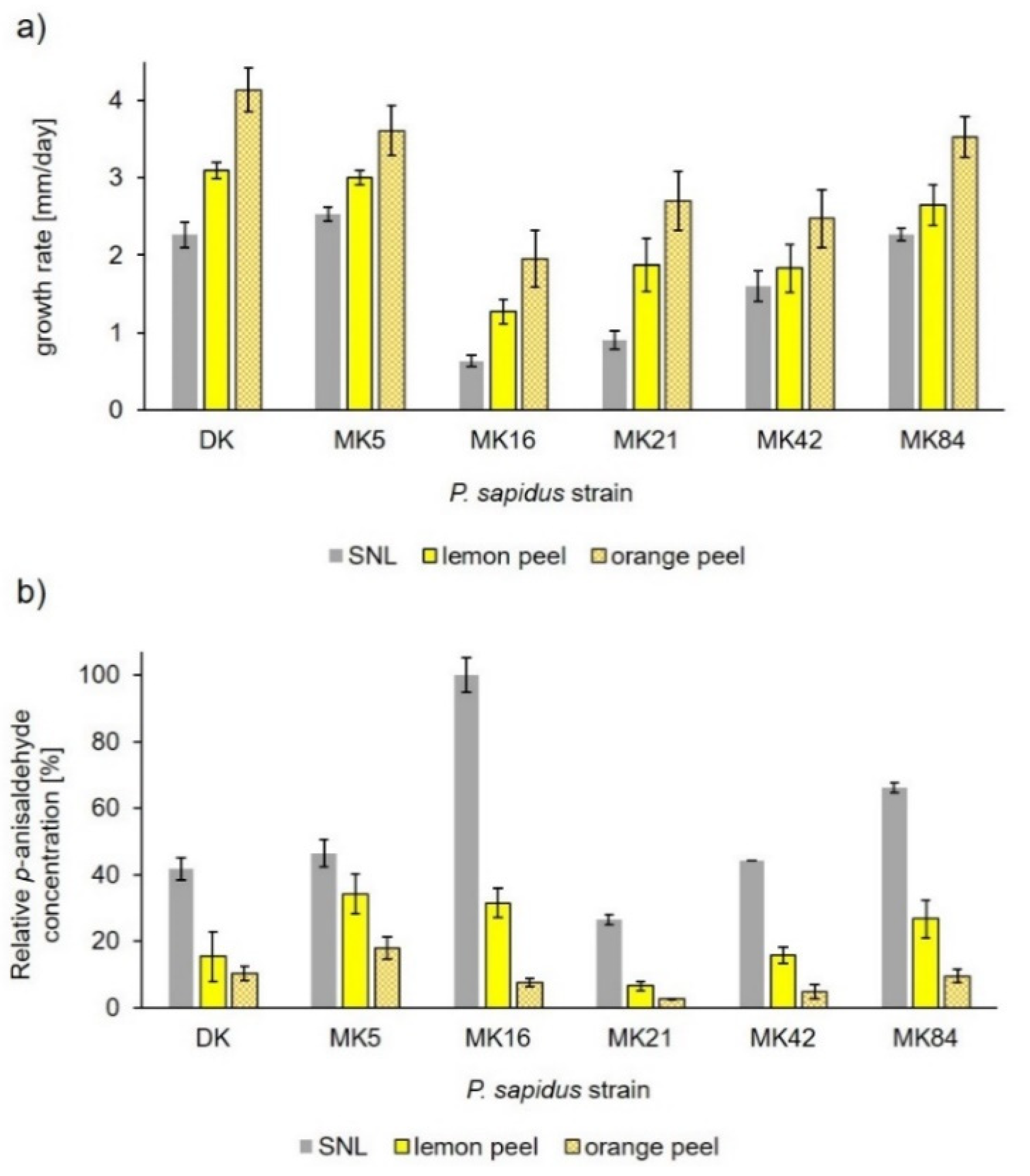
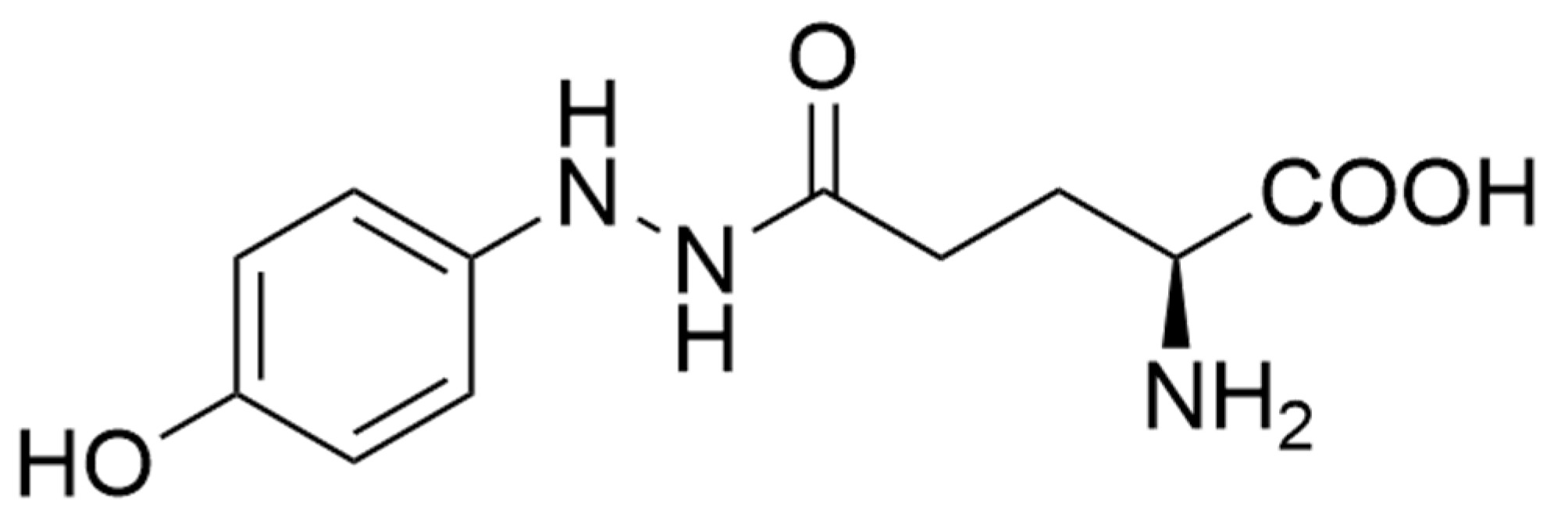
| Side Stream | Reference(s) |
|---|---|
| Remainders from the production of fruiting bodies | [54] |
| Straw from cereals and leguminous plants | [55,56] |
| Sugar beet fibre | (own experiments, data not shown) |
| Bran from wheat, corn, rice | [57,58,59] |
| Peel from potato, apple, grape, citrus, banana | [23,60,61,62,63] |
| Cabbage cuttings | [64] |
| Press cake from oil production | [65] |
| Empty sunflower blossoms, peanut shells | [57,59,66] |
| Corn cobs | [55,56,67] |
| Draff and pomace from beer and winemaking | [62,68] |
| Sugar beet bagasse | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berger, R.G.; Bordewick, S.; Krahe, N.-K.; Ersoy, F. Mycelium vs. Fruiting Bodies of Edible Fungi—A Comparison of Metabolites. Microorganisms 2022, 10, 1379. https://doi.org/10.3390/microorganisms10071379
Berger RG, Bordewick S, Krahe N-K, Ersoy F. Mycelium vs. Fruiting Bodies of Edible Fungi—A Comparison of Metabolites. Microorganisms. 2022; 10(7):1379. https://doi.org/10.3390/microorganisms10071379
Chicago/Turabian StyleBerger, Ralf G., Sven Bordewick, Nina-Katharina Krahe, and Franziska Ersoy. 2022. "Mycelium vs. Fruiting Bodies of Edible Fungi—A Comparison of Metabolites" Microorganisms 10, no. 7: 1379. https://doi.org/10.3390/microorganisms10071379
APA StyleBerger, R. G., Bordewick, S., Krahe, N.-K., & Ersoy, F. (2022). Mycelium vs. Fruiting Bodies of Edible Fungi—A Comparison of Metabolites. Microorganisms, 10(7), 1379. https://doi.org/10.3390/microorganisms10071379






