Evaluation of Conditions to Improve Biomass Production by Submerged Culture of Ganoderma sp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source and Maintenance of the Strain of Ganoderma sp.
2.2. Optimization of Culture Medium
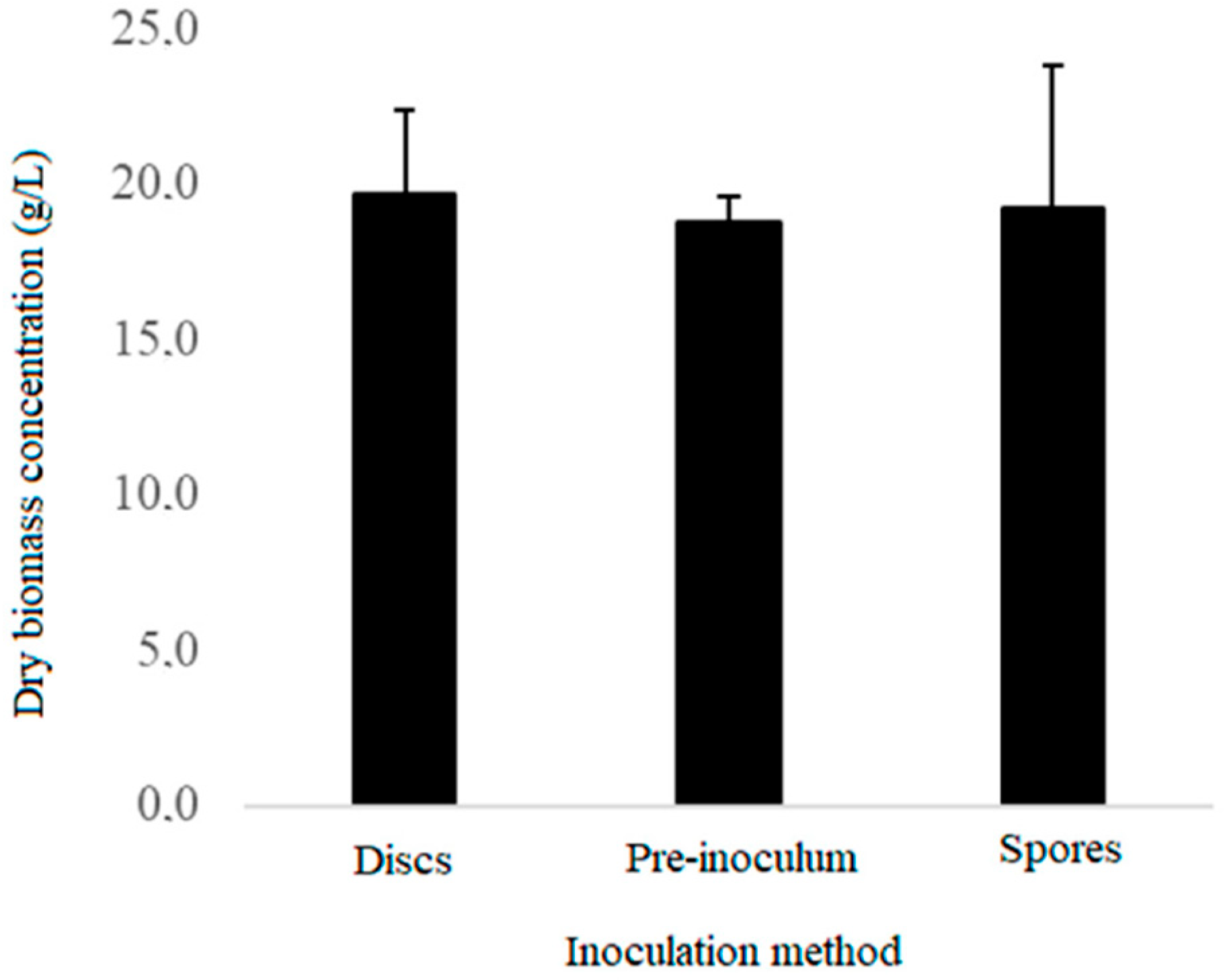
2.3. Selection of the Inoculation Method
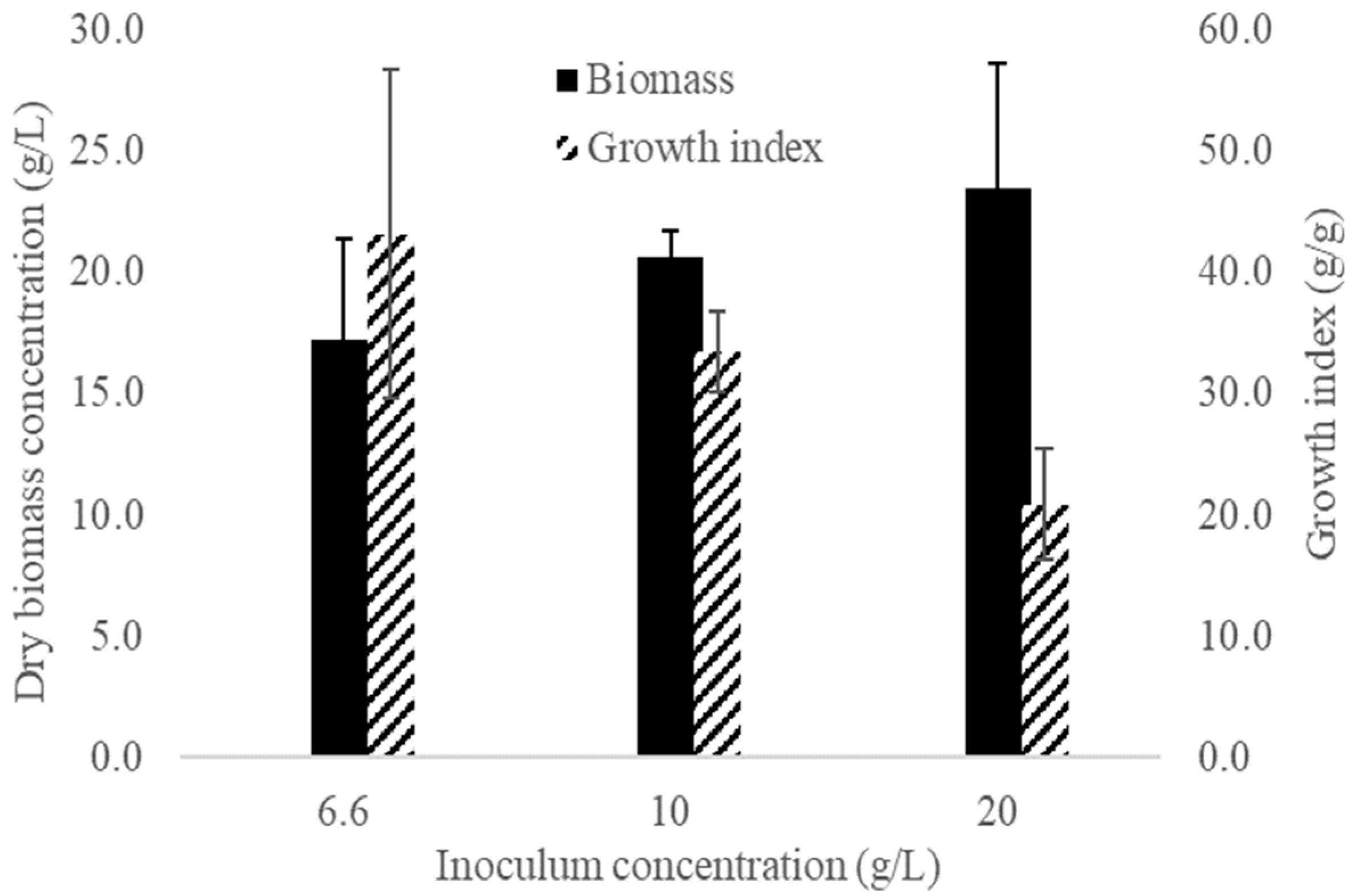
2.4. Determination of the Optimum Inoculum Concentration
- GI: growth index (g/g).
- Xf: final dry biomass weight (g).
- X0: initial dry biomass weight (g).
2.5. Growth Kinetics
2.6. Culture in the Bioreactor
2.7. Determination of Airflow Rate
2.8. Statistical Analyses
3. Results
3.1. Inoculation Method
3.2. Inoculum Concentration
3.3. Growth Kinetics
3.4. Growth in the Bioreactor Airflow Rate Tests
4. Discussion
4.1. Growth Kinetics
4.2. Growth in the Bioreactor
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Legarda, L.X.; Echevarria, A.C.; Sánchez, S.F. Producción de polisacáridos a partir de Ganoderma sp., aislado en la región andina. Rev. Colomb. Biotecnol. 2015, 17, 44–54. [Google Scholar] [CrossRef]
- López-Peña, D.; Samaniego-Rubiano, C.; Morales-Estrada, I.; Gutiérrez, A.; Gaitán-Hernández, R.; Esqueda, M. Características morfológicas de Ganoderma subincrustatum silvestre y cultivada de Sonora, México. Sci. Fungorum 2019, 49, e1213. [Google Scholar] [CrossRef]
- Ruiz-Boyer, A. The family Ganodermataceae (Aphyllophorales) in Costa Rica. La familia Ganodermataceae (Aphyllophorales) en Costa Rica. Tropical Diversity Origins, Maintenance, and Conservation. In Proceedings of the ATB & OTS Symposium and Annual Meeting Abstracts, San José, Costa Rica, 15–20 June 1997. [Google Scholar]
- Ruiz-Boyer, A.; Rodríguez-González, A. Lista preliminar de hongos (Ascomycota y Basidiomycota) y mixomicetos (Myxomycota) de la Isla del Coco, Puntarenas, Costa Rica. Rev. Biol. Trop. 2020, 68, S33–S56. [Google Scholar] [CrossRef]
- Cid-Martínez, M.A.; Gallardo-Velázquez, K.; Rosique-Gil, J.E.; Domínguez-Rodríguez, V.I.; Focil-Monterrubio, R.L. Cuantificación de las esporas de ganoderma del aire exterior en la ciudad de Villahermosa, Tabasco, México. Rev. Int. Contam. Ambient. 2019, 35, 501–508. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; He, R.; Sun, P.; Zhang, F.; Linhardt, R.J.; Zhang, A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int. J. Biol. Macromol. 2020, 150, 765–774. [Google Scholar] [CrossRef]
- Soccol, C.R.; Bissoqui, L.Y.; Rodrigues, C.; Rubel, R.; Sella, S.R.; Leifa, F.; Soccol, V.T. Pharmacological properties of biocompounds from spores of the lingzhi or reishi medicinal mushroom Ganoderma lucidum (Agaricomycetes): A review. Int. J. Med. Mushrooms 2016, 18, 757–767. [Google Scholar] [CrossRef]
- Soylu, E.M.; Soylu, S.; Kurt, S. Antimicrobial Activities of the Essential Oils of Various Plants against Tomato Late Blight Disease Agent Phytophthora infestans. Mycopathologia 2006, 161, 119–128. [Google Scholar] [CrossRef]
- Yang, F.-C.; Ke, Y.-F.; Kuo, S.-S. Effect of fatty acids on the mycelial growth and polysaccharide formation by Ganoderma lucidum in shake flask cultures. Enzym. Microb. Technol. 2000, 27, 295–301. [Google Scholar] [CrossRef]
- Xu, Y.-N.; Zhong, J.-J. Impacts of calcium signal transduction on the fermentation production of antitumor ganoderic acids by medicinal mushroom Ganoderma lucidum. Biotechnol. Adv. 2012, 30, 1301–1308. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Zhu, L.-W. Improvement of Ganoderic Acid and Ganoderma Polysaccharide Biosynthesis by Ganoderma lucidum Fermentation Under the Inducement of Cu2+. Biotechnol. Prog. 2010, 26, 417–423. [Google Scholar]
- Schnellbaecher, A.; Binder, D.; Bellmaine, S.; Zimmer, A. Vitamins in cell culture medium: Stability and stabilization strategies. Biotechnol. Bioeng. 2019, 116, 1537–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zárate-Chaves, C.A.; Romero-Rodríguez, M.C.; Niño-Arias, F.C.; Robles-Camargo, J.; Linares-Linares, M.; Rodríguez-Bocanegra, M.X.; Gutiérrez-Rojas, I. Optimizing a culture medium for biomass and phenolic compounds production using Ganoderma lucidum. Braz. J. Microbiol. 2013, 44, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, R.; Mitchell, D.A.; Sassaki, G.; Lopes de Almeida Amazonas, M.A.; Berovi, M. Current Techniques for the Cultivation of Ganoderma lucidum for the Production of Biomass, Ganoderic Acid and Polysaccharides. Food Technol. Biotechnol. 2003, 41, 371–382. Available online: https://www.ftb.com.hr/images/pdfarticles/2003/October-December/41-371.pdf (accessed on 28 January 2022).
- Fang, Q.-H.; Tang, Y.-J.; Zhong, J.-J. Significance of inoculation density control in production of polysaccharide and ganoderic acid by submerged culture of Ganoderma lucidum. Process. Biochem. 2002, 37, 1375–1379. [Google Scholar] [CrossRef]
- Chang, M.-Y.; Tsai, G.-J.; Houng, J.-Y. Optimization of the medium composition for the submerged culture of Ganoderma lucidum by Taguchi array design and steepest ascent method. Enzym. Microb. Technol. 2006, 38, 407–414. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Zhong, J.-J. Role of oxygen supply in submerged fermentation of Ganoderma lucidum for production of Ganoderma polysaccharide and ganoderic acid. Enzym. Microb. Technol. 2003, 32, 478–484. [Google Scholar] [CrossRef]
- Jo, W.-S.; Cho, Y.-J.; Cho, D.-H.; Park, S.-D.; Yoo, Y.-B.; Seok, S.-J. Culture Conditions for the Mycelial Growth ofGanoderma applanatum. Mycobiology 2009, 37, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Zhang, J.-Z.; Feng, N.; Yan, M.-Q.; Yang, Y.; Jia, W.; Lin, C.-C. A novel Ganoderma lucidum G0119 fermentation strategy for enhanced triterpenes production by statistical process optimization and addition of oleic acid. Eng. Life Sci. 2017, 17, 430–439. [Google Scholar] [CrossRef]
- Spiess, A.-N.; Neumeyer, N. An evaluation of R2 as an inadequate measure for nonlinear models in pharmacological and biochemical research: A Monte Carlo approach. BMC Pharmacol. 2010, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.M.; Kim, S.W.; Hwang, H.J.; Park, M.K.; Mahmoud, Y.A.-G.; Choi, J.W.; Yun, J.W. Influence of Agitation Intensity and Aeration Rate on Production of Antioxidative Exopolysaccharides from Submerged Mycelial Culture of Ganoderma resinaceum. J. Microbiol. Biotechnol. 2006, 16, 1240–1247. [Google Scholar]
- Salmon, D.N.; Fendrich, R.C.; Cruz, M.A.; Weingartner-Montibeller, V.; Vandenberghe, L.P.; Soccol, C.R.; Rigon-Spier, M. Bioprocess for phytase production by Ganoderma sp. MR-56 in different types of bioreactors through submerged cultivation. Biochem. Eng. J. 2016, 114, 288–297. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Zhong, J.-J. Fed-batch fermentation of Ganoderma lucidum for hyperproduction of polysaccharide and ganoderic acid. Enzym. Microb. Technol. 2002, 31, 20–28. [Google Scholar] [CrossRef]
- Lejeune, R.; Baron, G.V. Modeling the exponential growth of filamentous fungi during batch cultivation. Biotechnol. Bioeng. 1998, 60, 169–179. [Google Scholar] [CrossRef]
- Yorulmaz, A.; Erinc, H.; Tekin, A. Changes in Olive and Olive Oil Characteristics During Maturation. J. Am. Oil Chem. Soc. 2013, 90, 647–658. [Google Scholar] [CrossRef]
- Lv, G.-P.; Zhao, J.; Duan, J.-A.; Tang, Y.-P.; Li, S.-P. Comparison of sterols and fatty acids in two species of Ganoderma. Chem. Cent. J. 2012, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Fei, Y.; Li, N.; Zhang, D.-H.; Xu, J.-W. Increased production of ganoderic acids by overexpression of homologous farnesyl diphosphate synthase and kinetic modeling of ganoderic acid production in Ganoderma lucidum. Microb. Cell Factor. 2019, 18, 115. [Google Scholar] [CrossRef]
- Wei, Z.-H.; Duan, Y.-Y.; Qian, Y.-Q.; Guo, X.-F.; Li, Y.-J.; Jin, S.-H.; Zhou, Z.-X.; Shan, S.-Y.; Wang, C.-R.; Chen, X.-J.; et al. Screening of Ganoderma strains with high polysaccharides and ganoderic acid contents and optimization of the fermentation medium by statistical methods. Bioproc. Biosyst. Eng. 2014, 37, 1789–1797. [Google Scholar] [CrossRef]
- Torres-López, A.M.; Quintero-Díaz, J.C.; Atehortúa-Garcés, L. Efecto de nutrientes sobre la producción de biomasa del hongo medicinal Ganoderma lucidum. Rev. Colomb. De Biotecnol. 2011, 13, 103–109. [Google Scholar]
- Papagianni, M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol. Adv. 2004, 22, 189–259. [Google Scholar] [CrossRef]
- Guevara-Manzanares, V. Producción de Ácidos Ganodéricos y Beta-(1–3)-(1–6)-D-Glucanos en el Cultivo Líquido Sumergido de Ganoderma sp. de Interés Medicinal en la Industria Alimenticia de Costa Rica; Instituto Tecnológico de Costa Rica: Cartago, Costa Rica, 2015. [Google Scholar]



| Factors | Concentration (g/L) | Reference | ||
|---|---|---|---|---|
| Low (–) | High (+) | |||
| Olive oil | 0.00 * | 3.00 | [9] | |
| Salts | CaCl2 2H2O | 0.00 | 1.45 | [10] |
| CuSO4 5H2O | 0.00 | 0.25 | [11] | |
| Thiamine | 0.00 | 0.05 | [12] | |
| Medium | Variables | Dry Biomass Concentration (g/L) | Tukey’s Grouping | ||
|---|---|---|---|---|---|
| Olive Oil | Salts | Thiamine | |||
| A | + | + | + * | 19.87 ± 1.73 ** | a b |
| B | + | + | - | 19.05 ± 1.70 | a b c |
| C | + | - | + | 23.39 ± 2.44 | a |
| D | + | - | - | 14.24 ± 1.24 | c d |
| E | - | + | + | 12.23 ± 1.39 | d |
| F | - | + | - | 14.30 ± 0.98 | c d |
| G | - | - | + | 16.04 ± 0.94 | b c d |
| H | - | - | - | 12.56 ± 2.61 | d |
| Source | DF * | Adj SS ** | Adj MS *** | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 7 | 327.497 | 46.785 | 15.60 | 0.000 |
| Linear | 3 | 220.794 | 73.598 | 24.54 | 0.000 |
| Olive Oil | 1 | 171.949 | 171.949 | 57.33 | 0.000 |
| Salts | 1 | 0.224 | 0.224 | 0.07 | 0.788 |
| Thiamine | 1 | 48.621 | 48.621 | 16.21 | 0.001 |
| 2-Way Interactions | 3 | 103.818 | 34.606 | 11.54 | 0.000 |
| Olive Oil * Salts | 1 | 4.234 | 4.234 | 1.41 | 0.252 |
| Olive Oil * Thiamine | 1 | 27.478 | 27.478 | 9.16 | 0.008 |
| Salts * Thiamine | 1 | 72.107 | 72.107 | 24.04 | 0.000 |
| 3-Way Interactions | 1 | 2.884 | 2.884 | 0.96 | 0.341 |
| Olive Oil * Salts * Thiamine | 1 | 2.884 | 2.884 | 0.96 | 0.341 |
| Error | 16 | 47.992 | 3.000 | ||
| Total | 23 | 375.489 |
| Treatment Pairs | Level | Mean Dry Biomass Concentration (g/L) | Group | |||
|---|---|---|---|---|---|---|
| Olive Oil | Thiamine | |||||
| A-C | + | + | 21.63 ± 2.70 * | a | ||
| B-D | + | - | 16.64 ± 2.95 | b | ||
| E-G | - | + | 14.14 ± 2.34 | b | c | |
| F-H | - | - | 13.43 ± 2.01 | c | ||
| Means of Treatments | Concentration | Mean Dry Biomass Concentration (g/L) | Group | |||
|---|---|---|---|---|---|---|
| Salts | Thiamine | |||||
| C-G | - | + | 19.71 ± 4.35 * | a | ||
| B-F | + | - | 16.67 ± 2.88 | b | ||
| A-E | + | + | 16.05 ± 4.41 | b | c | |
| D-H | - | - | 13.40 ± 2.05 | c | ||
| Model | Specific Growth Rate (h−1) | Standard Error of the Regression (g/L) | AICc |
|---|---|---|---|
| Exponential | 0.0041 ± 0.0005 * | 2.42 | 42.54 |
| Logistic | 0.0139 ± 0.0026 | 1.76 | 28.54 |
| Gompertz | 0.0087 ± 0.0019 | 1.67 | 25.93 |
| Richards | 0.0087 ± 0.0108 | 1.71 | 28.56 |
| Culture Medium (g/L) | Operating Conditions | Bioreactor Configuration | Operation Mode | Dry Biomass Concentration (g/L) | Ref. |
|---|---|---|---|---|---|
| Glc 30, Pep 5, YE 5, KH2PO4 0.5, K2HPO4 0.5, MgSO4 7H2O 0.5, B1 0.05, OO 9.1 | 30 °C, pH 5.5, 0.5 g/L, 12 d | 2 L, 350 rpm, 0.25 vvm, MA-PI6-RT, PS | Batch | 22.6 (12 d) | This work |
| Glc 16, Pep 2.93, CF 20.93, SBP 6.44, KH2PO4 1.5, MgSO4 7H2O 1 | 30 °C, 2.0 g/L, 6 d | 35 L, 125 rpm, 0.6 vvm | Batch | 21.5 (5 d) | [18] |
| Glc 55, YE 14.3, KH2PO4 1, MgSO4 7H2O 0.26, Fe2(SO4)3 0.34, B1 0.05 | 30 °C, pH 5.5, 0.5 g/L, 12 d | 7 L, 300 rpm, 1.0 vvm, RT-RT, RS | Batch | 25.7 (12 d) | [19] |
| WB 200, YE 80 | 30 °C, pH 6.0, 14.7 g/L, 8 d | 4 L, 200 rpm, 1.0 vvm, RT | Batch | 28.2 (8 d) | [20] |
| Lac 35, Pep 5, YE 5, KH2PO4 1, MgSO4 7H2O 0.5, B1 0.05 | 30 °C, pH 5.5, pO2 20–35%, 0.6 g/L, 22 d | 2 L, 100–180 rpm, 0.25–0.5 vvm, RT-RT, RS | Fed batch | 21.9 (12 d) | [21] |
| Glc 35, Pep 5, YE 5, KH2PO4 1, MgSO4 7H2O 0.5 | 30 °C, pH 4.0, pO2 20%, 0.5 g/L, 10 d | 10 L, 300 rpm, 2.0 vvm (max) | Fed batch | 26.6 (10 d) | [22] |
| Lac 35, Pep 5, YE 5, KH2PO4 1, MgSO4 7H2O 0.5, B1 0.05 | 30 °C, pH 3.0–4.5, pO2 25–10%, 0.6 g/L, 18 d | 5.5 L, 50–400 rpm, 0.1–0.7 vvm, RT-RT-PI4, RS | Fed batch | 22.6 (12 d) | [23] |
| Glc 25, Suc 20, YE 14, KH2PO4 1, MgSO4 7H2O 0.26, B1 0.05 | 30 °C, pH 5.5, 0.5 g/L, 10 d | 7 L, 300 rpm, 1.0 vvm | Fed batch | 29.7 (9 d) | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosales-López, C.; Vargas-López, A.; Monge-Artavia, M.; Rojas-Chaves, M. Evaluation of Conditions to Improve Biomass Production by Submerged Culture of Ganoderma sp. Microorganisms 2022, 10, 1404. https://doi.org/10.3390/microorganisms10071404
Rosales-López C, Vargas-López A, Monge-Artavia M, Rojas-Chaves M. Evaluation of Conditions to Improve Biomass Production by Submerged Culture of Ganoderma sp. Microorganisms. 2022; 10(7):1404. https://doi.org/10.3390/microorganisms10071404
Chicago/Turabian StyleRosales-López, Catalina, Alejandro Vargas-López, Mariana Monge-Artavia, and Miguel Rojas-Chaves. 2022. "Evaluation of Conditions to Improve Biomass Production by Submerged Culture of Ganoderma sp." Microorganisms 10, no. 7: 1404. https://doi.org/10.3390/microorganisms10071404
APA StyleRosales-López, C., Vargas-López, A., Monge-Artavia, M., & Rojas-Chaves, M. (2022). Evaluation of Conditions to Improve Biomass Production by Submerged Culture of Ganoderma sp. Microorganisms, 10(7), 1404. https://doi.org/10.3390/microorganisms10071404







