Nasal Microbiota, Olfactory Health, Neurological Disorders and Aging—A Review
Abstract
:1. Introduction
2. Nasal Microbiota and Respiratory Tract Infections
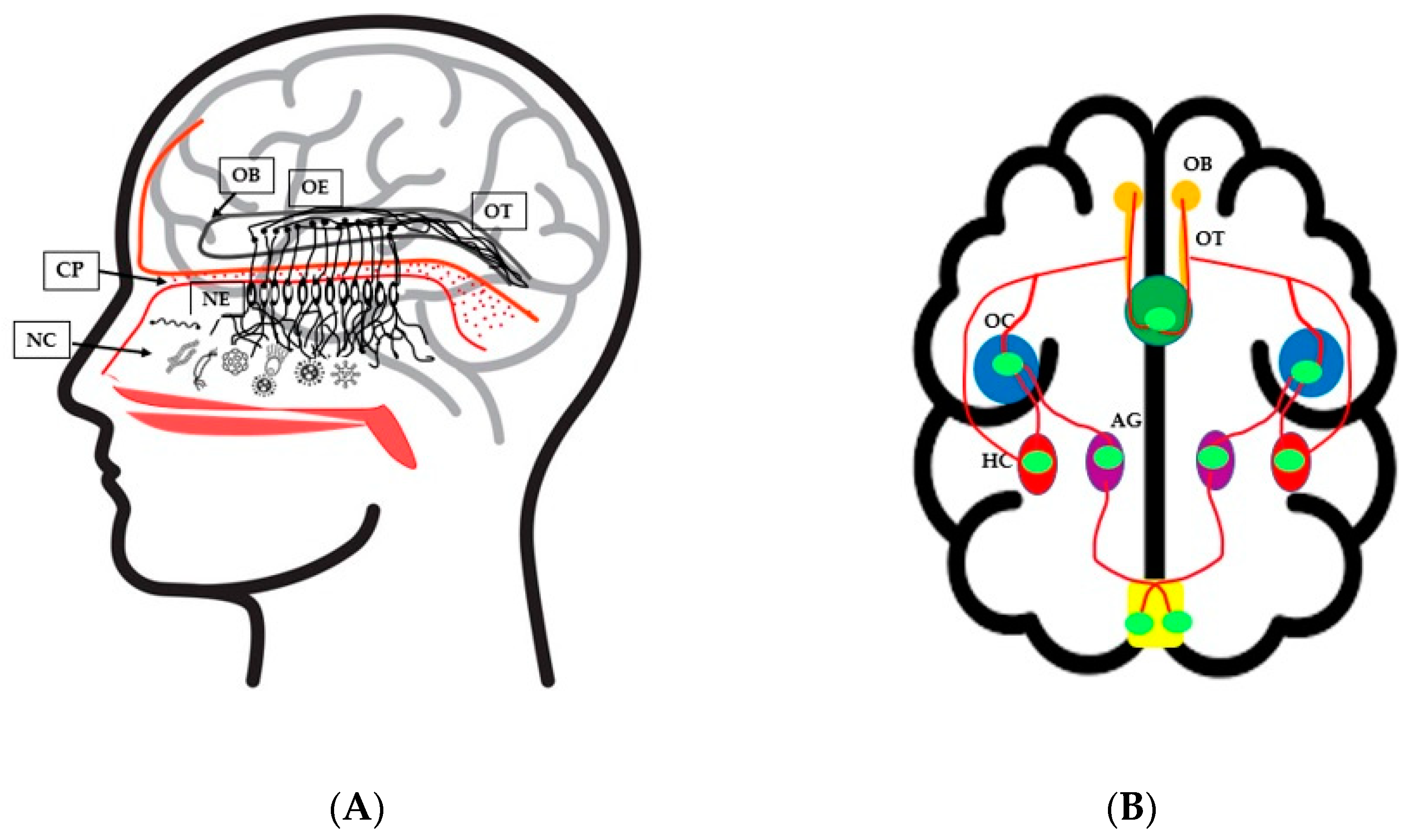
3. Nasal Microbiota and Olfactory Health
4. Olfactory Dysfunction and Neurological Disorders
5. Diet–Microbiota–Brain Interrelationship
6. Nasal Microbiota and COVID-19
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, M.J.; Schloter, M.; Berg, G.; Kostic, T.; Kinkel, L.L.; Eversole, K.; Macklin, J.A.; Schelkle, B.; Kazou, M.; Sarand, I.; et al. Development of microbiome biobanks-challenges and opportunities. Trends Microbiol. 2021, 29, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segata, N.; Haake, S.; Mannon, P.; Lemon, K.P.; Waldron, L.; Gevers, D.; Huttenhower, C.; Izard, J. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012, 13, R42. [Google Scholar] [CrossRef] [Green Version]
- Proctor, D.M.; Relman, D.A. The Landscape Ecology and Microbiota of the Human Nose, Mouth, and Throat. Cell Host Microbe 2017, 21, 421–432. [Google Scholar] [CrossRef] [Green Version]
- Ahern, P.P.; Maloy, K.J. Understanding immune-microbiota interactions in the intestine. Immunology 2020, 159, 4–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stapleton, A.E. The vaginal microbiota and urinary tract infection. Microbiol. Spectr. 2016, 4, 79–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota-host interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef]
- Hartstra, A.V.; Bouter, K.E.C.; Bäckhed, F.; Nieuwdorp, M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care 2015, 38, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Anwar, H.; Iftikhar, A.; Muzaffar, H.; Almatroudi, A.; Allemailem, K.S.; Navaid, S.; Saleem, S.; Khurshid, M. Biodiversity of gut microbiota: Impact of various host and environmental factors. Biomed Res. Int. 2021, 2021, 5575245. [Google Scholar] [CrossRef]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef] [Green Version]
- Faust, K.; Sathirapongsasuti, J.F.; Izard, J.; Segata, N.; Gevers, D.; Raes, J.; Huttenhower, C. Microbial Co-occurrence Relationships in the Human Microbiome. PLoS Comput. Biol. 2012, 8, e1002606. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Khan, Z.; Warnakulasuriya, S. Cancer-associated toll-like receptor modulation and insinuation in infection susceptibility: Association or coincidence? Ann. Oncol. 2016, 27, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Brugger, S.D.; Bomar, L.; Lemon, K.P. Commensal-Pathogen Interactions along the Human Nasal Passages. PLoS Pathog. 2016, 12, e1005633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, W.H.; de Steenhuijsen Piters, W.A.; Bogaert, D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017, 15, 259–270. [Google Scholar] [CrossRef]
- Bomar, L.; Brugger, S.D.; Lemon, K.P. Bacterial microbiota of the nasal passages across the span of human life. Curr. Opin. Microbiol. 2018, 41, 8–14. [Google Scholar] [CrossRef]
- Whelan, F.J.; Verschoor, C.P.; Stearns, J.C.; Rossi, L.; Luinstra, K.; Loeb, M.; Smieja, M.; Johnstone, J.; Surette, M.G.; Bowdish, D.M. The loss of topography in the microbial communities of the upper respiratory tract in the elderly. Ann. Am. Thorac. Soc. 2014, 11, 513–521. [Google Scholar] [CrossRef]
- Schenck, L.; Surette, M.G.; Bowdish, D.M.E. Composition and immunological significance of the upper respiratory tract microbiota. FEBS Lett. 2016, 590, 3705–3720. [Google Scholar] [CrossRef]
- Roghmann, M.C.; Lydecker, A.D.; Hittle, L.; DeBoy, R.T.; Nowak, R.G.; Johnson, J.K.; Mongodin, E.F. Comparison of the Microbiota of Older Adults Living in Nursing Homes and the Community. mSphere 2017, 2, e00210-17. [Google Scholar] [CrossRef] [Green Version]
- Van den Munckhof, E.; Hafkamp, H.C.; de Kluijver, J.; Kuijper, E.J.; de Koning, M.; Quint, W.; Knetsch, C.W. Nasal microbiota dominated by Moraxella spp. is associated with respiratory health in the elderly population: A case control study. Respir. Res. 2020, 21, 181. [Google Scholar] [CrossRef]
- Biesbroek, G.; Tsivtsivadze, E.; Sanders, E.A.; Montijn, R.; Veenhoven, R.H.; Keijser, B.J.; Bogaert, D. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am. J. Respir. Crit. Care Med. 2014, 190, 1283–1292. [Google Scholar] [CrossRef]
- Tsai, M.H.; Huang, Y.C.; Chen, C.J.; Lin, P.Y.; Chang, L.Y.; Chiu, C.H.; Tsao, K.C.; Huang, C.G.; Lin, T.Y. Chlamydial pneumonia in children requiring hospitalization: Effect of mixed infection on clinical outcome. J. Microbiol. Immunol. Infect. 2005, 38, 117–122. [Google Scholar] [PubMed]
- Emery, D.C.; Shoemark, D.K.; Batstone, T.E.; Waterfall, C.M.; Coghill, J.A.; Cerajewska, T.L.; Davies, M.; West, N.X.; Allen, S.J. 16S rRNA Next Generation Sequencing Analysis Shows Bacteria in Alzheimer’s Post-Mortem Brain. Front. Aging Neurosci. 2017, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, Y.; Kawakatsu, S.; Hayashi, H.; Kobayashi, R.; Suzuki, A.; Sato, C.; Otani, K. Comparison of entorhinal cortex atrophy between early-onset and late-onset Alzheimer’s disease using the VSRAD, a specific and sensitive voxel-based morphometry. Int. J. Geriatr. Psychiatry 2013, 28, 372–376. [Google Scholar] [CrossRef]
- Mu, L.; Chen, J.; Sobotka, S.; Nyirenda, T.; Benson, B.; Gupta, F.; Sanders, I.; Adler, C.H.; Caviness, J.N.; Shill, H.A. Alpha-Synuclein Pathology in Sensory Nerve Terminals of the Upper Aerodigestive Tract of Parkinson’s Disease Patients. Dysphagia 2015, 30, 404–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheperjans, F.; Aho, V.; Pereira, P.A.B.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef]
- Malek, N.; Swallow, D.; Grosset, K.A.; Anichtchik, O.; Spillantini, M.; Grosset, D.G. Alpha-synuclein in peripheral tissues and body fluids as a biomarker for Parkinson’s disease—A systematic review. Acta Neurol. Scand. 2014, 130, 59–72. [Google Scholar] [CrossRef]
- Crews, L.; Masliah, E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum. Mol. Genet. 2010, 19, R12–R20. [Google Scholar] [CrossRef]
- Knowles, R.B.; Wyart, C.; Buldyrev, S.V.; Cruz, L.; Urbanc, B.; Hasselmo, M.E.; Stanley, H.E.; Hyman, B.T. Plaque-induced neurite abnormalities: Implications for disruption of neural networks in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 1999, 96, 5274–5279. [Google Scholar] [CrossRef] [Green Version]
- Alemao, C.A.; Budden, K.F.; Gomez, H.M.; Rehman, S.F.; Marshall, J.E.; Shukla, S.D.; Donovan, C.; Forster, S.C.; Yang, I.A.; Keely, S.; et al. Impact of diet and the bacterial microbiome on the mucous barrier and immune disorders. Allergy 2021, 76, 714–734. [Google Scholar] [CrossRef]
- Jacka, F.N. Targeting the gut to achieve improved outcomes in mood disorders. Bipolar Disord. 2019, 21, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Suganthy, N.; Kesika, P.; Chaiyasut, C. The Role of Microbiome, Dietary Supplements, and Probiotics in Autism Spectrum Disorder. Int. J. Environ. Res. Public Health 2020, 17, 2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamberg, K.; Kolk, K.; Jaagura, M.; Vilu, R.; Adamberg, S. The composition and metabolism of faecal microbiota is specifically modulated by different dietary polysaccharides and mucin: An isothermal microcalorimetry study. Benef. Microbes 2018, 9, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef]
- Di Stadio, A.; Costantini, C.; Renga, G.; Pariano, M.; Ricci, G.; Romani, L. The Microbiota/Host Immune System Interaction in the Nose to Protect from COVID-19. Life 2020, 10, 345. [Google Scholar] [CrossRef]
- Rueca, M.; Fontana, A.; Bartolini, B.; Piselli, P.; Mazzarelli, A.; Copetti, M.; Binda, E.; Perri, F.; Gruber, C.; Nicastri; et al. Investigation of Nasal/Oropharyngeal Microbial Community of COVID-19 Patients by 16S rDNA Sequencing. Int. J. Environ. Res. Public Health. 2021, 18, 2174. [Google Scholar] [CrossRef]
- Khoruts, A. First microbial encounters. Nat. Med. 2016, 22, 231–232. [Google Scholar] [CrossRef]
- Biesbroek, G.; Bosch, A.A.; Wang, X.; Keijser, B.J.; Veenhoven, R.H.; Sanders, E.A.; Bogaert, D. The impact of breastfeeding on nasopharyngeal microbial communities in infants. Am. J. Respir. Crit. Care Med. 2014, 190, 298–308. [Google Scholar] [CrossRef]
- Sampaio-Maia, B.; Monteiro-Silva, F. Acquisition and maturation of oral microbiome throughout childhood: An update. J. Dent. Res. 2014, 11, 291–301. [Google Scholar]
- Teo, S.M.; Mok, D.; Pham, K.; Kusel, M.; Serralha, M.; Troy, N.; Holt, B.J.; Hales, B.J.; Walker, M.L.; Hollams, E.; et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 2015, 17, 704–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, M.S.; Goss, K.C.; Connett, G.J.; Kollamparambil, T.; Legg, J.P.; Thwaites, R.; Ashton, M.; Puddy, V.; Peacock, J.L.; Bruce, K.D. Molecular microbiological characterization of preterm neonates at risk of bronchopulmonary dysplasia. Pediatr. Res. 2010, 67, 412–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourani, P.M.; Harris, J.K.; Sontag, M.K.; Robertson, C.E.; Abman, S.H. Molecular identification of bacteria in tracheal aspirate fluid from mechanically ventilated preterm infants. PLoS ONE 2011, 6, e25959. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, P.; Luna, R.A.; Hollister, E.B.; Devaraj, S.; Mistretta, T.A.; Welty, S.E.; Versalovic, J. The airway microbiome of intubated premature infants: Characteristics and changes that predict the development of bronchopulmonary dysplasia. Pediatr. Res. 2014, 76, 294–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camarinha-Silva, A.; Jáuregui, R.; Chaves-Moreno, D.; Oxley, A.P.A.; Schaumburg, F.; Becker, K.; Wos-Oxley, M.L.; Pieper, D.H. Comparing the anterior nare bacterial community of two discrete human populations using Illumina amplicon sequencing. Environ. Microbiol. 2014, 16, 2939–2952. [Google Scholar] [CrossRef] [PubMed]
- Camarinha-Silva, A.; Wos-Oxley, M.L.; Jáuregui, R.; Becker, K.; Pieper, D.H. Validating T-RFLP as a sensitive and high-throughput approach to assess bacterial diversity patterns in human anterior nares. FEMS Microbiol. Ecol. 2011, 79, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Byrd, A.L.; Deming, C.B.; Conlan, S.; Kong, H.H.; Segre, J.A. Biogeography and individuality shape function in the human skin metagenome. Nature 2014, 514, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Gao, H.; Mihindukulasuriya, K.A.; Rosa, P.S.L.; Wylie, K.M.; Vishnivetskaya, T.; Podar, M.; Warner, B.; Tarr, P.I.; Nelson, D.E.; et al. Biogeography of the ecosystems of the healthy human body. Genome Biol. 2013, 14, R1. [Google Scholar] [CrossRef] [Green Version]
- De Boeck, I.; Wittouck, S.; Wuyts, S.; Oerlemans, E.F.; Van den Broek, M.F.; Vandenheuvel, D.; Vanderveken, O.; Lebeer, S. Comparing the healthy nose and nasopharynx microbiota reveals continuity as well as niche-specificity. Front. Microbiol. 2017, 8, 2372. [Google Scholar] [CrossRef] [Green Version]
- de Steenhuijsen Piters, W.A.; Sanders, E.A.; Bogaert, D. The role of the local microbial ecosystem in respiratory health and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140294. [Google Scholar] [CrossRef] [Green Version]
- Wenfang, H.; Yueyun, M.; Zhou, L.; Hao, X. The role of microbiome in respiratory disease. Chin. J. Lab. Med. 2016, 39, 322–325. [Google Scholar]
- Unger, S.A.; Bogaert, D. The respiratory microbiome and respiratory infections. J. Infect. 2017, 74 (Suppl. S1), S84–S88. [Google Scholar] [CrossRef]
- Kumpitsch, C.; Koskinen, K.; Schopf, V.; Moissl-Eichinger, C. The microbiome of the upper respiratory in health and disease. BMC Biol. 2019, 17, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashworth, M.; Charlton, J.; Ballard, K.; Latinovic, R.; Gulliford, M. Variations in antibiotic prescribing and consultation rates for acute respiratory infection in UK general practices 1995–2000. Br. J. Gen. Pract. 2005, 55, 603–608. [Google Scholar]
- Feinberg, M.J.; Knebl, J.; Tully, J.; Segall, L. Aspiration and the elderly. Dysphagia 1990, 5, 61–71. [Google Scholar] [CrossRef]
- Scannapieco, F.A. Role of oral bacteria in respiratory infection. J. Periodontol. 2013, 70, 793–802. [Google Scholar] [CrossRef]
- Bassis, C.M.; Erb-Downward, J.R.; Dickson, R.P.; Freeman, C.M.; Schmidt, T.M.; Young, V.B.; Beck, J.M.; Curtis, J.L.; Huffnagle, G.B. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio 2015, 6, e00037. [Google Scholar] [CrossRef] [Green Version]
- Frank, D.N.; Feazel, L.M.; Bessesen, M.T.; Price, C.S.; Janoff, E.N.; Pace, N.R. The human nasal microbiota and Staphylococcus aureus carriage. PLoS ONE 2010, 5, e10598. [Google Scholar] [CrossRef]
- Zhou, Y.; Mihindukulasuriya, K.A.; Gao, H.; La Rosa, P.S.; Wylie, K.M.; Martin, J.C.; Kota, K.; Shannon, W.D.; Mitreva, M.; Sodergren, E.; et al. Exploration of bacterial community classes in major human habitats. Genome Biol. 2014, 15, R66. [Google Scholar] [CrossRef]
- Wos-Oxley, M.L.; Chaves-Moreno, D.; Jáuregui, R.; Oxley, A.P.; Kaspar, U.; Plumeier, I.; Kahl, S.; Rudack, C.; Becker, K.; Pieper, D.H. Exploring the bacterial assemblages along the human nasal passage. Environ. Microbiol. 2016, 18, 2259–2271. [Google Scholar] [CrossRef]
- Bosch, A.A.T.M.; Levin, E.; van Houten, M.A.; Hasrat, R.; Kalkman, G.; Biesbroek, G.; de Steenhuijsen Piters, W.A.A.; de Groot, P.C.M.; Pernet, P.; Keijser, B.J.F.; et al. Development of Upper Respiratory Tract Microbiota in Infancy is Affected by Mode of Delivery. EBioMedicine 2016, 9, 336–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlson, E.S.; Chen, J.; Custers-Allen, R.; Bittinger, K.; Li, H.; Sinha, R.; Hwang, J.; Bushman, F.D.; Collman, R.G. Disordered microbial communities in the upper respiratory tract of cigarette smokers. PLoS ONE 2010, 5, e15216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eidi, S.; Kamali, S.A.; Hajari, Z.; Fata, F.; Hosseini, R.F.; Naseri, A.; Bakhshaee, M. Nasal and indoors fungal contamination in healthy subjects. Health Scope 2016, 5, e30033. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, N.; Li, Y.; Lu, R.; Wang, H.; Liu, G.; Zou, X.; Xie, Z.; Tan, W. Metagenomic analysis of viral genetic diversity in respiratory samples from children with severe acute respiratory infection in China. Clin. Microbiol. Infect. 2016, 22, 458.e1–458.e9. [Google Scholar] [CrossRef] [Green Version]
- Marsh, R.L.; Kaestli, M.; Chang, A.B.; Binks, M.J.; Pope, C.E.; Hoffman, L.R.; Smith-Vaughan, H.C. The microbiota in bronchoalveolar lavage from young children with chronic lung disease includes taxa present in both the oropharynx and nasopharynx. Microbiome 2016, 4, 37. [Google Scholar] [CrossRef] [Green Version]
- Segal, L.N.; Alekseyenko, A.V.; Clemente, J.C.; Kulkarni, R.; Wu, B.; Gao, Z.; Chen, H.; Berger, K.I.; Goldring, R.M.; Rom, W.N.; et al. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome 2013, 1, 19. [Google Scholar] [CrossRef] [Green Version]
- Stearns, J.C.; Davidson, C.J.; McKeon, S.; Whelan, F.J.; Fontes, M.E.; Schryvers, A.B.; Bowdish, D.M.; Kellner, J.D.; Surette, M.G. Culture and molecular-based profiles show shifts in bacterial communities of the upper respiratory tract that occur with age. ISME J. 2015, 9, 1246–1259. [Google Scholar] [CrossRef] [Green Version]
- Tai, J.; Han, M.S.; Kwak, J.; Kim, T.H. Association Between Microbiota and Nasal Mucosal Diseases in terms of Immunity. Int. J. Mol. Sci. 2021, 22, 4744. [Google Scholar] [CrossRef]
- Anand, V.K. Epidemiology and economic impact of rhinosinusitis. Ann. Otol. Rhinol. Laryngol. Suppl. 2004, 193, 3–5. [Google Scholar] [CrossRef]
- Gliklich, R.E.; Metson, R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol. Head Neck Surg. 1995, 113, 104–109. [Google Scholar] [CrossRef]
- Harrass, S.; Yi, C.; Chen, H. Chronic Rhinosinusitis and Alzheimer’s Disease-A Possible Role for the Nasal Microbiome in Causing Neurodegeneration in the Elderly. Int. J. Mol. Sci. 2021, 22, 11207. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, M.; Keshavarzian, A.; Tobin, M.C.; Landay, A.L.; Schleimer, R.P. A comprehensive review of the nasal microbiome in chronic rhinosinusitis (CRS). Clin. Exp. Allergy 2016, 46, 21–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andualem, Z.; Gizaw, Z.; Bogale, L.; Dagne, H. Indoor bacterial load and its correlation to physical indoor air quality parameters in public primary schools. Multidiscip. Respir. Med. 2019, 14, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridley, C.; Thornton, D.J. Mucins: The frontline defence of the lung. Biochem. Soc. Trans. 2018, 46, 1099–1106. [Google Scholar] [CrossRef] [Green Version]
- Jones, N. The nose and paranasal sinuses physiology and anatomy. Adv. Drug Deliv. Rev. 2001, 51, 5–19. [Google Scholar] [CrossRef]
- Ballester, B.; Milara, J.; Cortijo, J. Mucins as a New Frontier in Pulmonary Fibrosis. J. Clin. Med. 2019, 8, 1447. [Google Scholar] [CrossRef] [Green Version]
- Mellert, T.K.; Getchell, M.L.; Sparks, L.; Getchell, T.V. Characterization of the immune barrier in human olfactory mucosa. Otolaryngol. Head Neck Surg. 1992, 106, 181–188. [Google Scholar] [CrossRef]
- Koskinen, K.; Reichert, J.L.; Hoier, S.; Schachenreiter, J.; Duller, S.; Moissl-Eichinger, C.; Schöpf, V. The nasal microbiome mirrors and potentially shapes olfactory function. Sci. Rep. 2018, 8, 1296. [Google Scholar] [CrossRef] [Green Version]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [Green Version]
- Bushdid, C.; Magnasco, M.O.; Vosshall, L.B.; Keller, A. Humans can discriminate more than 1 trillion olfactory stimuli. Science 2014, 343, 1370–1372. [Google Scholar] [CrossRef] [Green Version]
- Moran, D.T.; Rowley, J.C., III; Jafek, B.W.; Lovell, M.A. The fine structure of the olfactory mucosa in man. J. Neurocytol. 1982, 11, 721–746. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, E.H.; Brämerson, A.; Stjärne, P.; Nordin, S. Consequences of olfactory loss and adopted coping strategies. Rhinology 2004, 42, 189–194. [Google Scholar] [PubMed]
- Frasnelli, J.; Hummel, T. Olfactory dysfunction and daily life. Eur. Arch. Otorhinolaryngol. 2005, 262, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Kollndorfer, K.; Reichert, J.; Braunsteiner, J.; Schöpf, V. Assessment of olfactory memory in olfactory dysfunction. Perception 2017, 46, 516–529. [Google Scholar] [CrossRef]
- Ferris, A.M.; Duffy, V.B. Effect of olfactory deficits on nutritional status. Does age predict persons at risk? Ann. N. Y. Acad. Sci. 1989, 561, 113–123. [Google Scholar] [CrossRef]
- Deems, D.A.; Doty, R.L.; Settle, R.G.; Moore-Gillon, V.; Shaman, P.; Mester, A.F.; Kimmelman, C.P.; Brightman, V.J.; Snow, J.B., Jr. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch. Otolaryngol. Head. Neck Surg. 1991, 117, 519–528. [Google Scholar] [CrossRef]
- Morris, A.; Beck, J.M.; Schloss, P.D.; Campbell, T.B.; Crothers, K.; Curtis, J.L.; Flores, S.C.; Fontenot, A.P.; Ghedin, E.; Huang, L.; et al. Comparison of the respiratory microbiome in healthy non-smokers and smokers. Am. J. Respir. Crit. Care Med. 2013, 187, 1067–1075. [Google Scholar] [CrossRef] [Green Version]
- Jokinen, C.; Heiskanen, L.; Juvonen, H.; Kallinen, S.; Karkola, K.; Korppi, M.; Kurki, S.; Ronnberg, P.R.; Seppa, A.; Soimakallio, S. Incidence of community-acquired pneumonia in the population of four municipalities in eastern Finland. Am. J. Epidemiol. 1993, 137, 977–988. [Google Scholar] [CrossRef]
- Ahmed, R.; Oldstone, M.B.A.; Palese, P. Protective immunity and susceptibility to infectious diseases: Lessons from the 1918 influenza pandemic. Nat. Immunol. 2007, 8, 1188–1193. [Google Scholar] [CrossRef]
- Benten, I.J.; Drunen, C.M.; Koopman, L.P.; Middelkoop, B.C.; Hop, W.C.J.; Osterhaus, A.D.M.E.; Neijens, H.J.; Fokkens, W.J. Age- and infection related maturation of the nasal immune response in 0–2-year-old children. Allergy 2005, 60, 226–232. [Google Scholar] [CrossRef]
- Oh, J.; Conlan, S.; Polley, E.C.; Segre, J.A.; Kong, H.H. Shifts in human skin and nares microbiota of healthy children and adults. Genome Med. 2012, 4, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Royet, J.P.; Plailly, J. Lateralization of Olfactory Processes. Chem. Sens. 2004, 29, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Summanen, P.; Finegold, S.M.; Summanen, P.; Finegold, S.M. Bergey’s Manual of Systematics of Archaea and Bacteria 1–14; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Croy, I.; Hoffmann, H.; Philpott, C.; Rombaux, P.; Welge-Luessen, A.; Vodicka, J.; Konstantinidis, I.; Morera, E.; Hummel, T. Retronasal testing of olfactory function: An investigation and comparison in seven countries. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Bharathi, M.; Kunaviktikul, W.; Klunklin, A.; Chanthapoon, C.; Chaiyasut, C. Essential Oils, Phytoncides, Aromachology, and Aromatherapy—A Review. Appl. Sci. 2022, 12, 4495. [Google Scholar] [CrossRef]
- Attems, J.; Walker, L.; Jellinger, K.A. Olfaction and aging: A mini-review. Gerontology 2015, 61, 485–490. [Google Scholar] [CrossRef]
- Markovic, K.; Reulbach, U.; Vassiliadu, A.; Lunkenheimer, J.; Lunkenheimer, B.; Spannenberger, R.; Thuerauf, N. Good news for elderly persons: Olfactory pleasure increases at later stages of the life span. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 1287–1293. [Google Scholar] [CrossRef] [Green Version]
- Schubert, C.R.; Cruickshanks, K.J.; Klein, B.E.; Klein, R.; Nondahl, D.M. Olfactory impairment in older adults: Five-year incidence and risk factors. Laryngoscope 2011, 121, 873–878. [Google Scholar] [CrossRef] [Green Version]
- Barresi, M.; Ciurleo, R.; Giacoppo, S.; Foti Cuzzola, V.; Celi, D.; Bramanti, P.; Marino, S. Evaluation of olfactory dysfunction in neurodegenerative diseases. J. Neurol. Sci. 2012, 323, 16–24. [Google Scholar] [CrossRef]
- Bianchi, A.J.; Guépet-Sordet, H.; Manckoundia, P. Changes in olfaction during ageing and in certain neurodegenerative diseases: Up-to-date. Rev. Med. Interne. 2015, 36, 31–37. [Google Scholar] [CrossRef]
- François, A.; Grebert, D.; Rhimi, M.; Mariadassou, M.; Naudon, L.; Rabot, S.; Meunier, N. Olfactory epithelium changes in germfree mice. Sci. Rep. 2016, 6, 24687. [Google Scholar] [CrossRef] [PubMed]
- Lécuyer, H.; Audibert, J.; Bobigny, A.; Eckert, C.; Jannière-Nartey, C.; Buu-Hoï, A.; Mainardi, J.L.; Podglajen, I. Dolosigranulum pigrum causing nosocomial pneumonia and septicemia. J. Clin. Microbiol. 2007, 45, 3474–3475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, J.S.; Spencer, J.I.; Yates, R.L.; Yee, S.A.; Jacobs, B.M.; DeLuca, G.C. Invited Review: From nose to gut—The role of the microbiome in neurological disease. Neuropathol. Appl. Neurobiol. 2019, 5, 195–215. [Google Scholar] [CrossRef] [Green Version]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [Green Version]
- Shipley, M.T.; McLean, J.H.; Ennis, M. Olfactory system. In Rat Nervous System; Paxinos, G., Ed.; Academic Press: San Diego, CA, USA, 1995; pp. 899–926. [Google Scholar]
- Saiz-Sánchez, D.; de la Rosa-Prieto, C.; Úbeda-Bañón, I.; Martínez-Marcos, A. Neural Basis of Hyposmia in Alzheimer’s Disease. In The Clinical Spectrum of Alzheimer’s Disease—The Charge Toward Comprehensive Diagnostic and Therapeutic Strategies; De La Monte, S., Ed.; IntechOpen: London, UK, 2011. [Google Scholar]
- Doty, R.L.; Kamath, V. The influences of age on olfaction: A review. Front. Psychol. 2014, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Arnold, S.E.; Lee, E.B.; Moberg, P.J.; Stutzbach, L.; Kazi, H.; Han, L.Y.; Lee, V.M.; Trojanowski, J.Q. Olfactory epithelium amyloid-beta and paired helical filament-tau pathology in Alzheimer disease. Ann. Neurol. 2010, 67, 462–469. [Google Scholar] [CrossRef]
- Panitch, H.B. Respiratory implications of pediatric neuromuscular disease. Respir Care. 2017, 62, 826–848. [Google Scholar] [CrossRef]
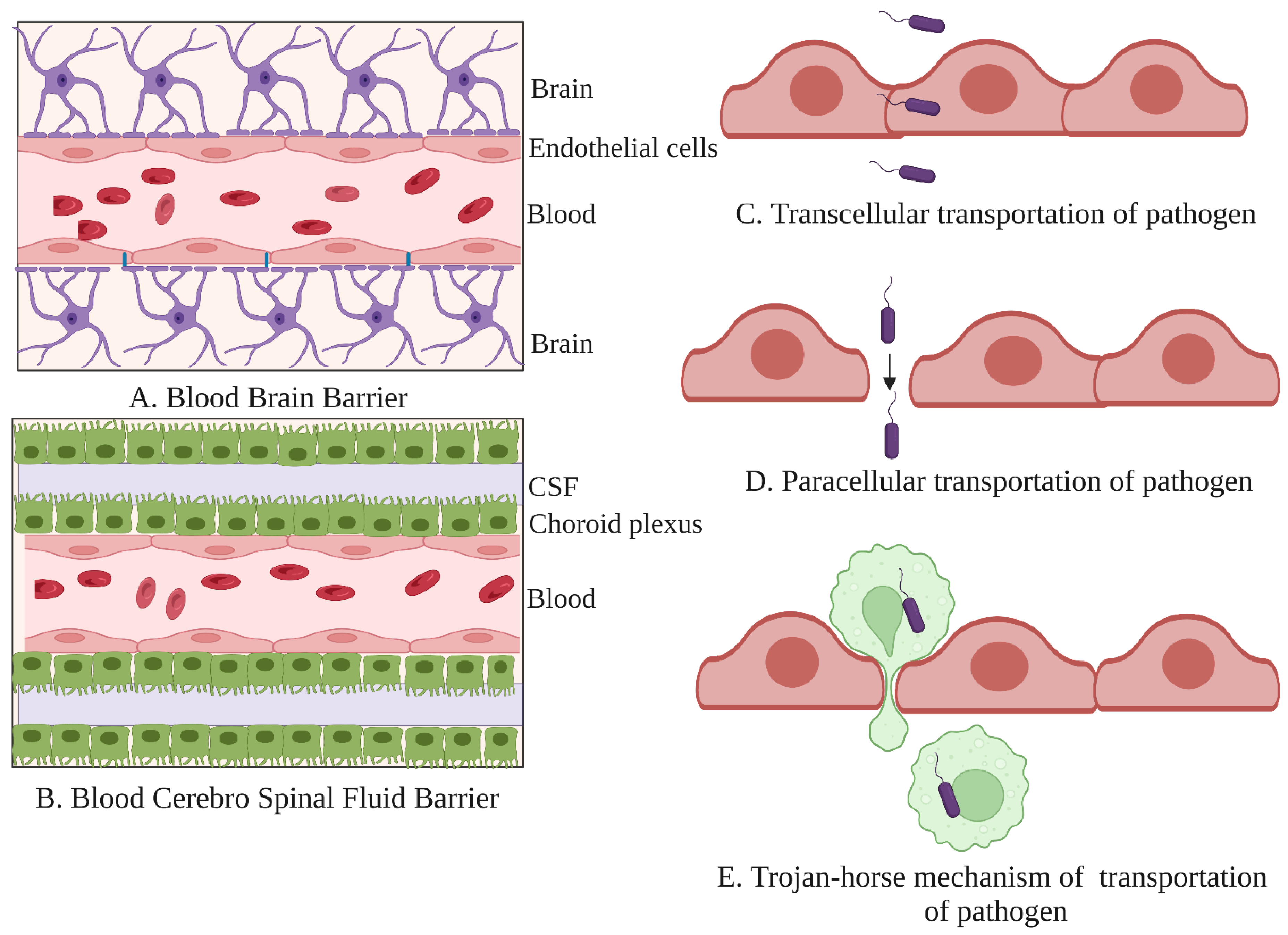
- Kristensson, K. Microbes’ roadmap to neurons. Nat. Rev. Neurosci. 2011, 12, 345–357. [Google Scholar] [CrossRef]
- Engelhardt, B.; Sorokin, L. The blood-brain and the blood-cerebrospinal fluid barriers: Function and dysfunction. Semin. ImmunoPathol. 2009, 31, 497–511. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.S. Mechanisms of microbial traversal of the blood-brain barrier. Nat. Rev. Microbiol. 2008, 6, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Lane, A.P. Chronic rhinosinusitis as a multifactorial inflammatory disorder. Curr. Infect. Dis. Rep. 2011, 13, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Dando, S.J.; Mackay-Sim, A.; Norton, R.; Currie, B.J.; St John, J.A.; Ekberg, J.A.; Batzloff, M.; Ulett, G.C.; Beacham, I.R. Pathogens penetrating the central nervous system: Infection pathways and the cellular and molecular mechanisms of invasion. Clin. Micr. Biol. Rev. 2014, 27, 691–726. [Google Scholar] [CrossRef] [Green Version]
- Hedner, M.; Larsson, M.; Arnold, N.; Zucco, G.M.; Hummel, T. Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J. Clin. Exp. Neuropsychol. 2010, 32, 1062–1067. [Google Scholar] [CrossRef]
- Croy, I.; Nordin, S.; Hummel, T. Olfactory Disorders and Quality of Life—An Updated Review. Chem. Sens. 2014, 39, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Boesveldt, S.; Postma, E.M.; Boak, D.; Welge-Luessen, A.; Schöpf, V.; Mainland, J.D.; Martens, J.; Ngai, J.; Duffy, V.B. Anosmia-A Clinical Review. Chem. Sens. 2017, 42, 513–523. [Google Scholar] [CrossRef]
- Miwa, T.; Furukawa, M.; Tsukatani, T.; Costanzo, R.M.; DiNardo, L.J.; Reiter, E.R. Impact of olfactory impairment on quality of life and disability. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Temmel, A.F.P.; Quint, C.; Schickinger-Fischer, B.; Klimek, L.; Stoller, E.; Hummel, T. Characteristics of olfactory disorders in relation to major causes of olfactory loss. AOHNS 2002, 128, 635–641. [Google Scholar] [CrossRef] [Green Version]
- Matsui, T.; Arai, H.; Nakajo, M.; Maruyama, M.; Ebihara, S.; Sasaki, H.; Yoshida, Y. Role of Chronic Sinusitis in Cognitive Functioning in the Elderly. J. Am. Geriatr. Soc. 2003, 51, 1818–1819. [Google Scholar] [CrossRef]
- Tarasidis, G.S.; De Conde, A.S.; Mace, J.C.; Ashby, S.; Smith, T.L.; Orlandi, R.R.; Alt, J.A. Cognitive dysfunction associated with pain and quality of life in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2015, 5, 1004–1009. [Google Scholar] [CrossRef]
- Jafari, A.; de Lima Xavier, L.; Bernstein, J.D.; Simonyan, K.; Bleier, B.S. Association of Sinonasal Inflammation with functional brain connectivity. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 534–543. [Google Scholar] [CrossRef]
- Jung, H.J.; Lee, J.Y.; Choi, Y.S.; Choi, H.G.; Wee, J.H. Chronic rhinosinusitis and progression of cognitive impairment in dementia. Eur. Ann. Otorhino Laryngol. Head Neck Dis. 2021, 138, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Cells Producing Immunoglobulins and other Immune Factors in Human Nasal Mucosa. In Protides of the Biological Fluids; Peeters, H., Ed.; Elsevier: Amsterdam, The Netherlands, 1985; Volume 32, pp. 363–366. [Google Scholar]
- Brescia, G.; Barion, U.; Zanotti, C.; Giacomelli, L.; Martini, A.; Marioni, G. The prognostic role of serum eosinophil and basophil levels in sinonasal polyposis. Int. Forum Allergy Rhinol. 2017, 7, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Hulse, K.E.; Stevens, W.W.; Tan, B.K.; Schleimer, R.P. Pathogenesis of nasal polyposis. Clin. Exp. Allergy 2015, 45, 328–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, J.H.; Liang, K.L.; May, L.; Lane, A.P. Tumor necrosis factor alpha inhibits olfactory regeneration in a transgenic model of chronic rhinosinusitis-associated olfactory loss. Am. J. Rhinol. Allergy 2010, 24, 336–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, R.C. Chronic sinusitis and anosmia: Pathologic changes in the olfactory mucosa. Laryngoscope 2000, 110, 1071–1077. [Google Scholar] [CrossRef]
- Litvack, J.R.; Fong, K.; Mace, J.; James, K.E.; Smith, T.L. Predictors of olfactory dysfunction in patients with chronic rhinosinusitis. Laryngoscope 2008, 118, 2225–2230. [Google Scholar] [CrossRef] [Green Version]
- Snowdon, D.A.; Greiner, L.H.; Mortimer, J.A.; Riley, K.P.; Greiner, P.A.; Markesbery, W.R. Brain Infarction and the Clinical Expression of Alzheimer Disease: The Nun Study. JAMA 1997, 277, 813–817. [Google Scholar] [CrossRef]
- Bature, F.; Guinn, B.-A.; Pang, D.; Pappas, Y. Signs and symptoms preceding the diagnosis of Alzheimer’s disease: A systematic scoping review of literature from 1937 to 2016. BMJ Open 2017, 7, e015746. [Google Scholar] [CrossRef] [Green Version]
- Panza, F.; Seripa, D.; D’Onofrio, G.; Frisardi, V.; Solfrizzi, V.; Mecocci, P.; Pilotto, A. Neuropsychiatric Symptoms, Endophenotypes, and Syndromes in Late-Onset Alzheimer’s Disease: Focus on APOE Gene. Int. J. Alzheimers Dis. 2011, 2011, 721457. [Google Scholar] [CrossRef] [Green Version]
- Li, X.L.; Hu, N.; Tan, M.S.; Yu, J.T.; Tan, L. Behavioral and psychological symptoms in Alzheimer’s disease. BioMed Res. Int. 2014, 2014, 927804. [Google Scholar] [CrossRef]
- Rowan, N.R.; Schlosser, R.J.; Storck, K.A.; Ganjaei, K.G.; Soler, Z.M. The impact of medical therapy on cognitive dysfunction in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2019, 9, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Manabe, T.; Fujikura, Y.; Mizukami, K.; Akatsu, H.; Kudo, K. Pneumonia-associated death in patients with dementia: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0213825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, J.M.; Wroblewski, K.E.; Kern, D.W.; Schumm, L.P.; McClintock, M.K. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS ONE 1991, 9, e107541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, A.; Brewer, W.J.; Yung, A.R.; Nelson, B.; Pantelis, C.; Wood, S.J. Olfactory identification deficits at identification as ultra-high risk for psychosis are associated with poor functional outcome. Schizophr. Res. 2015, 161, 156–162. [Google Scholar] [CrossRef]
- Pereira, P.A.B.; Aho, V.T.E.; Paulin, L.; Pekkonen, E.; Auvinen, P.; Scheperjans, F. Oral and nasal microbiota in Parkinson’s disease. Parkinsonism Relat. Disord. 2017, 38, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Mulak, A.; Bonaz, B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 2015, 21, 10609–10620. [Google Scholar] [CrossRef]
- Khan, F.; Oloketuyi, S.F. A Future perspective on neurodegenerative diseases: Nasopharyngeal and gut microbiota. J. Appl. Microbiol. 2016, 122, 306–320. [Google Scholar] [CrossRef] [Green Version]
- Braak, H.; Del Tredici, K.; Rüb, U.; Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Doty, R.L. Handbook of Olfaction and Gustation, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Lema Tomé, C.M.; Tyson, T.; Rey, N.L.; Grathwohl, S.; Britschgi, M.; Brundin, P. Inflammation and alpha-synuclein’s prion-like behavior in Parkinson’s Disease—Is there a link? Mol. Neurobiol. 2013, 47, 561–574. [Google Scholar] [CrossRef] [Green Version]
- Pal, G.; Ramirez, V.; Engen, P.A.; Naqib, A.; Forsyth, C.B.; Green, S.J.; Mahdavinia, M.; Batra, P.S.; Tajudeen, B.A.; Keshavarzian, A. Deep nasal sinus cavity microbiota dysbiosis in Parkinson’s disease. NPJ Parkinsons Dis. 2021, 7, 111. [Google Scholar] [CrossRef]
- Diaz, H.R.; Wang, S.; Anuar, F.; Qian, Y.; Bjorkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar]
- Lu, J.; Synowiec, S.; Lu, L.; Yu, Y.; Bretherick, T.; Takada, S.; Yarnykh, V.; Caplan, J.; Caplan, M.; Claud, E.C.; et al. Microbiota influence the development of the brain and behaviors in C57BL/6J mice. PLoS ONE 2018, 13, e0201829. [Google Scholar] [CrossRef]
- Wu, G.D.; Compher, C.; Chen, E.Z.; Smith, S.A.; Shah, R.D.; Bittinger, K.; Chehoud, C.; Albenberg, L.G.; Nessel, L.; Gilroy, E.; et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016, 65, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Park, W. Gut microbiomes and their metabolites shape human and animal health. J. Microbiol. 2018, 56, 151–153. [Google Scholar] [CrossRef] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef] [Green Version]
- Bassis, C.M.; Tang, A.L.; Young, V.B.; Pynnonen, M.A. The nasal cavity microbiota of healthy adults. Microbiome 2014, 2, 27. [Google Scholar] [CrossRef] [Green Version]
- Shilts, M.H.; Rosas-Salazar, C.; Tovchigrechko, A.; Larkin, E.K.; Toralba, M.; Akopov, A.; Halpin, R.; Peebles, R.S.; Moore, M.L.; Anderson, L.J.; et al. Minimally Invasive sampling method identifies differences in taxonomic richness of nasal microbiomes in young infants associated with mode of delivery. Microb. Ecol. 2016, 71, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Branton, W.G.; Ellestad, K.K.; Maingat, F.; Wheatley, B.M.; Rud, E.; Warren, R.L.; Holt, R.A.; Surette, M.G.; Power, C. Brain microbial populations in HIV/AIDS: A-proteobacteria predominate independent of host immune status. PLoS ONE 2013, 8, e54673. [Google Scholar] [CrossRef] [Green Version]
- Branton, W.G.; Lu, J.Q.; Surette, M.G.; Holt, R.A.; Lind, J.; Laman, J.D.; Power, C. Brain microbiota disruption within inflammatory demyelinating lesions in multiple sclerosis. Sci. Rep. 2016, 6, 37344. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.R.; Banerjee, S. Gut microbiota in neurodegenerative disorders. J. Neuroimmunol. 2019, 328, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.P.; Colette, C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295, 168–1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, J.L.; Erickson, J.M.; Lloyd, B.B.; Slavin, J.L. Health effects and sources of prebiotic dietary fiber. Curr. Dev. Nutr. 2018, 2, nzy005. [Google Scholar] [CrossRef] [Green Version]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrêa-Oliveira, R.; Fachi, J.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [Green Version]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Schwiertz, A.; Hold, G.L.; Duncan, S.H.; Gruhl, B.; Collins, M.D.; Lawson, P.A.; Flint, H.J.; Blaut, M. Anaerostipes caccae gen. nov., sp. nov., a new saccharolytic, acetate-utilising, butyrate-producing bacterium from human faeces. Syst. Appl. Microbiol. 2002, 25, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Dupraz, L.; Magniez, A.; Rolhion, N.; Rolhion, N.; Richard, M.L.; Da Costa, G.; Touch, S.; Mayeur, C.; Planchais, J.; Agus, A.; et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell Rep. 2021, 36, 109332. [Google Scholar] [CrossRef]
- Requena, T.; Martínez-Cuesta, M.C.; Peláez, C. Diet and microbiota linked in health and disease. Food Func. 2018, 9, 688–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Quintela, A.; Milton-Laskibar, I.; Trepiana, J.; Gómez-Zorita, S.; Kajarabille, N.; Léniz, A.; González, M.; Portillo, M.P. Key Aspects in Nutritional Management of COVID-19 Patients. J. Clin. Med. 2020, 9, 2589. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.P.; Busl, K.M. Neurologic Manifestations of Severe Respiratory Viral Contagions. Crit. Care Explor. 2020, 2, e0107. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, G.G.; Zhao, J.; LiPuma, J.J.; Downey, D.G.; Tunney, M.M.; Elborn, J.S. Community analysis and co-occurrence patterns in airway microbial communities during health and disease. ERJ Open Res. 2019, 5, 00128–02017. [Google Scholar] [CrossRef]
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M. Update on neurological manifestations of COVID-19. Life Sci. 2020, 257, 118063. [Google Scholar] [CrossRef]
- Arbour, N.; Day, R.; Newcombe, J.; Talbot, P.J. Neuro invasion by human respiratory coronaviruses. J. Virol. 2000, 74, 8913–8921. [Google Scholar] [CrossRef] [Green Version]
- Lau, K.K.; Yu, W.C.; Chu, C.M.; Lau, S.T.; Sheng, B.; Yuen, K.Y. Possible central nervous system infection by SARS coronavirus. Emerg. Infect. Dis. 2004, 10, 342–344. [Google Scholar] [CrossRef]
- Yeh, E.A.; Collins, A.; Cohen, M.E.; Duffner, P.K.; Faden, H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics 2004, 113, e73–e76. [Google Scholar]
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Liu, C.; Yang, C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020, 87, 18–22. [Google Scholar] [CrossRef]
- Keni, R.; Alexander, A.; Nayak, P.G.; Mudgal, J.; Nandakumar, K. COVID-19: Emergence, spread, possible treatments, and global burden. Front. Public Health 2020, 8, 216. [Google Scholar] [CrossRef]
- Murta, V.; Villarreal, A.; Ramos, A.J. Severe acute respiratory syndrome coronavirus 2 impact on the Central nervous system: Are astrocytes and microglia Main players or merely bystanders? ASN Neuro 2020, 12, 1759091420954960. [Google Scholar] [CrossRef]
- Zhou, Z.; Kang, H.; Li, S.; Zhao, X. Understanding the neurotropic characteristics of SARS-CoV-2: From neurological manifestations of COVID-19 to potential neurotropic mechanisms. J. Neurol. 2020, 267, 2179–2184. [Google Scholar] [CrossRef]
- De Maio, F.; Posteraro, B.; Ponziani, F.R.; Cattani, P.; Gasbarrini, A.; Sanguinetti, M. Nasopharyngeal Microbiota Profiling of SARS-CoV-2 Infected Patients. Biol. Proced. Online 2020, 22, 18. [Google Scholar] [CrossRef]
- Ventero, M.P.; Cuadrat, R.; Vidal, I.; Andrade, B.; Molina-Pardines, C.; Haro-Moreno, J.M.; Coutinho, F.H.; Merino, E.; Regitano, L.; Silveira, C.B.; et al. Nasopharyngeal Microbial Communities of Patients Infected With SARS-CoV-2 That Developed COVID-19. Front. Microbiol. 2021, 12, 637430. [Google Scholar] [CrossRef]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Yang, X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020, 53, 368–370. [Google Scholar] [CrossRef]
- Khan, A.A.; Khan, Z. COVID-2019-associated overexpressed Prevotella proteins mediated host-pathogen interactions and their role in coronavirus outbreak. Bioinformatics 2020, 36, 4065–4069. [Google Scholar] [CrossRef]
- Gu, S.; Chen, Y.; Wu, Z.; Chen, Y.; Gao, H.; Lv, L.; Guo, F.; Zhang, X.; Luo, R.; Huang, C.; et al. Alterations of the Gut Microbiota in Patients with COVID-19 or H1N1 Influenza. Clin. Infect. Dis. 2020, 71, 2669–2678. [Google Scholar] [CrossRef]
- Shen, Z.; Xiao, Y.; Kang, L.; Ma, W.; Shi, L.; Zhang, L.; Zhou, Z.; Yang, J.; Zhong, J.; Yang, D.; et al. Genomic Diversity of Severe Acute Respiratory Syndrome-Coronavirus 2 in Patients with Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 713–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatiwada, S.; Subedi, A. Lung microbiome and coronavirus disease 2019 (COVID-19): Possible link and implications. Hum. Microbiome J. 2020, 17, 100073. [Google Scholar] [CrossRef] [PubMed]
- Rabaan, A.A.; Al-Ahmed, S.H.; Haque, S.; Sah, R.; Tiwari, R.; Malik, Y.S.; Dhama, K.; Yatoo, M.I.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. SARS-CoV-2, SARS-CoV, and MERS-CoV: A comparative overview. Infez. Med. 2020, 28, 174–184. [Google Scholar]
- Fotuhi, M.; Mian, A.; Meysami, S.; Raji, C.A. Neurobiology of COVID-19. J. Alzheimers Dis. 2020, 76, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.D.; de Freitas, G.R.; Pettigrew, L.C.; Ay, H.; Liebeskind, D.S.; Kase, C.S.; Del Brutto, O.H.; Hankey, J.; Venketasubramanian, N. Mechanisms of stroke in COVID-19. Cerebrovasc. Dis. 2020, 49, 451–458. [Google Scholar] [CrossRef]
- Brown, E.E.; Kumar, S.; Rajji, T.K.; Pollock, B.G.; Mulsant, B.H. Anticipating and mitigating the impact of the COVID-19 pandemic on Alzheimer’s disease and related dementias. Am. J. Geriatr. Psychiatry 2020, 28, 712–721. [Google Scholar] [CrossRef]
- Zhang, Q.; Schultz, J.L.; Aldridge, G.M.; Simmering, E.; Narayanan, N.S. Coronavirus disease 2019 case fatality and Parkinson’s disease. Mov. Disord. 2020, 35, 1914–1915. [Google Scholar] [CrossRef]
- Van der Heide, A.; Meinders, M.J.; Bloem, B.R.; Helmich, R.C. The impact of the COVID-19 pandemic on psychological distress, physical activity, and symptom severity in parkinson’s disease. J. Parkinsons Dis. 2020, 10, 1355–1364. [Google Scholar] [CrossRef]
- Guo, D.; Han, B.; Lu, Y.; Lv, C.; Fang, X.; Zhang, Z.; Liu, Z.; Wang, X. Influence of the COVID-19 pandemic on quality of life of patients with parkinson’s disease. Parkinsons Dis. 2020, 2020, 1216568. [Google Scholar] [CrossRef]
- Besnard, S.; Nardin, C.; Lyon, E.; Debroucker, T.; Arjmand, R.; Moretti, R.; Pochat, H. Electroencephalographic abnormalites in SARS-CoV-2 patients. Front. Neurol. 2020, 11, 582794. [Google Scholar] [CrossRef]
- Antony, A.R.; Haneef, Z. Systematic review of EEG findings in 617 patients diagnosed with COVID-19. Seizure 2020, 83, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594–600. [Google Scholar] [CrossRef]

| S. No. | Samples | Experimental Subjects | Study Methodology | Commensal Microbiota | Reference |
|---|---|---|---|---|---|
| 1 | Anterior nares and Oropharynx | Elderly participants (age 68 to 96 years) | 16S rRNA gene sequencing | Propionibacterium spp., Corynebacterium spp., Staphylococcus spp., Veillonella spp., Streptococcus spp. | [16] |
| 2 | Nasal and Oropharynx | Elderly participants (age ≥ 65 years); 152 controls and 152 patients with RTIs. | 16S rRNA gene sequencing, quantitative real-time PCR, and culture. | In the nasal passage: Corynebacterium, Staphylococcus, Moraxella, Dolosigranulum, Streptococcus, Haemophilus, Peptoniphilus, Cutibacterium, Anaerococcus, and Enterobacteriaceae. Less abundantly: Pseudomonas and Neisseria. In the oropharynx: Prevotella, Veillonella, Streptococcus, Neisseria, Fusobacterium, Leptotrichia, Haemophilus, Rothia, Porphyromonas, Actinobacillus, Lactobacillus, Staphylococcus. | [19] |
| 3 | URT, Nasopharynx | Healthy children (n = 60); Age 1.5, 6, 12, and 24 months. | 16S rRNA-based pyrosequencing | At 1.5 months of age: Staphylococcus sp., Corynebacterium sp., Moraxella sp. At 1.5 to 6 months of age: M. catarrhalis, Dolosigranulum sp., Corynebacterium sp. At 6 months of age: Staphylococcus aureus In the first 2 years of age: Moraxella sp., Dolosigranulum sp., Corynebacterium sp., Haemophilus sp., Streptococcus sp. | [20] |
| 4 | URT, Nasopharynx | Infants exclusively breastfed (n = 101) and exclusively fed formula (n = 101); Age 6 weeks to 6 months. | 16S-based GS-FLX-titanium-pyrosequencing | Breastfed infants: Abundance of Dolosigranulum sp., Corynebacterium, pseudodiphteriticum, C. propinquum, C. accolens, C. fastidiosum, or C. segmentosum, Decreased abundance of Staphylococcus spp., Prevotella sp., Veilonella sp. Formula-fed infants: Dolosigranulum sp., Corynebacterium sp. | [39] |
| 5 | URT, Nasopharynx | A cohort of 234 children, including healthy infants and infants who had experienced acute respiratory infections once. | Microbial profiling using 16S rRNA gene deep sequencing | Staphylococcus sp., Corynebacterium sp., Alloiococcus sp., Moraxella sp., Haemophilus sp. | [41] |
| 6 | URT-anterior nares (left and right) | Healthy adults and hospitalized patients. S. aureus carriers (n = 26) and non-carriers (n = 16). | Culture-independent analysis of 16S rRNA sequencing | Actinobacteria (Propionibacterium sp., Corynebacterium sp.) Firmicutes (Staphylococcus spp.), Proteobacteria (Enterobacter sp.) | [58] |
| 7 | URT-anterior nares | A healthy cohort of 236 subjects from the Human Microbiome Project. | 16S rRNA gene sequencing | Moraxella sp., Corynebacterium sp., Propionibacterium sp., Staphylococcus sp. | [59] |
| 8 | URT-anterior and posterior vestibule, inferior and middle meatuses of the nasal passage | A cohort of CR and CR-free individuals (n = 79). | Illumina paired-end sequencing of the V1-V2 variable regions of the 16S rRNA gene. | Staphylococcusaureus, Moraxella sp., Finegoldia magna, Prevotella sp., Staphylococcus epidermidis, Haemophilus influenzae. | [60] |
| 9 | URT, Nasopharynx | The unselected birth cohort of healthy children born by Cesarean (n = 40) and vaginal birth (n = 62); Age (birth to 6 months). | Constructing the phylogenetic library by amplifying hypervariable v4 region of 6s rRNA gene | Staphylococcus aureus, Streptococcus viridans, S. pneumoniae, Corynebacterium pseudodiphteriticum, C. propinquum, Dolosigranulum pigrum, Moraxella catarrhalis, M. nonliquefaciens, Haemophilus influenzae | [61] |
| 10 | The right and left nasopharynx and oropharynx | Smoking (n = 29) and non-smoking (n = 33) healthy asymptomatic adults | 16S rRNA-based pyrosequencing | The nasopharynx is dominated by Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria, and Campylobacter sp. Streptococcus, Shigella, Acinetobacter, and Corynebacterium sp. The oropharynx is dominated by Streptococcus Bacteroidetes, Firmicutes, Proteobacteria, and Fusobacteria. Prevotella, Fusobacterium, Neisseria, Leptotrichia, and Veillonella sp. | [62] |
| 11 | Nasal cavity and dust samples | A cohort of healthy volunteers (n = 50) without a history of respiratory system diseases. | Standard mycological techniques based on gross cultural and microscopic morphology | Aspergillus, Penicillium, Yeast, Alternaria and Rhizopus | [63] |
| 12 | Nasopharynx | Children (Age < 6 years; n = 135) with and without severe acute respiratory infections (SARI) | Metagenomic analysis based on Next-Generation Sequencing | In children with SARI: Members of the Paramyxoviridae, Coronaviridae, Parvoviridae, Orthomyxoviridae, Picornaviridae, Anelloviridae and Adenoviridae In children without SARI: Members of Anelloviridae | [64] |
| 13 | Oropharynx Nasopharynx Bronchoalveolar | Children with or without lung infection (n = 78) | 16S rRNA gene sequencing | Moraxella, Haemophilus, Staphylococcus, Streptococcus, Neisseria, Prevotella and Corynebacterium spp. | [65] |
| 14 | Bronchoalveolar | Asymptomatic subjects. Never-smokers (n = 9), former-smokers (n = 14), and current-smokers (n = 6). | 16S rRNA gene sequencing | Propionibacterium, Staphylococcus, Corynebacterium, Stenotrophomonas, Pseudomonas, Prevotella, Veillonella, Streptococcus, Fusobacterium, Porphyromonas, Sphingomonas, Tropheryma, Acidovorax, and Asticcacaulis | [66] |
| 15 | oropharynx and nasopharynx | Healthy children (Age 1 to 4.5 years; n =51) and accompanying parents (n = 19). | Molecular profiling of the bacterial 16S rRNA gene | Oropharynx of both children and adults: Streptococcus sp., Rothia sp., Prevotella sp. Gemella sp., Veillonella sp., Fusobacteria sp., Haemophilus spp., Neisseria sp. Nasopharynx of adults: Firmicutes sp., Staphylococcus sp., Streptococcus sp., Bacteriodetes sp., Prevotella sp., Actinobacteria sp., Corynebacterium sp., Rothia sp., and Propionibacterium sp., Nasopharynx of children: Moraxella spp., Enterobacteriaceae sp., Haemophilus sp., Enterococcus sp. | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Bharathi, M.; Chaiyasut, C. Nasal Microbiota, Olfactory Health, Neurological Disorders and Aging—A Review. Microorganisms 2022, 10, 1405. https://doi.org/10.3390/microorganisms10071405
Thangaleela S, Sivamaruthi BS, Kesika P, Bharathi M, Chaiyasut C. Nasal Microbiota, Olfactory Health, Neurological Disorders and Aging—A Review. Microorganisms. 2022; 10(7):1405. https://doi.org/10.3390/microorganisms10071405
Chicago/Turabian StyleThangaleela, Subramanian, Bhagavathi Sundaram Sivamaruthi, Periyanaina Kesika, Muruganantham Bharathi, and Chaiyavat Chaiyasut. 2022. "Nasal Microbiota, Olfactory Health, Neurological Disorders and Aging—A Review" Microorganisms 10, no. 7: 1405. https://doi.org/10.3390/microorganisms10071405






