Are the Effects of Malnutrition on the Gut Microbiota–Brain Axis the Core Pathologies of Anorexia Nervosa?
Abstract
1. Case Vignette
2. Anorexia Nervosa
2.1. Treatment
2.2. Severe and Enduring Disease
2.3. Epidemiology
2.4. Genome-Wide Association Studies on Anorexia Nervosa
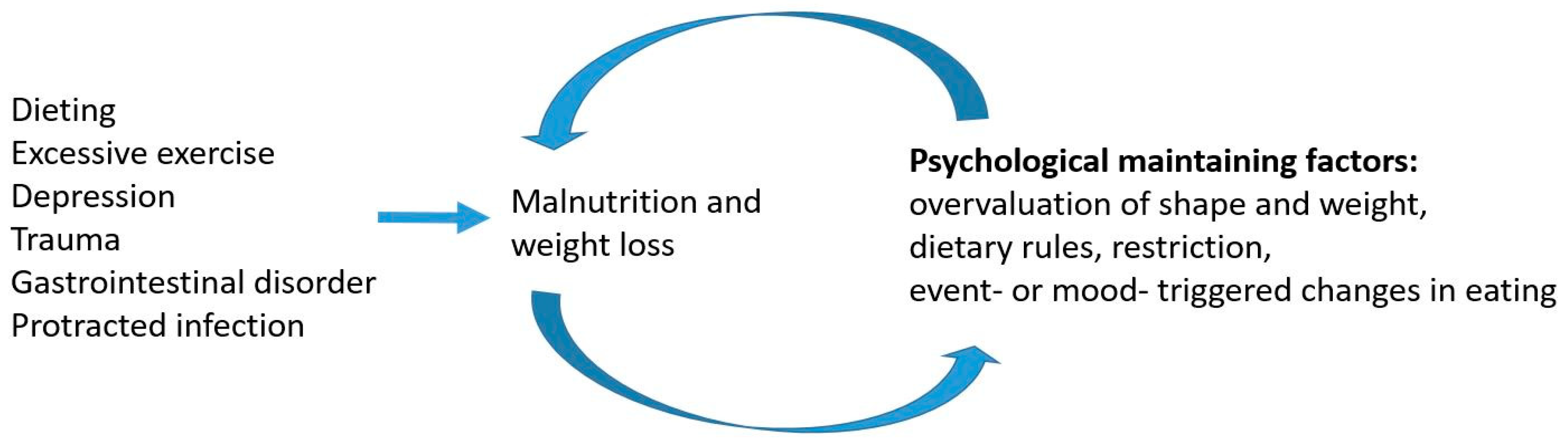
2.5. Predisposing and Precipitating Factors
2.6. Comorbidities and Complications
3. What Is the Core Pathology of Anorexia Nervosa?
Can Studies on Treatment Help Identify the Core Pathology?
4. The Gut Microbiota–Brain Axis
4.1. The Neuroendocrine Pathway
4.2. The Vagus Nerve Pathway
4.3. Immune Pathways
5. Barriers to Gut Microbiota–Brain Signaling
5.1. The Intestinal Barrier
5.2. The Blood–Brain Barrier
6. The Gut Microbiota–Brain Axis and Anorexia Nervosa
6.1. The Neuroendocrine Interaction in the Gut Microbiota–Brain Axis in Anorexia Nervosa
6.2. Appetite Regulation in Anorexia Nervosa
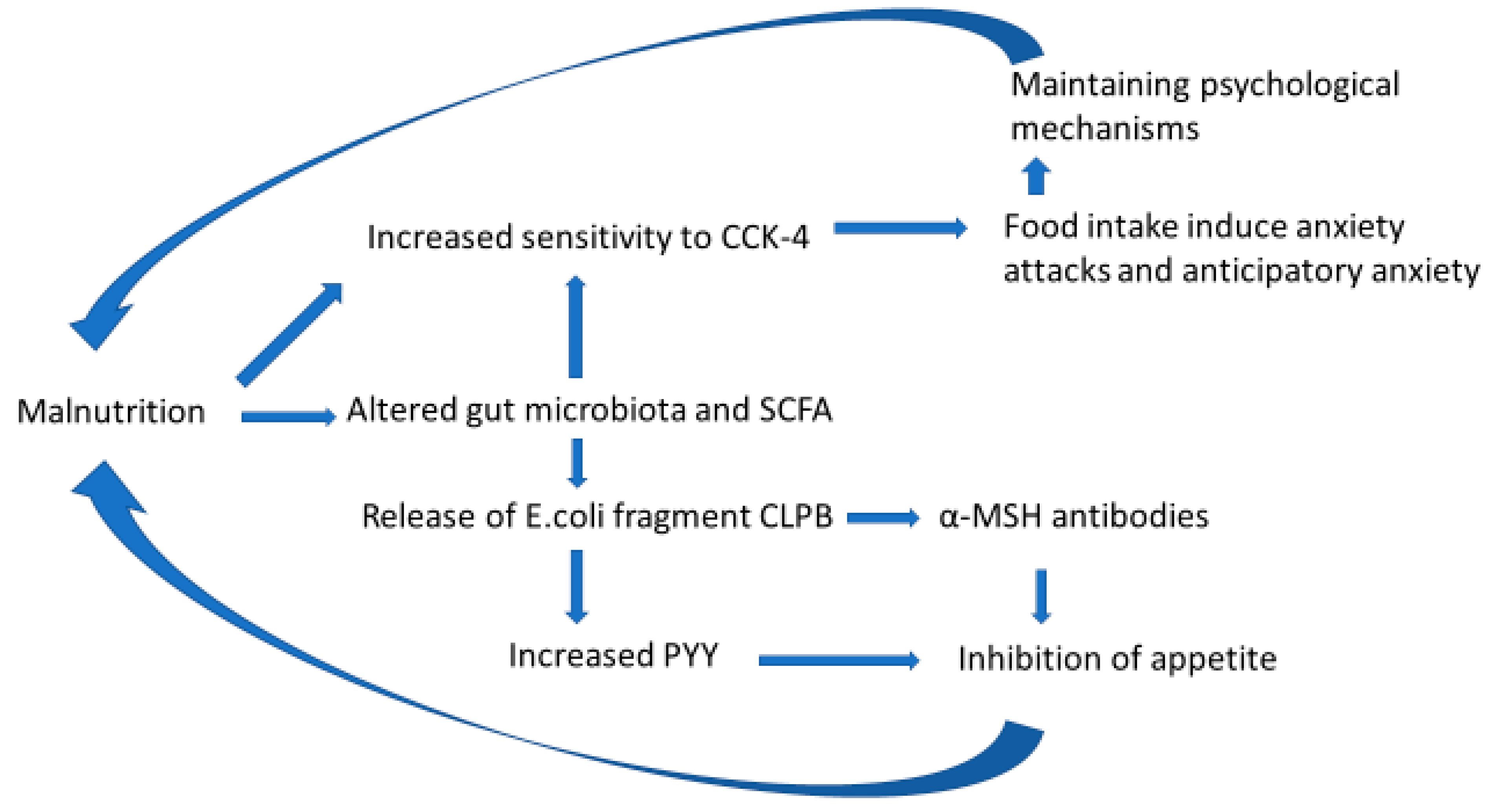
7. Hypothesis: Malnutrition-Induced Alterations in the Gut Microbiota–Brain Axis Are the Core Pathologies in Anorexia Nervosa
8. Discussion
9. Future Research
Funding
Acknowledgments
Conflicts of Interest
References
- Mitchell, J.E.; Peterson, C.B. Anorexia Nervosa. N. Engl. J. Med. 2020, 382, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Treasure, J.; Duarte, T.A.; Schmidt, U. Eating disorders. Lancet 2020, 395, 899–911. [Google Scholar] [CrossRef]
- Frostad, S.; Bentz, M. Anorexia nervosa: Outpatient treatment and medical management. World J. Psychiatry 2022, 12, 558–579. [Google Scholar] [CrossRef] [PubMed]
- Heruc, G.A.; Little, T.J.; Kohn, M.; Madden, S.; Clarke, S.; Horowitz, M.; Feinle-Bisset, C. Appetite perceptions, gastrointestinal symptoms, ghrelin, peptide YY and state anxiety are disturbed in adolescent females with anorexia nervosa and only partially restored with short-term refeeding. Nutrients 2018, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Brozek, J.; Henshel, A.; Mickelson, O.; Taylor, H.L. The Biology of Human Starvation; University of Minnesota Press: Minneapolis, MN, USA, 1950. [Google Scholar]
- Dalle Grave, R.; Calugi, S. Introduction and overview. In Cognitive Behavior Therapy for Adolescents with Eating Disorders; Dalle Grave, R., Calugi, S., Eds.; The Guilford Press: London, UK, 2020. [Google Scholar]
- Fairburn, C.G. Cognitive Behavior Therapy and Eating Disorders; Guilford Press: New York, NY, USA, 2008. [Google Scholar]
- Godart, N.; Flament, M.; Lecrubier, Y.; Jeammet, P. Anxiety disorders in anorexia nervosa and bulimia nervosa: Co-morbidity and chronology of appearance. Eur. Psychiatry 2000, 15, 38–45. [Google Scholar] [CrossRef]
- Dalle Grave, R.; Calugi, S. Cognitive Behavior Therapy for Adolescents with Eating Disorders; Guilford Press: New York, NY, USA, 2020. [Google Scholar]
- McClelland, J.; Hodsoll, J.; Brown, A.; Lang, K.; Boysen, E.; Flynn, M.; Mountford, V.; Glennon, D.; Schmidt, U. A pilot evaluation of a novel First Episode and Rapid Early Intervention service for Eating Disorders (FREED). Eur. Eat. Disord. Rev. 2018, 26, 129–140. [Google Scholar] [CrossRef]
- Royal College of Psychiatrists. Position Statement on Early Intervention for Eating Disorders; Royal College of Psychiatrists: London, UK, 2019; Available online: https://www.rcpsych.ac.uk/docs/default-source/improving-care/better-mh-policy/position-statements/ps03_19.pdf?sfvrsn=b1283556_2 (accessed on 15 March 2019).
- Bell, C.; Bulik, C.; Clayton, P.; Crow, S.; Davis, D.M.; DeMaso, D.R.; Dogin, J.; Fairburn, C.G.; Fink, A.H.; Fisher, M.; et al. Practice guideline for the treatment of patients with eating disorders (revision). American Psychiatric Association Work Group on Eating Disorders. Am. J. Psychiatry 2000, 157, 1–39. [Google Scholar]
- Zimmerman, J.; Fisher, M. Avoidant/restrictive food intake disorder (ARFID). Curr. Probl. Pediatr. Adolesc. Health Care 2017, 47, 95–103. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5); American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Eating Disorders: Recognition and Treatment [Internet]; National Institute for Health and Care Excellence: London, UK, 2017. Available online: https://www.nice.org.uk/guidance/ng69 (accessed on 18 June 2022).
- Bulik, C.M.; Flatt, R.; Abbaspour, A.; Carroll, I. Reconceptualizing anorexia nervosa. Psychiatry Clin. Neurosci. 2019, 73, 518–525. [Google Scholar] [CrossRef]
- Sjögren, M.; Kizilkaya, I.; Støving, R.K. Inpatient weight restoration treatment is associated with decrease in post-meal anxiety. J. Pers. Med. 2021, 11, 1079. [Google Scholar] [CrossRef]
- Frostad, S.; Danielsen, Y.S.; Rekkedal, G.; Jevne, C.; Grave, R.; Rø, Ø.; Kessler, U. Implementation of enhanced cognitive behaviour therapy (CBT-E) for adults with anorexia nervosa in an outpatient eating-disorder unit at a public hospital. J. Eat. Disord. 2018, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Frostad, S.; Rozakou-Soumalia, N.; Dârvariu, Ş; Foruzesh, B.; Azkia, H.; Larsen, M.P.; Rowshandel, E.; Sjögren, J.M. BMI at discharge from treatment predicts relapse in anorexia nervosa: A systematic scoping review. J. Pers. Med. 2022, 12, 836. [Google Scholar] [CrossRef] [PubMed]
- Wonderlich, S.A.; Bulik, C.M.; Schmidt, U.; Steiger, H.; Hoek, H.W. Severe and enduring anorexia nervosa: Update and observations about the current clinical reality. Int. J. Eat. Disord. 2020, 53, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Udo, T.; Grilo, C.M. Prevalence and Correlates of DSM-5-Defined Eating Disorders in a Nationally Representative Sample of U.S. Adults. Biol. Psychiatry 2018, 84, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Nagata, J.M.; Ganson, K.T.; Austin, S.B. Emerging trends in eating disorders among sexual and gender minorities. Curr. Opin. Psychiatry 2020, 33, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Litmanen, J.; Frojd, S.; Marttunen, M.; Isomaa, R.; Kaltiala-Heino, R. Are eating disorders and their symptoms increasing in prevalence among adolescent population? Nord. J. Psychiatry 2017, 71, 61–66. [Google Scholar] [CrossRef]
- Javaras, K.N.; Runfola, C.D.; Thornton, L.M.; Agerbo, E.; Birgegård, A.; Norring, C.; Yao, S.; Råstam, M.; Larsson, H.; Lichtenstein, P.; et al. Sex- and age-specific incidence of healthcare-register-recorded eating disorders in the complete swedish 1979–2001 birth cohort. Int. J. Eat. Disord. 2015, 48, 1070–1081. [Google Scholar] [CrossRef]
- Galmiche, M.; Déchelotte, P.; Lambert, G.; Tavolacci, M.P. Prevalence of eating disorders over the 2000–2018 period: A systematic literature review. Am. J. Clin. Nutr. 2019, 109, 1402–1413. [Google Scholar] [CrossRef]
- Sullivan, P.F.; Agrawal, A.; Bulik, C.M.; Andreassen, O.A.; Børglum, A.D.; Breen, G.; Cichon, S.; Edenberg, H.J.; Faraone, S.V.; Gelernter, J.; et al. Psychiatric genomics: An update and an agenda. Am. J. Psychiatry 2018, 175, 15–27. [Google Scholar] [CrossRef]
- Watson, H.J.; Yilmaz, Z.; Thornton, L.M.; Hübel, C.; Coleman, J.R.I.; Gaspar, H.A.; Bryois, J.; Hinney, A.; Leppä, V.M.; Mattheisen, M.; et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 2019, 51, 1207–1214. [Google Scholar] [CrossRef]
- Godart, N.; Perdereau, F.; Rein, Z.; Berthoz, S.; Wallier, J.; Jeammet, P.; Flament, M. Comorbidity studies of eating disorders and mood disorders. Critical review of the literature. J. Affect. Disord. 2007, 97, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Pallister, E.; Waller, G. Anxiety in the eating disorders: Understanding the overlap. Clin. Psychol. Rev. 2008, 28, 366–386. [Google Scholar] [CrossRef]
- Longo, P.; Marzola, E.; De Bacco, C.; Demarchi, M.; Abbate-Daga, G. Young patients with anorexia nervosa: The contribution of post-traumatic stress disorder and traumatic events. Medicina 2020, 57, 2. [Google Scholar] [CrossRef] [PubMed]
- Breithaupt, L.; Köhler-Forsberg, O.; Larsen, J.T.; Benros, M.E.; Thornton, L.M.; Bulik, C.M.; Petersen, L. Association of exposure to infections in childhood with risk of eating Disorders in adolescent girls. JAMA Psychiatry 2019, 76, 800–809. [Google Scholar] [CrossRef]
- Mårild, K.; Størdal, K.; Bulik, C.M.; Rewers, M.; Ekbom, A.; Liu, E.; Ludvigsson, J.F. Celiac disease and anorexia nervosa: A nationwide study. Pediatrics 2017, 139. [Google Scholar] [CrossRef] [PubMed]
- Syed, F.Z. Type 1 diabetes mellitus. Ann. Intern. Med. 2022, 175, ITC33–ITC48. [Google Scholar] [CrossRef]
- Russell-Jones, D.; Khan, R. Insulin-associated weight gain in diabetes-causes, effects and coping strategies. Diabetes Obes. Metab. 2007, 9, 799–812. [Google Scholar] [CrossRef]
- Winston, A.P. Eating disorders and diabetes. Curr. Diab Rep. 2020, 20, 32. [Google Scholar] [CrossRef]
- Hall, R.; Keeble, L.; Sünram-Lea, S.-I.; To, M. A review of risk factors associated with insulin omission for weight loss in type 1 diabetes. Clin. Child Psychol. Psychiatry 2021, 26, 606–616. [Google Scholar] [CrossRef]
- Hudson, J.I.; Hiripi, E.; Pope, H.G., Jr.; Kessler, R.C. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol. Psychiatry 2007, 61, 348–358. [Google Scholar] [CrossRef]
- Stewart, C.S.; McEwen, F.S.; Konstantellou, A.; Eisler, I.; Simic, M. Impact of ASD traits on treatment outcomes of eating disorders in girls. Eur. Eat. Disord. Rev. 2017, 25, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.K.; Goldschmidt, A.B.; Labuschagne, Z.; Loeb, K.L.; Sawyer, S.M.; Le Grange, D. Eating disorders with and without comorbid depression and anxiety: Similarities and differences in a clinical sample of children and adolescents. Eur. Eat. Disord. Rev. 2013, 21, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Herpertz-Dahlmann, B.; Dempfle, A.; Egberts, K.M.; Kappel, V.; Konrad, K.; Vloet, J.A.; Bühren, K. Outcome of childhood anorexia nervosa-The results of a five- to ten-year follow-up study. Int. J. Eat. Disord. 2018, 51, 295–304. [Google Scholar] [CrossRef]
- Eskild–Jensen, M.; Støving, R.K.; Flindt, C.F.; Sjogren, M. Comorbid depression as a negative predictor of weight gain during treatment of anorexia nervosa: A systematic scoping review. Eur. Eat. Disord. Rev. 2020, 28, 605–619. [Google Scholar] [CrossRef]
- Hjern, A.; Lindberg, L.; Lindblad, F. Outcome and prognostic factors for adolescent female in-patients with anorexia nervosa: 9- to 4-year follow-up. Br. J. Psychiatry 2006, 189, 428–432. [Google Scholar] [CrossRef]
- Hetterich, L.; Mack, I.; Giel, K.E.; Zipfel, S.; Stengel, A. An update on gastrointestinal disturbances in eating disorders. Mol. Cell. Endocrinol. 2018, 497, 110318. [Google Scholar] [CrossRef] [PubMed]
- Schalla, M.A.; Stengel, A. Gastrointestinal alterations in anorexia nervosa–A systematic review. Eur. Eat. Disord. Rev. 2019, 27, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Hay, P.J.; Sachdev, P. Brain dysfunction in anorexia nervosa: Cause or consequence of under-nutrition? Curr. Opin. Psychiatry 2011, 24, 251–256. [Google Scholar] [CrossRef]
- Micali, N.; Dahlgren, C.L. All that glisters is not an endophenotype: Rethinking endophenotypes in anorexia nervosa. Eur. Child Adolesc. Psychiatry 2016, 25, 1149–1150. [Google Scholar] [CrossRef]
- Van Noort, B.M.; Pfeiffer, E.; Ehrlich, S.; Lehmkuhl, U.; Kappel, V. Cognitive performance in children with acute early-onset anorexia nervosa. Eur. Child Adolesc. Psychiatry 2016, 25, 1233–1244. [Google Scholar] [CrossRef]
- Mayhew, A.J.; Pigeyre, M.; Couturier, J.; Meyre, D. An Evolutionary Genetic Perspective of Eating Disorders. Neuroendocrinology 2017, 106, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Alcock, J.; Maley, C.C.; Aktipis, C.A. Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. BioEssays 2014, 36, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.; Le Grange, D. Treatment Manual for Anorexia Nervosa: A Family-Based Approach; Guilford Press: New York, NY, USA, 2015. [Google Scholar]
- Dalle Grave, R.; Eckhardt, S.; Calugi, S.; Le Grange, D. A conceptual comparison of family-based treatment and enhanced cognitive behavior therapy in the treatment of adolescents with eating disorders. J. Eat. Disord. 2019, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, M.; Bjørnelv, S.; Weider, S.; Myklebust, T.; Lundh, H.; Rø, Ø. The outcome at follow-up after inpatient eating disorder treatment: A naturalistic study. J. Eat. Disord. 2020, 8, 67. [Google Scholar] [CrossRef]
- Södersten, P.; Brodin, U.; Sjöberg, J.; Zandian, M.; Bergh, C. Treatment outcomes for eating disorders in Sweden: Data from the national quality registry. BMJ Open 2019, 9, e024179. [Google Scholar] [CrossRef]
- Bergh, C.; Brodin, U.; Lindberg, G.; Södersten, P. Randomized controlled trial of a treatment for anorexia and bulimia nervosa. Proc. Natl. Acad. Sci. USA 2002, 99, 9486–9491. [Google Scholar] [CrossRef]
- Van Elburg, A.A.; Hillebrand, J.J.; Huyser, C.; Snoek, M.; Kas, M.J.; Hoek, H.W.; Adan, R.A. Mandometer treatment not superior to treatment as usual for anorexia nervosa. Int. J. Eat. Disord. 2012, 45, 193–201. [Google Scholar] [CrossRef]
- Calugi, S.; Sartirana, M.; Frostad, S.; Grave, R.D. Enhanced cognitive behavior therapy for severe and extreme anorexia nervosa: An outpatient case series. Int. J. Eat. Disord. 2020, 54, 305–312. [Google Scholar] [CrossRef]
- Couturier, J.; Lock, J. What is recovery in adolescent anorexia nervosa? Int. J. Eat. Disord. 2006, 39, 550–555. [Google Scholar] [CrossRef]
- Wei, Y.; Peng, S.; Lian, C.; Kang, Q.; Chen, J. Anorexia nervosa and gut microbiome: Implications for weight change and novel treatments. Expert Rev. Gastroenterol. Hepatol. 2022, 16, 321–332. [Google Scholar] [CrossRef]
- Mendez-Figueroa, V.; Biscaia, J.M.; Mohedano, R.B.; Blanco-Fernandez, A.; Bailen, M.; Bressa, C.; Larrosa, M.; Gonzalez-Soltero, R. Can Gut Microbiota and Lifestyle Help Us in the Handling of Anorexia Nervosa Patients? Microorganisms 2019, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. The gut-brain axis and the microbiome: Mechanisms and clinical implications. Clin. Gastroenterol. Hepatol. 2019, 17, 322–332. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wei, J.; Chen, T. Editorial: Gut microbiota in the occurrence, development and treatment of gut-brain disorders. Front. Cell. Infect. Microbiol. 2021, 11, 808454. [Google Scholar] [CrossRef]
- Carlson, P.E., Jr. Regulatory considerations for fecal microbiota transplantation products. Cell Host Microbe 2020, 27, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339–1353. [Google Scholar] [CrossRef]
- Vaishnava, S.; Behrendt, C.L.; Ismail, A.S.; Eckmann, L.; Hooper, L.V. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. USA 2008, 105, 20858–20863. [Google Scholar] [CrossRef]
- Zhang, X.; Grosfeld, A.; Williams, E.; Vasiliauskas, D.; Barretto, S.; Smith, L.; Mariadassou, M.; Philippe, C.; Devime, F.; Melchior, C.; et al. Fructose malabsorption induces cholecystokinin expression in the ileum and cecum by changing microbiota composition and metabolism. FASEB J. 2019, 33, 7126–7142. [Google Scholar] [CrossRef]
- Larraufie, P.; Martin-Gallausiaux, C.; Lapaque, N.; Dore, J.; Gribble, F.M.; Reimann, F.; Blottiere, H.M. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep. 2018, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Rogier, R.; Koenders, M.I.; Abdollahi-Roodsaz, S. Toll-like receptor mediated modulation of T cell response by commensal Intestinal microbiota as a Trigger for autoimmune arthritis. J. Immunol. Res. 2015, 2015, 527696. [Google Scholar] [CrossRef] [PubMed]
- Herpertz-Dahlmann, B.; Seitz, J.; Baines, J. Food matters: How the microbiome and gut–brain interaction might impact the development and course of anorexia nervosa. Eur. Child Adolesc. Psychiatry 2017, 26, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- De Punder, K.; Pruimboom, L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front. Immunol. 2015, 6, 223. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Jésus, P.; Ouelaa, W.; François, M.; Riachy, L.; Guérin, C.; Aziz, M.; Rego, J.-C.D.; Déchelotte, P.; Fetissov, S.O.; Coëffier, M. Alteration of intestinal barrier function during activity-based anorexia in mice. Clin. Nutr. 2014, 33, 1046–1053. [Google Scholar] [CrossRef]
- Logsdon, A.F.; Erickson, M.; Rhea, E.M.; Salameh, T.S.; A Banks, W. Gut reactions: How the blood–brain barrier connects the microbiome and the brain. Exp. Biol. Med. 2017, 243, 159–165. [Google Scholar] [CrossRef]
- Navarro-Tapia, E.; Almeida-Toledano, L.; Sebastiani, G.; Serra-Delgado, M.; García-Algar, Ó; Andreu-Fernández, V. Effects of microbiota imbalance in anxiety and eating disorders: Probiotics as novel therapeutic approaches. Int. J. Mol. Sci. 2021, 22, 2351. [Google Scholar] [CrossRef]
- Seitz, J.; Belheouane, M.; Schulz, N.; Dempfle, A.; Baines, J.F.; Herpertz-Dahlmann, B. The Impact of starvation on the microbiome and gut-brain interaction in anorexia nervosa. Front. Endocrinol. 2019, 10, 41. [Google Scholar] [CrossRef]
- Seitz, J.; Dahmen, B.; Keller, L.; Herpertz-Dahlmann, B. Gut feelings: How microbiota might impact the development and course of anorexia nervosa. Nutrients 2020, 12, 3295. [Google Scholar] [CrossRef]
- Carr, J.; Kleiman, S.C.; Bulik, C.M.; Bulik-Sullivan, E.C.; Carroll, I.M. Can attention to the intestinal microbiota improve understanding and treatment of anorexia nervosa? Expert Rev. Gastroenterol. Hepatol. 2016, 10, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Voderholzer, U.; Haas, V.; Correll, C.U.; Korner, T. Medical management of eating disorders: An update. Curr. Opin. Psychiatry 2020, 33, 542–543. [Google Scholar] [CrossRef] [PubMed]
- Kleiman, S.C.; Carroll, I.M.; Tarantino, L.M.; Bulik, C.M. Gut feelings: A role for the intestinal microbiota in anorexia nervosa? Int. J. Eat. Disord. 2015, 48, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Karakula-Juchnowicz, H.; Pankowicz, H.; Juchnowicz, D.; Valverde Piedra, J.L.; Malecka-Massalska, T. Intestinal microbiota–a key to understanding the pathophysiology of anorexia nervosa? Psychiatr. Pol. 2017, 51, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.Y.; Maguire, S.; Palacios, T.; Caterson, I.D. Are the gut bacteria telling us to eat or not to eat? Reviewing the role of gut microbiota in the etiology, disease progression and treatment of eating disorders. Nutrients 2017, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Morita, C.; Tsuji, H.; Hata, T.; Gondo, M.; Takakura, S.; Kawai, K.; Yoshihara, K.; Ogata, K.; Nomoto, K.; Miyazaki, K.; et al. Gut dysbiosis in patients with anorexia nervosa. PLoS ONE 2015, 10, e0145274. [Google Scholar] [CrossRef]
- Di Lodovico, L.; Mondot, S.; Dore, J.; Mack, I.; Hanachi, M.; Gorwood, P. Anorexia nervosa and gut microbiota: A systematic review and quantitative synthesis of pooled microbiological data. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 106, 110114. [Google Scholar] [CrossRef]
- Mason, B.L. Feeding Systems and the Gut Microbiome: Gut-brain interactions with relevance to psychiatric conditions. Psychosomatics 2017, 58, 574–580. [Google Scholar] [CrossRef]
- Borgo, F.; Riva, A.; Benetti, A.; Casiraghi, M.C.; Bertelli, S.; Garbossa, S.; Anselmetti, S.; Scarone, S.; Pontiroli, A.E.; Morace, G.; et al. Microbiota in anorexia nervosa: The triangle between bacterial species, metabolites and psychological tests. PLoS ONE 2017, 12, e0179739. [Google Scholar] [CrossRef] [PubMed]
- Roubalová, R.; Procházková, P.; Papežová, H.; Smitka, K.; Bilej, M.; Tlaskalová-Hogenová, H. Anorexia nervosa: Gut microbiota-immune-brain interactions. Clin. Nutr. 2019, 39, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Kleiman, S.C.; Watson, H.J.; Bulik-Sullivan, E.C.; Huh, E.Y.; Tarantino, L.M.; Bulik, C.M.; Carroll, I.M. The intestinal microbiota in acute anorexia nervosa and during renourishment: Relationship to depression, anxiety, and eating disorder psychopathology. Psychosom. Med. 2015, 77, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Monteleone, P.; Carratù, R.; Cartenì, M.; Generoso, M.; Lamberti, M.; De Magistris, L.; Brambilla, F.; Colurcio, B.; Secondulfo, M.; Maj, M. Intestinal permeability is decreased in anorexia nervosa. Mol. Psychiatry 2003, 9, 76–80. [Google Scholar] [CrossRef]
- Breton, J.; Déchelotte, P.; Ribet, D. Intestinal microbiota and anorexia nervosa. Clin. Nutr. Exp. 2019, 28, 11–21. [Google Scholar] [CrossRef]
- Ms, M.M.K.; Brønstad, I.; Lied, G.A.; Danielsen, Y.; Rekkedal, G.; Kessler, U. Intestinal barrier integrity in anorexia nervosa (a pilot study). Int. J. Eat. Disord. 2022, 55, 703–708. [Google Scholar] [CrossRef]
- Bauer, P.V.; Hamr, S.C.; Duca, F.A. Regulation of energy balance by a gut–brain axis and involvement of the gut microbiota. Cell. Mol. Life Sci. 2016, 73, 737–755. [Google Scholar] [CrossRef]
- Rehfeld, J.F. Cholecystokinin—From local gut hormone to ubiquitous messenger. Front. Endocrinol. 2017, 8, 47. [Google Scholar] [CrossRef]
- Schunck, T.; Erb, G.; Mathis, A.; Gilles, C.; Namer, I.J.; Hode, Y.; Demaziere, A.; Luthringer, R.; Macher, J.-P. Functional magnetic resonance imaging characterization of CCK-4-induced panic attack and subsequent anticipatory anxiety. NeuroImage 2006, 31, 1197–1208. [Google Scholar] [CrossRef]
- Eser, D.; Leicht, G.; Lutz, J.; Wenninger, S.; Kirsch, V.; Schüle, C.; Karch, S.; Baghai, T.; Pogarell, O.; Born, C.; et al. Functional neuroanatomy of CCK-4-induced panic attacks in healthy volunteers. Hum. Brain Mapp. 2007, 30, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Zwanzger, P.; Domschke, K.; Bradwejn, J. Neuronal network of panic disorder: The role of the neuropeptide cholecystokinin. Depress. Anxiety 2012, 29, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Rehfeld, J.F. Cholecystokinin and Panic Disorder: Reflections on the History and Some Unsolved Questions. Molecules 2021, 26, 5657. [Google Scholar] [CrossRef] [PubMed]
- Javanmard, M.; Shlik, J.; Kennedy, S.; Vaccarino, F.J.; Houle, S.; Bradwejn, J. Neuroanatomic correlates of CCK-4-induced panic attacks in healthy humans: A comparison of two time points. Biol. Psychiatry 1999, 45, 872–882. [Google Scholar] [CrossRef]
- Wang, Q.-P.; Guan, J.-L.; Pan, W.; Kastin, A.J.; Shioda, S. A diffusion barrier between the area postrema and nucleus tractus solitarius. Neurochem. Res. 2008, 33, 2035–2043. [Google Scholar] [CrossRef]
- Cuntz, U.; Enck, P.; Frühauf, E.; Lehnert, P.; Riepl, R.L.; Fichter, M.M.; Otto, B. Cholecystokinin revisited: CCK and the hunger trap in anorexia nervosa. PLoS ONE 2013, 8, e54457. [Google Scholar] [CrossRef]
- Koszycki, D.; Copen, J.; Bradwejn, J. Sensitivity to cholecystokinin-tetrapeptide in major depression. J. Affect. Disord. 2004, 80, 285–290. [Google Scholar] [CrossRef]
- Koszycki, D.; Torres, S.; Swain, J.; Bradwejn, J. Central cholecystokinin activity in irritable bowel syndrome, panic disorder, and healthy controls. Psychosom. Med. 2005, 67, 590–595. [Google Scholar] [CrossRef]
- Le Mellédo, J.M.; Merani, S.; Koszycki, D.; Bellavance, F.; Palmour, R.; Gutkowska, J.; Steinberg, S.; Bichet, D.G.; Bradwejn, J. Sensitivity to CCK-4 in women with and without premenstrual dysphoric disorder (PMDD) during their follicular and luteal phases. Neuropsychopharmacology 1999, 20, 81–91. [Google Scholar] [CrossRef][Green Version]
- Lai, J.; Jiang, J.; Zhang, P.; Xi, C.; Wu, L.; Gao, X.; Fu, Y.; Zhang, D.; Chen, Y.; Huang, H.; et al. Impaired blood-brain barrier in the microbiota-gut-brain axis: Potential role of bipolar susceptibility gene TRANK1. J. Cell. Mol. Med. 2021, 25, 6463–6469. [Google Scholar] [CrossRef]
- Mishra, A.K.; Dubey, V.; Ghosh, A.R. Obesity: An overview of possible role(s) of gut hormones, lipid sensing and gut microbiota. Metabolism 2015, 65, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Scharner, S.; Stengel, A. Animal models for anorexia nervosa—A systematic review. front. Hum. Neurosci. 2020, 14, 596381. [Google Scholar] [CrossRef] [PubMed]
- Legrand, R.; Lucas, N.; Breton, J.; Azhar, S.; Do Rego, J.C.; Déchelotte, P.; Coëffier, M.; Fetissov, S.O. Ghrelin treatment prevents development of activity based anorexia in mice. Eur. Neuropsychopharmacol. 2016, 26, 948–958. [Google Scholar] [CrossRef]
- Ueno, H.; Yamaguchi, H.; Mizuta, M.; Nakazato, M. The role of PYY in feeding regulation. Regul. Pept. 2008, 145, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut hormone PYY3-36 physiologically inhibits food intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Dominique, M.; Lucas, N.; Legrand, R.; Bouleté, I.-M.; Bôle-Feysot, C.; Deroissart, C.; Léon, F.; Nobis, S.; Rego, J.-C.D.; Lambert, G.; et al. Effects of bacterial CLPB protein fragments on food intake and PYY secretion. Nutrients 2021, 13, 2223. [Google Scholar] [CrossRef] [PubMed]
- Painsipp, E.; Herzog, H.; Holzer, P. The gut-mood axis: A novel role of the gut hormone peptide YY on emotional-affective behaviour in mice. BMC Pharmacol. 2009, 9, A13. [Google Scholar] [CrossRef]
- Tennoune, N.; Chan, P.; Breton, J.; Legrand, R.; Chabane, Y.N.; Akkermann, K.; Järv, A.; Ouelaa, W.; Takagi, K.; Ghouzali, I.; et al. Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide alpha-MSH, at the origin of eating disorders. Transl. Psychiatry 2014, 4, e458. [Google Scholar] [CrossRef]
- Fairburn, C.G. Ending Well. In Cognitive Behavior Therapy and Eating Disorders; Fairburn, C.G., Ed.; The Guilford Press: New York, NY, USA, 2008; pp. 183–193. [Google Scholar]
- Bulik, C.M.; Carroll, I.M.; Mehler, P. Reframing anorexia nervosa as a metabo-psychiatric disorder. Trends Endocrinol. Metab. 2021, 32, 752–761. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Gröbner, E.M.; Zeiler, M.; Fischmeister, F.P.S.; Kollndorfer, K.; Schmelz, S.; Schneider, A.; Haid-Stecher, N.; Sevecke, K.; Wagner, G.; Keller, L.; et al. The effects of probiotics administration on the gut microbiome in adolescents with anorexia nervosa-A study protocol for a longitudinal, double-blind, randomized, placebo-controlled trial. Eur. Eat. Disord. Rev. 2022, 30, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Kamintsky, L.; Cairns, K.A.; Veksler, R.; Bowen, C.; Beyea, S.D.; Friedman, A.; Calkin, C. Blood-brain barrier imaging as a potential biomarker for bipolar disorder progression. NeuroImage Clin. 2019, 26, 102049. [Google Scholar] [CrossRef] [PubMed]
| Diagnostic Criteria |
| Restriction of energy intake relative to requirements in anorexia nervosa leads to a significantly low body weight for the patient’s age, sex, developmental trajectory and physical health. A significantly low weight is defined as a weight that is less than the minimal normal weight or (in children and adolescents) less than the minimum expected weight. Intense fear of gaining weight or becoming fat, or persistent behaviour that interferes with weight gain, even though the patient has a significantly low weight. Disturbance in the way in which one’s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or persistent lack of recognition of the seriousness of the current low body weight. |
| Subtype Designation |
| Restricting subtype: During the past 3 months, the patient has not engagd in recurrent episodes of binge-eating or purging behaviour (i.e., self-induced vomiting or the misuse of laxatives, diuretics or enemas). Weight loss is primarily caused through dieting, fasting, excessive exercise, or all of these methods. Binge-eating/purging subtype: During the past 3 months, the patient has engaged in recurrent episodes of binge-eating or purging behavior (i.e., self-induced vomiting or the misuse of laxatives, diuretics or enemas). |
| Current Severity |
| Mildly severe low body weight is defined as a BMI ≥ 17.00 kg/m2 Moderately severe low body weight is defined as a BMI of 16.00–16.99 kg/m2 Severe low body weight is defined as a BMI of 15.00–15.99 kg/m2 Extremely severe low body weight is defined as a BMI < 15.00 kg/m2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frostad, S. Are the Effects of Malnutrition on the Gut Microbiota–Brain Axis the Core Pathologies of Anorexia Nervosa? Microorganisms 2022, 10, 1486. https://doi.org/10.3390/microorganisms10081486
Frostad S. Are the Effects of Malnutrition on the Gut Microbiota–Brain Axis the Core Pathologies of Anorexia Nervosa? Microorganisms. 2022; 10(8):1486. https://doi.org/10.3390/microorganisms10081486
Chicago/Turabian StyleFrostad, Stein. 2022. "Are the Effects of Malnutrition on the Gut Microbiota–Brain Axis the Core Pathologies of Anorexia Nervosa?" Microorganisms 10, no. 8: 1486. https://doi.org/10.3390/microorganisms10081486
APA StyleFrostad, S. (2022). Are the Effects of Malnutrition on the Gut Microbiota–Brain Axis the Core Pathologies of Anorexia Nervosa? Microorganisms, 10(8), 1486. https://doi.org/10.3390/microorganisms10081486






