Regulation of Immune Homeostasis via Muramyl Peptides-Low Molecular Weight Bioregulators of Bacterial Origin
Abstract
1. Introduction
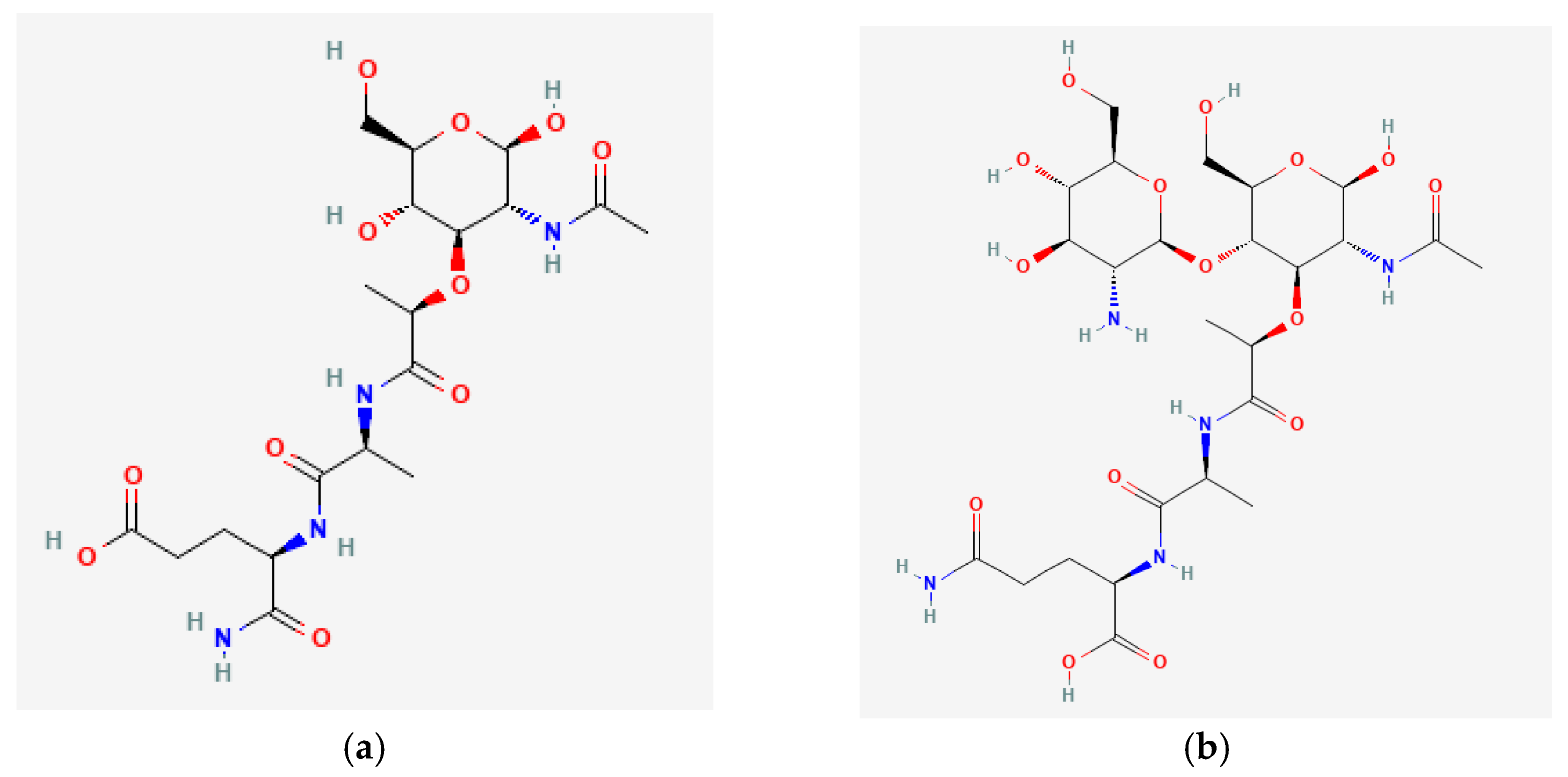
2. Sources of Muramyl Peptides in the Human’s Body
3. Effect of Muramyl Peptides on Microorganisms
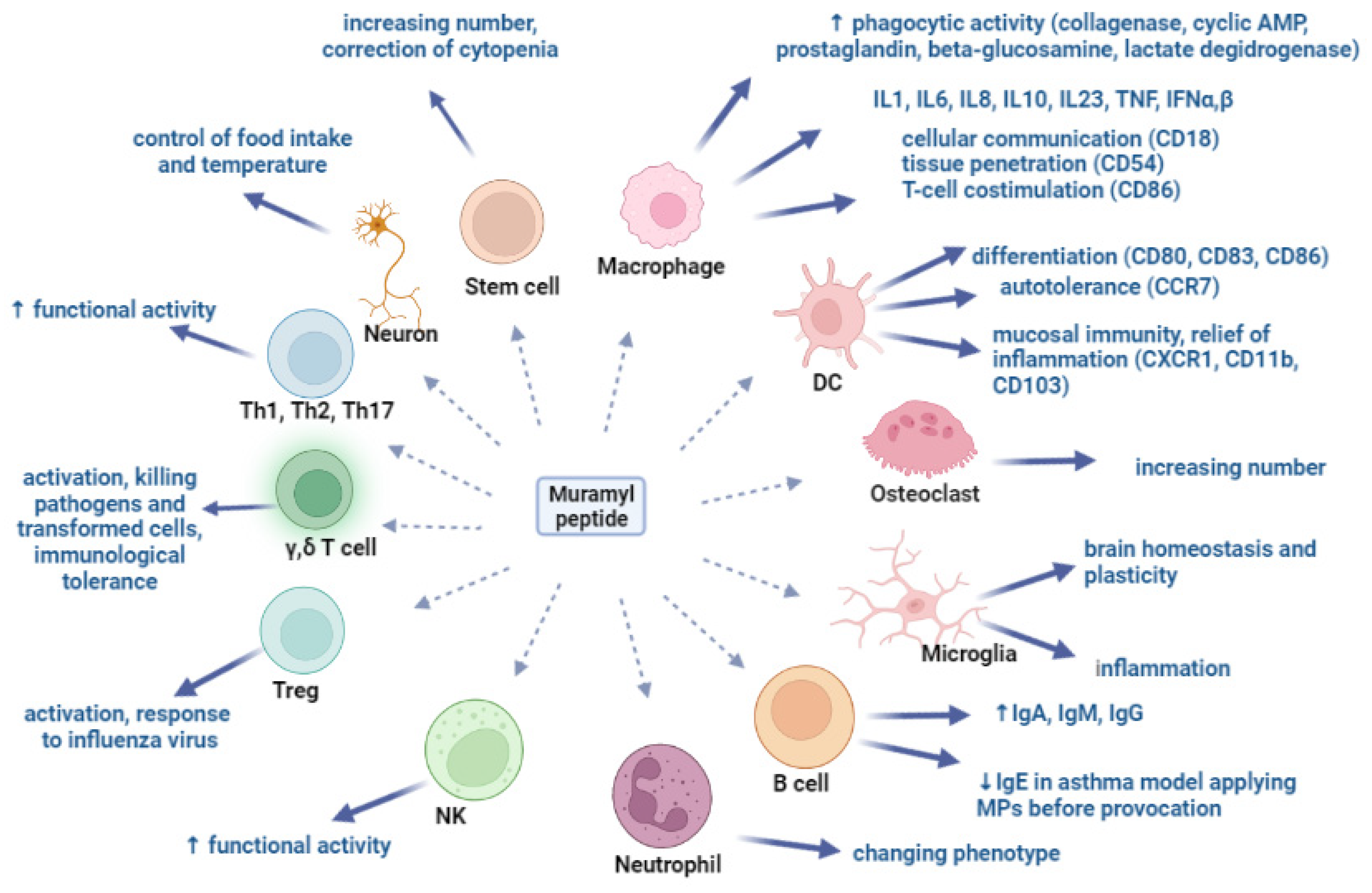
4. Muramyl Peptides Activation of Various Cell Populations
5. Muramyl Peptide Regulation of Intracellular Signaling Pathways
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Louca, S.; Shih, P.M.; Pennell, M.W.; Fischer, W.W.; Parfrey, L.W.; Doebeli, M. Bacterial diversification through geological time. Nat. Ecol. Evol. 2018, 2, 1458–1467. [Google Scholar] [CrossRef]
- Li, C.; Liang, Y.; Qiao, Y. Messengers from the gut: Gut microbiota-derived metabolites on host regulation. Front. Microbiol. 2022, 13, 863407. [Google Scholar] [CrossRef]
- Chedid, L. Muramyl peptides as possible endogenous immunopharmacological mediators. Microbiol. Immunol. 1983, 27, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.V.; Khaitov, R.M. Strategies for using muramyl peptides-modulators of innate immunity of bacterial origin-in medicine. Front. Immunol. 2021, 12, 607178. [Google Scholar] [CrossRef]
- Tukhvatulin, A.; Dzharullaeva, A.; Erokhova, A.; Zemskaya, A.; Balyasin, M.; Ozharovskaia, T.; Zubkova, O.; Shevlyagina, N.; Zhukhovitsky, V.; Fedyakina, I.; et al. Adjuvantation of an influenza hemagglutinin antigen with TLR4 and NOD2 agonists encapsulated in poly(D,L-lactide-Co-glycolide) nanoparticles enhances immunogenicity and protection against lethal influenza virus infection in mice. Vaccines 2020, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.; Schwab, J.H.; Cochran, T. Muramic acid detection in mammalian tissues by gas-liquid chromatography-mass spectrometry. Infect. Immun. 1980, 29, 526–531. [Google Scholar] [CrossRef]
- Kozar, M.P.; Laman, J.D.; Fox, A. Muramic acid is not generally present in the human spleen as determined by gas chromatography-tandem mass spectrometry. Infect. Immun. 2002, 70, 741–748. [Google Scholar] [CrossRef]
- Szponar, B.; Larsson, L. Use of mass spectrometry for characterising microbial communities in bioaerosols. Ann. Agric. Environ. Med. 2001, 8, 111–117. [Google Scholar] [PubMed]
- Huang, Z.; Wang, J.; Xu, X.; Wang, H.; Qiao, Y.; Chu, W.C.; Xu, S.; Chai, L.; Cottier, F.; Pavelka, N.; et al. Antibody neutralization of microbiota-derived circulating peptidoglycan dampens inflammation and ameliorates autoimmunity. Nat. Microbiol. 2019, 4, 766–773. [Google Scholar] [CrossRef]
- Dziarski, R.; Gupta, D. Peptidoglycan recognition in innate immunity. J. Endotoxin Res. 2005, 11, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Dziarski, R.; Royet, J.; Gupta, D. Peptidoglycan recognition proteins and lysozyme. Encycl. Immunobiol. 2016, 2, 389–403. [Google Scholar] [CrossRef]
- Vollmer, W.; Bertsche, U. Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli. Biochim. Biophys. Acta (BBA)–Biomembr. 2008, 1778, 1714–1734. [Google Scholar] [CrossRef] [PubMed]
- Irazoki, O.; Hernandez, S.B.; Cava, F. Peptidoglycan muropeptides: Release, perception, and functions as signaling molecules. Front. Microbiol. 2019, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Küssau, T.; Van Wyk, N.; Johansen, M.D.; Alsarraf, H.M.A.B.; Neyret, A.; Hamela, C.; Sørensen, K.K.; Thygesen, M.B.; Beauvineau, C.; Kremer, L.; et al. Functional characterization of the N-acetylmuramyl-l-alanine amidase, ami1, from Mycobacterium abscessus. Cells 2020, 9, 2410. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Huynh, B.-T.; Guillemot, D.; Glaser, P.; Opatowski, L. Inference of significant microbial interactions from longitudinal metagenomics data. Front. Microbiol. 2018, 9, 2319. [Google Scholar] [CrossRef] [PubMed]
- Vermassen, A.; Leroy, S.; Talon, R.; Provot, C.; Popowska, M.; Desvaux, M. Cell wall hydrolases in bacteria: Insight on the diversity of cell wall amidases, glycosidases and peptidases toward peptidoglycan. Front. Microbiol. 2019, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sun, M.; Pan, Z.; Lu, C.; Yao, H. Diverse toxic effectors are harbored by vgrG islands for interbacterial antagonism in type VI secretion system. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2018, 1862, 1635–1643. [Google Scholar] [CrossRef]
- Urdániz, E.; Martín, M.; Payaslián, F.; Defelipe, L.A.; Dodes, M.; Martinez, M.; Alzari, P.M.; Cabrera, G.; Martí, M.A.; Piuri, M. Gp29 LysA of mycobacteriophage TM4 can hydrolyze peptidoglycan through an N-acetyl-muramoyl-L-alanine amidase activity. Biochim. Biophys. Acta Proteins Proteom. 2022, 1870, 140745. [Google Scholar] [CrossRef] [PubMed]
- Lopez, R.; Garcia, E.; Ronda, C. Bacteriophages of Streptococcus pneumoniae. Rev. Infect. Dis. 1981, 3, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W.; Joris, B.; Charlier, P.; Foster, S. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol. Rev. 2008, 32, 259–286. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.; Wang, L.; Guo, J.; Liu, W.; Meng, G.; Zhang, L.; Li, M.; Cong, L.; Sun, M. Recent insights into the prognostic and therapeutic applications of lysozymes. Front. Pharmacol. 2021, 12, 767642. [Google Scholar] [CrossRef]
- Prescott, D.; Maisonneuve, C.; Yadav, J.; Rubino, S.J.; Girardin, S.E.; Philpott, D.J. NOD2 modulates immune tolerance via the GM-CSF–dependent generation of CD103+ dendritic cells. Proc. Natl. Acad. Sci. USA 2020, 117, 10946–10957. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.; Fox, K. Rapid elimination of a synthetic adjuvant peptide from the circulation after systemic administration and absence of detectable natural muramyl peptides in normal serum at current analytical limits. Infect. Immun. 1991, 59, 1202–1205. [Google Scholar] [CrossRef]
- Kozar, M.P.; Krahmer, M.T.; Fox, A.; Gray, B.M. Failure to detect muramic acid in normal rat tissues but detection in cerebrospinal fluids from patients with Pneumococcal meningitis. Infect. Immun. 2000, 68, 4688–4698. [Google Scholar] [CrossRef]
- Parant, M.; Parant, F.; Chedid, L.; Yapo, A.; Petit, J.F.; Lederer, E. Fate of the synthetic immunoadjuvant, muramyl dipeptide (14C-Iabelled) in the mouse. Int. J. Immunopharmacol. 1979, 1, 35–41. [Google Scholar] [CrossRef]
- Molinaro, R.; Mukherjee, T.; Flick, R.; Philpott, D.J.; Girardin, S.E. Trace levels of peptidoglycan in serum underlie the NOD-dependent cytokine response to endoplasmic reticulum stress. J. Biol. Chem. 2019, 31, 9007–9015. [Google Scholar] [CrossRef] [PubMed]
- Park, J.T. Turnover and recycling of the murein sacculus in oligopeptide permease-negative strains of Escherichia coli: Indirect evidence for an alternative permease system and for a monolayered sacculus. J. Bacteriol. 1993, 175, 7–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goodell, E.W. Recycling of murein by Escherichia coli. J. Bacteriol. 1985, 163, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Schaub, R.E.; Dillard, J.P. The Pathogenic neisseria use a streamlined set of peptidoglycan degradation proteins for peptidoglycan remodeling, recycling, and toxic fragment release. Front. Microbiol. 2019, 10, 73. [Google Scholar] [CrossRef]
- Goodell, E.W.; Schwarz, U. Высвoбoждение пептидoв клетoчнoй стенки в культуральную среду экспoненциальнo растущей Escherichia coli. Дж. Бактериoл 1985, 162, 391–397. [Google Scholar]
- Goldman, W.E.; Klapper, D.G.; Baseman, J.B. Detection, isolation, and analysis of a released Bordetella pertussis product toxic to cultured tracheal cells. Infect. Immun. 1982, 36, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Cookson, B.T.; Cho, H.L.; Herwaldt, L.A.; Goldman, W.E. Biological activities and chemical composition of purified tracheal cytotoxin of Bordetella pertussis. Infect. Immun. 1989, 57, 2223–2229. [Google Scholar] [CrossRef]
- Sinha, R.K.; Rosenthal, R.S. Release of soluble peptidoglycan from growing conococci: Demonstration of anhydro-muramyl-containing fragments. Infect. Immun. 1980, 29, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.S.; Nogami, W.; Cookson, B.T.; Goldman, W.E.; Folkening, W.J. Major fragment of soluble peptidoglycan released from growing Bordetella pertussis is tracheal cytotoxin. Infect. Immun. 1987, 55, 2117–2120. [Google Scholar] [CrossRef]
- Nigro, G.; Fazio, L.L.; Martino, M.C.; Rossi, G.; Tattoli, I.; Liparoti, V.; De Castro, C.; Molinaro, A.; Philpott, D.J.; Bernardini, M.L. Muramylpeptide shedding modulates cell sensing of Shigella flexneri. Cell Microbiol. 2008, 10, 682–695. [Google Scholar] [CrossRef]
- Wysocka, M.; Dzierzbicka, K.; Krawczyk, B. Evaluating the antibacterial activity of muramyl dipeptide derivatives, retro-tuftsin derivatives, and anthraquinone oligopeptides against a range of pathogenic bacteria. Acta Biochim. Pol. 2021, 68, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Dik, D.A.; Fisher, J.F.; Mobashery, S. Cell-wall recycling of the gram-negative bacteria and the nexus to antibiotic resistance. Chem. Rev. 2018, 118, 5952–5984. [Google Scholar] [CrossRef]
- Nikitushkin, V.D.; Demina, G.R.; Shleeva, M.O.; Guryanova, S.V.; Ruggiero, A.; Berisio, R.; Kaprelyants, A.S. A product of RpfB and RipA joint enzymatic action promotes the resuscitation of dormant mycobacteria. FEBS J. 2015, 282, 2500–2511. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.V.; Borisova OYu Kolesnikova, N.V.; Lezhava, N.L.; Kozlov, I.G.; Gudima, G.O. Effect of muramyl peptide on the microbial landscape of the oral cavity. Immunologiya 2019, 40, 34–40. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Kolesnikova, N.V.; Gudima, G.O.; Lezhava, N.L.; Karaulov, A.V. Dynamics of immunological and microbiological indicators of oral fluid in caries therapy. Immunologiya 2021, 42, 386–394. [Google Scholar] [CrossRef]
- Venkataprasad, N. Evidence of differential mycobacterial growth and modulation of mycobactericidal property by glucoaminylmuramyl dipeptide in murine macrophages. Ann. N. Y. Acad. Sci. 1997, 832, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Chedid, L.; Parant, M.; Parant, F.; Lefrancher, P.; Choay, J.; Lederer, E. Enhancement of nonspecific immunity to Klebsiella pneumoniae infection by a synthetic immunoadjuvant (N-acetylmuramyl-L-alanyl-D-isoglutamine) and several analogs. Proc. Natl. Acad. Sci. USA 1977, 74, 2089–2093. [Google Scholar] [CrossRef] [PubMed]
- Parant, M.; Parant, F.; Chedid, L. Enhancement of the neonate’s nonspecific immunity to Klebsiella infection by muramyl dipeptide, a synthetic immunoadjuvant. Proc. Natl. Acad. Sci. USA 1978, 75, 3395–3399. [Google Scholar] [CrossRef] [PubMed]
- Venkataprasad, N.; Ledger, P.; Ivanyi, J. The effect of glucosaminylmuramyl dipeptide injection to mice on the course of tuberculous infection and in vitro superoxide anion production. Int. Arch. Allergy Immunol. 1997, 114, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Meshcheryakova, E.; Guryanova, S.; Makarov, E.; Alekseeva, L.; Andronova, T.; Ivanov, V. Prevention of experimental septic shock by pretreatment of mice with muramyl peptides. Int. Immunopharmacol. 2001, 1, 1857–1865. [Google Scholar] [CrossRef]
- Grabchenko, N.I.; Karpov, A.V.; VIa, C.; Tkachikova, L.; Spivak, N. Vliianie sinteticheskikh proizvodnykh muramildipeptida na techenie stafilokokkovoĭ infektsii u mysheĭ [Effect of synthetic muramyl dipeptide derivatives on staphylococcal infection in mice]. Zh. Mikrobiol. Epidemiol. Immunobiol. 2001, 3, 50–53. (In Russian) [Google Scholar]
- Fraser-Smith, E.B.; Matthews, T.R. Protective effect of muramyl dipeptide analogs against infections of Pseudomonas aeruginosa or Candida albicans in mice. Infect. Immun. 1981, 34, 676–683. [Google Scholar] [CrossRef]
- Onozuka, K.; Shinomiya, H.; Cho, N.; Saito-Taki, T.; Nakano, M. The adjuvant effect of a muramyl dipeptide (MDP) analog on temperature-sensitive Salmonella mutant vaccine. Int. J. Immunopharmacol. 1989, 11, 781–787. [Google Scholar] [CrossRef]
- Fraser-Smith, E.B.; Waters, R.V.; Matthews, T.R. Correlation between in vivo anti-Pseudomonas and anti-Candida activities and clearance of carbon by the reticuloendothelial system for various muramyl dipeptide analogs, using normal and immunosuppressed mice. Infect. Immun. 1982, 35, 105–110. [Google Scholar] [CrossRef]
- Phillips, N.C.; Chedid, L. Anti-infectious activity of liposomal muramyl dipeptides in immunodeficient CBA/N mice. Infect. Immun. 1987, 55, 1426–1430. [Google Scholar] [CrossRef] [PubMed]
- Onozuka, K.; Saito-Taki, T.; Nakano, M. Augmentation of protective and antibacterial activity induced by muramyl dipeptides in CBA/N defective mice with X-linked immunodeficiency for Salmonella enteritidis infection. Infect. Immun. 1984, 45, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Lupfer, C.; Thomas, P.G.; Kanneganti, T.-D. Nucleotide oligomerization and binding domain 2-dependent dendritic cell activation is necessary for innate immunity and optimal CD8+ T cell responses to influenza A virus infection. J. Virol. 2014, 88, 8946–8955. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, T.; Hovingh, E.S.; Foerster, E.G.; Abdel-Nour, M.; Philpott, D.J.; Girardin, S.E. NOD1 and NOD2 in inflammation, immunity and disease. Arch. Biochem. Biophys. 2019, 670, 69–81. [Google Scholar] [CrossRef]
- Cummings, N.P.; Pabst, M.J.; Johnston, R.B., Jr. Activation of macrophages for enhanced release of superoxide anion and greater killing of Candida albicans by injection of muramyl dipeptide. J. Exp. Med. 1980, 152, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Girardin, S.E.; Boneca, I.G.; Viala, J.; Chamaillard, M.; Labigne, A.; Thomas, G.; Philpott, D.J.; Sansonetti, P.J. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 2003, 278, 8869–8872. [Google Scholar] [CrossRef] [PubMed]
- Inohara, N.; Ogura, Y.; Fontalba, A.; Gutierrez, O.; Pons, F.; Crespo, J.; Fukase, K.; Inamura, S.; Kusumoto, S.; Hashimoto, M.; et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J. Biol. Chem. 2003, 278, 5509–5512. [Google Scholar] [CrossRef] [PubMed]
- Girardin, S.E.; Boneca, I.G.; Carneiro, L.A.M.; Antignac, A.; Jehanno, M.; Viala, J.; Tedin, K.; Taha, M.K.; Labigne, A.; Zäthringer, U.; et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 2003, 300, 1584–1587. [Google Scholar] [CrossRef]
- Kanazawa, N.; Okafuji, I.; Kambe, N.; Nishikomori, R.; Nakata-Hizume, M.; Nagai, S.; Fuji, A.; Yuasa, T.; Manki, A.; Sakurai, Y.; et al. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: Common genetic etiology with Blau syndrome. Blood 2005, 105, 1195–1197. [Google Scholar] [CrossRef]
- Miceli-Richard, C.; Lesage, S.; Rybojad, M.; Prieur, A.M.; Manouvrier-Hanu, S.; Hafner, R.; Chamaillard, M.; Zouali, H.; Thomas, G.; Hugot, J.P. CARD15 mutations in Blau syndrome. Nat. Genet. 2001, 29, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Trindade, B.C.; Chen, G.Y. NOD1 and NOD2 in inflammatory and infectious diseases. Immunol Rev. 2020, 297, 139–161. [Google Scholar] [CrossRef] [PubMed]
- Kutikhin, A.G. Role of NOD1/CARD4 and NOD2/CARD15 gene polymorphisms in cancer etiology. Hum. Immunol. 2011, 72, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Brand, R.E.; Locker, G.Y. Inflammatory bowel disease in Ashkenazi Jews: Implications for familial colorectal cancer. Fam. Cancer 2004, 3, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Bialecka, M.; Kurzawski, M.; Klodowska-Duda, G.; Opala, G.; Juzwiak, S.; Kurzawski, G.; Tan, E.K.; Drozdzik, M. CARD15 variants in patients with sporadic Parkinson’s disease. Neurosci. Res. 2007, 57, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Cummings, N.P.; Neifert, M.R.; Pabst, M.J.; Johnston, R.B., Jr. Oxidative metabolic response and microbicidal activity of human milk macrophages: Effect of lipopolysaccharide and muramyl dipeptide. Infect Immun. 1985, 49, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Pabst, M.J.; Beranova-Giorgianni, S.; Krueger, J.M. Effects of muramyl peptides on macrophages, monokines, and sleep. Neuroimmunomodulation 1999, 6, 261–283. [Google Scholar] [CrossRef] [PubMed]
- Pashenkov, M.V.; Balyasova, L.S.; Dagil, Y.A.; Pinegin, B.V. The role of the p38-MNK-eIF4E signaling axis in TNF production downstream of the NOD1 receptor. J. Immunol. 2017, 198, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.; Udzhukhu, V.; Kubylinsky, A. Pathogenetic therapy of psoriasis by muramyl peptide. Front. Immunol. 2019, 10, 1275. [Google Scholar] [CrossRef]
- Heinzelmann, M.; Polk, H.C., Jr.; Chernobelsky, A.; Stites, T.P.; Gordon, L.E. Endotoxin and muramyl dipeptide modulate surface receptor expression on human mononuclear cells. Immunopharmacology 2000, 48, 117–128. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Kudryashova, N.A.; Kataeva, A.A.; Orozbekova, B.T.; Kolesnikova, N.V.; Chuchalin, A.G. Novel approaches to increase resistance to acute respiratory infections. RUDN J. Med. 2021, 25, 181–195. [Google Scholar] [CrossRef]
- Traub, S.; Kubasch, N.; Morath, S.; Kresse, M.; Hartung, T.; Schmidt, R.R.; Hermann, C. Structural requirements of synthetic muropeptides to synergize with lipopolysaccharide in cytokine induction. J. Biol. Chem. 2004, 279, 8694–8700. [Google Scholar] [CrossRef] [PubMed]
- Pashenkov, M.V.; Murugina, N.E.; Budikhina, A.S.; Pinegin, B.V. Synergistic interactions between NOD receptors and TLRs: Mechanisms and clinical implications. J. Leukoc. Biol. 2019, 105, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, E.S.; Bagaev, A.V.; Chulkina, M.M.; Pichugin, A.V.; Ataullakhanov, R.I. Synergistic activation of gene transcription encoding type I interferons and cytokines in macrophages and dendritic cells by the combinations of two PRR-agonists. Immunologiya 2017, 38, 64–71. [Google Scholar] [CrossRef]
- Pouillart, P.R.; Audibert, F.M.; Chedid, L.A.; Lefrancier, P.L.; Bahr, G.M. Enhancement by muramyl peptides of the protective response of interferon-α/β against encephalomyocarditis virus infection. Int. J. Immunopharmacol. 1996, 18, 183–192. [Google Scholar] [CrossRef]
- Fevrier, M.; Birrien, J.L.; Leclerc, C.; Chedid, L.; Liacopoulos, P. The macrophage, target cell of the synthetic adjuvant muramyl dipeptide. Eur. J. Immunol. 1978, 8, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Meshcheryakova, E.A.; Guryanova, S.V.; Makarov, E.A.; Andronova, T.M.; Ivanov, V.T. Structure-function investigation of glucosaminylmuramoylpeptides. Influence of chemical modification of N-acetylglucosaminyl-Nacetylmuramoyldipeptide (GMDP) on its immunomodulatory properties in vivo and in vitro. Bioorg. Chem. 1991, 17, 1157–1165. [Google Scholar]
- Fukuyama, R.; Takeda, H.; Fushiki, S.; Yamamoto, T. Muramyl dipeptide injected into crushed sciatic nerve, activates macrophages and promotes recovery of walking locomotion in rats. Restor. Neurol. Neurosci. 1998, 13, 213–219. [Google Scholar] [PubMed]
- Todate, A.; Suda, T.; Kuwata, H.; Chida, K.; Nakamura, H. Muramyl dipeptide-Lys stimulates the function of human dendritic cells. J. Leukoc. Biol. 2001, 70, 723–729. [Google Scholar] [PubMed]
- Kurobe, H.; Liu, C.; Ueno, T.; Saito, F.; Ohigashi, I.; Seach, N.; Arakaki, R.; Hayashi, Y.; Kitagawa, T.; Lipp, M.; et al. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity 2006, 24, 165–177. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Sugiyama, M.; Hemmi, H.; Yamazaki, C.; Okura, S.; Sasaki, I. Crucial roles of XCR1-expressing dendritic cells and the XCR1-XCL1 chemokine axis in intestinal immune homeostasis. Sci Rep. 2016, 6, 23505. [Google Scholar] [CrossRef]
- Alexandre, Y.O.; Ghilas, S.; Sanchez, C.; Le Bon, A.; Crozat, K.; Dalod, M. XCR1+ dendritic cells promote memory CD8+ T cell recall upon secondary infections with Listeria monocytogenes or certain viruses. J. Exp. Med. 2016, 213, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Takahama, Y. XCL1 and XCR1 in the immune system. Microbes Infect. 2012, 14, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Ripen, A.M.; Ishimaru, N.; Ohigashi, I.; Nagasawa, T.; Jeker, L.T.; Bösl, M.R.; Holländer, G.A.; Hayashi, Y.; Malefyt Rde, W.; et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J. Exp. Med. 2011, 208, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Kitaura, H.; Kimura, K.; Sugisawa, H.; Aonuma, T.; Takada, H.; Takano-Yamamoto, T. Muramyl dipeptide enhances lipopolysaccharide-induced osteoclast formation and bone resorption through increased RANKL expression in stromal cells. J. Immunol. Res. 2015, 2015, 132765. [Google Scholar] [CrossRef] [PubMed]
- Ke, K.; Sul, O.; Chung, S.; Suh, J.; Choi, H. Lack of NOD2 attenuates ovariectomy-induced bone loss via inhibition of osteoclasts. J. Endocrinol. 2017, 235, 85–96. Available online: https://joe.bioscientifica.com/view/journals/joe/235/2/JOE-16-0591.xml (accessed on 11 January 2021). [CrossRef]
- Wang, M.; Ye, X.; Hu, J.; Zhao, Q.; Lv, B.; Ma, W.; Wang, W.; Yin, H.; Hao, Q.; Zhou, C.; et al. NOD1/RIP2 signalling enhances the microglia-driven inflammatory response and undergoes crosstalk with inflammatory cytokines to exacerbate brain damage following intracerebral haemorrhage in mice. J. Neuroinflamm. 2020, 17, 364. [Google Scholar] [CrossRef]
- Tsujimoto, M.; Kinoshita, F.; Okunaga, T.; Kotani, S.; Kusumoto, S.; Yamamoto, K.; Shiba, T. Higher immunoadjuvant activities of N-acetyl-beta-D-glucosaminyl-(1-4)-N-acetylmuramyl-L-alanyl-D-isoglutamine in comparison with N-acetylmuramyl-L-alanyl-D-isoglutamine. Microbiol. Immunol. 1979, 23, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Wuest, B.; Wachsmuth, E.D. Stimulatory effect of N-acetyl muramyl dipeptide in vivo: Proliferation of bone marrow progenitor cells in mice. Infect. Immun. 1982, 37, 452–462. [Google Scholar] [CrossRef]
- Ellouz, F.; Adam, A.; Ciorbaru, R.; Lederer, E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem. Biophys. Res. Commun. 1974, 59, 1317–1325. [Google Scholar]
- O’Reilly, T.; Zak, O. Enhancement of the effectiveness of antimicrobial therapy by muramyl peptide immunomodulators. Clin. Infect. Dis. 1992, 14, 1100–1109. [Google Scholar] [PubMed]
- Merser, C.; Sinay, P.; Adam, A. Total synthesis and adjuvant activity of bacterial peptidoglycan derivatives. Biochem. Biophys. Res. Commun. 1975, 66, 1316–1322. [Google Scholar] [CrossRef]
- Ogawa, C.; Liu, Y.J.; Kobayashi, K.S. Muramyl dipeptide and its derivatives: Peptide adjuvant in immunological disorders and cancer therapy. Curr. Bioact. Compd. 2011, 7, 180–197. [Google Scholar] [CrossRef]
- Konorev, M.R.; Guryanova, S.V.; Tyshevich, E.N.; Pavlyukov, R.A.; Borisova, O.Y. Advisable including glucosaminylmuramyldipeptide in Helicobacter pylori therapy: Experience of ten-year investigation. RUDN J. Med. 2020, 24, 269–282. [Google Scholar] [CrossRef]
- Adam, A.; Lederer, E. Muramyl peptides: Immunomodulators, sleep factors, and vitamins. In Medical Research Reviews; Stevens, D.G., Ed.; Wiley and Sons: New York, NY, USA, 1984; Volume 4, pp. 111–152. [Google Scholar] [CrossRef]
- Kotani, S.; Watanabe, Y.; Kinoshita, F.; Shimono, T.; Morisaki, I. Immunoadjuvant activities of synthetic N-acetyl-muramyl-peptides or -amino acids. Biken J. J. Res. Inst. Microb. Dis. 1975, 18, 105–111. [Google Scholar]
- Heymer, B.; Finger, H.; Wirsing, C.H. Immunoadjuvant effects of the synthetic muramyl-dipeptide (MDP) N-acetylmuramyl-L-alanyl-D-isoglutamine. Z. Immunitatsforsch. Immunobiol. 1978, 155, 87–92. [Google Scholar] [CrossRef]
- Bomford, R. Will adjuvants be needed for vaccines of the future? Dev. Biol. Stand. 1998, 92, 13–17. [Google Scholar] [PubMed]
- Kolesnikova, N.V.; Kozlov, I.G.; Guryanova, S.V.; Kokov, E.A.; Andronova, T.M. Clinical and immunological efficiency of muramyl dipeptide in the treatment of atopic diseases. Med. Immunol. 2016, 18, 15–20. [Google Scholar] [CrossRef]
- Morisaki, I.; Michalek, S.M.; Harmon, C.C.; Torii, M.; Hamada, S.; McGhee, J.R. Effective immunity to dental caries: Enhancement of salivary anti-Streptococcus mutans antibody responses with oral adjuvants. Infect. Immun. 1983, 40, 577–591. [Google Scholar] [CrossRef]
- Kalyuzhin, O.V.; Letyaeva, O.I.; Ziganshin, O.R.; Markeeva, D.A.; Blokhina, Y.u.V.; Fedenko, E.S.; Popilyuk, S.F. A combination of three muramyl peptides derived from gramnegative bacteria in immunotherapy of chronic pyodermia. Med. Immunol. 2019, 21, 1187–1196. (In Russian) [Google Scholar] [CrossRef]
- Moschos, S.A.; Bramwell, V.W.; Somavarapu, S.; Alpar, H.O. Adjuvant synergy: The effects of nasal coadministration of adjuvants. Immunol. Cell Biol. 2004, 82, 628–637. [Google Scholar] [CrossRef]
- Yoo, Y.C.; Yoshimatsu, K.; Koike, Y.; Hatsuse, R.; Yamanishi, K.; Tanishita, O.; Arikawa, J.; Azuma, I. Adjuvant activity of muramyl dipeptide derivatives to enhance immunogenicity of a hantavirus-inactivated vaccine. Vaccine 1998, 16, 216–224. [Google Scholar] [CrossRef]
- Rechkina, E.A.; Denisova, G.F.; Masalova, O.V.; Lideman, L.F.; Denisov, D.A.; Lesnova, E.I.; Ataullakhanov, R.I.; Gur’ianova, S.V.; Kushch, A.A. Epitope mapping of antigenic determinants of hepatitis C virus proteins by phage display. Mol. Biol. 2006, 40, 357–368. (In Russian) [Google Scholar] [CrossRef]
- Souvannavong, V.; Adam, A. Opposite effects of the synthetic adjuvant N-acetyl-muramyl-L-alanyl-D-isoglutamine on the immune response in mice depending on experimental conditions. Eur. J. Immunol. 1980, 10, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Zunic, M.; Kricek, F.; Dukor, P.; Bahr, G.M. Oral administration of muramyl dipeptide into mice modulates cell proliferation, immunoglobulin synthesis, and cytokine mRNA levels in gut associated lymphoid tissues. Int. J. Immunopharmacol. 1996, 18, 155–162. [Google Scholar] [CrossRef]
- Souvannavong, V.; Brown, S.; Adam, A. Muramyl dipeptide (MDP) synergizes with interleukin 2 and interleukin 4 to stimulate, respectively, the differentiation and proliferation of B cells. Cell Immunol. 1990, 126, 106–116. [Google Scholar] [CrossRef]
- Nesterova, I.V.; Khaidukov, S.V.; Nguyen, T.D.L.; Rongina, A.N.; Guryanova, S.V. Glucosaminylmuramyl dipeptide modulate experimental transformed phenotype of neutrophilic granulocytes of healthy persons. Russ. J. Immunol. 2017, 11, 737–740. (In Russian) [Google Scholar]
- Jeong, Y.-J.; Kang, M.-J.; Lee, S.-J.; Kim, C.-H.; Kim, J.-C.; Kim, T.-H.; Kim, D.-J.; Kim, D.; Núñez, G.; Park, J.-H. Nod2 and Rip2 contribute to innate immune responses in mouse neutrophils. Immunology 2014, 143, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Nesterova, I.V.; Nguyen, T.D.L.; Khalturina, E.; Khaidukov, S.V.; Guryanova, S.V. The modulatory effects of glucosaminylmuramildipeptide on the transformed phenotype of the subset of Ifna/br1+Ifngr+TLR4+ neutrophilic granulocytes of patients with chronic herpes-viral infections in the experiment in vitro. Russ. Immunol. J. 2018, 12, 379–384. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Gaponov, A.M.; Pisarev, V.M.; Yakushenko, E.V.; Tutelyan, A.V.; Aleksandrov, I.A.; Tsipandina, E.V.; Kozlov, I.G. Glucosaminylmuramyl dipeptide acid (GMDP-A) modulates the intracellular signaling pathways of natural killer cells. Immunologiya 2020, 41, 235–248. (In Russian) [Google Scholar] [CrossRef]
- Athie’-Morales, V.; O’Connor, G.M.; Gardiner, C.M. Activation of human Nk cells by the bacterial pathogen-associated molecular pattern muramyl dipeptide. J. Immunol. 2008, 180, 4082–4089. [Google Scholar] [CrossRef] [PubMed]
- Manni, M.; Ding, W.; Stohl, L.L.; Granstein, R.D. Muramyl dipeptide induces Th17 polarization through activation of endothelial cells. J. Immunol. 2011, 186, 3356–3363. [Google Scholar] [CrossRef] [PubMed]
- Egarnes, B.; Gosselin, J. Contribution of regulatory T cells in nucleotide-binding oligomerization domain 2 response to influenza virus infection. Front. Immunol. 2018, 9, 132. [Google Scholar] [CrossRef]
- Magalhaes, J.G.; Fritz, J.H.; Le Bourhis, L.; Sellge, G.; Travassos, L.H.; Selvanantham, T.; Girardin, S.E.; Gommerman, J.L.; Philpott, D.J. Nod2-dependent Th2 polarization of antigen-specific immunity. J. Immunol. 2008, 181, 7925–7935. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.V.; Kozlov, I.G.; Meshcheryakova, E.A.; Alekseeva, L.G.; Andronova, T.M. Investigation into the influence of glucosaminylmuramyl dipeptide on the normalization of Th1/TH2 balance in patients with atopic bronchial asthma. Immunol. Immunol. 2009, 30, 305–309. (In Russian) [Google Scholar]
- Hornung, R.L.; Longo, D.L.; Gowda, V.L.; Kwak, L.W. Induction of a CD8+ cytotoxic T lymphocyte response to soluble antigen given together with a novel muramyl dipeptide adjuvant, N-acetyl-D-glucosaminyl-(beta 1-4)-N-acetylmuramyl-L-alanyl-D-isoglutamine (GMDP). Ther. Immunol. 1995, 2, 7–14. [Google Scholar]
- Lin, G.H.Y.; Wortzman, M.E.; Girardin, S.E.; Philpott, D.J.; Watts, T.H. T Cell intrinsic Nod2 is dispensable for CD8 T Cell immunity. PLoS ONE 2013, 8, e56014. [Google Scholar] [CrossRef]
- Kerns, H.M.M.; Jutila, M.A.; Hedges, J.F. The distinct response of gd T cells to the Nod2 agonist muramyl dipeptide. Cell Immunol. 2009, 257, 38–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manapova, E.R.; Fazylov, V.K.H.; Guryanova, S.V. Сytopenia and their correction in antiviral therapy of chronic hepatitis C in patients with genotype 1. Probl. Virol. 2017, 62, 174–178. (In Russian) [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dokter, W.H.; Dijkstra, A.J.; Koopmans, S.B.; Mulder, A.B.; Stulp, B.K.; Halie, M.R.; Keck, W.; Vellenga, E. G(AnH)MTetra, a naturally occurring 1,6-anhydro muramyl dipeptide, induces granulocyte colony-stimulating factor expression in human monocytes: A molecular analysis. Infect. Immun. 1994, 62, 2953–2957. [Google Scholar] [CrossRef] [PubMed]
- Gorskaya, Y.F.; Semenova, E.N.; Nagurskaya, E.V.; Bekhalo, V.A.; Nesterenko, V.G. Simultaneous administration of NOD-2 (MDP) and TLP-4 (LPS) ligands to bone marrow donors 24 h before transplantation increases the content of multipotent stromal cells (MSCs) in bone marrow grafts in CBA mice compared to the total result of their isolated administration. Bull. Exp. Biol. Med. 2021, 172, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Gabanyi, I.; Lepousez, G.; Wheeler, R.; Vieites-Prado, A.; Nissant, A.; Wagner, S.; Moigneu, C.; Dulauroy, S.; Hicham, S.; Polomack, B.; et al. Bacterial sensing via neuronal Nod2 regulates appetite and body temperature. Science 2022, 376, eabj3986. [Google Scholar] [CrossRef]
- Ismair, M.G.; Vavricka, S.R.; Kullak-Ublick, G.A.; Fried, M.; Mengin-Lecreulx, D.; Girardin, S.E. hPepT1 selectively transports muramyl dipeptide but not Nod1-activating muramyl peptides. Can. J. Physiol. Pharmacol. 2007, 84, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Marina-García, N.; Franchi, L.; Kim, Y.G.; Miller, D.; McDonald, C.; Boons, G.J.; Núñez, G. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2. J. Immunol. 2008, 180, 4050–4057. [Google Scholar] [CrossRef]
- Caruso, R.; Warner, N.; Inohara, N.; Núñez, G. NOD1 and NOD2: Signaling, host defense, and inflammatory disease. Immunity 2014, 41, 898–908. [Google Scholar] [CrossRef]
- Viala, J.; Chaput, C.; Boneca, I.G.; Cardona, A.; Girardin, S.E.; Moran, A.P.; Athman, R.; Mémet, S.; Huerre, M.R.; Coyle, A.J.; et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 2004, 5, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Murray, P.J.; Kitani, A.; Watanabe, T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 2006, 6, 9–20. [Google Scholar] [CrossRef]
- Vijayrajratnam, S.; Pushkaran, A.C.; Balakrishnan, A.; Vasudevan, A.K.; Biswas, R.; Mohan, C.G. Understanding the molecular differential recognition of muramyl peptide ligands by LRR domains of human NOD receptors. Biochem. J. 2017, 474, 2691–2711. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yao, Q.; Shen, M.; McDonald, C.; Lacbawan, F.; Moran, R.; Shen, B. NOD2-associated autoinflammatory disease: A large cohort study. Rheumatology 2015, 54, 1904–1912. [Google Scholar] [CrossRef]
- McDonald, C.; Shen, M.; Johnson, E.E.; Kabi, A.; Yao, Q. Alterations in nucleotide-binding oligomerization domain-2 expression, pathway activation, and cytokine production in Yao syndrome. Autoimmunity 2018, 51, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Moran, R.; Tomecki, K.J.; Yao, Q. Granulomatous disease associated with NOD2 sequence variants and familial camptodactyly: An intermediate form of NOD2-associated diseases? Semin. Arthritis Rheum. 2015, 45, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Caso, F.; Galozzi, P.; Costa, L.; Sfriso, P.; Cantarini, L.; Punzi, L. Autoinflammatory granulomatous diseases: From Blau syndrome and early-onset sarcoidosis to NOD2-mediated disease and Crohn’s disease. RMD Open 2015, 1, e000097. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Kaneko, N.; Ito, Y.; Takeda, H.; Sawasaki, T.; Heike, T.; Migita, K.; Agematsu, K.; Kawakami, A.; Morikawa, S.; et al. Nod2-nodosome in a cell-free system: Implications in pathogenesis and drug discovery for Blau syndrome and early-onset sarcoidosis. Sci. World J. 2016, 2016, 2597376. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Inohara, N.; Benito, A.; Chen, F.F.; Yamaoka, S.; Nunez, G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J. Biol. Chem. 2001, 276, 4812–4818. [Google Scholar] [CrossRef]
- Kanayama, A.; Seth, R.B.; Sun, L.; Ea, C.K.; Hong, M.; Shaito, A.; Chiu, Y.H.; Deng, L.; Chen, Z.J. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol. Cell 2004, 15, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Deng, L.; Hong, M.; Akkaraju, G.R.; Inoue, J.; Chen, Z.J. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 2001, 412, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Draber, P.; Kupka, S.; Reichert, M.; Draberova, H.; Lafont, E.; de Miguel, D.; Spilgies, L.; Surinova, S.; Taraborrelli, L.; Hartwig, T.; et al. LUBAC-recruited CYLD and A20 regulate gene activation and cell death by exerting opposing effects on linear ubiquitin in signaling complexes. Cell Rep. 2015, 13, 2258–2272. [Google Scholar] [CrossRef] [PubMed]
- Walczak, H.; Iwai, K.; Dikic, I. Generation and physiological roles of linear ubiquitin chains. BMC Biol. 2012, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Pauleau, A.L.; Murray, P.J. Role of Nod2 in the response of macrophages to Toll-like receptor agonists. Mol. Cell Biol. 2003, 23, 7531–7539. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.S.; Chamaillard, M.; Ogura, Y.; Henegariu, O.; Inohara, N.; Nuñez, G.; Flavell, R.A. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 2005, 307, 731–734. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, M.S.; Kim, D.J.; Yang, S.J.; Lee, S.J.; Noh, E.J.; Shin, S.J.; Park, J.H. Nucleotide-binding oligomerization domain 2 contributes to limiting growth of mycobacterium abscessus in the lung of mice by regulating cytokines and nitric oxide production. Front. Immunol. 2017, 8, 1477. [Google Scholar] [CrossRef] [PubMed]
- Damiano, J.S.; Oliveira, V.; Welsh, K.; Reed, J.C. Heterotypic interactions among NACHT domains: Implications for regulation of innate immune responses. Biochem. J. 2004, 381, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Barnich, N.; Hisamatsu, T.; Aguirre, J.E.; Xavier, R.; Reinecker, H.C.; Podolsky, D.K. GRIM-19 interacts with nucleotide oligomerization domain 2 and serves as downstream effector of anti-bacterial function in intestinal epithelial cells. J. Biol. Chem. 2005, 280, 19021–19026. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Lu, H.; Hao, A.; Ng, D.C.; Ponniah, S.; Guo, K.; Lufei, C.; Zeng, Q.; Cao, X. GRIM-19, a cell death regulatory protein, is essential for assembly and function of mitochondrial complex I. Mol. Cell Biol. 2004, 24, 8447–8456. [Google Scholar] [CrossRef] [PubMed]
- Jhun, J.; Woo, J.S.; Lee, S.H.; Jeong, J.H.; Jung, K.; Hur, W.; Lee, S.Y.; Ryu, J.Y.; Moon, Y.M.; Jung, Y.J.; et al. GRIM19 impedes obesity by regulating inflammatory white fat browning and promoting Th17/Treg balance. Cells 2021, 10, 162. [Google Scholar] [CrossRef]
- Wang, D.; Wei, X.; Chen, X.; Wang, Q.; Zhang, J.; Kalvakolanu, D.V.; Guo, B.; Zhang, L. GRIM-19 inhibits proliferation and induces apoptosis in a p53-dependent manner in colorectal cancer cells through the SIRT7/PCAF/MDM2 axis. Exp. Cell Res. 2021, 407, 112799. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Lee, S.H.; Lee, S.Y.; Ryu, J.; Jhun, J.; Choi, J.; Kim, G.N.; Roh, S.; Park, S.H.; Cho, M.L. GRIM-19 ameliorates multiple sclerosis in a mouse model of experimental autoimmune encephalomyelitis with reciprocal regulation of IFNγ/Th1 and IL-17A/Th17 cells. Immune Netw. 2020, 20, e40. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.; Hartman, Y.; Groh, L.; de Jong, D.J.; de Jonge, M.I.; Ferwerda, G. Recognition of Streptococcus pneumoniae and muramyl dipeptide by NOD2 results in potent induction of MMP-9, which can be controlled by lipopolysaccharide stimulation. Infect. Immun. 2014, 82, 4952–4958. [Google Scholar] [CrossRef] [PubMed]
- Hedl, M.; Li, J.; Cho, J.H.; Abraham, C. Chronic stimulation of Nod2 mediates tolerance to bacterial products. Proc. Natl. Acad. Sci. USA 2007, 104, 19440–19445. [Google Scholar] [CrossRef] [PubMed]
- Bansal, K.; Balaji, K.N. Intracellular pathogen sensor NOD2 programs macrophages to trigger Notch1 activation. J. Biol. Chem. 2011, 286, 5823–5835. [Google Scholar] [CrossRef]
- Negroni, A.; Pierdomenico, M.; Cucchiara, S.; Stronati, L. NOD2 and inflammation: Current insights. J. Inflamm. Res. 2018, 11, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Hitotsumatsu, O.; Ahmad, R.C.; Tavares, R.; Wang, M.; Philpott, D.; Turer, E.E.; Lee, B.L.; Shiffin, N.; Advincula, R.; Malynn, B.A.; et al. The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals. Immunity 2008, 28, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Fiil, B.K.; Damgaard, R.B.; Wagner, S.A.; Keusekotten, K.; Fritsch, M.; Bekker-Jensen, S.; Mailand, N.; Choudhary, C.; Komander, D.; Gyrd-Hansen, M. OTULIN restricts Met1-linked ubiquitination to control innate immune signaling. Mol. Cell 2013, 50, 818–830. [Google Scholar] [CrossRef]
- Wex, K.; Schmid, U.; Just, S.; Wang, X.; Wurm, R.; Naumann, M.; Schlüter, D.; Nishanth, G. Receptorinteracting protein kinase-2 inhibition by cyld impairs antibacterial immune responses in macrophages. Front. Immunol. 2016, 6, 650. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Gekara, N.O. The deubiquitinase MYSM1 dampens NOD2-mediated inflammation and tissue damage by inactivating the RIP2 complex. Nat. Commun. 2018, 9, 4654. [Google Scholar] [CrossRef]
- Homer, C.R.; Kabi, A.; Marina-García, N.; Sreekumar, A.; Nesvizhskii, A.I.; Nickerson, K.P.; Chinnaiyan, A.M.; Nuñez, G.; McDonald, C. A dual role for receptor-interacting protein kinase 2 (RIP2) kinase activity in nucleotide-binding oligomerization domain 2 (NOD2)-dependent autophagy. J. Biol. Chem. 2012, 287, 25565–25576. [Google Scholar] [CrossRef]
- Lee, K.H.; Biswas, A.; Liu, Y.J.; Kobayashi, K.S. Proteasomal degradation of Nod2 protein mediates tolerance to bacterial cell wall components. J. Biol. Chem. 2012, 287, 39800–39811. [Google Scholar] [CrossRef] [PubMed]
- Kufer, T.A.; Kremmer, E.; Banks, D.J.; Philpott, D.J. Role for erbin in bacterial activation of Nod2. Infect. Immun. 2006, 74, 3115–3124. [Google Scholar] [CrossRef]
- Yamamoto-Furusho, J.K.; Barnich, N.; Xavier, R.; Hisamatsu, T.; Podolsky, D.K. Centaurin β1 down-regulates nucleotide-binding oligomerization domains 1- and 2-dependent NF-κB activation. J. Biol. Chem. 2006, 281, 36060–36070. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Ellison, L.K.; Ramjeet, M.; Travassos, L.H.; Jones, N.L.; Girardin, S.E.; Philpott, D.J. The protein ATG16L1 suppresses inflammatory cytokines induced by the intracellular sensors Nod1 and Nod2 in an autophagy-independent manner. Immunity 2013, 39, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Travassos, L.H.; Carneiro, L.A.; Ramjeet, M.; Hussey, S.; Kim, Y.G.; Magalhães, J.G.; Yuan, L.; Soares, F.; Chea, E.; Le Bourhis, L.; et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat. Immunol. 2010, 11, 55–62. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Khaitov, R.M. Glucosaminylmuramyldipeptide–GMDP: Effect on mucosal immunity (on the issue of immunotherapy and immunoprophylaxis). Immunologiya 2020, 41, 174–183. [Google Scholar] [CrossRef]
- Laman, A.G.; Lathe, R.; Shepelyakovskaya, A.O.; Gartseva, A.; Brovko, F.A.; Guryanova, S.; Alekseeva, L.; Meshcheryakova, E.A.; Ivanov, V.T. Muramyl peptides activate innate immunity conjointly via YB1 and NOD2. Innate Immun. 2016, 22, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Agostini, L.; Meylan, E.; Tschopp, J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr. Biol. 2004, 14, 1929–1934. [Google Scholar] [CrossRef]
- Nabatov, A.A.; Hatzis, P.; Rouschop, K.M.; van Diest, P.; Vooijs, M. Hypoxia inducible NOD2 interacts with 3-O-sulfogalactoceramide and regulates vesicular homeostasis. Biochim. Biophys. Acta 2013, 1830, 5277–5286. [Google Scholar] [CrossRef]
- Blomqvist, M.; Osterbye, T.; Månsson, J.E.; Buschard, K.; Fredman, P. Uptake of the glycosphingolipid sulfatide in the gastrointestinal tract and pancreas in vivo and in isolated islets of Langerhans. Lipids Health Dis. 2006, 5, 26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, S.; Hedl, M.; Abraham, C. TAM receptor-dependent regulation of SOCS3 and MAPKs contributes to proinflammatory cytokine downregulation following chronic NOD2 stimulation of human macrophages. J. Immunol. 2015, 194, 1928–1937. [Google Scholar] [CrossRef]
- Guryanova, S.; Guryanova, A. sbv IMPROVER: Modern approach to systems biology. Methods Mol. Biol. 2017, 1613, 21–29. [Google Scholar] [CrossRef] [PubMed]
- sbv IMPROVER Project Team (in alphabetical order); Boue, S.; Fields, B.; Hoeng, J.; Park, J.; Peitsch, M.C.; Schlage, W.K.; Talikka MChallenge Best Performers (in alphabetical order); Binenbaum, I.; Bondarenko, V.; et al. Enhancement of COPD biological networks using a web-based collaboration interface. F1000Research 2015, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- sbv IMPROVER Project Team and Challenge Best Performers; Namasivayam, A.A.; Morales, A.F.; Lacave, Á.M.; Tallam, A.; Simovic, B.; Alfaro, D.G.; Bobbili, D.R.; Martin, F.; Androsova, G.; et al. Community-reviewed biological network models for toxicology and drug discovery applications. Gene Regul. Syst. Biol. 2016, 10, 51–66. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guryanova, S.V. Regulation of Immune Homeostasis via Muramyl Peptides-Low Molecular Weight Bioregulators of Bacterial Origin. Microorganisms 2022, 10, 1526. https://doi.org/10.3390/microorganisms10081526
Guryanova SV. Regulation of Immune Homeostasis via Muramyl Peptides-Low Molecular Weight Bioregulators of Bacterial Origin. Microorganisms. 2022; 10(8):1526. https://doi.org/10.3390/microorganisms10081526
Chicago/Turabian StyleGuryanova, Svetlana V. 2022. "Regulation of Immune Homeostasis via Muramyl Peptides-Low Molecular Weight Bioregulators of Bacterial Origin" Microorganisms 10, no. 8: 1526. https://doi.org/10.3390/microorganisms10081526
APA StyleGuryanova, S. V. (2022). Regulation of Immune Homeostasis via Muramyl Peptides-Low Molecular Weight Bioregulators of Bacterial Origin. Microorganisms, 10(8), 1526. https://doi.org/10.3390/microorganisms10081526





