Abstract
Recent multidrug resistance in Pseudomonas aeruginosa has favoured the adaptation and dissemination of worldwide high-risk strains. In June 2018, 15 P. aeruginosa strains isolated from patients and a contaminated multi-dose meropenem vial were characterized to assess their association to an outbreak in a Mexican paediatric hospital. The strains were characterized by antibiotic susceptibility profiling, virulence factors’ production, and biofilm formation. The clonal relationship among isolates was determined with pulse-field gel electrophoresis (PFGE) and multi-locus sequence typing (MLST) sequencing. Repressor genes for the MexAB-OprM efflux pump were sequenced for haplotype identification. Of the strains, 60% were profiled as extensively drug-resistant (XDR), 33% as multidrug-resistant (MDR), and 6.6% were classified as sensitive (S). All strains presented intermediate resistance to colistin, and 80% were sensitive to aztreonam. Pyoverdine was the most produced virulence factor. The PFGE technique was performed for the identification of the outbreak, revealing eight strains with the same electrophoretic pattern. ST235 and ten new sequence types (STs) were identified, all closely related to ST233. ST3241 predominated in 26.66% of the strains. Twenty-five synonymous and seventeen nonsynonymous substitutions were identified in the regulatory genes of the MexAB-OprM efflux pump, and nalC was the most variable gene. Six different haplotypes were identified. Strains from the outbreak were metallo-β-lactamases and phylogenetically related to the high-risk clone ST233.
1. Introduction
Pseudomonas aeruginosa is a free-living microorganism that can survive and grow in a nutrient limitation microenvironment [1]. This bacterium is considered as one of the main opportunist pathogens in hospital wards, capable of contaminating a wide variety of objects, solutions, medicines, and even disinfectants, which can all lead to infection in immunocompromised patients [2]. P. aeruginosa stands out worldwide as one of the main causes of an important rate of healthcare-associated infections (HAIs), defined as infections acquired within a healthcare setting during patients’ stays and not present during hospital admission [3,4].
A multicentre study carried out in Mexico showed that P. aeruginosa causes 24% of HAIs in paediatric patients, showing special resistance to carbapenems, quinolones, and third generation cephalosporins, with a major frequency in intensive care units (ICUs) and highly involved in outbreaks [4].
Epidemic HAIs usually occur during outbreaks [5], defined as an unusual increase in the number of infections (two or more) related to each other, and caused by the same pathogen with geotemporal associations [6]. When researching suspected outbreaks, a list of potential patients and several hospital environmental factors must be considered, such as the clinical records of the patients, including treatments, devices, or material used in their care, geographic location of hospital wards, possible interactions among patients, common cleaning supplies, common health personnel, and the moment where the disease is taking place, among others [6,7].
Although epidemic outbreaks are infrequent, they represent an important problem due to the increase in morbidity and mortality rates [6]. The genetic relationship of the suspected strains associated within an outbreak must be confirmed or refuted, to determine the source of infection, possible reservoirs, routes of transmission, and therefore control their spread [6,8]. Next-generation whole genome sequencing (WGS) has become the new gold standard for bacterial typing due to its accuracy for not only epidemiological research in hospital environments, but also different sources, and its capability to generate highly supported phylogenetic trees. However, clustering of epidemiologically linked isolates obtained by WGS is difficult to set due to the typing method and the bacterium, additional to sharp discrimination of clonal populations and sequencing costs. Pulsed-field gel electrophoresis (PFGE) has shown to be as accurate as WGS-based typing by revealing to be less affected than WGS-based typing by accelerated genetic drift, which usually occurs in epidemic P. aeruginosa, demonstrating that the technique can still be employed for identification of outbreak-associated strains, additional to the multi-locus sequence typing (MLST) of the P. aeruginosa core genome genes [9].
In recent decades, multidrug-resistant P. aeruginosa high-risk clones have been frequently implicated in hospital outbreaks [7], being responsible for the increasing rates of morbidity and mortality worldwide [10]. High-risk clones exhibit a great capability to accumulate mutations, resistance genes (extended-spectrum β-lactamases or carbapenemases-encoding genes), and exotoxins, allowing their intrahospital persistence, transmission, and their association within multidrug-resistance dissemination among bacterial species [11,12,13,14]. Worldwide, a group of successful high-risk clones (ST111, ST175, ST235, ST233, and ST253) stands out due to the characteristics mentioned above [15,16,17,18], additionally to multidrug resistance, high biofilm production, low pyocyanin and pyoverdine production, and motility, possibly due to the physiological cost that the expression of resistance determinants produces to the bacteria [10,19]. In Mexico, our group first reported the ST233 and ST1725 clones, both registered in the periods of 2007 and 2013, which stand out as the first identification of an extensively drug resistant ST233 clone [18].
The identification and notification of main sequence types (STs) involved in outbreaks in different parts of the world apport relevant information to their spread control, to determine the sources of infection, reservoirs, and possible routes of transmission [8]. In addition, it has been observed that identical STs, or phylogenetically related STs, share similar characteristics of multidrug resistance, virulence factors, and haplotypes (mutational patterns) in the repressor genes of the constitutive MexAB-OprM efflux pump (mexR, nalC, and nalD) [20] characteristics that could be used for their control and eradication.
According to the history of circulation of multidrug-resistant P. aeruginosa clones in a paediatric hospital, the aim of this study was to characterize the P. aeruginosa isolates associated with an outbreak, in different wards of a third-level healthcare institute in Mexico in June 2018, to establish the source and identify the clone or clones that caused this event.
2. Materials and Methods
2.1. Biological Samples
In this study, a total of 15 isolates, which were associated with an outbreak from the Hospital Infantil de México Federico Gómez (HIMFG) reported in June 2018, were analysed: 14 strains were isolated from 10 paediatric patients, and 1 from a multi-dose meropenem vial. The patients were in different and distant ward rooms: emergency room, surgical therapy (STx), neonatal intensive care unit (NICU), oncology, and infectious diseases outpatient. Patients who were associated to the outbreak were found to be geographically distant from each other. Identification of the isolates was preliminarily carried out by culturing in both selective and differential media (Mueller-Hinton, Cetrimide (BD BBL, Sparks, MD, USA)), macroscopic and microscopic morphology, and traditional biochemical tests. Confirmation of bacterial identity was performed with the MALDI-TOF automated system (Biomerieux, Marcy l‘Etoile, France).
2.2. Susceptibility Profile
The susceptibility profiles to 14 antibiotics from 9 different categories were evaluated by the minimal inhibitory concentration method (MIC), through the microdilution technique, according to the manual of the Clinical Laboratory Standard Institute (CLSI) [21]. Fosfomycin was evaluated by the plate dilution technique. The antibiotics tested in this study were: Gentamicin (GEN), Tobramycin (TOB), Amikacin (AK), Meropenem (MEM), Imipenem (IMI), Ceftazidime (CAZ), Cefepime (CPM), Ciprofloxacin (CIP), Levofloxacin (LEV), Carbenicillin (CB), Piperacillin/Tazobactam (P/T), Aztreonam (AZT), Fosfomycin (FOS), and Colistin (CS). All antibiotics employed were from Sigma-Aldrich, St Louis, MO, USA. The Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25922 (American Type Culture Collection, Manassas, VA, USA) reference strains were both employed for validation of all techniques. The breakpoint values (µg/mL) employed for the interpretation of the MIC for the P. aeruginosa clinical strains were according to the CLSI, 2021 [21].
Once the susceptibility profiles were obtained, all strains were classified as sensitive (S), intermediate (I), resistant (R), multidrug-resistant (MDR), extensively drug-resistant (XDR), or pan drug-resistant (PDR), as established by Magiorakos et al. [22].
2.3. Phenotypic Screening and Detection of Carbapenemases
Detection and typing of carbapenemases (serine carbapenemase or metallo-β-lactamase) was performed using the modified carbapenems inactivation method (mCIM) and the EDTA-modified carbapenem inactivation method (eCIM), both described by the CLSI, 2021 [21].
2.4. Phenotypic Production of Pyocyanin and Pyoverdine
Phenotypic production of pyocyanin and pyoverdine was determined by measuring the absorbance of both pigments at 520 and 407 nm, respectively, in an Epoch Microplate Spectrophotometer (BioTek, software Gen5TM, Winooski, VT, USA). Briefly, P. aeruginosa strains were grown on blood agar at 37 °C for 24 h. Colonies were recovered and cultured in Mueller-Hinton broth and adjusted to 1 × 106 colony forming units per millilitre (CFU/mL) (with and without antibiotic), placing 1 mL in a 24-well microplate, and incubated at 37 °C for 24 h. Before incubation, absorbance measuring at 520 and 407 nm (initial absorbance) was performed. Then, 800 μL of these cultures and 480 μL of chloroform (Sigma-Aldrich) were both added to a tube and homogenized, and the organic phase (pyoverdine) was measured at 407 mn (final absorbance). From the same tube, 300 μL of the aqueous phase was placed in a new tube with 800 μL of 0.2 N HCl (Sigma-Aldrich) and homogenized. Finally, the absorbance of the aqueous phase at 520 nm (pyocyanin) was measured (final absorbance). Three replicates were performed per strain. The initial absorbance was subtracted from the final absorbance.
The concentration of antibiotic tested corresponded to the dilution prior to the MIC value determined for each antibiotic, for each of the evaluated strains. A heatmap diagram was built with the obtained results, using the RStudio v. 0.01 program (Vienna, Austria) [23]. The concentration of pyocyanin was calculated in µg/mL, by multiplying the obtained absorbance by the factor 17.072 [24,25].
2.5. Phenotypic Production of Biofilm
Phenotypic production of biofilm was determined by the crystal violet staining method [26]. Briefly, P. aeruginosa strains were grown in TSA broth (BD BBL) for 24 h at 37 °C. A 1:100 dilution was performed, to place 100 μL per well in a 96-well microplate, previously filled with 100 μL of TSA per well (with and without antibiotic). Absorbance at 550 nm (initial absorbance) was measured before incubation at 37 °C for 24 h. Then, the microplates were washed with 200 μL of PBS, 1X per well. The supernatant was removed and fixed with heat for a second wash, as described above. The microplate was then stained with 200 μL of 0.5% crystal violet for 10 min, and two washes were performed with PBS 1X. Finally, 200 μL of alcohol–acetone 1:1 was added to quantify the absorbance at 550 nm. Eight replicates were performed per strain. The initial absorbance was subtracted from the final absorbance. The concentration of antibiotic tested was the dilution prior to the MIC value for each antibiotic, for each evaluated strain. A heatmap diagram was built with the obtained results, using the RStudio v. 0.01 program (Vienna, Austria) [23].
2.6. Pulsed-Field Gel Electrophoresis (PFGE)
All P. aeruginosa strains were cultured on blood agar for 18 h at 37 °C. The isolates were treated with lysozyme and proteinase K (Sigma-Aldrich), embedded in 1.2% agarose blocks, and stored in cell lysis buffer (Tris 1 M pH = 8.0, EDTA 0.5 M pH = 8.0, Sarcosil 10%) and proteinase K overnight. Enzymatic digestion was performed with the SpeI enzyme (Jena Bioscience, Jena, Germany), at 37 °C for 2.5 h. Electrophoretic running was performed by pulsed-field in a high-fidelity 1.2% agarose gel, with the CHEF-DRII equipment (Bio-Rad Life Science Research, Hercules, CA, USA), described as follows: an initial pulse of 5 s, final pulse of 40 s, 5.2 V/cm2, and maintaining a constant temperature of 14 °C for 22 h. After ethidium bromide (BrEt) (Sigma-Aldrich) staining and washing, the gel was visualized on an iBright CL1000 photo documenter (Thermo Fischer Scientific Inc., Waltham, MA, USA).
For the analysis of the electrophoretic patterns, a binary matrix (presence–absence of bands) was created. A phylogenetic tree was built applying the unweighted pair group method with arithmetic mean (UPGMA) in RStudio v.0.01 (Vienna, Austria) [23], a similarity ≥90% was established as the breakpoint value to consider the clusters reliable. In addition, according to criteria of Tenover et al. [27], based on the differences in the number of bands, the electrophoretic patterns were categorized as indistinguishable, closely related, possibly related, or unrelated to the outbreak.
2.7. Multi-Locus Sequence Typing (MLST)
Isolation of bacterial genomic deoxyribonucleic acid (DNA) was performed using the Wizard Genomic DNA purification kit (Promega, Madison, WI, USA) following the manufacturer’s instructions. For the multi-locus sequence typing (MLST) technique, the amplification and sequencing of seven housekeeping genes (acsA, aroE, guaA, mutL, nuoD, ppsA, and trpE) were both performed with primers and conditions described by Curran et al. [28]. Amplicon sequencing was performed with the BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fischer Scientific Inc., Waltham, MA, USA) followed by the BigDye Xterminator Purification Kit (ThermoFischer Scientific Inc., Waltham, MA, USA), and using an ABI 310 Genetic Analyser (Applied Biosystems, Foster City, CA, USA). The resulting electropherograms were manually analysed with the FinchTV 1.5 (Geospiza, Inc.; Seattle, WA, USA; http://www.geospiza.com) [29], ClustalX 2.1 (Conway Institute, UCD, Ireland) [30], and Seaview 3.2 (Institut Français de Bioinformatique, France) [31] programs.
The consensus sequences obtained for each gene of each strain were individually typed in the PubMLST database (http://pubmlst.org/paeruginosa/, Warwick, UK; accessed on 18 May 2022) for the assignment of an allelic number based on worldwide reports. Once the allelic profile was obtained, the ST of each strain was determined. New alleles and STs were added to the global P. aeruginosa PubMLST database (accessed on 18 May 2022).
2.8. MexAB-OprM Efflux Pump Repressor Genes’ Characterization
The amplification and subsequent sequencing of the MexAB-OprM efflux pump repressor genes mexR, nalC, and nalD were performed with the primers and conditions reported by Aguilar-Rodea et al. [20] for the identification of substitutions and determination of haplotypes in all strains of P. aeruginosa.
2.9. Phylogenetic Analysis
The genetic relationship among P. aeruginosa strains and its possible association with an outbreak was determined by building a phylogenetic network based on the ST of the strains with the eBURST v3 algorithm (available in the global P. aeruginosa PubMLST database, Warwick, UK) [32].
3. Results
3.1. Isolation, Identification, and Characterization of Pseudomonas aeruginosa from Biological Samples
A total of 15 isolates possibly associated with an outbreak in a Mexican paediatric hospital during the month of June 2018 were recovered: 14 from 10 paediatric patients, and 1 from a multi-dose meropenem vial. All strains were identified as P. aeruginosa. The strains were isolated from patients of different hospital wards, including emergency (21.43%, n = 3), neonatal intensive care unit (NICU) (21.43%, n = 3), infectious diseases outpatient (14.29%, n = 2), oncology (28.57%, n = 4), and surgical therapy (STx) (14.29%, n = 2). Up to 71.43% of the strains (n = 10) were isolated from blood, while 28.57% were recovered from urine (n = 4) (Table 1). More than one morphotype was identified in three patients: Patient 1 (P1), strains 4 and 13 with three days apart, Patient 2 (P2), strains 11, 10, and 3 identified in the same blood culture, and Patient 10 (P10), strains 7 and 6 with 14 days apart (Table 1, Figure 1, and Supplementary Table S1). The clinical features of the patients are shown in Supplementary Table S1.

Table 1.
Susceptibility profile of the P. aeruginosa strains and their classification.
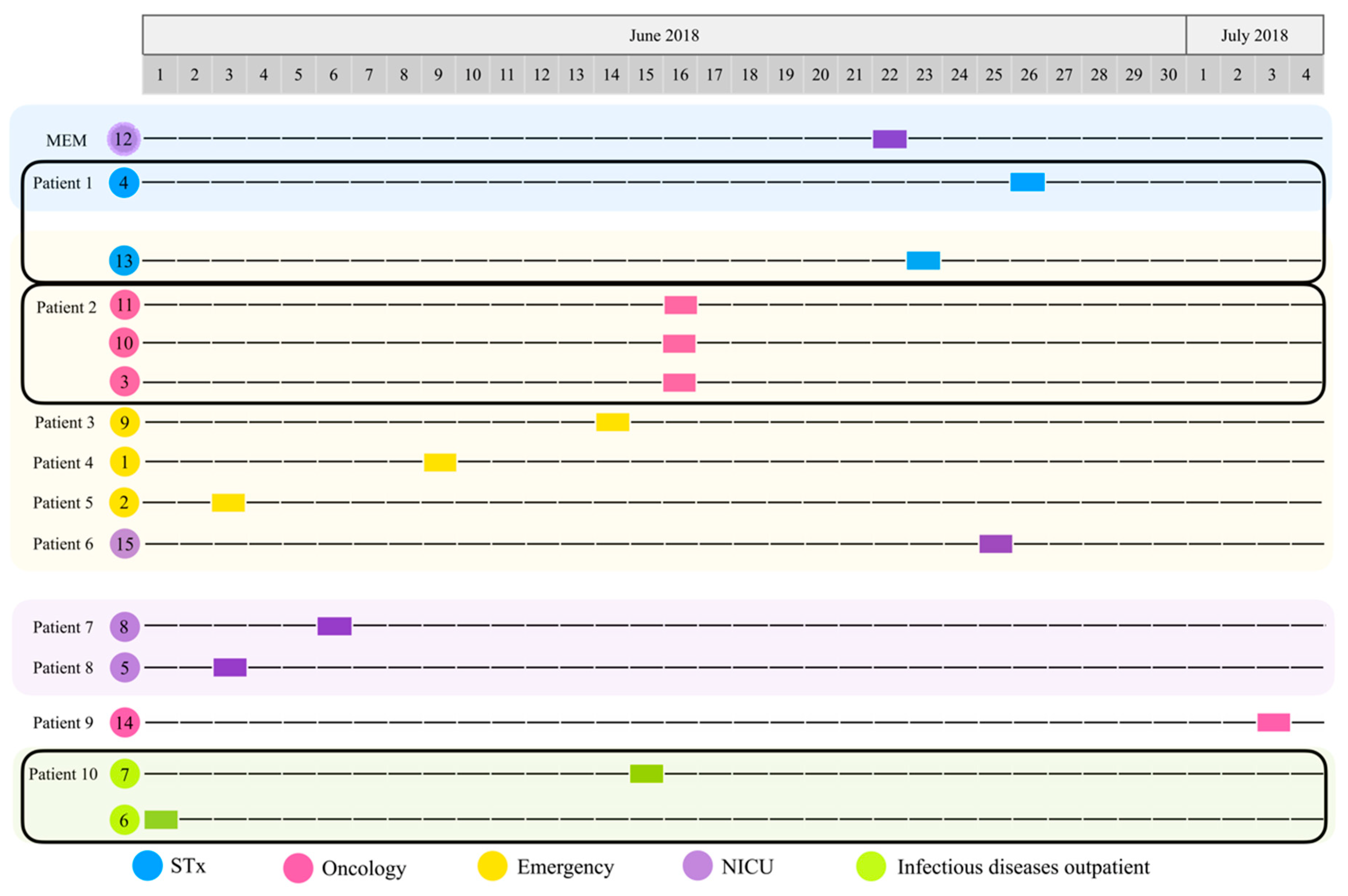
Figure 1.
Timeline of the isolation of P. aeruginosa strains. Hospital ward: surgical therapy (STx, blue), oncology (pink), emergency (yellow), neonatal intensive care unit (NICU, purple), infectious diseases outpatient (I, green). Patients with two or more isolated strains are highlighted with a black rectangle (Patient 1: strains 4 and 13; Patient 2: strains 11, 10, and 3; Patient 10: strains 7 and 6).
According to the timeline (Figure 1), strain 6 was the first culture isolated from patient 10 (P10) in the infectious diseases outpatient room (1 June 2018); then, strains 2 (P5) and 5 (P8) (3 June 2018), which were obtained from emergency and NICU, followed by strain 8 (P7) (6 June 2018) isolated from the NICU. Three days later, strain 1 (P4) was identified in emergency (9 June 2018). Five days later (14 June 2018), strain 9 (P3) was also isolated from emergency. The next day (15 June 2018), strain 7 was isolated from the infectious diseases outpatient room from the same patient (P10) where strain 6 was previously isolated. Strains 3, 10, and 11 were all identified in oncology (16 June 18) and were isolated from the same patient (P2). Around this time, while investigating the cases and the environment, the Department of Epidemiology identified strain 12 in a multi-dose meropenem vial from the NICU (22 June 2018). The next day, strain 13 (P1) was isolated from STx (23 June 2018); two days later, strain 15 (P6) was identified in the NICU (25 June 2018). Strain 4 was isolated from STx (26 June 2018) from the same patient (P1) where strain 13 was isolated as well. Finally, strain 14 (P9) was isolated from oncology (3 July 2018) (Figure 1 and Table 1).
3.2. P. aeruginosa Strains’ Susceptibility Profile
Of the strains, 60% (n = 9) were classified as XDR, 33% (n = 5) as MDR, and 6.6% (n = 1) were classified as S. All strains were classified as intermediate to CS, while 80% were susceptible to AZT. In contrast, 80% were classified as resistant to aminoglycosides (GEN and AK) and β-lactams (P/T), and 93.3% as resistant to cephalosporins (CAZ and CPM), carbapenems (IMI and MEM), fluoroquinolones (CIP and LEV), penicillin (CB), and the aminoglycoside TOB (Table 1).
3.3. Carbapenemases’ Production
3.4. Phenotypic Production of Pyocyanin and Pyoverdine
Pyoverdine production by P. aeruginosa strains in the absence of antibiotics was determined in an interval of λAbs = 1.77–2.62 (Figure 2B). The lowest production of pyoverdine was determined in the presence of GEN (strain 3, λAbs = 0.37), while the highest production was recorded in the absence of antibiotics (strain 5, λAbs = 2.62) (Figure 2B).
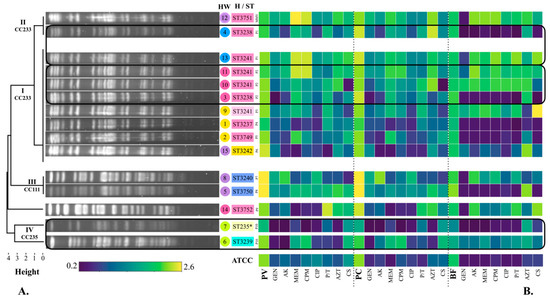
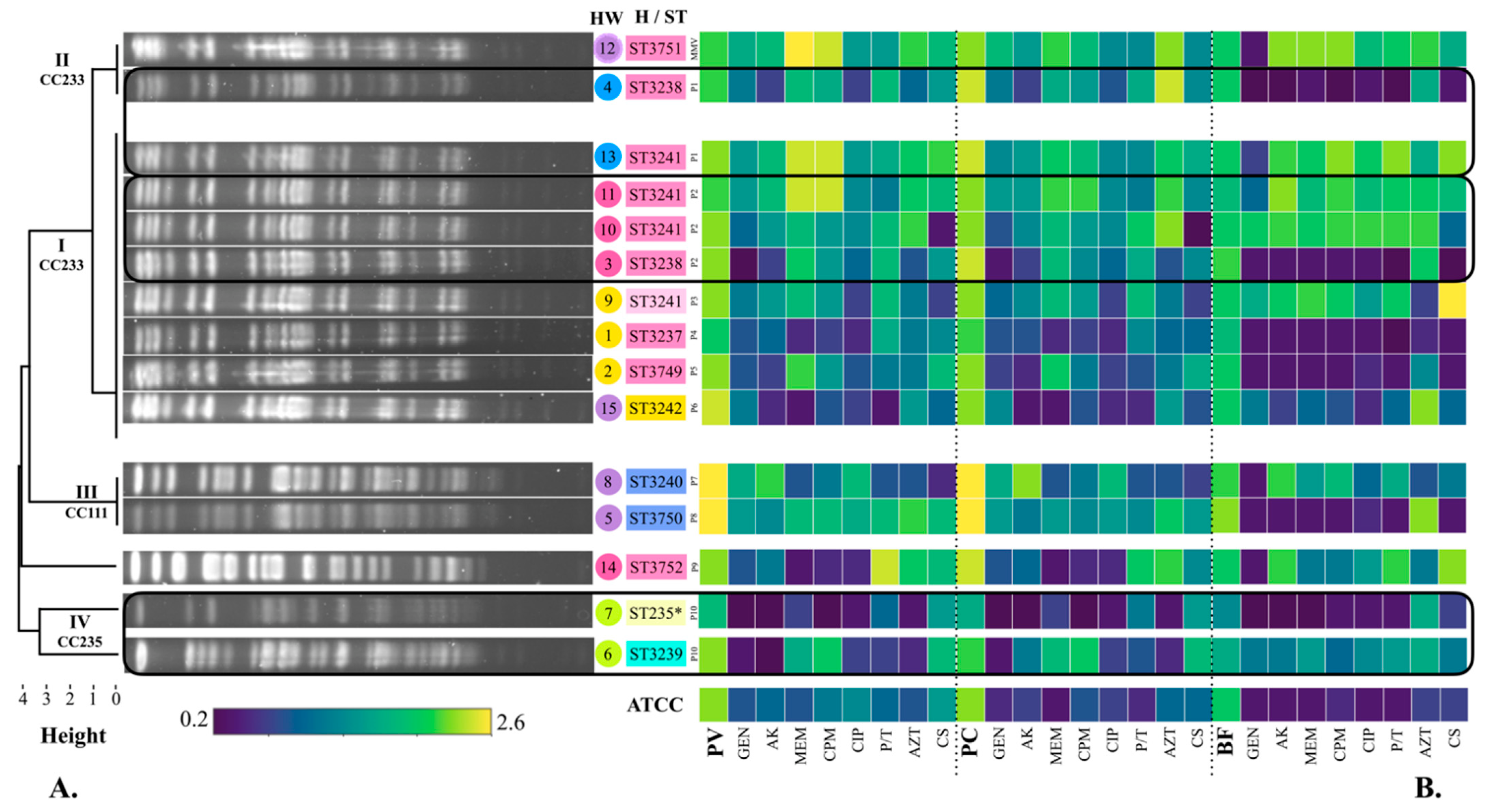
Figure 2.
Phylogenetic tree of the 15 P. aeruginosa strains based on their electrophoretic pattern (PFGE), phenotypic production of virulence factors, and sequence type (ST). (A) Pulsed-field gel electrophoresis (PFGE). Electrophoretic patterns were obtained by PFGE with the Spe I enzyme. The phylogenetic tree was built with the unweighted pair group method with arithmetic mean (UPGMA) according to the PFGE electrophoretic patterns. Clades I, II, III, and IV were obtained. Strains from clades I and II were designated as closely related and considered part of the outbreak. CC: Clonal complex. The strain number followed by its identified ST is shown. Colour of the strain is given by the hospital ward (HW) according to Figure 1: Yellow: emergency (E), Blue: surgical therapy (STx), Pink: oncology (O), Purple: neonatal intensive care unit (NICU), Green: infectious diseases outpatient (I). Colour of the ST is given by the mexR-nalC-nalD haplotype (H): H12 (pink), H28 (blue), H29 (light blue), H30 (light green), H31 (light pink), H32 (yellow). High-risk clone ST235 is highlighted with a *. Number of patients is shown as well (P1–P10). MMV: Multidose MEM vial. (B) Phenotypic production of pyoverdine, pyocyanin, and biofilm. Virulence factors: PV: Pyoverdine, PC: Pyocyanin, BF: Biofilm. Antibiotic categories: 1. Aminoglycosides: GEN: gentamicin, AK: amikacin, 2. Carbapenems: MEM: meropenem, 3. Cephalosporins: CPM: cefepime, 4. Fluoroquinolones: CIP: ciprofloxacin, 5. Penicillins + β-lactamase inhibitors: P/T: piperacillin-tazobactam, 6. Monobactams: AZT: aztreonam, 7. Polymyxins: CS: colistin.
The production of pyocyanin in the absence of antibiotics was determined in an interval of λAbs = 1.18–1.80 (20.16–30.68 µg/mL) (Figure 2B). The lowest production of pyocyanin was determined in the presence of CS (strain 10, λAbs = 0.24, 4.11 µg/mL), while the highest production was recorded in the absence of antibiotics (strain 5, λAbs = 1.80, 30.68 µg/mL).
Pyoverdine was the virulence factor that showed the highest phenotypic production in the studied strains, with an average λAbs = 1.47, while pyocyanin showed an average λAbs = 0.93 (15.88 µg/mL). Up to 73.33% of the strains produced higher amounts of pyoverdine in the absence of antibiotics, 20% showed higher production in the presence of MEM and CPM (strains 12, 11, and 13), and 6.66% of the strains with P/T (strain 14). Additionally, all strains showed a higher production of pyocyanin in the absence of antibiotics (Figure 2B).
3.5. Phenotypic Production of Biofilm
The production of biofilm by P. aeruginosa strains in the absence of antibiotics was determined in an interval of λAbs = 1.14–1.77 (Figure 2B). The lowest biofilm production was determined in the presence of MEM and CPM (strain 4, λAbs = 0.207), and the highest production was observed with CS (strain 9, λAbs = 2.055) (Figure 2B). On average, the strains showed a biofilm production of λAbs = 1.00. Up to 40% of the strains produced a greater amount of biofilm in the absence of antibiotics. On the other hand, 40% showed higher biofilm production in the presence of AK, P/T (strains 12, 9, 10, 11, 13, and 14), and AZT (strains 12, 10, 11, 15, 5, and 7), 33.33% with CPM (strains 12, 9, 10, 11, and 13), 26.66% with MEM (strains 12, 9, 10, and 13), 20% with CS (strains 9, 11, and 13), and 13.33% of the strains with CIP (strains 10 and 13) (Figure 2B).
3.6. Pulsed-Field Gel Electrophoresis (PFGE)
Six distinct PFGE patterns were identified among the 15 P. aeruginosa isolates (Figure 2A). Eight strains were designated as identical or indistinguishable (1, 2, 3, 9, 10, 11, 13, 15), and formed clade I. All strains isolated from the multi-dose meropenem vial (12) and strain 4 were grouped in clade II. Clade III included strains 5 and 8, while clade IV included strains 6 and 7. Clades III and IV presented a similarity coefficient < 90%, and were designated as unrelated to the outbreak, as well as the strain 14, which belongs to either to clade II or III. The difference between clade I and II was a single band, so the strains were designated as closely related and were considered part of the outbreak, according to the criteria established by Tenover et al. [27] (Figure 2A).
3.7. Multi-Locus Sequence Typing (MLST)
Genotypic characterization of P. aeruginosa strains by MLST is shown in Table 2. A total of eleven different STs were identified: ST235, ST3237, ST3238, ST3239, ST3240, ST3241, ST3242, ST3749, ST3750, ST3751, and ST3752, and all new STs were uploaded into the worldwide P. aeruginosa PubMLST database (Supplementary Table S2), except for the previously reported ST235. ST3241 was the most frequent (n = 4) among the analysed strains.

Table 2.
Allelic profile, sequence types, and haplotypes of the MexAB-OprM efflux pump repressor genes (mexR, nalC, nalD) of the analysed P. aeruginosa strains.
3.8. Characterization of the MexAB-OprM Efflux Pump Repressor Genes (mexR, nalC, nalD)
A total of 42 nucleotide substitutions, 25 synonymous and 17 nonsynonymous, were identified in the MexAB-OprM efflux pump repressor genes (mexR, nalC, nalD) (Table 3).

Table 3.
Substitutions identified in the mexR, nalC, and nalD repressor genes in P. aeruginosa strains.
In the mexR gene, five synonymous and two nonsynonymous substitutions were identified in 26.6% of the strains. In the nalC gene, 15 synonymous substitutions were identified in 20% of the strains, and 15 nonsynonymous substitutions: 11 identified in strain 9. The 212G→A substitution was identified in 100% of the strains, followed by the 459G→T and 556G→A mutations, both identified in 60% of the strains. In the nalD gene, five synonymous substitutions were identified in 26.6% of the strains.
A haplotype number was designated to a specific set of mutations in the repressor genes (mexR, nalC, nalD) [20]. A total of six different haplotypes were determined. Haplotype 12 was the most frequent, being identified in 60% of the strains (Table 4).

Table 4.
Identified haplotypes of the MexAB-OprM efflux pump repressor genes in P. aeruginosa strains.
3.9. Phylogenetic Analysis
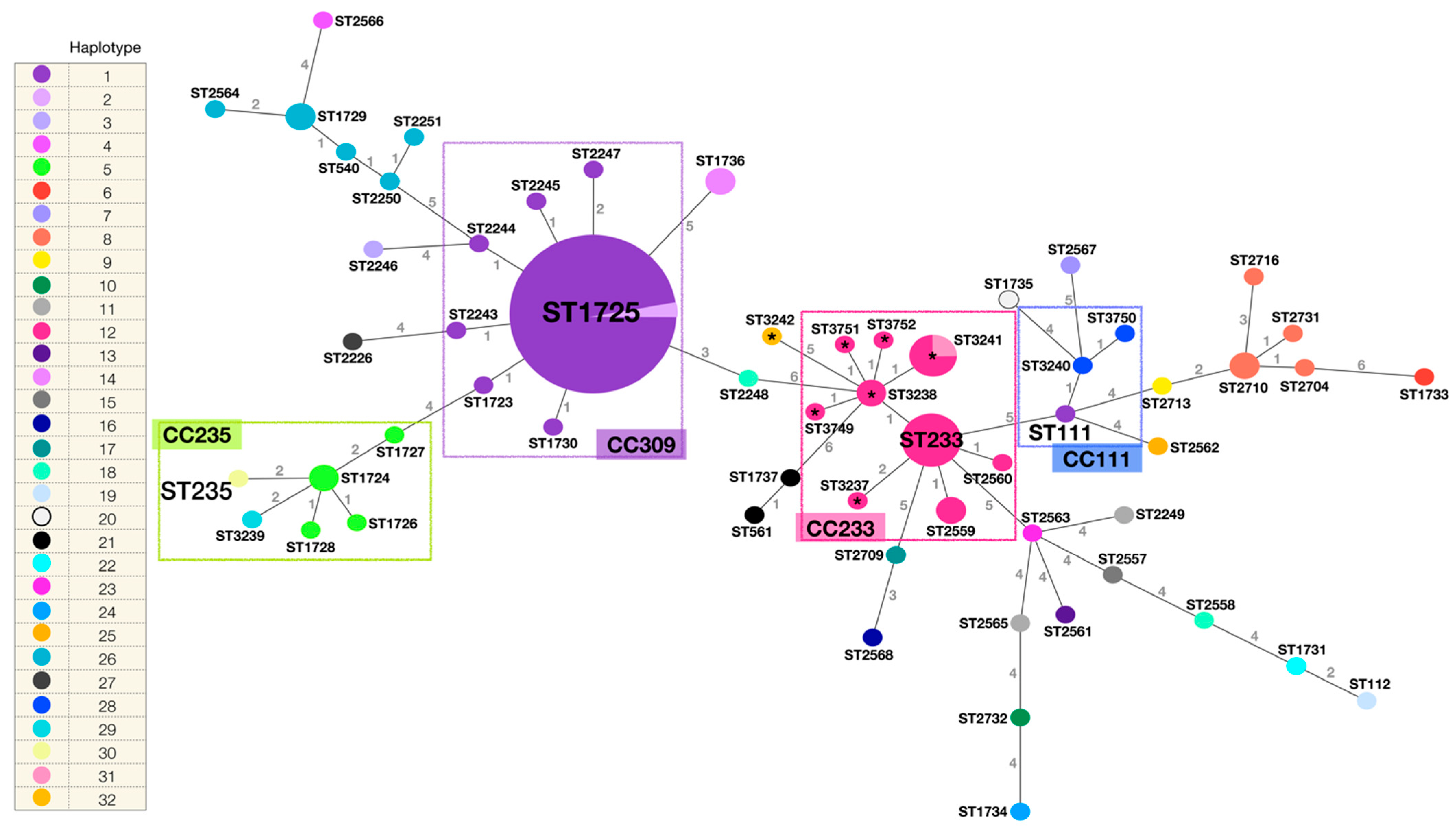
The phylogenetic network built from the identified STs by the eBURST v3 algorithm (PubMLST Pseudomonas aeruginosa) [32] and their genetic relationship is described in Figure 3. The STs of the 15 strains analysed in this study, and in addition all the STs identified in the HIMFG by the working group from 2007 to 2015 [18,20] are depicted as well. The identified haplotype (mexR, nalC, nalD) in each strain is indicated. Fifty-nine different STs were incorporated (n = 106 strains). ST1725 was the most frequent ST (n = 34), followed by ST233 (n = 6), ST3241 (n = 4), ST1724, ST1729, ST1736, ST2559, ST2710, and ST3238 (n = 2). The remaining STs were identified in a single strain. Clonal complexes (CC) were defined as the set of STs that descended from the same founding genotype, which in most of the cases corresponded to the high-risk clones reported worldwide. Members of a given CC shared identical alleles (at least five of the seven loci).
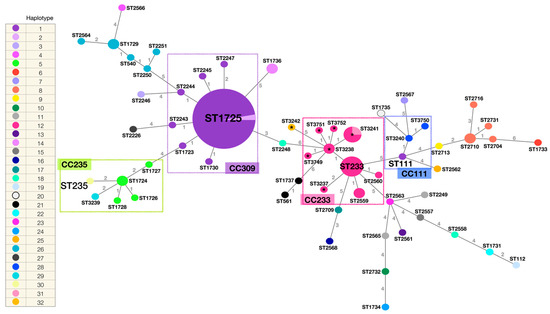
Figure 3.
Phylogenetic network of the P. aeruginosa STs identified in the HIMFG. STs are indicated. The size of the circumferences is given by the ST frequency, and the numbers in the lines indicate the differences in the allelic profile between the strains (n = 7 genes, maximum number of differences). The colour of the ST is given by the identified haplotype (mexR, nalC, nalD). Total number of haplotypes = 32. Total number of STs = 59. Total number of strains = 106. Relevant clonal complexes (CC) are highlighted with rectangles (CC235, CC309, CC233, and CC111). Strains related to the outbreak are highlighted with *.
Clade I (ST3237, ST3238, ST3241, ST3242, and ST3749) and clade II (ST3751, isolated from the multi-dose meropenem vial, and ST3238), determined by PFGE, were closely related to the previously identified high-risk clone ST233 (CC233). Most of the STs in CC233 presented haplotype 12 (mexR-nalC-nalD) (except for ST3241 and ST3242). Clade III (ST3240 and ST3750, both with haplotype 28) were related to the high-risk clone ST111 (CC111), haplotype 1 [23]. The latter presented variations, additional to those identified in haplotype 28, including nalC mutations: A4A, S5S, A23A, I41I, R43R, G49G, E59E, S118S, Y137Y, A145A, A148A, P149P, and S209R, and nalD: L99L. Finally, clade IV (ST3239, haplotype 29, and ST235, haplotype 30) were part of CC235 and closely related to previously identified haplotype 5 STs [23]. The only difference between haplotype 29 and 5 is an additional A148A variation in the nalC gene (identified in haplotype 5), and between haplotype 30 and 5, an additional variation in the mexR gene (L131Q) (identified in haplotype 30), and the lack of the A148A variation in the nalC gene.
Additionally, a second phylogenetic analysis was performed among different P. aeruginosa STs, which included ten previously isolated and reported STs in the HIMFG, and the strains reported in this study (Supplementary Figure S1).
4. Discussion
Continued use of antibiotics exerts selection pressure for MDR strains, such as Pseudomonas aeruginosa high-risk clones. Despite hygienic measures to prevent and control HAIs, dissemination and persistence of MDR strains (mainly high-risk clones) in healthcare institutes remain as the principal causes of outbreaks due to their genetic and phenotypic factors [10,33,35].
The HIMFG is a third-level healthcare institute, where almost 40% of patients are admitted for different types of cancer, who usually go through long hospital stays and receive several antibiotic treatments, and this represents a high-risk factor for the HAIs’ establishment, even more so with the hospital history of MDR P. aeruginosa strains’ (ST1725 and ST233) dissemination in different wards, prevailing since 2007. These STs were last identified in 2013, significantly increasing the mortality rate up to 17.39% [18]. In June 2018, an outbreak caused by P. aeruginosa was identified in the paediatric hospital. A total of 14 P. aeruginosa strains from ten paediatric patients, and one strain recovered from a multi-dose meropenem vial, were analysed.
High microbial resistance rates were observed. Of the strains, 60% were classified as XDR, and 13.33% as MDR, where AZT and CS were the most effective antibiotics against the P. aeruginosa strains isolated from the outbreak. Nevertheless, in the HIMFG, AZT-resistant strains have been previously reported [18,20,36]. CS is considered the last treatment option for patients, due to its neuro and nephrotoxic properties [19,35,37]; however, in recent years, it has emerged as the only therapeutic option for PDR infections, showing greater efficacy in combination with other antibiotics [38,39]. Although AZT has been tested to be effective against P. aeruginosa clinical strains (86.6% in vitro effectiveness), this sensitivity can be attributed to the lack of genes or plasmids that code for the production of extended-spectrum β-lactamases (ESBLs); in addition, as a consequence of the unavailability of this antibiotic in Mexico, there is no selection of resistant strains containing plasmids, unlike other countries such as Brazil or India, where 40.8% and 41.38% of resistant rates are respectively reported [40,41,42].
Studies from several countries have highlighted the importance of characterization of outbreaks by pathogenic bacteria within healthcare institutes [4,43,44], where several MDR P. aeruginosa strains stand out for their association with high dissemination rates [45,46]. Various studies around the world report an increase in P. aeruginosa MDR strains that cause HAIs, and these reports range from 28.2% to 69.8% [40,47,48,49]. For this reason, it is not surprising that MDR strains are the cause of outbreaks due to their ability to spread and resist the hospital environment. In our study, 93.3% of the isolates were classified as MDR.
Several virulence factors in P. aeruginosa, which are also expressed in unfavourable conditions, play an important role in infectious processes where specific mechanisms for its survival are activated (i.e., pyocyanin and pyoverdine). Nevertheless, in this study, we observed the highest production of both pyocyanin and pyoverdine in the absence of antibiotics, which suggests that the stress generated by antibiotics does not necessarily trigger the production of these virulence factors. On the contrary, their production diminished in the presence of antibiotics. Schalk et al. reported that the presence of gentamycin diminished the production of pyoverdine, probably associated with the inhibition of its transporter proteins [42]. On the other hand, biofilm is produced to help the bacteria to persist in several infectious processes; in our study, most strains diminished biofilm production in the presence of antibiotics. Contrary to this fact, two clones involved in the outbreak increased their production in the presence of antibiotics, representing a high-risk rate in therapy failures. Additionally, AZT improved the production of biofilm in most strains. In fact, Javed et al. [50] reported that strains of P. aeruginosa exposed to CS produced higher biofilm rates. Recent studies have demonstrated that MDR strains of P. aeruginosa are higher producers of pyocyanin, similar to our study [51,52].
On the other hand, Mullet et al. reported in 2013 [10] that MDR and XDR strains, both high-risk clones, were significantly associated with a reduced production of pyocyanin and a higher production of biofilm. Contrary to this, Horcajada et al. in 2019 [53] suggested that some virulence factors are not related to their susceptibility profile. In our study, we identified the high-risk clone ST235, which interestingly revealed a reduced production of both pigments, as well as biofilm production, maintaining lower levels than those once determined in the reference strain (P. aeruginosa ATCC 27853). The results of our study suggest that the employment of antibiotics can affect the production of virulence and resistance factors, such as pyoverdine, pyocyanin, and biofilm.
MDR P. aeruginosa strains possess multiple antibiotic-resistant genes, with the efflux pumps as one of the main factors that contribute to P. aeruginosa resistance, mainly due to the MexAB-OprM overexpression. This study agrees with several other authors [54,55], and the modulation of the efflux pump by the mexR, nalC, and nalD regulator genes and its respective mutations, which can affect their function [34,56,57,58]. Some studies have analysed the three repressor genes of the MexAB-OprM efflux pump as a whole set; in one of these, the researchers showed that not all MDR strains overexpress the MexAB-OprM operon, because of the continuous transcription of both nalC and nalD genes as a consequence of the mutations in these regulators [55]. In this study, however, six different haplotypes (mexR-nalC-nalD nucleotide substitutions) were identified: haplotype 12 stands out as previously described in isolates recovered from patients in the HIMFG, during the period of 2007 and 2015 [20], and haplotypes related to the previously identified haplotype 5 that has been associated with fatal outcomes in patients; however, no patient deaths were reported in the present study.
Worldwide, the sequence types ST111, ST175, ST235, ST233, and ST253 stand out, being considered as of special caution for their persistence and ability to spread in multiple environments [15,16,17,18]. In Mexico, the report of the ST233 and ST1725 clones registered between 2007 and 2013 stands out, where the XDR ST233 clone was identified for the first time, showing resistance to CS [18]. In this study, six different electrophoretic patterns were identified by PFGE, and eleven different STs were determined by MLST within the analysed strains. The phylogenetic tree based on the electrophoretic patterns and the phylogenetic network built with the obtained STs showed correlation: both graphics identified a close phylogenetic relationship between nine of the analysed strains and the strain isolated from the multi-dose MEM vial, all considered as variants of the previously identified ST233 and consequently members of the CC233. In addition, ST3240 and ST3750, both closely related strains to the ST111 (CC111), a ST235 strain, and a related ST (ST3239, CC235) are highlighted for being previously identified as high-risk clones as well; nevertheless, they were considered as not related to the outbreak.
It is remarkable that strains from CC235 (strains 6 and 7, ST235 and ST3239, respectively) occurred exclusively in a patient from the infectious disease outpatient ward, 14 days apart, although both strains were phylogenetically closely related, suggesting the association of the use of antibiotics in the success of ST235 over ST3239. Recent whole genome sequencing studies have identified ST235 high-risk clones with novel genetic characteristics, such as the Type IV Secretion Systems, that allows the uptake of foreign genetic material, contributing to the competition among P. aeruginosa isolates [59]. In addition, despite that ST235 is considered a high-risk clone, the patient reported no symptoms, which strongly correlates with the low phenotypic expression of virulence factors.
The NICU is located on the fourth floor, where P. aeruginosa isolates conforming CC111 (strains 8 and 5, ST3240 and ST3750, respectively) were recovered and identified. These STs were related to the ST111, but distant from the ST235. The NICU is distantly located from other services by a floodgate, and handwashing with chlorhexidine upon admission is mandatory. We suggest that this geographic isolation from other areas of the hospital explains the independent clonal evolution; however, 16 days apart, an isolate was obtained from a multi-dose MEM vial, and 3 days apart another strain was identified in a patient (P6) (both related to the outbreak). Inquiring, the multi-dose MEM vial was an antibiotic vial shared within the emergency room, where the vial could have been contaminated, explaining the relationship between the isolates of patient 6 (P6, NICU) and patient 1 (P1, STx) with the outbreak. In addition, NICU and emergency patients are frequently visited by surgeons, nurses, paediatricians, or rehabilitation personnel, who also admit STx patients. In fact, strain 15 (P6) was identified in the NICU, after the closely related isolation in STx (strain 13, P1).
The remaining isolates were first identified in patients from the emergency room, and later in patient 2 (P2) from oncology. We must highlight that P2 was first admitted to emergency before being translated to oncology, and these isolates occurred at intermediate points between those described above and were associated with the ST233 (CC233). According to the bed location in this service (which are very close to each other), we suggest both direct and indirect transmission events between patients because of contamination of surfaces. However, given that CC233 has already been found in different countries and in this hospital was predominantly found in emergency patients, it is difficult to rule out if the strains were imported from other hospitals or were colonizing readmitted patients.
The sequence types ST111, ST233, and ST235 have been reported as high-risk clones with high mortality and spread rates throughout the world in several countries, such as Spain, Brazil, Venezuela, Greece, Russia, Italy, Japan, Sweden, France, and Turkey, among others [10,12,18,60,61,62]. In 2014, our research group reported the isolation and characterization of ST111 from a water source, classifying the isolate as resistant to CB, FOS, and CS [20]. In this study, two variants of this ST (ST3240 and ST3750), isolated from patients and resistant to TOB, IMI, MEM, CAZ, CPM, CIP, LEV, and CB, were identified, suggesting the adaptation of MDR variants to both outside and clinical environments, representing a significant risk due to its possible transmission to patients, constant circulation and prevalence, and the emergence of new, higher resistant and better adapted STs.
There is a record of the ST233 identified in the period of 2007 to 2015 in the HIMFG, exclusively sensitive to AZT [18]. In this study, ten more strains were closely related to this ST, identified as the main cause of the outbreak, which presented an intermediate value of resistance to CS and were classified as sensitive to AZT. Additionally, haplotype 12 (mexR-nalC-nalD) (previously reported in ST233) was determined in eight of these strains, suggesting a relationship between the phylogenetically related strains and the haplotype, as previously described [20]. The identification of these variants after the first report of ST233 evidenced the high capability of adaptation and persistence of these clones to the hospital environment, which although they have not yet produced deaths as reported worldwide, they do represent a latent risk in the hospital and may be the cause of future outbreaks [4], HAIs, and resistance reservoirs. In this regard, it should be noted that P. aeruginosa infections can be caused by a single ST or by more than one ST belonging to different CCs, hence the importance of determining the STs involved, since they can differ genotypically and phenotypically. Effective treatment to eradicate one ST will not necessarily be functional to eliminate another ST, since virulence and resistance factors can be transferred between bacteria.
In the HIMFG, a permanent hand washing program exists, which was intensified during the outbreak, as well as the continuous hospital wards’ disinfection reinforced with exhaustive washing and UV irradiation, specifically where the contaminated MEM vial was detected. Nursing sessions were implemented to enhance correct disinfection and avoid contamination during intravenous drugs’ preparation, and the process was monitored. The actions taken by the Department of Epidemiology to identify and contain the outbreak prevented fatal outcomes in the patients and successfully controlled the outbreak.
This is the first report in Mexico of the ST235 and its variant ST3239, both classified as XDR, with an intermediate value of resistance to CS and with haplotypes 30 and 29 (mexR-nalC-nalD), respectively. Both haplotypes are closely related to previously identified haplotype 5 STs, which were associated with death in paediatric patients, compared to other haplotypes [20].
5. Conclusions
Ten out of 15 strains of P. aeruginosa were associated with the outbreak by PFGE. Although most strains were identified as new STs, all were closely related to previously identified high-risk clones (ST233) in the HIMFG. This is valuable, especially when high-risk clones identified in the outbreaks are usually considered more important than new variants; however, new variants could be as important as these high-risk clones, by showing similar characteristics, such as the presence of metallo-β-lactamases, the same haplotype of the MexAB-OprM efflux pump, increased production of biofilm, and a decrease in the production of virulence factors (specifically in the presence of antibiotics), favouring better adaptation and persistence mechanisms in the hospital environment. The diversification of high-risk clones is a latent problem, especially in non-optimal environments for this bacterium. For this reason, outbreaks will always be considered as an alarm due to the presence of some of them.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10081533/s1, Supplementary Figure S1: Phylogenetic tree of 25 P. aeruginosa strains identified in the HIMFG based on their electrophoretic pattern (PFGE). Supplementary Table S1: Clinical patients’ features. Supplementary Table S2: Accession numbers (ID) of the P. aeruginosa strains analyzed in this work.
Author Contributions
Conceptualization, P.A.-R., N.V.-G. and I.R.-P.; methodology, E.L.E.-J., V.J.-R., B.A.R.-E., B.L.-M. and I.F.H.; formal analysis, P.A.-R., U.G.-R. and C.G.N.-R.; investigation, C.A.; resources, D.d.l.R.Z. and G.C.-V.; writing—original draft preparation, P.A-R.; writing—review and editing, G.E.R., S.M.-E., A.E.G.-A., N.V.-G. and I.R.-P.; supervision, R.M.-S., M.T.G. and I.P.-O.; project administration, N.V.-G.; funding acquisition, N.V.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Federal Resources of the Hospital Infantil de México Federico Gomez, grant number HIM/2019/040 of the Secretaría de Salud (SSA) 1619, México. This work is part of the dissertation of P.A.-R., a second-year National Postdoctoral fellow supported by CONACyT (385706).
Institutional Review Board Statement
This study was approved by the Research Committee (Dr. Juan Garduño Espinosa), Ethics Committee (Dr. Luis Jasso Gutiérrez), and Biosecurity Committee (Dr. Marcela Salazar García) of HIMFG under the following protocols: HIM/2019/040. SSA 1619.
Informed Consent Statement
Not applicable.
Data Availability Statement
Some of the nucleotide sequences identified in this study were previously reported and are available in the GenBank database under the following accession numbers: mexR gene sequences, MT188163 and MT188164; nalC gene sequences, MT188183 and MT188186. The new nucleotide sequences obtained in this study were deposited in the GenBank database under the following accession numbers: mexR gene sequence, ON015859; nalC gene sequences, ON052748 and ON052749; nalD gene sequences, ON052750–ON052752. Accession numbers for the Pseudomonas aeruginosa isolates used in this work are available in the public database Pseudomonas aeruginosa PubMLST (See Supplementary Table S2).
Acknowledgments
We thank CONACyT, and the Programa de Posgrado en Ciencias de la Tierra, Centro de Ciencias de la Atmósfera, Laboratorio de Aerobiología, UNAM, and the Hospital Infantil de México Federico Gómez, Research Unit in Infectious Diseases: Area of Bacterial Genetics, for making possible the development of this research project. We thank Leticia Martínez Romero and Eva Salinas Cortés (Centro de Ciencias de la Atmósfera, Laboratorio de Aerobiología, UNAM) and Biol. Juan Carlos Vigueras Galindo for technical support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Murray, P.R.; Rosenthal, K.S.; Pfaller, M.A. Microbiología Médica, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Ryan, K.J.; Ray, C.G.; Sherris. Microbiología Médica, 5th ed.; McGraw-Hill Interamericana: Mexico City, Mexico, 2011. [Google Scholar]
- NOM-045-SSA2-2005; Norma Oficial Mexicana: Para la Vigilancia Epidemiológica, Prevención y Control de las Infecciones Nosocomiales. Estados Unidos Mexicanos-Secretaria de Salud: Mexico City, Mexico, 2005.
- Gutiérrez, M.J.; Morayta, R.C.A.; Martínez, B.M.E.; Coria, L.J.J.; Armenta, G.L.; Ayala, F.J.R.; Bernal, G.S.M.; Flores, Z.F.J.; García, P.F.E.; Monjardín, R.J.A.; et al. Estudio multicéntrico de resistencias bacterianas nosocomiales en México. Rev. Latinoam. Infectología Pediátrica 2017, 30, 68–75. [Google Scholar]
- Organización Mundial de la Salud (OMS). Prevención de las Infecciones Nosocomiales: Guía Práctica [Internet]. Ducel, G., Fabray, J., Nicolle, L., Eds.; 2nd ed 2002. Available online: https://apps.who.int/iris/handle/10665/67877 (accessed on 22 July 2022).
- S Sundermann, A.J.; Chen, J.; Miller, J.K.; Saul, M.I.; Shutt, K.A.; Griffith, M.P.; Mustapha, M.M.; Ezeonwuka, C.; Waggle, K.; Srinivasa, V.; et al. Outbreak of Pseudomonas aeruginosa infections from a contaminated gastroscope detected by whole genome sequencing surveillance. Clin. Infect. Dis. 2021, 73, e638–e642. [Google Scholar] [CrossRef]
- Horcajada, J.P.; Padilla, B. Endemia y epidemia: Investigación de un brote epidémico nosocomial. Enferm. Infecc. Microbiol. Clínica 2013, 31, 181–186. [Google Scholar] [CrossRef]
- Parcell, B.J.; Oravcova, K.; Pinheiro, M.; Holden, M.; Phillips, G.; Turton, J.F.; Gillespie, S.H. Pseudomonas aeruginosa intensive care unit outbreak: Winnowing of transmissions with molecular and genomic typing. J. Hosp. Infect. 2018, 98, 282–288. [Google Scholar] [CrossRef]
- Martak, D.; Meunier, A.; Sauget, M.; Cholley, P.; Thouverez, M.; Bertrand, X.; Valot, B.; Hocquet, D. Comparison of pulsed-field gel electrophoresis and whole-genome-sequencing-based typing confirms the accuracy of pulsed-field gel electrophoresis for the investigation of local Pseudomonas aeruginosa outbreaks. J. Hosp. Infect. 2020, 105, 643–647. [Google Scholar] [CrossRef]
- Mulet, X.; Cabot, G.; Ocampo-Sosa, A.A.; Domínguez, M.A.; Zamorano, L.; Juan, C.; Tubau, F.; Rodríguez, C.; Moyà, B.; Peña, C.; et al. Biological markers of Pseudomonas aeruginosa epidemic high-risk clones. Spanish Network for Research in Infectious Diseases (REIPI). Antimicrob. Agents Chemother. 2013, 57, 5527–5535. [Google Scholar] [CrossRef]
- Correa, A.; del Campo, R.; Perenguez, M.; Blanco, V.M.; Rodríguez-Baños, M.; Perez, F.; Maya, J.J.; Rojas, L.; Cantón, R.; Arias, C.A.; et al. Dissemination of high-risk clones of extensively drug-resistant Pseudomonas aeruginosa in Colombia. Antimicrob. Agents Chemother. 2015, 59, 2421–2425. [Google Scholar] [CrossRef]
- Oliver, A. Epidemiología y mecanismos de resistencia a carbapenemas en Pseudomonas aeruginosa: Papel de los clones de alto riesgo en la multirresistencia. Enferm. Infecc. Microbiol. Clínica 2017, 35, 137–138. [Google Scholar] [CrossRef]
- Hu, Y.; Peng, W.; Wu, Y.; Li, H.; Wang, Q.; Yi, H.; Zhang, R.; Shao, B.; Zhu, K. A potential high-risk clone of Pseudomonas aeruginosa ST463. Front. Microbiol. 2021, 12, 670202. [Google Scholar] [CrossRef]
- Del Barrio-Tpfiño, E.; López-Causapé, C.; Oliver, A. Pseudomonas aeruginosa epidemic high-risk clones and their association with horizontally-acquired β-lactamases: 2020 update. Int. J. Antimicrob. Agents 2020, 56, 106196. [Google Scholar] [CrossRef]
- Maatallah, M.; Cheriaa, J.; Backhrouf, A.; Iversen, A.; Grundmann, H.; Do, T.; Lanotte, P.; Mastouri, M.; Elghmati, M.S.; Rojo, F.; et al. Population structure of Pseudomonas aeruginosa from five Mediterranean countries: Evidence for frequent recombination and epidemic occurrence of CC235. PLoS ONE 2011, 6, e25617. [Google Scholar] [CrossRef] [PubMed]
- López-Causapé, C.; Rojo-Molinero, E.; Mulet, X.; Cabot, G.; Moyà, B.; Figuerola, J.; Togores, B.; Pérez, J.L.; Oliver, A. Clonal dissemination, emergence of mutator lineages and antibiotic resistance evolution in Pseudomonas aeruginosa cystic fibrosis chronic lung infection. PLoS ONE 2013, 8, e71001. [Google Scholar] [CrossRef]
- Oliver, A.; Mulet, X.; López-Causapé, C.; Juan, C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist. Updates 2015, 21, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Rodea, P.; Zúñiga, G.; Rodríguez-Espino, B.A.; Olivares Cervantes, A.L.; Gamiño Arroyo, A.E.; Moreno-Espinosa, S.; de la Rosa Zamboni, D.; López Martínez, B.; Castellanos-Cruz, M.D.; Parra-Ortega, I.; et al. Identification of extensive drug resistant Pseudomonas aeruginosa strains: New clone ST1725 and high-risk clone ST233. PLoS ONE 2017, 12, e0172882. [Google Scholar] [CrossRef]
- Sánchez, A.; Salso, S.; Culebras, E.; Picazo, J.J. Carbapenem resistance determined by metalloenzymes in clinical isolates of Pseudomonas aeruginosa. Rev. Española Quimioter. 2004, 17, 336–340. [Google Scholar]
- Aguilar-Rodea, P.; Zúñiga, G.; Cerritos, R.; Rodríguez-Espino, B.A.; Gomez-Ramirez, U.; Nolasco-Romero, C.G.; López-Marceliano, B.; Rodea, G.E.; Mendoza-Elizalde, S.; Reyes-López, A.; et al. Nucleotide substitutions in the mexR, nalC and nalD regulator genes of the MexAB-OprM efflux pump are maintained in Pseudomonas aeruginosa genetic lineages. PLoS ONE 2022, 17, e0266742. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; Wayne, P.A., Ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2021. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; R Studio, Inc.: Boston, MA, USA, 2015. [Google Scholar]
- Wongsaroj, L.; Saninjuk, K.; Romsang, A.; Duang-Nkern, J.; Trinachartvanit, W.; Vattanaviboon, P.; Mongkolsuk, S. Pseudomonas aeruginosa glutathione biosynthesis genes play multiple roles in stress protection, bacterial virulence and biofilm formation. PLoS ONE 2018, 13, e0205815. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Rudden, M.; Smyth, T.J.; Dooley, J.; Marchant, R.; Banat, I.M. Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Appl. Microbiol. Biotechnol. 2019, 103, 3521–3535. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 30, 2437. [Google Scholar] [CrossRef]
- Tenover, F.C.; Arbeit, R.D.; Goering, R.V.; Mickelsen, P.A.; Murray, B.E.; Persing, D.H.; Swaminathan, B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J. Clin. Microbiol. 1995, 33, 2233–2239. [Google Scholar] [CrossRef]
- Curran, B.; Jonas, D.; Grundmann, H.; Pitt, T.; Dowson, C.G. Development of a multi locus sequence typing scheme for the opportunistic pathogen Pseudomonas aeruginosa. J. Clin. Microbiol. 2004, 42, 5644–5649. [Google Scholar] [CrossRef]
- FinchTV 1. 4.0: A Brilliant Trace Viewer; Geospiza, Inc.: Seattle, WA, USA, 2006; Available online: http://www.geospiza.com (accessed on 22 July 2022).
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R. ClustalW and ClustalX version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef]
- Ribeiro-Gonçalves, B.; Francisco, A.P.; Vaz, C.; Ramirez, M.; Carriço, J.A. PHYLOViZ Online: Web-based tool for visualization, phylogenetic inference, analysis and sharing of minimum spanning trees. Nucleic Acids Res. 2016, 44, W246–W251. Available online: https://online.phyloviz.net/ (accessed on 22 July 2022). [CrossRef]
- Quale, J.; Bratu, S.; Gupta, J.; Landman, D. Interplay of efflux system, ampC, and oprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 2006, 50, 1633–1641. [Google Scholar] [CrossRef]
- Suresh, M.; Nithya, N.; Jayasree, P.R.; Vimal, K.P.; Manish Kumar, P.R. Mutational analyses of regulatory genes, mexR, nalC, nalD and mexZ of mexAB-oprM and mexXY operons, in efflux pump hyperexpressing multidrug-resistant clinical isolates of Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2018, 34, 83. [Google Scholar] [CrossRef]
- Pankuch, G.A.; Lin, G.; Seifert, H.; Appelbaum, P.C. Activity of meropenem with and without ciprofloxacin and colistin against Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob. Agents Chemother. 2008, 52, 333–336. [Google Scholar] [CrossRef]
- Ochoa, S.A.; Cruz, A.C.; Rodea, G.E.; Cázares-Domínguez, V.; Escalona, G.; Arellano-Galindo, J.; Hernández-Castro, R.; Reyes-López, A.; Xicohtencatl-Cortes, J. Phenotypic characterization of multidrug-resistant Pseudomonas aeruginosa strains isolated from pediatric patients associated to biofilm formation. Microbiol. Res. 2015, 172, 68–78. [Google Scholar] [CrossRef]
- Almangour, T.A.; Aljabri, A.; Musawa, M.; Almohaizeie, A.; Almuhisen, S.; Damfu, N.; Alfozan, A.; Alraddadi, B.M.; Alattas, M.; Qutub, M.; et al. Ceftolozane-tazobactam vs. colistin for the treatment of infections due to multidrug-resistant Pseudomonas aeruginosa: A multicentre cohort study. J. Glob. Antimicrob. Resist. 2022, 28, 288–294. [Google Scholar] [CrossRef]
- Biswas, S.; Brunel, J.M.; Dubus, J.C.; Reynaud-Gaubert, M.; Rolain, J.M. Colistin: An update on the antibiotic of the 21st century. Expert Rev. Anti-Infect. Ther. 2012, 10, 917–934. [Google Scholar] [CrossRef]
- Angles-Yanqui, E.; Chumbes-Pérez, J.; Huaringa-Marcelo, J. Colistina en el tratamiento de infecciones por Pseudomonas aeruginosa y Acinetobacter baumanii extensamente resistentes (XDR) en un hospital de tercer nivel. Rev. Infect. 2020, 24, 201–207. [Google Scholar] [CrossRef]
- Ribeiro, Á.; Crozatti, M.; Silva, A.; Macedo, R.S.; Machado, A.; Silva, A. Pseudomonas aeruginosa in the ICU: Prevalence, resistance profile, and antimicrobial consumption. Rev. Soc. Bras. Med. Trop. 2019, 53, 1–6. [Google Scholar] [CrossRef]
- Biswal, I.; Arora, B.S.; Kasana, D.; Neetushree. Incidence of multidrug resistant Pseudomonas aeruginosa isolated from burn patients and environment of teaching institution. J. Clin. Diagn. Res. 2014, 8, DC26–DC29. [Google Scholar] [CrossRef]
- Schalk, I.J.; Guillon, L. Pyoverdine biosynthesis and secretion in Pseudomonas aeruginosa: Implications for metal homeostasis. Environ. Microbiol. 2013, 15, 161–173. [Google Scholar] [CrossRef]
- Hansen, S.; Stamm-Balderjahn, S.; Zuschneid, I.; Behnke, M.; Rüden, H.; Vonberg, R.P.; Gastmeier, P. Closure of medical departments during nosocomial outbreaks: Data from a systematic analysis of the literature. J. Hosp. Infect. 2007, 65, 348–353. [Google Scholar] [CrossRef]
- Seyman, D.; Inan, D.; Ozen, N.S.; Ogunc, E. Un brote epidémico de endocarditis por Pseudomonas aeruginosa secundario a angiografía coronaria. Rev. Chil. Infectología 2014, 31, 261–267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deplano, A.; Denis, O.; Poirel, L.; Hocquet, D.; Nonhoff, C.; Byl, B.; Nordmann, P.; Vincent, J.L.; Struelens, M.J. Molecular characterization of an epidemic clone of panantibiotic-resistant Pseudomonas aeruginosa. J. Clin. Microbiol. 2005, 43, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F.; Coque, T.M.; de la Cruz, F. Ecology and evolution as targets: The need for novel eco-evo drugs and strategies to fight antibiotic resistance. Antimicrob. Agents Chemother. 2011, 55, 3649–3660. [Google Scholar] [CrossRef] [PubMed]
- Micek, S.T.; Wunderink, R.G.; Kollef, M.H.; Chen, C.; Rello, J.; Chastre, J.; Antonelli, M.; Welte, T.; Clair, B.; Ostermann, H.; et al. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: Impact of multidrug resistance. Crit. Care 2015, 19, 219. [Google Scholar] [CrossRef]
- González-Olvera, E.M.; Pérez-Morales, R.; González, A.; Castro-Escarpulli, G.; Palma-Martínez, I.; Alba-Romero, J.J. Antibiotic resistance, virulence factors and genotyping of Pseudomonas aeruginosa in public hospitals of northeastern Mexico. J. Infect. Dev. Ctries. 2019, 13, 374–383. [Google Scholar] [CrossRef]
- Uc-Cachón, A.H.; Gracida-Osorno, C.; Luna-Chi, I.G.; Jiménez-Guillermo, J.G.; Molina-Salinas, G.M. High prevalence of antimicrobial resistance among Gram-negative isolated bacilli in intensive care units at a tertiary-care hospital in Yucatán Mexico. Medicina 2019, 55, 588. [Google Scholar] [CrossRef]
- Javed, M.; Jentzsch, B.; Heinrich, M.; Ueltzhoeffer, V.; Peter, S.; Schoppmeier, U.; Angelov, A.; Schwarz, S.; Willmann, M. Transcriptomic basis of serum resistance and virulence related traits in XDR P. aeruginosa evolved under antibiotic pressure in a morbidostat device. Front. Microbiol. 2021, 11, 619542. [Google Scholar] [CrossRef]
- Rodulfo, H.; Arcia, A.; Hernández, A.; Michelli, E.; Martinez, D.; Guzman, M.; Sharma, A.; Donato, M. Virulence factors and integrons are associated with MDR and XDR phenotypes in nosocomial strains of Pseudomonas aeruginosa in a Venezuelan university hospital. Rev. Inst. Med. Trop. Sao Paulo 2019, 61, e20. [Google Scholar] [CrossRef]
- Gajdács, M.; Baráth, Z.; Kárpáti, K.; Szabó, D.; Usai, D.; Zanetti, S.; Donadu, M.G. No correlation between biofilm formation, virulence factors, and antibiotic resistance in Pseudomonas aeruginosa: Results from a laboratory-based in vitro study. Antibiotics 2021, 10, 1134. [Google Scholar] [CrossRef]
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorlí, L.; Luque, S.; Gómez-Zorrilla, S.; Benito, N.; Grau, S. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin. Microbiol. Rev. 2019, 32, e00031-e19. [Google Scholar] [CrossRef]
- Pesingi, P.V.; Singh, B.R.; Pesingi, P.K.; Bhardwaj, M.; Singh, S.V.; Kumawat, M.; Sinha, D.K.; Gandham, R.K. MexAB-OprM efflux pump of Pseudomonas aeruginosa offers resistance to carvacrol: A herbal antimicrobial agent. Front. Microbiol. 2019, 10, 2664. [Google Scholar] [CrossRef]
- Hwnag, W.; Yoon, S.S. Virulence characteristics and an action mode of antibiotic resistance in multidrug-resistant Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 487. [Google Scholar] [CrossRef]
- Daigle, D.M.; Cao, L.; Fraud, S.; Wilke, M.S.; Pacey, A.; Klinoski, R.; Strynadka, N.C.; Dean, C.R.; Poole, K. Protein modulator of multidrug efflux gene expression in Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 5441–5451. [Google Scholar] [CrossRef]
- Ghosh, S.; Cremers, C.M.; Jakob, U.; Love, N.G. Chlorinated phenols control the expression of the multidrug resistance efflux pump MexAB-OprM in Pseudomonas aeruginosa by interacting with NalC. Mol. Microbiol. 2011, 79, 1547–1556. [Google Scholar] [CrossRef]
- Chen, W.; Wang, D.; Zhou, W.; Sang, H.; Liu, X.; Ge, Z.; Zhang, J.; Lan, L.; Yang, C.G.; Chen, H.; et al. Novobiocin binding to NalD induces the expression of the MexAB-OprM pump in Pseudomonas aeruginosa. Mol. Microbiol. 2016, 100, 749–758. [Google Scholar] [CrossRef]
- Miyoshi-Akiyama, T.; Tada, T.; Ohmagari, N.; Viet Hung, N.; Tharavichitkul, P.; Pokhrel, B.M.; Gniadkowski, M.; Shimojima, M.; Kirikae, T. Emergence and spread of epidemic multidrug-resistant Pseudomonas aeruginosa. Genome Biol. Evol. 2017, 9, 3238–3245. [Google Scholar] [CrossRef]
- Woodford, N.; Turton, J.F.; Livermore, D.M. Multiresistant gram-negative bacteria: The role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 2011, 35, 736–755. [Google Scholar] [CrossRef]
- García-Castillo, M.; del Campo, R.; Morosini, M.I.; Riera, E.; Cabot, G.; Willems, R.; van Mansfeld, R.; Oliver, A.; Cantón, R. Wide dispersion of ST175 clone despite high genetic diversity of carbapenem-nonsusceptible Pseudomonas aeruginosa clinical strains in 16 Spanish hospitals. J. Clin. Microbiol. 2011, 49, 2905–2910. [Google Scholar] [CrossRef]
- Pragasam, A.; Veeraraghavan, B.; Anandan, S.; Narasiman, V.; Sistla, S.; Kapil, A.; Mathur, P.; Ray, P.; Wattal, C.; Bhattacharya, S.; et al. Dominance of international high-risk clones in carbapenemase producing Pseudomonas aeruginosa: Multicentric molecular epidemiology report from India. Indian J. Med. Microbiol. 2018, 36, 344–351. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).