A Novel Approach of Antiviral Drugs Targeting Viral Genomes
Abstract
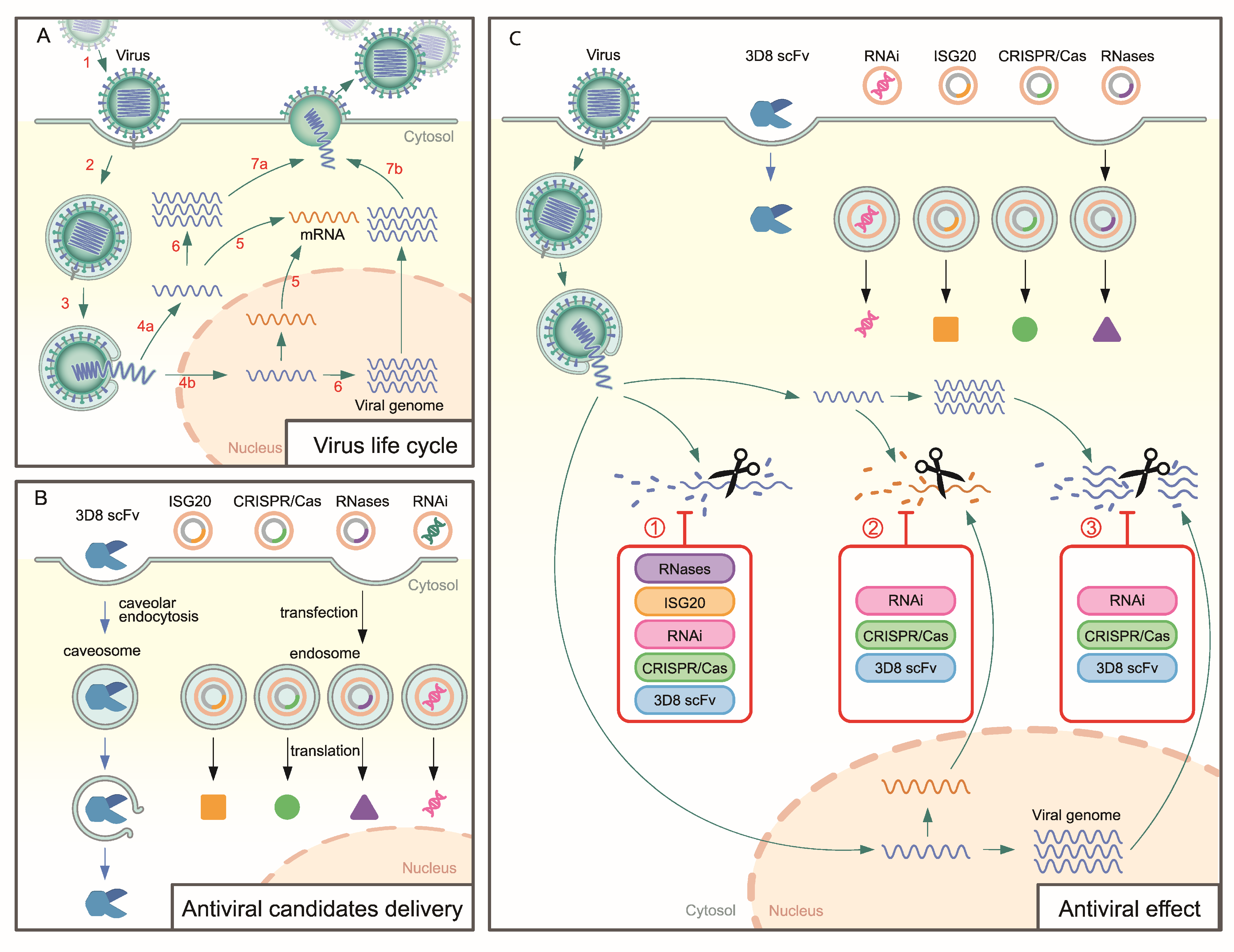
:1. Introduction
2. Antiviral Approach Targeting Viral Genomes
2.1. RNases
2.2. RNAi
2.3. ISG20
| ISG20 Derivation | Virus | Genome | Replication | Genus | Family | Ref |
|---|---|---|---|---|---|---|
| Ectopic expression | HBV | Double-stranded, relaxed circular DNA (rcDNA) | Nucleus | Orthohepadnavirus | Hepadnaviridae | [56,57,58] |
| Ectopic expression | YFV | (+)ssRNA | Cytoplasm | Flavivirus | Flaviviridae | [62] |
| Transgenic cell line | BVDV | (+)ssRNA | Cytoplasm | Pestivirus | Flaviviridae | |
| Transgenic cell line | HAV | (+)ssRNA | Cytoplasm | Hepatovirus | Picornaviriade | |
| Transgenic cell line | VSV | (−)ssRNA | Cytoplasm | Vesiculovirus | Rhabdoviridae | [63] |
| Transgenic cell line | Influenza | (−)ssRNA | Nucleus | Alphainfluenzavirus | Orthomyxoviridae | |
| Transgenic cell line | EMCV | (+)ssRNA | Cytoplasm | Cardiovirus | Picornaviriade | |
| Transgenic cell line | WNV | (+)ssRNA | Cytoplasm | Flavivirus | Flaviviridae | [64] |
| Transgenic cell line | DENV | (+)ssRNA | Cytoplasm | Flavivirus | Flaviviridae | |
| Transgenic cell line | HCV | (+)ssRNA | Cytoplasm | Hepacivirus | Flaviviridae | [65] |
2.4. CRISPR/Cas System
| Cas | Experiment | Target Gene | Virus | Genome | Replication | Genus | Family | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cas9 | WSL-gRp30 cell | p30 gene (CP204L) | ASFV (BA71V) | dsDNA | Cytoplasm | Asfivirus | Asfarviridae | [92] |
| Vero, ICP0-complementing L7 cell line 27, TC620 | ICP0, ICP4, and ICP27 genes | HSV-1 | dsDNA | Nucleus | Simplexvirus | Herpesviridae | [93] | |
| Vero, 239T, and BALB/c mice | UL7 genes | [94] | ||||||
| Vero cell | UL15, UL27, UL29, UL30, UL36, UL37, UL42, UL5, UL52, UL8, UL54, UL9, US3, and US8 | [95] | ||||||
| Vero cell | UL8, UL29, and UL52 | [96] | ||||||
| 297T, HaCaT, HaCaT IFNAR2-knockout, THP-1, primary mouse corneal stromal cell, and C57BL/6J mice | UL8 and UL29 genes | [97] | ||||||
| HEK293T, HeLa, and Jurkat c5 and c19 cells | TLR | HIV-1 | (−)ssRNA | Reverse transcription in cytoplasm Replication in nucleus | Lentivirus | Retrovirus | [82] | |
| CHME5 cell, HeLa-derived TZM-bI cells, promonocytic U-937 cell subclone U1 | TLR-U3 | [83] | ||||||
| Transgenic mice | 5′-LTR and Gag gene | [98] | ||||||
| Human T-lymphoid cell, Jurkat 2D10, PBMCs | LTR-U3 | [99] | ||||||
| NRG mice | LTR | [71] | ||||||
| HEK293T, Jurkat C11, and TZM-bl cells | LTR7, LTR8, and structural region (env5, vif2, rev3, gag8, pol6, and pol7) | [100] | ||||||
| HEK293T | LTR, gag, and pol | [84] | ||||||
| Tg26 transgenic mice, BLT mice, NCr nude mice | LTR, gag, and pol | [101] | ||||||
| HEK293FT, primary human monocytes | LTR, gag, env, ref, tat | [102] | ||||||
| Cas12a | HEK293T cell | LTR, gag, env, pol, tat, rev, nef, vpr | [70] | |||||
| Cas13a | HEK293T cell, HEK293 cell | LTR, gag, tat, and rev | [103] | |||||
| Cas9 | HepG2.2.15 cell | HBV DNA sequences | HBV | Double-stranded, relaxed circular DNA (rcDNA) | Nucleus | Orthohepadnavirus | Hepadnaviridae | [72] |
| HepG2 cell, and Balb/c mice | Conserved regions of HBV | [74] | ||||||
| Huh7 cell, HepG2.2.15 cell, and Balb/c mice | 20 nucleotide HBV DNA sequences | [104] | ||||||
| HepG2 cell | Conserved regions of HBV | [105] | ||||||
| Huh7 cell, and C57BL/6 mice | Conserved regions of HBV | [106] | ||||||
| PK-15 cell | UL30 | PRV | dsDNA | Nucleus | Varicellovirus | Herpesviridae | [73] | |
| Vero cell | Essential and nonessential genes | [107] | ||||||
| Vero cell | EBNA1, OriP | EBV | DNA | Nucleus | Lymphocryptovirus | Herpesviridae | [95] | |
| Vero cell | UL54, UL44, UL57, UL70, UL105, UL86, and UL84 | HCMV | DNA | Nucleus | Cytomegalovirus | Herpesviridae | [95] | |
| Cas9 | Hela cell, Caski, HEK293T, Jurkat, Hela-FLAG16E7MYC cell, and Rag1 mice | E6 and E7 | HPV | dsDNA | Nucleus | Alphapapillomavirus | Papovaviridae | [108] |
| SiHa, C33-A, and BALB/c nude mice | E6 and E7 | [109] | ||||||
| SiHa cell, and nude mice | E7 | [110] | ||||||
| Hela, HCS-2, SKG-I, 293, and BALB/c nude mice | E6 | [111] | ||||||
| Hela, 293T, and SiHa cell | E6 and E7 | HPV | dsDNA | Nucleus | Alphapapillomavirus | Papovaviridae | [112] | |
| Cas12a | BmN-SWU1 cell, and transgenic silkworm | ei-1 gene | BmNPV | dsDNA | Nucleus | Alphabaculovirus | Baculovirudae | [69] |
| Cas13a | Mice | PB1 and PB2 genes of influenza | IAV-H1N1 (A/WSN/33) | (−)ssRNA | Nucleus | Alphainfluenzavirus | Orthomyxoviridae | [89] |
| MDCK cell | Conserved regions of H1N1 | IAV-H1N1 (A/Puerto Rico/8/1934) | (−)ssRNA | Nucleus | Alphainfluenzavirus | Orthomyxoviridae | [81] | |
| A549 cell | Conserved regions of H1N1 | IAV-H1N1 | (−)ssRNA | Nucleus | Alphainfluenzavirus | Orthomyxoviridae | [77] | |
| Hamsters | Replicase and nucleocapsid genes of SARS | SARS-CoV2 | (+)ssRNA | Cytoplasm | Betacoronavirus | Coronaviridae | [89] | |
| HepG2 cell, and AT2 cell | S gene | SARS-CoV2 | [113] | |||||
| A549 cell, and HEK293T cell | RdRp (ORF1ab) and N gene | SARS-CoV2 | (+)ssRNA | Cytoplasm | Betacoronavirus | Coronaviridae | [77] | |
| - | Replicase and transcriptase (ORFab) and S gene | SARS-CoV2 | (+)ssRNA | Cytoplasm | Betacoronavirus | Coronaviridae | [114] | |
| HEK293FT cell | Conserved regions of LCMV | Wild type-LCMV Armstrong | (−)ssRNA | Cytoplasm | Mammarenavirus | Arenaviridae | [81] | |
| HEK293FT cell | Conserved regions of VSV | VSV | (−)ssRNA | Cytoplasm | Vesiculovirus | Rhabdoviridae | [81] | |
| HEK293T, HEK293FT, and MARC-145 cell | ORF5 and ORF7 | PRRSV | (+)ssRNA | Cytoplasm | Porartevirus | Arteriviridae | [115] |
2.5. 3D8 Single-Chain Variable Fragment (3D8 scFv)
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Coronavirus Disease (COVID-19) Dashboard; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- WHO. Summary of Probable SARS Cases with Onset of Illness from 1 November 2002 to 31 July 2003; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- WHO. Middle East Respiratory Syndrome Coronavirus (MERS-CoV)—Saudi Arabia; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- WHO. Ebola in Democratic Republic of Congo—North Kivu, Ituri 2018–2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. Ebola Virus Disease-Democratic Republic of Congo; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- WHO. Latest HIV Estimates and Updates on HIV Policies Uptake, July 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. Global Hepatitis Report, 2017; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Dawood, F.S.; Iuliano, A.D.; Reed, C.; Martin, I.; Meltzer, D.K. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: A modelling study. Lancet Infect. Dis. 2012, 12, 687–695. [Google Scholar] [CrossRef] [Green Version]
- WHO. Avian Influenza A (H5N1)—United States of America; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Tompa, D.R.; Immanuel, A.; Srikanth, S.; Kadhirvel, S. Trends and strategies to combat viral infections: A review on FDA approved antiviral drugs. Int. J. Biol. Macromol. 2021, 172, 524–541. [Google Scholar] [CrossRef]
- Reyes, E.D.; Kulej, K.; Pancholi, N.J.; Akhta, L.N.; Avgousti, D.C.; Kim, E.U.; Bricker, D.K.; Spruce, L.A.; Koniski, S.A.; Seeholzer, S.H.; et al. Identifying Host Factors Associated with DNA Replicated During Virus Infection. Mol. Cell. Proteom. 2017, 16, 2079–2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boersma, S.; Rabouw, H.H.; Bruurs, L.J.M.; Pavlovic, T.; Vliet, A.L.W.; Beumer, J.; Clevers, H.; Kuppeveld, F.J.M.; Tanenbaum, M.E. Translation and Replication Dynamics of Single RNA Viruses. Cell 2020, 183, 1930–1945. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Su, S.; Yang, H.; Jiang, S. Antivirals with common targets against highly pathogenic viruses. Cell 2021, 184, 1604–1620. [Google Scholar] [CrossRef]
- Dou, D.; Revol, R.; Östbye, H.; Wang, H.; Daniels, R. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef] [PubMed]
- Clercq, E.D.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef] [Green Version]
- Strasfeld, L.; Chou, S. Antiviral drug resistance: Mechanisms and clinical implications. Infect. Dis. Clin. N. Am. 2010, 24, 413–437. [Google Scholar] [CrossRef] [Green Version]
- Lampejo, T. Influenza and antiviral resistance: An overview. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Van Poelvoorde, L.A.E.; Saelens, X.; Thomas, I.; Roosens, N.H. Next-Generation Sequencing: An Eye-Opener for the Surveillance of Antiviral Resistance in Influenza. Trends Biotechnol. 2020, 38, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Kuroda, K.; Dhangar, K.; Mazumder, P.; Sonne, C.; Rinklebe, J.; Kitajima, M. Potential Emergence of Antiviral-Resistant Pandemic Viruses via Environmental Drug Exposure of Animal Reservoirs. Environ. Sci. Technol. 2020, 54, 8503–8505. [Google Scholar] [CrossRef] [PubMed]
- Durmuş, S.; Ülgen, K. Comparative interactomics for virus-human protein-protein interactions: DNA viruses versus RNA viruses. FEBS Open Bio 2017, 7, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.H. Viral encounters with 2’,5’-oligoadenylate synthetase and RNase L during the interferon antiviral response. J. Virol. 2007, 81, 12720–12729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Boix, E. Host Defence RNases as Antiviral Agents against Enveloped Single Stranded RNA Viruses. Virulence 2021, 12, 444–469. [Google Scholar] [CrossRef] [PubMed]
- Ilinskaya, O.N.; Mahmud, R.S. Ribonucleases as antiviral agents. Mol. Biol. 2014, 48, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Lee-Huang, S.; Huang, P.L.; Sun, Y.; Huang, P.L.; Kung, H.F.; Blithe, D.L.; Chen, H.C. Lysozyme and RNases as anti-HIV components in beta-core preparations of human chorionic gonadotropin. Proc. Natl. Acad. Sci. USA 1999, 96, 2678–2681. [Google Scholar] [CrossRef] [Green Version]
- Cooper, D.A.; Banerjee, S.; Chakrabarti, A.; García-Sastre, A.; Hesselberth, J.R.; Silverman, R.H.; Barton, D.J. RNase L targets distinct sites in influenza A virus RNAs. J. Virol. 2015, 89, 2764–2776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherbik, S.V.; Paranjape, J.M.; Stockman, B.M.; Silverman, R.H.; Brinton, M.A. RNase L plays a role in the antiviral response to West Nile virus. J. Virol. 2006, 80, 2987–2999. [Google Scholar] [CrossRef] [Green Version]
- Samuel, M.A.; Whitby, K.; Keller, B.C.; Marri, A.; Barchet, W.; Williams, B.R.; Silverman, R.H.; Gale, M., Jr.; Diamond, M.S. PKR and RNase L contribute to protection against lethal West Nile Virus infection by controlling early viral spread in the periphery and replication in neurons. J. Virol. 2006, 80, 7009–7019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, R.J.; Chien, H.L.; Lin, S.Y.; Chang, B.L.; Yu, H.P.; Tang, W.C.; Lin, Y.L. MCPIP1 ribonuclease exhibits broad-spectrum antiviral effects through viral RNA binding and degradation. Nucleic Acids Res. 2013, 41, 3314–3326. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.J.; Chu, J.S.; Chien, H.L.; Tseng, C.H.; Ko, P.C.; Mei, Y.Y.; Tang, W.C.; Kao, Y.T.; Cheng, H.Y.; Liang, Y.C.; et al. MCPIP1 suppresses hepatitis C virus replication and negatively regulates virus-induced proinflammatory cytokine responses. J. Immunol. 2014, 193, 4159–4168. [Google Scholar] [CrossRef] [Green Version]
- Sorgeloos, F.; Jha, B.K.; Silverman, R.H.; Michiels, T. Evasion of Antiviral Innate Immunity by Theiler’s Virus L* Protein through Direct Inhibition of RNase L. PLoS Pathog. 2013, 9, e1003474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, J.M.; Gilchrist, A.R.; Sawyer, S.L.; Parker, R. RNase L limits host and viral protein synthesis via inhibition of mRNA export. Sci. Adv. 2021, 7, eabh2479. [Google Scholar] [CrossRef] [PubMed]
- Makarov, A.A.; Ilinskaya, O.N. Cytotoxic ribonucleases: Molecular weapons and their targets. FEBS Lett. 2003, 540, 15–20. [Google Scholar] [CrossRef]
- Qureshi, A.; Tantray, V.G.; Kirmani, A.R.; Ahangar, A.G. A review on current status of antiviral siRNA. Rev. Med. Virol. 2018, 28, e1976. [Google Scholar] [CrossRef]
- Singh, S.K. RNA interference and its therapeutic potential against HIV infection. Expert Opin. Biol. Ther. 2008, 8, 449–461. [Google Scholar] [CrossRef]
- Rossi, J. RNAi as a treatment for HIV-1 infection. BioTechniques 2006, 40, S25–S29. [Google Scholar] [CrossRef]
- Ge, Q.; McManus, M.T.; Nguyen, T.; Shen, C.-H.; Sharp, P.A.; Eisen, H.N.; Chen, J. RNA interference of influenza virus production by directly targeting mRNA for degradation and indirectly inhibiting all viral RNA transcription. Proc. Natl. Acad. Sci. USA 2003, 100, 2718–2723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakazawa, M.; Kadowaki, S.-e.; Watanabe, I.; Kadowaki, Y.; Takei, M.; Fukuda, H. PA subunit of RNA polymerase as a promising target for anti-influenza virus agents. Antivir. Res. 2008, 78, 194–201. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, D.H.; Xiong, J.; Jia, J.; Huang, B.; Jin, Y.X. Inhibition of genes expression of SARS coronavirus by synthetic small interfering RNAs. Cell Res. 2005, 15, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Meng, B.; Lui, Y.-w.; Meng, S.; Cao, C.; Hu, Y. Identification of effective siRNA blocking the expression of SARS viral envelope E and RDRP genes. Mol. Biotechnol. 2006, 33, 141–148. [Google Scholar] [CrossRef]
- Konishi, M.; Wu, C.H.; Wu, G.Y. Inhibition of HBV replication by siRNA in a stable HBV-producing cell line. Hepatology 2003, 38, 842–850. [Google Scholar] [CrossRef]
- Chen, Y.; Mahato, R.I. siRNA pool targeting different sites of human hepatitis B surface antigen efficiently inhibits HBV infection. J. Drug Target. 2008, 16, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Bousarghin, L.; Touzé, A.; Gaud, G.; Iochmann, S.; Alvarez, E.; Reverdiau, P.; Gaitan, J.; Jourdan, M.-L.; Sizaret, P.-Y.; Coursaget, P. Inhibition of cervical cancer cell growth by human papillomavirus virus-like particles packaged with human papillomavirus oncoprotein short hairpin RNAs. Mol. Cancer Ther. 2009, 8, 357–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Lee, S.K.; Shankar, P.; Manjunath, N. A single siRNA suppresses fatal encephalitis induced by two different flaviviruses. PLoS Med. 2006, 3, e96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, A.; Michler, T.; Merkel, O.M. siRNA Therapeutics against Respiratory Viral Infections-What Have We Learned for Potential COVID-19 Therapies? Adv. Healthc Mater. 2021, 10, e2001650. [Google Scholar] [CrossRef]
- Gish, R.G.; Satishchandran, C.; Young, M.; Pachuk, C. RNA Interference and Its Potential Applications to Chronic HBV Treatment: Results of a Phase I Safety and Tolerability Study. Antivir. Ther. 2011, 16, 547–554. [Google Scholar] [CrossRef] [Green Version]
- DeVincenzo, J.; Lambkin-Williams, R.; Wilkinson, T.; Cehelsky, J.; Nochur, S.; Walsh, E.; Meyers, R.; Gollob, J.; Vaishnaw, A. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc. Natl. Acad. Sci. USA 2010, 107, 8800–8805. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.H.; Croyle, M.A. Emerging targets and novel approaches to Ebola virus prophylaxis and treatment. BioDrugs 2013, 27, 565–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espert, L.; Rey, C.; Gonzalez, L.; Degols, G.; Chelbi-Alix, M.K.; Mechti, N.; Gongora, C. The exonuclease ISG20 is directly induced by synthetic dsRNA via NF-kappaB and IRF1 activation. Oncogene 2004, 23, 4636–4640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gongora, C.; David, G.; Pintard, L.; Tissot, C.; Hua, T.D.; Dejean, A.; Mechti, N. Molecular cloning of a new interferon-induced PML nuclear body-associated protein. J. Biol. Chem. 1997, 272, 19457–19463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imaizumi, T.; Mechti, N.; Matsumiya, T.; Sakaki, H.; Kubota, K.; Yoshida, H.; Kimura, H.; Satoh, K. Expression of interferon-stimulated gene 20 in vascular endothelial cells. Microbiol. Immunol. 2008, 52, 30–35. [Google Scholar] [CrossRef]
- Moser, M.J.; Holley, W.R.; Chatterjee, A.; Mian, I.S. The proofreading domain of Escherichia coli DNA polymerase I and other DNA and/or RNA exonuclease domains. Nucleic Acids Res. 1997, 25, 5110–5118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.H.; Espert, L.; Mechti, N.; Wilson, D.M., 3rd. The human interferon- and estrogen-regulated ISG20/HEM45 gene product degrades single-stranded RNA and DNA in vitro. Biochemistry 2001, 40, 7174–7179. [Google Scholar] [CrossRef] [PubMed]
- Horio, T.; Murai, M.; Inoue, T.; Hamasaki, T.; Tanaka, T.; Ohgi, T. Crystal structure of human ISG20, an interferon-induced antiviral ribonuclease. FEBS Lett. 2004, 577, 111–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.; Wang, L.; Pan, J. Interferon-stimulated gene 20-kDa protein (ISG20) in infection and disease: Review and outlook. Intractable Rare Dis. Res. 2017, 6, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Yang, E.; Li, M.M.H. All About the RNA: Interferon-Stimulated Genes That Interfere With Viral RNA Processes. Front. Immunol. 2020, 11, 605024. [Google Scholar] [CrossRef]
- Leong, C.R.; Funami, K.; Oshiumi, H.; Mengao, D.; Takaki, H.; Matsumoto, M.; Aly, H.H.; Watashi, K.; Chayama, K.; Seya, T. Interferon-stimulated gene of 20 kDa protein (ISG20) degrades RNA of hepatitis B virus to impede the replication of HBV in vitro and in vivo. Oncotarget 2016, 7, 68179–68193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imam, H.; Kim, G.W.; Mir, S.A.; Khan, M.; Siddiqui, A. Interferon-stimulated gene 20 (ISG20) selectively degrades N6-methyladenosine modified Hepatitis B Virus transcripts. PLoS Pathog. 2020, 16, e1008338. [Google Scholar] [CrossRef] [PubMed]
- Imam, H.; Khan, M.; Gokhale, N.S.; McIntyre, A.B.R.; Kim, G.W.; Jang, J.Y.; Kim, S.J.; Mason, C.E.; Horner, S.M.; Siddiqui, A. N6-methyladenosine modification of hepatitis B virus RNA differentially regulates the viral life cycle. Proc. Natl. Acad. Sci. USA 2018, 115, 8829–8834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaccara, S.; Jaffrey, S.R. A Unified Model for the Function of YTHDF Proteins in Regulating m(6)A-Modified mRNA. Cell 2020, 181, 1582–1595.e18. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Nguyen, X.N.; Wang, L.; Appourchaux, R.; Zhang, C.; Panthu, B.; Gruffat, H.; Journo, C.; Alais, S.; Qin, J.; et al. The interferon stimulated gene 20 protein (ISG20) is an innate defense antiviral factor that discriminates self versus non-self translation. PLoS Pathog. 2019, 15, e1008093. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.M.; Trobaugh, D.W.; Sun, C.; Lucas, T.M.; Diamond, M.S.; Ryman, K.D.; Klimstra, W.B. The Interferon-Induced Exonuclease ISG20 Exerts Antiviral Activity through Upregulation of Type I Interferon Response Proteins. mSphere 2018, 3, e00209-18. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Wang, N.; Woodson, S.E.; Dong, Q.; Wang, J.; Liang, Y.; Rijnbrand, R.; Wei, L.; Nichols, J.E.; Guo, J.T.; et al. Antiviral activities of ISG20 in positive-strand RNA virus infections. Virology 2011, 409, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Espert, L.; Degols, G.; Gongora, C.; Blondel, D.; Williams, B.R.; Silverman, R.H.; Mechti, N. ISG20, a new interferon-induced RNase specific for single-stranded RNA, defines an alternative antiviral pathway against RNA genomic viruses. J. Biol. Chem. 2003, 278, 16151–16158. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Weidner, J.M.; Qing, M.; Pan, X.B.; Guo, H.; Xu, C.; Zhang, X.; Birk, A.; Chang, J.; Shi, P.Y.; et al. Identification of five interferon-induced cellular proteins that inhibit west nile virus and dengue virus infections. J. Virol. 2010, 84, 8332–8341. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Guo, H.; Xu, C.; Chang, J.; Gu, B.; Wang, L.; Block, T.M.; Guo, J.T. Identification of three interferon-inducible cellular enzymes that inhibit the replication of hepatitis C virus. J. Virol. 2008, 82, 1665–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Oost, J.; Westra, E.R.; Jackson, R.N.; Wiedenheft, B. Unravelling the structural and mechanistic basis of CRISPR-Cas systems. Nat. Rev. Microbiol. 2014, 12, 479–492. [Google Scholar] [CrossRef]
- Hille, F.; Charpentier, E. CRISPR-Cas: Biology, mechanisms and relevance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150496. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, H.J.E.; Isalan, M. The Application of CRISPR/Cas Systems for Antiviral Therapy. Front. Genome Ed. 2021, 3, 745559. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Qin, Q.; Hu, Z.; Zhang, X.; Miao, J.; Huang, L.; Chen, P.; Lu, C.; Pan, M. CRISPR/Cas12a Mediated Genome Editing Enhances Bombyx mori Resistance to BmNPV. Front. Bioeng. Biotechnol. 2020, 8, 841. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Fan, M.; Das, A.T.; Herrera-Carrillo, E.; Berkhout, B. Extinction of all infectious HIV in cell culture by the CRISPR-Cas12a system with only a single crRNA. Nucleic Acids Res. 2020, 48, 5527–5539. [Google Scholar] [CrossRef]
- Bella, R.; Kaminski, R.; Mancuso, P.; Young, W.-B.; Chen, C.; Sariyer, R.; Fischer, T.; Amini, S.; Ferrante, P.; Jacobson, J.M.; et al. Removal of HIV DNA by CRISPR from Patient Blood Engrafts in Humanized Mice. Mol. Ther. -Nucleic Acids 2018, 12, 275–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanan, V.; Shlomai, A.; Cox, D.B.T.; Schwartz, R.E.; Michailidis, E.; Bhatta, A.; Scott, D.A.; Zhang, F.; Rice, C.M.; Bhatia, S.N. CRISPR/Cas9 cleavage of viral DNA efficiently suppresses hepatitis B virus. Sci. Rep. 2015, 5, 10833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Z.; Ouyang, T.; Pang, D.; Ma, T.; Chen, X.; Guo, N.; Chen, F.; Yuan, L.; Ouyang, H.; Ren, L. Pseudorabies virus can escape from CRISPR-Cas9-mediated inhibition. Virus Res. 2016, 223, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, R.; Chen, S.; Guo, D.; Chen, Y. Inhibition of hepatitis B virus by the CRISPR/Cas9 system via targeting the conserved regions of the viral genome. J. Gen. Virol. 2015, 96, 2252–2261. [Google Scholar] [CrossRef] [PubMed]
- Nishimasu, H.; Ran, F.A.; Hsu, P.D.; Konermann, S.; Shehata, S.I.; Dohmae, N.; Ishitani, R.; Zhang, F.; Nureki, O. Crystal Structure of Cas9 in Complex with Guide RNA and Target DNA. Cell 2014, 156, 935–949. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Newbigging, A.M.; Tao, J.; Cao, Y.; Peng, H.; Le, C.; Wu, J.; Pang, B.; Li, J.; Tyrrell, D.L.; et al. CRISPR technology incorporating amplification strategies: Molecular assays for nucleic acids, proteins, and small molecules. Chem. Sci. 2021, 12, 4683–4698. [Google Scholar] [CrossRef]
- Abbott, T.R.; Dhamdhere, G.; Liu, Y.; Lin, X.; Goudy, L.; Zeng, L.; Chemparathy, A.; Chmura, S.; Heaton, N.S.; Debs, R.; et al. Development of CRISPR as an Antiviral Strategy to Combat SARS-CoV-2 and Influenza. Cell 2020, 181, 865–876.e12. [Google Scholar] [CrossRef]
- Woolhouse, M.E.J.; Brierley, L. Epidemiological characteristics of human-infective RNA viruses. Sci. Data 2018, 5, 180017. [Google Scholar] [CrossRef]
- Woolhouse, M.; Scott, F.; Hudson, Z.; Howey, R.; Chase-Topping, M. Human viruses: Discovery and emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2864–2871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Li, X.; Ma, J.; Li, Z.; You, L.; Wang, J.; Wang, M.; Zhang, X.; Wang, Y. The Molecular Architecture for RNA-Guided RNA Cleavage by Cas13a. Cell 2017, 170, 714–726.e10. [Google Scholar] [CrossRef] [PubMed]
- Freije, C.A.; Myhrvold, C.; Boehm, C.K.; Lin, A.E.; Welch, N.L.; Carter, A.; Metsky, H.C.; Luo, C.Y.; Abudayyeh, O.O.; Gootenberg, J.S.; et al. Programmable Inhibition and Detection of RNA Viruses Using Cas13. Mol. Cell 2019, 76, 826–837.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebina, H.; Misawa, N.; Kanemura, Y.; Koyanagi, Y. Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci. Rep. 2013, 3, 2510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Kaminski, R.; Yang, F.; Zhang, Y.; Cosentino, L.; Li, F.; Luo, B.; Alvarez-Carbonell, D.; Garcia-Mesa, Y.; Karn, J.; et al. RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proc. Natl. Acad. Sci. USA 2014, 111, 11461–11466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, C.; Zhang, T.; Li, F.; Yang, F.; Putatunda, R.; Young, W.-B.; Khalili, K.; Hu, W.; Zhang, Y. Functional screening of guide RNAs targeting the regulatory and structural HIV-1 viral genome for a cure of AIDS. AIDS 2016, 30, 1163–1174. [Google Scholar] [CrossRef] [Green Version]
- Bayoumi, M.; Munir, M. Potential Use of CRISPR/Cas13 Machinery in Understanding Virus–Host Interaction. Front. Microbiol. 2021, 12, 743580. [Google Scholar] [CrossRef] [PubMed]
- Abudayyeh, O.O.; Gootenberg, J.S.; Essletzbichler, P.; Han, S.; Joung, J.; Belanto, J.J.; Verdine, V.; Cox, D.B.T.; Kellner, M.J.; Regev, A.; et al. RNA targeting with CRISPR–Cas13. Nature 2017, 550, 280–284. [Google Scholar] [CrossRef] [Green Version]
- Myhrvold, C.; Freije, C.A.; Gootenberg, J.S.; Abudayyeh, O.O.; Metsky, H.C.; Durbin, A.F.; Kellner, M.J.; Tan, A.L.; Paul, L.M.; Parham, L.A.; et al. Field-deployable viral diagnostics using CRISPR-Cas13. Science 2018, 360, 444–448. [Google Scholar] [CrossRef] [Green Version]
- Konermann, S.; Lotfy, P.; Brideau, N.J.; Oki, J.; Shokhirev, M.N.; Hsu, P.D. Transcriptome Engineering with RNA-Targeting Type VI-D CRISPR Effectors. Cell 2018, 173, 665–676.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanchard, E.L.; Vanover, D.; Bawage, S.S.; Tiwari, P.M.; Rotolo, L.; Beyersdorf, J.; Peck, H.E.; Bruno, N.C.; Hincapie, R.; Michel, F.; et al. Treatment of influenza and SARS-CoV-2 infections via mRNA-encoded Cas13a in rodents. Nat. Biotechnol. 2021, 39, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Bawage, S.S.; Tiwari, P.M.; Santangelo, P.J. Synthetic mRNA expressed Cas13a mitigates RNA virus infections. bioRxiv 2018. [Google Scholar] [CrossRef] [Green Version]
- Ai, Y.; Liang, D.; Wilusz, J.E. CRISPR/Cas13 effectors have differing extents of off-target effects that limit their utility in eukaryotic cells. Nucleic Acids Res. 2022, 50, e65. [Google Scholar] [CrossRef]
- Hübner, A.; Petersen, B.; Keil, G.M.; Niemann, H.; Mettenleiter, T.C.; Fuchs, W. Efficient inhibition of African swine fever virus replication by CRISPR/Cas9 targeting of the viral p30 gene (CP204L). Sci. Rep. 2018, 8, 1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roehm, P.C.; Shekarabi, M.; Wollebo, H.S.; Bellizzi, A.; He, L.; Salkind, J.; Khalili, K. Inhibition of HSV-1 Replication by Gene Editing Strategy. Sci. Rep. 2016, 6, 23146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Fan, S.; Zhou, J.; Zhang, Y.; Che, Y.; Cai, H.; Wang, L.; Guo, L.; Liu, L.; Li, Q. The mutated tegument protein UL7 attenuates the virulence of herpes simplex virus 1 by reducing the modulation of α-4 gene transcription. Virol. J. 2016, 13, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Diemen, F.R.; Kruse, E.M.; Hooykaas, M.J.G.; Bruggeling, C.E.; Schürch, A.C.; van Ham, P.M.; Imhof, S.M.; Nijhuis, M.; Wiertz, E.J.H.J.; Lebbink, R.J. CRISPR/Cas9-Mediated Genome Editing of Herpesviruses Limits Productive and Latent Infections. PLoS Pathog. 2016, 12, e1005701. [Google Scholar] [CrossRef] [PubMed]
- Karpov, D.S.; Karpov, V.L.; Klimova, R.R.; Demidova, N.A.; Kushch, A.A. A Plasmid-Expressed CRISPR/Cas9 System Suppresses Replication of HSV Type I in a Vero Cell Culture. Mol. Biol. 2019, 53, 70–78. [Google Scholar] [CrossRef]
- Yin, D.; Ling, S.; Wang, D.; Dai, Y.; Jiang, H.; Zhou, X.; Paludan, S.R.; Hong, J.; Cai, Y. Targeting herpes simplex virus with CRISPR–Cas9 cures herpetic stromal keratitis in mice. Nat. Biotechnol. 2021, 39, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, R.; Bella, R.; Yin, C.; Otte, J.; Ferrante, P.; Gendelman, H.E.; Li, H.; Booze, R.; Gordon, J.; Hu, W.; et al. Excision of HIV-1 DNA by gene editing: A proof-of-concept in vivo study. Gene Ther. 2016, 23, 690–695. [Google Scholar] [CrossRef]
- Kaminski, R.; Chen, Y.; Fischer, T.; Tedaldi, E.; Napoli, A.; Zhang, Y.; Karn, J.; Hu, W.; Khalili, K. Elimination of HIV-1 Genomes from Human T-lymphoid Cells by CRISPR/Cas9 Gene Editing. Sci. Rep. 2016, 6, 22555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Liu, S.; Liu, Z.; Ke, Z.; Li, C.; Yu, X.; Chen, S.; Guo, D. Genome scale screening identification of SaCas9/gRNAs for targeting HIV-1 provirus and suppression of HIV-1 infection. Virus Res. 2018, 250, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Zhang, T.; Qu, X.; Zhang, Y.; Putatunda, R.; Xiao, X.; Li, F.; Xiao, W.; Zhao, H.; Dai, S.; et al. In Vivo Excision of HIV-1 Provirus by saCas9 and Multiplex Single-Guide RNAs in Animal Models. Mol. Ther. 2017, 25, 1168–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herskovitz, J.; Hasan, M.; Patel, M.; Blomberg, W.R.; Cohen, J.D.; Machhi, J.; Shahjin, F.; Mosley, R.L.; McMillan, J.; Kevadiya, B.D.; et al. CRISPR-Cas9 Mediated Exonic Disruption for HIV-1 Elimination. EBioMedicine 2021, 73, 103678. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhao, F.; Sun, H.; Wang, Z.; Huang, Y.; Zhu, W.; Xu, F.; Mei, S.; Liu, X.; Zhang, D.; et al. CRISPR-Cas13a Inhibits HIV-1 Infection. Mol. Ther. Nucleic Acids 2020, 21, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Qu, L.; Wang, H.; Wei, L.; Dong, Y.; Xiong, S. Targeting hepatitis B virus cccDNA by CRISPR/Cas9 nuclease efficiently inhibits viral replication. Antivir. Res. 2015, 118, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kostyushev, D.; Brezgin, S.; Kostyusheva, A.; Zarifyan, D.; Goptar, I.; Chulanov, V. Orthologous CRISPR/Cas9 systems for specific and efficient degradation of covalently closed circular DNA of hepatitis B virus. Cell. Mol. Life Sci. 2019, 76, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Feng, J.; Liu, X.; Wang, H.; Li, Q.; Li, J.; Xu, T.; Sajid, M.; Ullah, H.; Zhou, L.; et al. Inhibition of Hepatitis B Virus by AAV8-Derived CRISPR/SaCas9 Expressed From Liver-Specific Promoters. Front. Microbiol. 2021, 12, 665184. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-D.; Liu, J.-T.; Wang, T.-Y.; Sun, M.-X.; Tian, Z.-J.; Cai, X.-H. CRISPR/Cas9-mediated multiple single guide RNAs potently abrogate pseudorabies virus replication. Arch. Virol. 2017, 162, 3881–3886. [Google Scholar] [CrossRef]
- Jubair, L.; Fallaha, S.; McMillan, N.A.J. Systemic Delivery of CRISPR/Cas9 Targeting HPV Oncogenes Is Effective at Eliminating Established Tumors. Mol. Ther. 2019, 27, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Hua, L.; Takahashi, Y.; Narita, S.; Liu, Y.-H.; Li, Y. In vitro and in vivo growth suppression of human papillomavirus 16-positive cervical cancer cells by CRISPR/Cas9. Biochem. Biophys. Res. Commun. 2014, 450, 1422–1426. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jin, Z.; Tan, X.; Zhang, C.; Zou, C.; Zhang, W.; Ding, J.; Das, B.C.; Severinov, K.; Hitzeroth, I.I.; et al. Hyperbranched poly(β-amino ester) based polyplex nanopaticles for delivery of CRISPR/Cas9 system and treatment of HPV infection associated cervical cancer. J. Control. Release 2020, 321, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Yoshiba, T.; Saga, Y.; Urabe, M.; Uchibor, R.; Matsubara, S.; Fujiwara, H.; Mizukami, H. CRISPR/Cas9-mediated cervical cancer treatment targeting human papillomavirus E6. Oncol Lett. 2019, 17, 2197–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, E.M.; Kornepati, A.V.R.; Goldstein, M.; Bogerd, H.P.; Poling, B.C.; Whisnant, A.W.; Kastan, M.B.; Cullen, B.R. Inactivation of the human papillomavirus E6 or E7 gene in cervical carcinoma cells by using a bacterial CRISPR/Cas RNA-guided endonuclease. J. Virol. 2014, 88, 11965–11972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhou, J.; Wang, Q.; Wang, Y.; Kang, C. Rapid design and development of CRISPR-Cas13a targeting SARS-CoV-2 spike protein. Theranostics 2021, 11, 649–664. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Zhang, Y.; Pandolfi, P.P. Virus against virus: A potential treatment for 2019-nCov (SARS-CoV-2) and other RNA viruses. Cell Res. 2020, 30, 189–190. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Techakriengkrai, N.; Nedumpun, T.; Suradhat, S. Abrogation of PRRSV infectivity by CRISPR-Cas13b-mediated viral RNA cleavage in mammalian cells. Sci. Rep. 2020, 10, 9617. [Google Scholar] [CrossRef]
- Kwon, M.H.; Lee, M.S.; Kim, K.H.; Park, S.; Shin, H.J.; Jang, Y.J.; Kim, H.I. Production and characterization of an anti-idiotypic single chain Fv that recognizes an anti-DNA antibody. Immunol. Investig. 2002, 31, 205–218. [Google Scholar] [CrossRef]
- Kim, Y.R.; Kim, J.S.; Lee, S.H.; Lee, W.R.; Sohn, J.N.; Chung, Y.C.; Shim, H.K.; Lee, S.C.; Kwon, M.H.; Kim, Y.S. Heavy and light chain variable single domains of an anti-DNA binding antibody hydrolyze both double- and single-stranded DNAs without sequence specificity. J. Biol. Chem. 2006, 281, 15287–15295. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.; Cho, S.; Hoang, P.M.; Kim, D.; Lee, Y.; Kil, E.J.; Byun, S.J.; Lee, T.K.; Kim, D.H.; Kim, S.; et al. Therapeutic Strategy for the Prevention of Pseudorabies Virus Infection in C57BL/6 Mice by 3D8 scFv with Intrinsic Nuclease Activity. Mol. Cells 2015, 38, 773–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.; Yu, J.; Cho, S.; Byun, S.J.; Kim, D.H.; Lee, T.K.; Kwon, M.H.; Lee, S. A nucleic-acid hydrolyzing single chain antibody confers resistance to DNA virus infection in hela cells and C57BL/6 mice. PLoS Pathog. 2014, 10, e1004208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Hoang, P.T.; Kim, D.; Ayun, R.Q.; Luong, Q.X.T.; Na, K.; Kim, T.; Oh, Y.; Kim, W.-K.; Lee, S. A Therapeutically Active Minibody Exhibits an Antiviral Activity in Oseltamivir-Resistant Influenza-Infected Mice via Direct Hydrolysis of Viral RNAs. Viruses 2022, 14, 1105. [Google Scholar] [CrossRef]
- Hoang, P.M.; Cho, S.; Kim, K.E.; Byun, S.J.; Lee, T.K.; Lee, S. Development of Lactobacillus paracasei harboring nucleic acid-hydrolyzing 3D8 scFv as a preventive probiotic against murine norovirus infection. Appl. Microbiol. Biotechnol. 2015, 99, 2793–2803. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Rhee, Y.; Auh, C.K.; Shim, H.; Choi, J.J.; Kwon, S.T.; Yang, J.S.; Kim, D.; Kwon, M.H.; Kim, Y.S.; et al. Production of recombinant single chain antibodies (scFv) in vegetatively reproductive Kalanchoe pinnata by in planta transformation. Plant Cell Rep. 2009, 28, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, M.; Seo, Y.; Lee, Y.; Park, H.; Byun, S.J.; Kwon, M.H. The catalytic activity of a recombinant single chain variable fragment nucleic acid-hydrolysing antibody varies with fusion tag and expression host. Arch. Biochem. Biophys. 2017, 633, 110–117. [Google Scholar] [CrossRef]
- Park, H.; Kim, M.; Kim, H.J.; Lee, Y.; Seo, Y.; Pham, C.D.; Lee, J.; Byun, S.J.; Kwon, M.H. Heparan sulfate proteoglycans (HSPGs) and chondroitin sulfate proteoglycans (CSPGs) function as endocytic receptors for an internalizing anti-nucleic acid antibody. Sci. Rep. 2017, 7, 14373. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Jeong, J.G.; Jun, H.R.; Lee, S.C.; Kim, J.S.; Kim, Y.S.; Kwon, M.H. A nucleic acid-hydrolyzing antibody penetrates into cells via caveolae-mediated endocytosis, localizes in the cytosol and exhibits cytotoxicity. Cell Mol. Life Sci. 2009, 66, 1985–1997. [Google Scholar] [CrossRef]
- Cho, S.; Youn, H.N.; Hoang, P.M.; Cho, S.; Kim, K.E.; Kil, E.J.; Lee, G.; Cho, M.J.; Hong, J.; Byun, S.J.; et al. Preventive Activity against Influenza (H1N1) Virus by Intranasally Delivered RNA-Hydrolyzing Antibody in Respiratory Epithelial Cells of Mice. Viruses 2015, 7, 5133–5144. [Google Scholar] [CrossRef]
- Jun, H.R.; Pham, C.D.; Lim, S.I.; Lee, S.C.; Kim, Y.S.; Park, S.; Kwon, M.H. An RNA-hydrolyzing recombinant antibody exhibits an antiviral activity against classical swine fever virus. Biochem. Biophys. Res. Commun. 2010, 395, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Lee, J.W.; Choi, H.; Jung, S.K.; Kim, J.S.; Kim, K.W.; Oh, K.B.; Yang, H.; Byun, S.J. Survival of Escherichia coli harboring nucleic acid-hydrolyzing 3D8 scFv during RNA virus infection. Regul. Toxicol. Pharmacol. 2018, 94, 286–292. [Google Scholar] [CrossRef]
- Sureshkumar, S.; Jung, S.K.; Kim, D.; Oh, K.B.; Yang, H.; Lee, H.C.; Jo, Y.J.; Lee, H.S.; Lee, S.; Byun, S.J. Administration of L. salivarius expressing 3D8 scFv as a feed additive improved the growth performance, immune homeostasis, and gut microbiota of chickens. Anim. Sci. J. 2020, 91, e13399. [Google Scholar] [CrossRef] [PubMed]
- Sureshkumar, S.; Jung, S.K.; Kim, D.; Oh, K.B.; Yang, H.; Lee, H.C.; Jin, J.Y.; Sun, L.H.; Lee, S.; Byun, S.J. Oral administration of Lactobacillus reuteri expressing a 3D8 single-chain variable fragment (scFv) enhances chicken growth and conserves immune homeostasis. 3 Biotech. 2019, 9, 282. [Google Scholar] [CrossRef]
- Cho, S.; Kim, D.; Lee, Y.; Kil, E.J.; Cho, M.J.; Byun, S.J.; Cho, W.K.; Lee, S. Probiotic Lactobacillus Paracasei Expressing a Nucleic Acid-Hydrolyzing Minibody (3D8 Scfv) Enhances Probiotic Activities in Mice Intestine as Revealed by Metagenomic Analyses. Genes 2018, 9, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.S.; Lee, S.H.; Kim, J.S.; Lee, S.C.; Kwon, M.H.; Kim, Y.S. Generation of humanized anti-DNA hydrolyzing catalytic antibodies by complementarity determining region grafting. Biochem. Biophys. Res. Commun. 2009, 379, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Byun, S.J.; Seo, Y.; Kim, M.; Lee, J.H.; Kim, S.; Lee, Y.; Lee, K.W.; Kim, J.K.; Kwon, M.H. Generation of a chickenized catalytic anti-nucleic acid antibody by complementarity-determining region grafting. Mol. Immunol. 2015, 63, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Lee, W.R.; Joung, Y.K.; Kwon, M.H.; Kim, Y.S.; Park, K.D. Optimized stability retention of a monoclonal antibody in the PLGA nanoparticles. Int. J. Pharm. 2009, 368, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Shim, H.-K.; Kwon, M.-H.; Son, S.-H.; Kim, K.-Y.; Park, E.-Y.; Yang, J.-K.; Lee, T.-K.; Auh, C.-K.; Kim, D.; et al. RNA virus accumulation is inhibited by ribonuclease activity of 3D8 scFv in transgenic Nicotiana tabacum. Plant Cell Tissue Organ. Cult. (PCTOC) 2013, 115, 189–197. [Google Scholar] [CrossRef]
- Tran, D.T.; Cho, S.; Hoang, P.M.; Kim, J.; Kil, E.-J.; Lee, T.-K.; Rhee, Y.; Lee, S. A Codon-Optimized Nucleic Acid Hydrolyzing Single-Chain Antibody Confers Resistance to Chrysanthemums Against Chrysanthemum Stunt Viroid Infection. Plant Mol. Biol. Rep. 2016, 34, 221–232. [Google Scholar] [CrossRef]
- June Byun, S.; Yuk, S.-s.; Jang, Y.-J.; Choi, H.; Jeon, M.-H.; Erdene-Ochir, T.O.; Kwon, J.-H.; Noh, J.-Y.; Sun Kim, J.; Gyu Yoo, J.; et al. Transgenic Chickens Expressing the 3D8 Single Chain Variable Fragment Protein Suppress Avian Influenza Transmission. Sci. Rep. 2017, 7, 5938. [Google Scholar] [CrossRef]
- Lee, G.; Choi, H.; Sureshkumar, S.; Jung, S.K.; Kim, J.S.; Oh, K.B.; Kim, K.W.; Yang, H.; Kim, D.H.; Byun, S.J. The 3D8 single chain variable fragment protein suppress infectious bronchitis virus transmission in the transgenic chickens. Res. Vet. Sci. 2019, 123, 293–297. [Google Scholar] [CrossRef]
- Byun, S.J.; Choi, H.; Sureshkumar, S.; Yuk, S.-S.; Kwon, J.-H.; Noh, J.-Y.; Jung, S.K.; Kim, J.S.; Oh, K.B.; Yang, H.; et al. The 3D8 single chain variable fragment protein suppresses Newcastle disease virus transmission in transgenic chickens. BMC Vet. Res. 2020, 16, 273. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Budhathoki, S.; Lee, G.Y.; Oh, K.J.; Ham, Y.K.; Kim, Y.J.; Lim, Y.R.; Hoang, P.T.; Lee, Y.; Lim, S.W.; et al. Broad-Spectrum Antiviral Activity of 3D8, a Nucleic Acid-Hydrolyzing Single-Chain Variable Fragment (scFv), Targeting SARS-CoV-2 and Multiple Coronaviruses In Vitro. Viruses 2021, 13, 650. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Shim, H.-K.; Kwon, M.-H.; Son, S.-H.; Kim, K.-Y.; Park, E.-Y.; Lee, T.-K.; Lee, W.-R.; Auh, C.-K.; Kim, D.; et al. A nucleic acid hydrolyzing recombinant antibody confers resistance to curtovirus infection in tobacco. Plant Cell Tissue Organ. Cult. (PCTOC) 2013, 115, 179–187. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, Y.; Xiao, Q.; He, B.; Geng, G.; Wang, Z.; Cao, B.; Dong, X.; Bai, W.; Wang, Y.; et al. Programmable RNA editing with compact CRISPR–Cas13 systems from uncultivated microbes. Nat. Methods 2021, 18, 499–506. [Google Scholar] [CrossRef]
| No. | Year 1 | Disease | Virus | Death 2 |
|---|---|---|---|---|
| 1 | 1520 | Smallpox | Variola virus | 500 million |
| 2 | ~1800 | Yellow fever | Yellow fever virus | >210,000 |
| 3 | 1918 | Spanish flu | Influenza A virus (H1N1) | 50 million |
| 4 | 1957 | Asian flu | Influenza A virus (H2N2) | 2 million |
| 5 | 1968 | Hong Kong flu | Influenza A virus (H3N2) | 1 million |
| 6 | 1976 | Ebola | Ebola virus | ~15,300 |
| 7 | 1981 | HIV/AIDS | HIV | ~37 million |
| 8 | 1990 | Dengue fever | Dengue virus | >680,000 |
| 9 | 2002 | SARS | SARS-CoV | 774 |
| 10 | 2009 | Swine flu | Influenza A virus (H1N1) | 284,000 |
| 11 | 2012 | MERS | MERS-CoV | 891 |
| 12 | 2014 | Chikungunya | Chikungunya virus | rare |
| 13 | 2015 | Zika | Zika virus | ~1000 |
| 14 | 2020 | COVID-19 | SARS-CoV-2 | ~6.2 million |
| Derivation | Experiment | Virus | Genome | Replication | Genus | Family | Ref. |
|---|---|---|---|---|---|---|---|
| Protein expressed in E. coli | PK-15 cells | CSFV | (+)ssRNA | Cytoplasm | Pestivirus | Flaviviridae | [127] |
| Transgenic cell line | |||||||
| Protein expressed in E. coli | Hela cells | VSV | (−)ssRNA | Cytoplasm | Vesiculovirus | Rhabdoviridae | [134] |
| Transgenic plants | N. tabacum | PMMoV | (+)ssRNA | Cytoplasm | Tobamovirus | Virgaviridae | [135] |
| TMGMV | |||||||
| ToMV | |||||||
| TMV | |||||||
| CMV | Cucumovirus | Bromoviridae | [135] | ||||
| Transgenic plants | Chrysanthemums | CSVd | (−)ssRNA | Nucleus | Pospiviroid | Pospiviroidae | [136] |
| Protein expressed in L. paracase | RAW264.7 cells | MNV1 | (+)ssRNA | Cytoplasm | Norovirus | Calciviridae | [121] |
| Transgenic bacteria L. paracase | Mice | ||||||
| Protein expressed in E. coli | MDCK cell | H1N1/NWS33 | (−)ssRNA | Nucleus | Influenzavirus A | Orthomyxoviridae | [120] |
| H9N2 | |||||||
| H1N1/PR8 | |||||||
| H3N2 | |||||||
| MDCK cell/Mice | H1N1/09pdm | ||||||
| Transgenic animal | Chickens | H9N2 | (−)ssRNA | Nucleus | Influenzavirus A | Orthomyxoviridae | [137] |
| Transgenic animal | Infectious bronchitis virus | (+)ssRNA | Cytoplasm | Gammacoronavirus | Coronaviridae | [138] | |
| Transgenic animal | Newcastle disease | (−)ssRNA | Cytoplasm | Avulavirus | Paramyxoviridae | [139] | |
| Protein expressed in E. coli | Vero E6 | SARS-CoV-2 | (+)ssRNA | Cytoplasm | Betacoronavirus | Coronaviridae | [140] |
| hCo-OC43 | Betacoronavirus | Coronaviridae | |||||
| PEDV | Alphacoronavirus | Coronaviridae | |||||
| Transgenic cell line | Hela | HSV1 | dsDNA | Nucleus | Simplexvirus | Herpesviridae | [119] |
| Transgenic cell line | PRV | dsDNA | Nucleus | Varicellovirus | Herpesviridae | [119] | |
| Transgenic animal | Mice | dsDNA | Nucleus | Varicellovirus | Herpesviridae | [118] | |
| Transgenic plants | N. tabacum | BCTV | ssDNA | Nucleus | Curtovirus | Geminiviridae | [141] |
| Transgenic plants | N. tabacum | BSCTV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang, P.T.; Luong, Q.X.T.; Ayun, R.Q.; Lee, Y.; Vo, T.T.B.; Kim, T.; Lee, S. A Novel Approach of Antiviral Drugs Targeting Viral Genomes. Microorganisms 2022, 10, 1552. https://doi.org/10.3390/microorganisms10081552
Hoang PT, Luong QXT, Ayun RQ, Lee Y, Vo TTB, Kim T, Lee S. A Novel Approach of Antiviral Drugs Targeting Viral Genomes. Microorganisms. 2022; 10(8):1552. https://doi.org/10.3390/microorganisms10081552
Chicago/Turabian StyleHoang, Phuong Thi, Quynh Xuan Thi Luong, Ramadhani Qurrota Ayun, Yongjun Lee, Thuy Thi Bich Vo, Taehyun Kim, and Sukchan Lee. 2022. "A Novel Approach of Antiviral Drugs Targeting Viral Genomes" Microorganisms 10, no. 8: 1552. https://doi.org/10.3390/microorganisms10081552
APA StyleHoang, P. T., Luong, Q. X. T., Ayun, R. Q., Lee, Y., Vo, T. T. B., Kim, T., & Lee, S. (2022). A Novel Approach of Antiviral Drugs Targeting Viral Genomes. Microorganisms, 10(8), 1552. https://doi.org/10.3390/microorganisms10081552






