Shewanella sp. T2.3D-1.1 a Novel Microorganism Sustaining the Iron Cycle in the Deep Subsurface of the Iberian Pyrite Belt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Shewanella sp. T2.3D-1.1 Isolation Context
2.2. Shewanellla sp. T2.3D-1.1 Genome Annotation
2.3. Shewanella spp. Genome Accessions Numbers
2.4. Phylogenomic Analysis and Taxonomic Classification
2.5. Variant Calling Analysis
2.6. BtuB Protein Analysis
2.7. Metal Tolerance
2.8. Anaerobic Culture and Electron Acceptors
2.9. Iron Reduction and Oxidation Assays
2.10. H2 Production and Measurements
3. Results and Discussion
3.1. Genomic Characterization
3.1.1. Genotypic Analysis
3.1.2. Gene Redundancy and Analysis of the Cobalamin Receptors
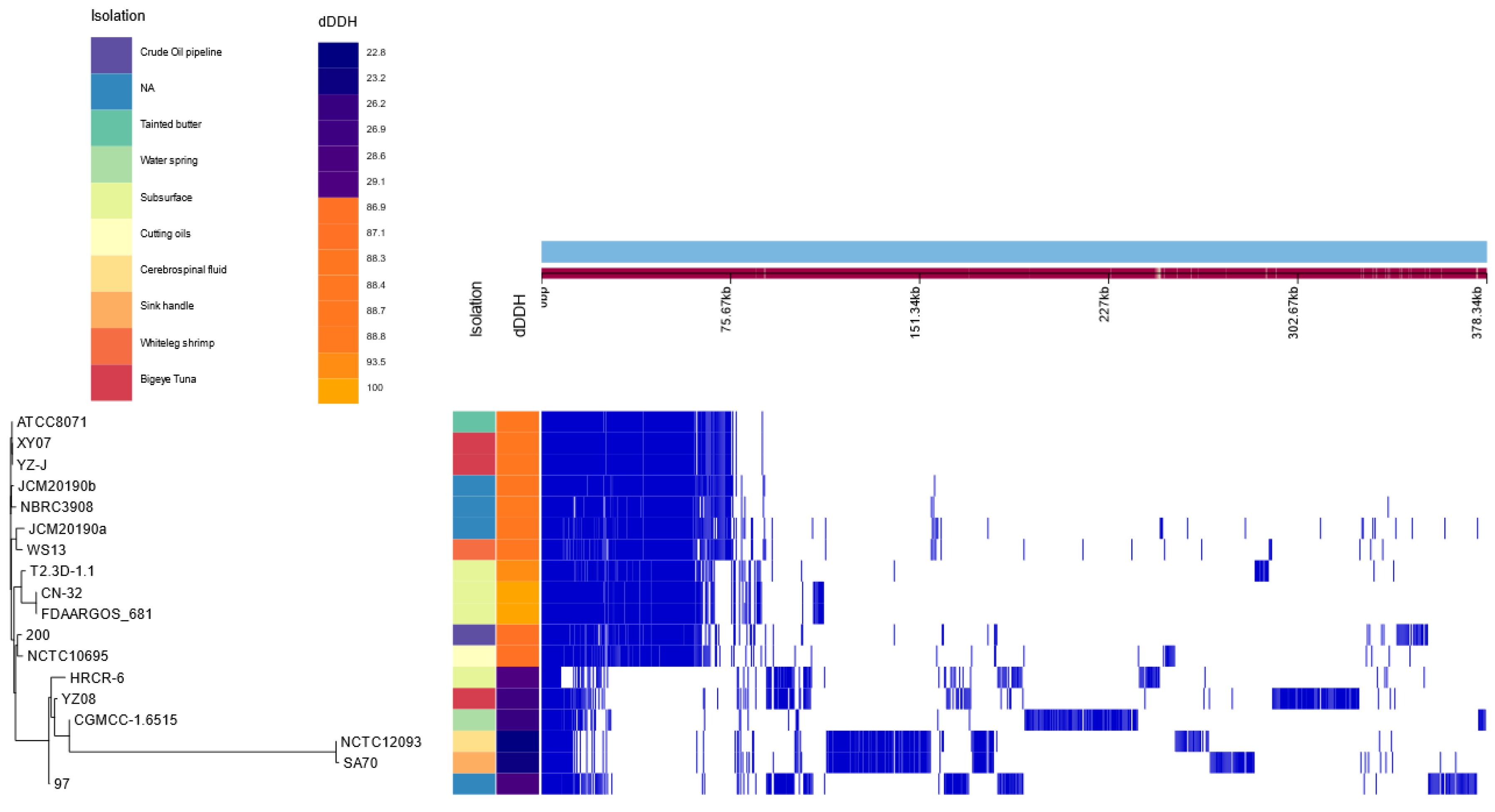
3.1.3. Phylogenomic Analyses
3.1.4. Variant Calling Analysis between Shewanella sp. T2.3D-1.1 and S. putrefaciens CN-32
3.2. Phenotypic Characterization
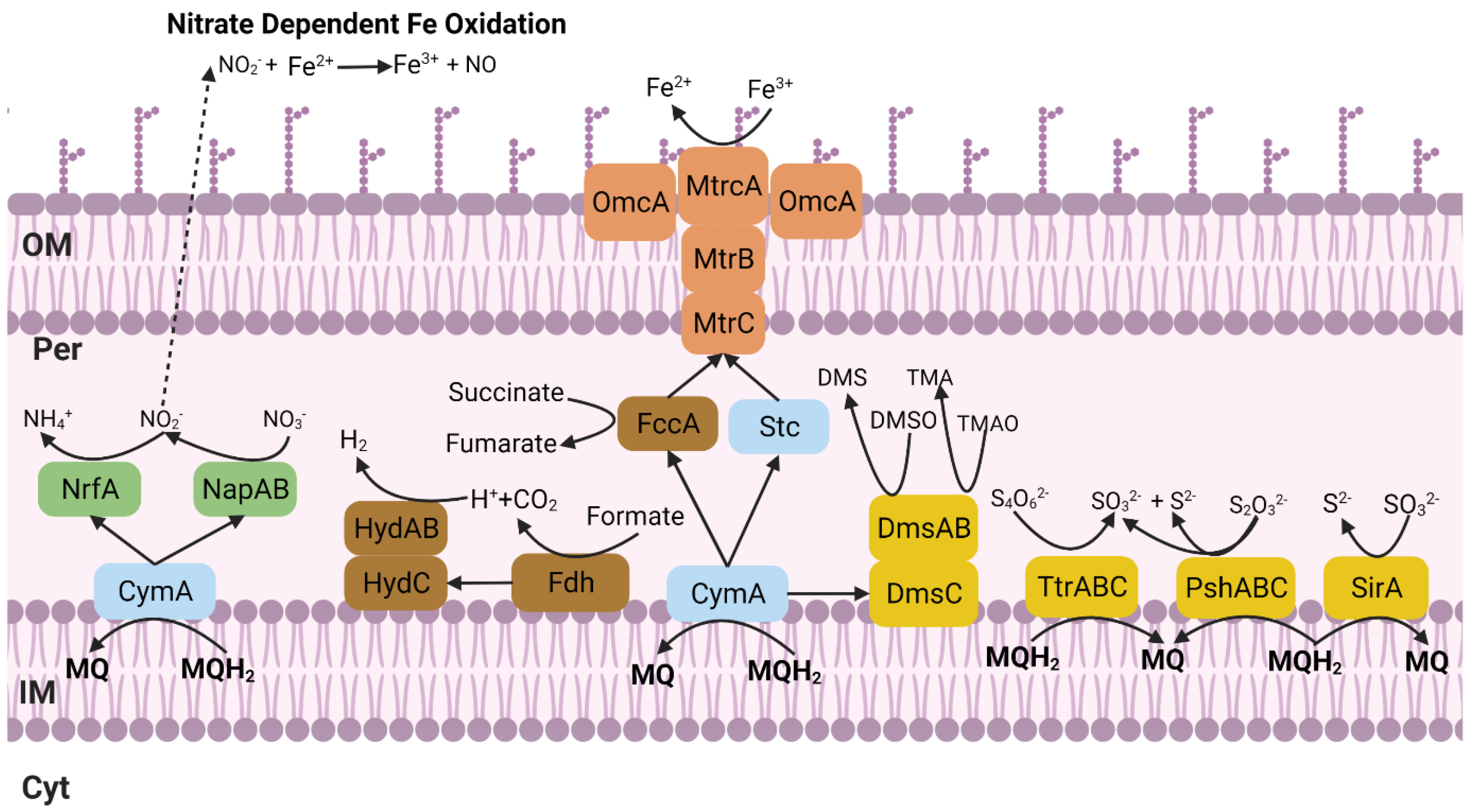
3.2.1. Metal Tolerance and Electron Acceptors
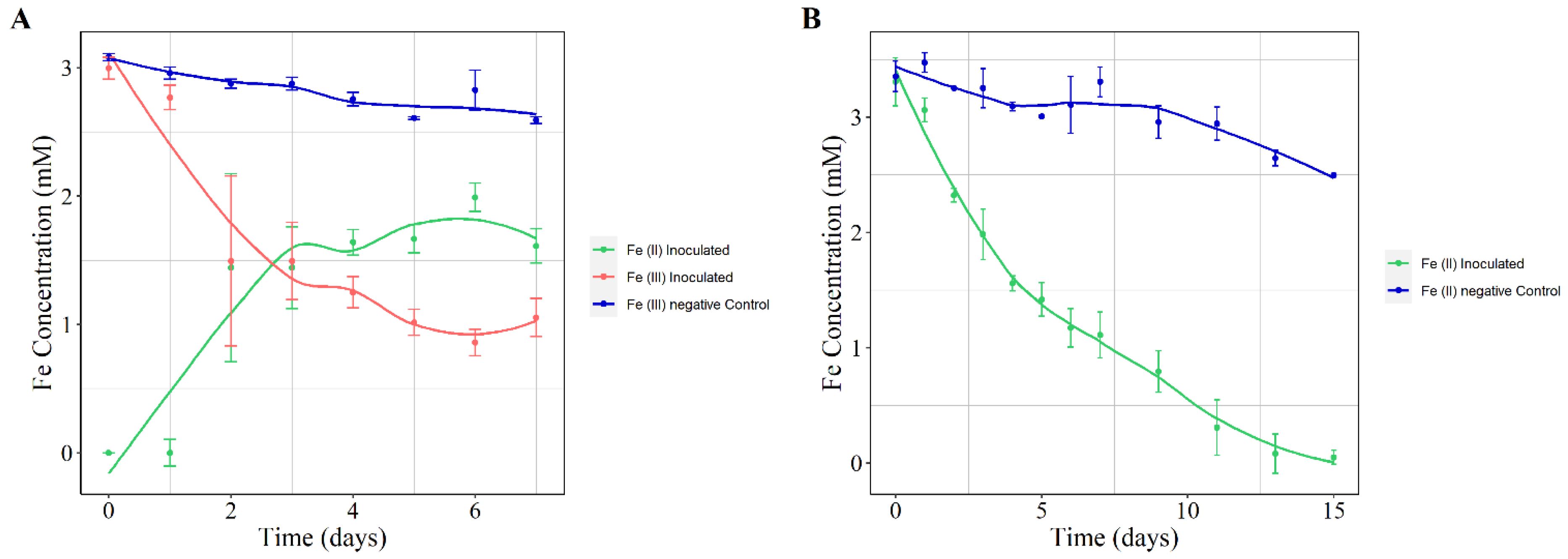
3.2.2. Iron Oxidation and Reduction Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Price, A.; Macey, M.C.; Pearson, V.K.; Schwenzer, S.P.; Ramkissoon, N.K.; Olsson-Francis, K. Oligotrophic Growth of Nitrate-Dependent Fe 2 -Oxidising Microorganisms Under Simulated Early Martian Conditions. Front. Microbiol. 2022, 13, 800219. [Google Scholar] [CrossRef] [PubMed]
- Amils, R.; Fernández-Remolar, D.; Ipbsl Team. Río Tinto: A geochemical and mineralogical terrestrial analogue of Mars. Life 2014, 4, 511–534. [Google Scholar] [CrossRef]
- Fernández-Remolar, D.C.; Morris, R.V.; Gruener, J.E.; Amils, R.; Knoll, A.H. The Río Tinto Basin, Spain: Mineralogy, sedimentary geobiology, and implications for interpretation of outcrop rocks at Meridiani Planum, Mars. Earth Planet. Sci. Lett. 2005, 240, 149–167. [Google Scholar] [CrossRef]
- Garber, A.I.; Cohen, A.B.; Nealson, K.H.; Ramírez, G.A.; Barco, R.A.; Enzingmüller-Bleyl, T.C.; Gehringer, M.M.; Merino, N. Metagenomic Insights into the Microbial Iron Cycle of Subseafloor Habitats. Front. Microbiol. 2021, 12, 667944. [Google Scholar] [CrossRef]
- Bell, E.; Lamminmäki, T.; Alneberg, J.; Andersson, A.F.; Qian, C.; Xiong, W.; Hettich, R.L.; Frutschi, M.; Bernier-Latmani, R. Active sulfur cycling in the terrestrial deep subsurface. ISME J. 2020, 14, 1260–1272. [Google Scholar] [CrossRef] [PubMed]
- Escudero, C.; Vera, M.; Oggerin, M.; Amils, R. Active microbial biofilms in deep poor porous continental subsurface rocks. Sci. Rep. 2018, 8, 1538. [Google Scholar] [CrossRef]
- Fernández-Remolar, D.C.; Gomez-Ortiz, D.; Huang, T.; Anglés, A.; Shen, Y.; Hu, Q.; Amils, R.; Rodríguez, N.; Escudero, C.; Banerjee, N.R. The molecular record of metabolic activity in the subsurface of the Rio Tinto Mars analog. Astrobiology 2021, 21, 1387–1405. [Google Scholar] [CrossRef]
- Escudero, C.; Del Campo, A.; Ares, J.R.; Sánchez, C.; Martínez, J.M.; Gómez, F.; Amils, R. Visualizing microorganism-mineral interaction in the Iberian Pyrite Belt subsurface: The Acidovorax case. Front. Microbiol. 2020, 11, 2833. [Google Scholar] [CrossRef]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef]
- Kallmeyer, J.; Pockalny, R.; Adhikari, R.R.; Smith, D.C.; D’Hondt, S. Global distribution of microbial abundance and biomass in subseafloor sediment. Proc. Natl. Acad. Sci. USA 2012, 109, 16213–16216. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.; Parnell, J. Weighing the deep continental biosphere. FEMS Microbiol. Ecol. 2014, 87, 113–120. [Google Scholar] [CrossRef]
- Amils, R.; Fernández-Remolar, D.; Parro, V.; Rodríguez-Manfredi, J.A.; Timmis, K.; Oggerin, M.; Sánchez-Román, M.; López, F.J.; Fernández, J.P.; Puente, F.; et al. Iberian Pyrite Belt Subsurface Life (IPBSL), a drilling project of biohydrometallurgical interest. Adv. Mater. Res. 2013, 825, 15–18. [Google Scholar] [CrossRef]
- Fernández-Remolar, D.C.; Banerjee, N.; Gómez-Ortiz, D.; Izawa, M.; Amils, R. A mineralogical archive of the biogeochemical sulfur cycle preserved in the subsurface of the Río Tinto system. Am. Mineral. J. Earth Planet. Mater. 2018, 103, 394–411. [Google Scholar] [CrossRef]
- De Francisco-Polanco, S.; Martínez, J.M.; Leandro, T.; Amils, R. Draft Genome Sequence of Shewanella sp. Strain T2. 3D-1.1, Isolated from 121.8 Meters Deep in the Subsurface of the Iberian Pyrite Belt. Microbiol. Resour. Announc. 2020, 9, e00190-20. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.P. Shewanella. In Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2015; pp. 1–22. [Google Scholar]
- Beblawy, S.; Bursac, T.; Paquete, C.; Louro, R.; Clarke, T.A.; Gescher, J. Extracellular reduction of solid electron acceptors by Shewanella oneidensis. Mol. Microbiol. 2018, 109, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, O.N.; Méjean, V.; Iobbi-Nivol, C. The Shewanella genus: Ubiquitous organisms sustaining and preserving aquatic ecosystems. FEMS Microbiol. Rev. 2020, 44, 155–170. [Google Scholar] [CrossRef]
- Amils, R. Lessons learned from thirty years of geomicrobiological studies of Río Tinto. Res. Microbiol. 2016, 167, 539–545. [Google Scholar] [CrossRef]
- Leandro, T.; Rodriguez, N.; Rojas, P.; Sanz, J.L.; Da Costa, M.S.; Amils, R. Study of methanogenic enrichment cultures of rock cores from the deep subsurface of the Iberian Pyritic Belt. Heliyon 2018, 4, e00605. [Google Scholar] [CrossRef]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018, 46, W246–W251. [Google Scholar] [CrossRef]
- Madden, T. The BLAST Sequence Analysis Tool. In The NCBI Handbook; NCBI: Bethesda, MD, USA, 2002; Volume 16. [Google Scholar]
- Caspi, R.; Billington, R.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Ong, W.K.; Paley, S.; Subhraveti, P.; Karp, P.D. The MetaCyc database of metabolic pathways and enzymes-a 2019 update. Nucleic Acids Res. 2020, 48, D445–D453. [Google Scholar] [CrossRef]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, J.; Croucher, N.J.; Goater, R.J.; Abudahab, K.; Aanensen, D.M.; Harris, S.R. Phandango: An interactive viewer for bacterial population genomics. Bioinformatics 2018, 34, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Na, S.; Kim, Y.O.; Yoon, S.; Sung-min, H.; Baek, I.; Chun, J. UBCG: Up-to-date bacterial core gene set and pipeline for phylogenomic tree reconstruction. J. Microbiol. 2018, 56, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.; Han, Y.; He, Q. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Carlson, M. KEGG.db, Version 3.1.2; A Set of Annotation Maps for KEGG.R Package; 2016. Available online: https://bioconductor.org/packages/2.7/data/annotation/html/KEGG.db.html (accessed on 2 August 2022).
- Kanehisa, M.; Sato, Y.; Kawashima, M. KEGG mapping tools for uncovering hidden features in biological data. Protein Sci. 2022, 31, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Balabanova, L.; Averianova, L.; Marchenok, M.; Son, O.; Tekutyeva, L. Microbial and genetic resources for cobalamin (vitamin B12) biosynthesis: From ecosystems to industrial biotechnology. Int. J. Mol. Sci. 2021, 22, 4522. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in bipolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1994, 2, 28–36. [Google Scholar]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.; Potter, S.C.; Finn, R.D. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef]
- Flynn, C.M.; Hunt, K.A.; Gralnick, J.A.; Srienc, F. Construction and elementary mode analysis of a metabolic model for Shewanella oneidensis MR-1. BioSystems 2012, 107, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Widdel, F.; Pfennig, N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. Arch. Microbiol. 1981, 129, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zachara, J.M.; Gorby, Y.A.; Szecsody, J.E.; Brown, C.F. Microbial reduction of Fe (III) and sorption/precipitation of Fe (II) on Shewanella putrefaciens strain CN32. Environ. Sci. Technol. 2001, 35, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Roden, E.E.; Schieber, J.; Picardal, F. Enhanced growth of Acidovorax sp. strain 2AN during nitrate-dependent Fe (II) oxidation in batch and continuous-flow systems. Appl. Env. Microbiol. 2011, 77, 8548–8556. [Google Scholar] [CrossRef]
- Kappler, A.; Schink, B.; Newman, D.K. Fe (III) mineral formation and cell encrustation by the nitrate-dependent Fe (II)-oxidizer strain BoFeN1. Geobiology 2005, 3, 235–245. [Google Scholar] [CrossRef]
- Easton, A.J. The colorimetric determination of iron. Chem. Anal. Silic. Rocks 1972, 95–112. [Google Scholar] [CrossRef]
- Escudero Parada, C. Fluorescence microscopy for the in situ study of the Iberian pyrite belt subsurface geomicrobiology. Front. Microbiol. 2018, 11, 572104. [Google Scholar]
- Schaedler, F.; Kappler, A.; Schmidt, C. A revised iron extraction protocol for environmental samples rich in nitrite and carbonate. Geomicrobiol. J. 2018, 35, 23–30. [Google Scholar] [CrossRef]
- Meshulam-Simon, G.; Behrens, S.; Choo, A.D.; Spormann, A.M. Hydrogen metabolism in Shewanella oneidensis MR-1. Appl. Env. Microbiol. 2007, 73, 1153–1165. [Google Scholar] [CrossRef]
- Cruz-García, C.; Murray, A.E.; Klappenbach, J.A.; Stewart, V.; Tiedje, J.M. Respiratory nitrate ammonification by Shewanella oneidensis MR-1. J. Bacteriol. 2007, 189, 656–662. [Google Scholar] [CrossRef]
- Gao, H.; Yang, Z.K.; Barua, S.; Reed, S.B.; Romine, M.F.; Nealson, K.H.; Fredrickson, J.K.; Tiedje, J.M.; Zhou, J. Reduction of nitrate in Shewanella oneidensis depends on atypical NAP and NRF systems with NapB as a preferred electron transport protein from CymA to NapA. ISME J. 2009, 3, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Oshiki, M.; Ali, M.; Shinyako-Hata, K.; Satoh, H.; Okabe, S. Hydroxylamine-dependent anaerobic ammonium oxidation (anammox) by “Candidatus Brocadia sinica”. Environ. Microbiol. 2016, 18, 3133–3143. [Google Scholar] [CrossRef]
- Venkateswaran, K.; Dollhopf, M.E.; Aller, R.; Stackebrandt, E.; Nealson, K.H. Shewanella amazonensis sp. nov., a novel metal-reducing facultative anaerobe from Amazonian shelf muds. Int. J. Syst. Evol. Microbiol. 1998, 48, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Brettar, I.; Christen, R.; Höfle, M.G. Shewanella denitrificans sp. nov., a vigorously denitrifying bacterium isolated from the oxic-anoxic interface of the Gotland Deep in the central Baltic Sea. Int. J. Syst. Evol. Microbiol. 2002, 52, 2211–2217. [Google Scholar] [PubMed]
- Yoon, S.; Cruz-García, C.; Sanford, R.; Ritalahti, K.M.; Löffler, F.E. Denitrification versus respiratory ammonification: Environmental controls of two competing dissimilatory NO3−/NO2− reduction pathways in Shewanella loihica strain PV-4. ISME J. 2015, 9, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, F. Insights on nitrate respiration by Shewanella. Front. Mar. Sci. 2015, 1, 80. [Google Scholar] [CrossRef]
- McGuirl, M.A.; Bollinger, J.A.; Cosper, N.; Scott, R.A.; Dooley, D.M. Expression, purification, and characterization of NosL, a novel Cu (I) protein of the nitrous oxide reductase (nos) gene cluster. JBIC J. Biol. Inorg. Chem. 2001, 6, 189–195. [Google Scholar] [CrossRef]
- Honisch, U.; Zumft, W.G. Operon structure and regulation of the nos gene region of Pseudomonas stutzeri, encoding an ABC-type ATPase for maturation of nitrous oxide reductase. J. Bacteriol. 2003, 185, 1895–1902. [Google Scholar] [CrossRef]
- Jiang, M.; Zheng, X.; Chen, Y. Enhancement of denitrification performance with reduction of nitrite accumulation and N2O emission by Shewanella oneidensis MR-1 in microbial denitrifying process. Water Res. 2020, 169, 115242. [Google Scholar] [CrossRef]
- García, R.; Martínez, J.M.; Leandro, T.; Amils, R. Draft genome sequence of Rhizobium sp. strain T2. 30D-1.1, isolated from 538.5 meters deep on the subsurface of the Iberian Pyrite Belt. Microbiol. Resour. Announc. 2018, 7, e01098–18. [Google Scholar] [CrossRef]
- Mariñán, N.; Martínez, J.M.; Leandro, T.; Amils, R. Draft genome sequence of Rhodoplanes sp. strain T2. 26MG-98, isolated from 492.6 meters deep on the subsurface of the Iberian Pyrite Belt. Microbiol. Resour. Announc. 2019, 8, e00070–19. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Escudero, C.; Leandro, T.; Mateos, G.; Amils, R. Draft Genome Sequence of Pseudomonas sp. Strain T2. 31D-1, Isolated from a Drilling Core Sample Obtained 414 Meters below Surface in the Iberian Pyrite Belt. Microbiol. Resour. Announc. 2021, 10, 1165. [Google Scholar] [CrossRef] [PubMed]
- Tornos, F. Environment of formation and styles of volcanogenic massive sulfides: The Iberian Pyrite Belt. Ore Geol. Rev. 2006, 28, 259–307. [Google Scholar] [CrossRef]
- Shirodkar, S.; Reed, S.; Romine, M.; Saffarini, D. The octahaem SirA catalyses dissimilatory sulfite reduction in Shewanella oneidensis MR-1. Environ. Microbiol. 2011, 13, 108–115. [Google Scholar] [CrossRef]
- Burns, J.L.; DiChristina, T.J. Anaerobic respiration of elemental sulfur and thiosulfate by Shewanella oneidensis MR-1 requires psrA, a homolog of the phsA gene of Salmonella enterica serovar typhimurium LT2. Appl. Environ. Microbiol. 2009, 75, 5209–5217. [Google Scholar] [CrossRef]
- Hensel, M.; Hinsley, A.P.; Nikolaus, T.; Sawers, G.; Berks, B.C. The genetic basis of tetrathionate respiration in Salmonella typhimurium. Mol. Microbiol. 1999, 32, 275–287. [Google Scholar] [CrossRef]
- Puente-Sánchez, F.; Arce-Rodríguez, A.; Oggerin, M.; García-Villadangos, M.; Moreno-Paz, M.; Blanco, Y.; Rodríguez, N.; Bird, L.; Lincoln, S.A.; Tornos, F. Viable cyanobacteria in the deep continental subsurface. Proc. Natl. Acad. Sci. USA 2018, 115, 10702–10707. [Google Scholar] [CrossRef]
- McAnulty, M.J.; Wood, T.K. YeeO from Escherichia coli exports flavins. Bioengineered 2014, 5, 386–392. [Google Scholar] [CrossRef]
- Chong, G.W.; Pirbadian, S.; El-Naggar, M.Y. Surface-induced formation and redox-dependent staining of outer membrane extensions in Shewanella oneidensis MR-1. Front. Energy Res. 2019, 7, 87. [Google Scholar] [CrossRef]
- Von Canstein, H.; Ogawa, J.; Shimizu, S.; Lloyd, J.R. Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl. Environ. Microbiol. 2008, 74, 615–623. [Google Scholar] [CrossRef]
- Daly, R.A.; Roux, S.; Borton, M.A.; Morgan, D.M.; Johnston, M.D.; Booker, A.E.; Hoyt, D.W.; Meulia, T.; Wolfe, R.A.; Hanson, A.J. Viruses control dominant bacteria colonizing the terrestrial deep biosphere after hydraulic fracturing. Nat. Microbiol. 2019, 4, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Rahlff, J.; Turzynski, V.; Esser, S.P.; Monsees, I.; Bornemann, T.L.; Figueroa-Gonzalez, P.A.; Schulz, F.; Woyke, T.; Klingl, A.; Moraru, C. Lytic archaeal viruses infect abundant primary producers in Earth’s crust. Nat. Commun. 2021, 12, 4642. [Google Scholar] [CrossRef]
- Cady, K.C.; Bondy-Denomy, J.; Heussler, G.E.; Davidson, A.R.; O’Toole, G.A. The CRISPR/Cas adaptive immune system of Pseudomonas aeruginosa mediates resistance to naturally occurring and engineered phages. J. Bacteriol. 2012, 194, 5728–5738. [Google Scholar] [CrossRef]
- Gil, J.F.; Mesa, V.; Estrada-Ortiz, N.; Lopez-Obando, M.; Gómez, A.; Plácido, J. Viruses in extreme environments, current overview, and biotechnological potential. Viruses 2021, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Boer, D.R.; Lorenzo-Díaz, F.; Russi, S.; Gómez, H.; Fernández-López, C.; Pérez-Luque, R.; Orozco, M.; Espinosa, M.; Coll, M. Structural basis of a histidine-DNA nicking/joining mechanism for gene transfer and promiscuous spread of antibiotic resistance. Proc. Natl. Acad. Sci. USA 2017, 114, E6526–E6535. [Google Scholar] [CrossRef] [PubMed]
- Noinaj, N.; Guillier, M.; Barnard, T.J.; Buchanan, S.K. TonB-dependent transporters: Regulation, structure, and function. Annu. Rev. Microbiol. 2010, 64, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Degnan, P.H.; Barry, N.A.; Mok, K.C.; Taga, M.E.; Goodman, A.L. Human gut microbes use multiple transporters to distinguish vitamin B12 analogs and compete in the gut. Cell Host Microbe 2014, 15, 47–57. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Zachara, J.M.; Kennedy, D.W.; Dong, H.; Onstott, T.C.; Hinman, N.W.; Li, S. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim. Cosmochim. Acta 1998, 62, 3239–3257. [Google Scholar] [CrossRef]
- Thorell, K.; Meier-Kolthoff, J.P.; Sjöling, Å.; Martín-Rodríguez, A.J. Whole-genome sequencing redefines Shewanella taxonomy. Front. Microbiol. 2019, 10, 1861. [Google Scholar] [CrossRef] [PubMed]
- Sichtig, H.; Minogue, T.; Yan, Y.; Stefan, C.; Hall, A.; Tallon, L.; Sadzewicz, L.; Nadendla, S.; Klimke, W.; Hatcher, E. FDA-ARGOS is a database with public quality-controlled reference genomes for diagnostic use and regulatory science. Nat. Commun. 2019, 10, 3313. [Google Scholar] [CrossRef]
- Kotloski, N.J.; Gralnick, J.A. Flavin electron shuttles dominate extracellular electron transfer by Shewanella oneidensis. MBio 2013, 4, 553. [Google Scholar] [CrossRef]
- Covington, E.D.; Gelbmann, C.B.; Kotloski, N.J.; Gralnick, J.A. An essential role for UshA in processing of extracellular flavin electron shuttles by Shewanella oneidensis. Mol. Microbiol. 2010, 78, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; So, J. Antibiotic and heavy metal resistance in Shewanella putrefaciens strains isolated from shellfishes collected from West Sea, Korea. Mar. Pollut. Bull. 2016, 112, 111–116. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Tian, T.; Wang, Z.; Jin, R.; Zhou, J. Role of extracellular polymeric substances in the immobilization of hexavalent chromium by Shewanella putrefaciens CN32 unsaturated biofilms. Sci. Total Environ. 2022, 810, 151184. [Google Scholar] [CrossRef]
- Wu, C.; Cheng, Y.; Li, B.; Li, W.; Li, D.; Yu, H. Electron acceptor dependence of electron shuttle secretion and extracellular electron transfer by Shewanella oneidensis MR-1. Bioresour. Technol. 2013, 136, 711–714. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, B.; Li, D.; Chen, J.; Li, W.; Tong, Z.; Wu, C.; Yu, H. Promotion of iron oxide reduction and extracellular electron transfer in Shewanella oneidensis by DMSO. PLoS ONE 2013, 8, e78466. [Google Scholar] [CrossRef] [PubMed]
- Van der Graaf, C.M.; Sánchez-España, J.; Yusta, I.; Ilin, A.; Shetty, S.A.; Bale, N.J.; Villanueva, L.; Stams, A.J.; Sánchez-Andrea, I. Biosulfidogenesis mediates natural attenuation in acidic mine pit lakes. Microorganisms 2020, 8, 1275. [Google Scholar] [CrossRef] [PubMed]
- Rickard, D. Kinetics of pyrite formation by the H2S oxidation of iron (II) monosulfide in aqueous solutions between 25 and 125 C: The rate equation. Geochim. Cosmochim. Acta 1997, 61, 115–134. [Google Scholar] [CrossRef]
- Pinchuk, G.E.; Geydebrekht, O.V.; Hill, E.A.; Reed, J.L.; Konopka, A.E.; Beliaev, A.S.; Fredrickson, J.K. Pyruvate and lactate metabolism by Shewanella oneidensis MR-1 under fermentation, oxygen limitation, and fumarate respiration conditions. Appl. Environ. Microbiol. 2011, 77, 8234–8240. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.L.; Brutinel, E.D.; Joo, H.; Maysonet, R.; VanDrisse, C.M.; Kotloski, N.J.; Gralnick, J.A. Formate metabolism in Shewanella oneidensis generates proton motive force and prevents growth without an electron acceptor. J. Bacteriol. 2016, 198, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.L.; Rodriguez, N.; Escudero, C.; Carrizo, D.; Amils, R. Biological Production of H2, CH4, and CO2 in the Deep Subsurface of the Iberian Pyrite Belt. Environ. Microbiol. 2021, 23, 3913–3922. [Google Scholar] [CrossRef] [PubMed]
- Escudero, C.; Oggerin, M.; Amils, R. The deep continental subsurface: The dark biosphere. Int. Microbiol. 2018, 21, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, C.; Wang, X.; Marshall, M.J.; Zachara, J.M.; Rosso, K.M.; Dupuis, M.; Fredrickson, J.K.; Heald, S.; Shi, L. Kinetics of reduction of Fe (III) complexes by outer membrane cytochromes MtrC and OmcA of Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 2008, 74, 6746–6755. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, S.; Wang, X.; Li, N. Biosynthesis of vivianite from microbial extracellular electron transfer and environmental application. Sci. Total Environ. 2021, 762, 143076. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Liu, T.; Li, F.; Shen, W. Competitive reduction of nitrate and iron oxides by Shewanella putrefaciens 200 under anoxic conditions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 445, 97–104. [Google Scholar] [CrossRef]
- Coby, A.J.; Picardal, F.W. Inhibition of NO3− and NO2− reduction by microbial Fe (III) reduction: Evidence of a reaction between NO2− and cell surface-bound Fe2. Appl. Environ. Microbiol. 2005, 71, 5267–5274. [Google Scholar] [CrossRef]
- Bryce, C.; Blackwell, N.; Schmidt, C.; Otte, J.; Huang, Y.; Kleindienst, S.; Tomaszewski, E.; Schad, M.; Warter, V.; Peng, C. Microbial anaerobic Fe (II) oxidation–Ecology, mechanisms and environmental implications. Environ. Microbiol. 2018, 20, 3462–3483. [Google Scholar] [CrossRef] [PubMed]
- Dopffel, N.; Jamieson, J.; Bryce, C.; Joshi, P.; Mansor, M.; Siade, A.; Prommer, H.; Kappler, A. Temperature dependence of nitrate-reducing Fe (II) oxidation by Acidovorax strain BoFeN1–evaluating the role of enzymatic vs. abiotic Fe (II) oxidation by nitrite. FEMS Microbiol. Ecol. 2021, 97, fiab155. [Google Scholar] [CrossRef]
- Coursolle, D.; Baron, D.B.; Bond, D.R.; Gralnick, J.A. The Mtr respiratory pathway is essential for reducing flavins and electrodes in Shewanella oneidensis. J. Bacteriol. 2010, 192, 467–474. [Google Scholar] [CrossRef]
- Vaughan, D.J. MINERALS|Sulphides. In Encyclopedia of Geology; Selley, R.C., Cocks, L.R.M., Plimer, I.R., Eds.; Elsevier: Oxford, UK, 2005; pp. 574–586. [Google Scholar]
- Fernández-Remolar, D.C.; Carrizo, D.; Harir, M.; Huang, T.; Amils, R.; Schmitt-Kopplin, P.; Sánchez-García, L.; Gomez-Ortiz, D.; Malmberg, P. Unveiling microbial preservation under hyperacidic and oxidizing conditions in the Oligocene Rio Tinto deposit. Sci. Rep. 2021, 11, 21543. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef] [PubMed]
| Type | Region | ||||
|---|---|---|---|---|---|
| Type | Count | Percent | Type | Count | Percent |
| Conservative inframe deletion | 8 | 0.00% | Downstream | 93.502 | 45.16% |
| Conservative inframe insertion | 19 | 0.01% | Exon | 17.776 | 8.59% |
| Disruptive inframe deletion | 24 | 0.01% | Intergenic | 2.893 | 1.40% |
| Disruptive inframe insertion | 25 | 0.01% | Splice site region | 10 | 0.01% |
| Downstream gene variant | 93.502 | 45.15% | Upstream | 92.872 | 44.85% |
| Upstream gene variant | 92.872 | 44.85% | Number of effects by impact | ||
| Intergenic region | 2.893 | 1.40% | Type | Count | Percent |
| Missense variant | 3.311 | 1.60% | High | 254 | 0.12% |
| Noncoding transcript exon variant | 12 | 0.01% | Low | 14.134 | 6.83% |
| Splice region variant | 16 | 0.01% | Moderate | 3.386 | 1.64% |
| Start lost | 5 | 0.00% | Modifier | 189.279 | 91.41% |
| Stop gained | 26 | 0.01% | Number of effects by functional class | ||
| Stop lost | 4 | 0.00% | Type | Count | Percent |
| Stop retained variant | 10 | 0.01% | Missense | 3.318 | 18.99% |
| Synonymous variant | 14.124 | 6.82% | Nonsense | 24 | 0.14% |
| Frameshift variant | 222 | 0.11% | Silent | 14.134 | 80.88% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateos, G.; Bonilla, A.M.; de Francisco de Polanco, S.; Martínez, J.M.; Escudero, C.; Rodríguez, N.; Sánchez-Andrea, I.; Amils, R. Shewanella sp. T2.3D-1.1 a Novel Microorganism Sustaining the Iron Cycle in the Deep Subsurface of the Iberian Pyrite Belt. Microorganisms 2022, 10, 1585. https://doi.org/10.3390/microorganisms10081585
Mateos G, Bonilla AM, de Francisco de Polanco S, Martínez JM, Escudero C, Rodríguez N, Sánchez-Andrea I, Amils R. Shewanella sp. T2.3D-1.1 a Novel Microorganism Sustaining the Iron Cycle in the Deep Subsurface of the Iberian Pyrite Belt. Microorganisms. 2022; 10(8):1585. https://doi.org/10.3390/microorganisms10081585
Chicago/Turabian StyleMateos, Guillermo, Adrián Martínez Bonilla, Sofía de Francisco de Polanco, José M. Martínez, Cristina Escudero, Nuria Rodríguez, Irene Sánchez-Andrea, and Ricardo Amils. 2022. "Shewanella sp. T2.3D-1.1 a Novel Microorganism Sustaining the Iron Cycle in the Deep Subsurface of the Iberian Pyrite Belt" Microorganisms 10, no. 8: 1585. https://doi.org/10.3390/microorganisms10081585
APA StyleMateos, G., Bonilla, A. M., de Francisco de Polanco, S., Martínez, J. M., Escudero, C., Rodríguez, N., Sánchez-Andrea, I., & Amils, R. (2022). Shewanella sp. T2.3D-1.1 a Novel Microorganism Sustaining the Iron Cycle in the Deep Subsurface of the Iberian Pyrite Belt. Microorganisms, 10(8), 1585. https://doi.org/10.3390/microorganisms10081585








