Combined Orthoplastic Approach in Fracture-Related Infections of the Distal Tibia
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panteli, M.; Giannoudis, P.V. Chronic osteomyelitis: What the surgeon needs to know. EFORT Open Rev. 2016, 1, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Ziran, B.H.; Rao, N.; Hall, R.A. A dedicated team approach enhances outcomes of osteomyelitis treatment. Clin. Orthop. Relat. Res. 2003, 414, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Sambri, A.; Fiore, M.; Tedeschi, S.; De Paolis, M. The Need for Multidisciplinarity in Modern Medicine: An Insight into Orthopaedic Infections. Microorganisms 2022, 10, 756. [Google Scholar] [CrossRef]
- Mediouni, M. A new generation of orthopaedic surgeons: “T-model”. Curr. Orthop. Practice 2019, 30, 444–445. [Google Scholar] [CrossRef]
- Swiontkowski, M.F.; Hanel, D.P.; Vedder, N.B.; Schwappach, J.R. A comparison of short- and long-term intravenous antibiotic therapy in the postoperative management of adult osteomyelitis. J. Bone Jt. Surg. Br. 1999, 81, 1046–1050. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McNally, M.A.; Small, J.O.; Tofighi, H.G.; Mollan, R.A. Two-stage management of chronic osteomyelitis of the long bones: The Belfast technique. J. Bone Jt. Surg. Br. 1993, 75, 375–380. [Google Scholar] [CrossRef]
- Sanders, J.; Mauffrey, C. Long bone osteomyelitis in adults: Fundamental concepts and current techniques. Orthopedics 2013, 36, 368–375. [Google Scholar] [CrossRef]
- Simpson, A.H.; Deakin, M.; Latham, J.M. Chronic osteomyelitis. The effect of the extent of surgical resection on infection-free survival. J. Bone Jt. Surg. Br. 2001, 83, 403–407. [Google Scholar] [CrossRef]
- Parsons, B.; Strauss, E. Surgical management of chronic osteomyelitis. Am. J. Surg. 2004, 188 (Suppl. 1A), 57–66. [Google Scholar] [CrossRef]
- Chan, J.K.K.; Ferguson, J.Y.; Scarborough, M.; McNally, M.A.; Ramsden, A.J. Management of Post-Traumatic Osteomyelitis in the Lower Limb: Current State of the Art. Indian J. Plast. Surg. 2019, 52, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Musharafieh, R.; Osmani, O.; Musharafieh, U.; Saghieh, S.; Atiyeh, B. Efficacy of microsurgical free-tissue transfer in chronic osteomyelitis of the leg and foot: Review of 22 cases. J. Reconstr. Microsurg. 1999, 15, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.G.; Yugueros, P.; Hanssen, A.D. Muscle flaps in osteomyelitis of the lower extremity: A 20-year account. Plast. Reconstr. Surg. 1999, 104, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Heller, L.; Levin, L.S. Lower extremity microsurgical reconstruction. Plast. Reconstr. Surg. 2001, 108, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Beals, R.K.; Bryant, R.E. The treatment of chronic open osteomyelitis of the tibia in adults. Clin. Orthop. Relat. Res. 2005, 433, 212–217. [Google Scholar] [CrossRef]
- Paluvadi, S.V.; Lal, H.; Mittal, D.; Vidyarthi, K. Management of fractures of the distal third tibia by minimally invasive plate osteosynthesis—A prospective series of 50 patients. J. Clin. Orthop. Trauma 2014, 5, 129–136. [Google Scholar] [CrossRef]
- Schottel, P.C.; Muthusamy, S.; Rozbruch, S.R. Distal tibial periarticular nonunions: Ankle salvage with bone transport. J. Orthop. Trauma 2014, 28, e146–e152. [Google Scholar] [CrossRef]
- Onsea, J.; Van Lieshout, E.M.M.; Zalavras, C.; Sliepen, J.; Depypere, M.; Noppe, N.; Ferguson, J.; Verhofstad, M.H.; Govaert, G.A.; IJpma, F.F.; et al. Validation of the diagnostic criteria of the consensus definition of fracture-related infection. Injury 2022, 53, 1867–1879. [Google Scholar] [CrossRef]
- Govaert, G.A.M.; Kuehl, R.; Atkins, B.L.; Trampuz, A.; Morgenstern, M.; Obremskey, W.T.; Verhofstad, M.H.; McNally, M.A.; Metsemakers, W.J. Diagnosing Fracture-Related Infection: Current Concepts and Recommendations. J. Orthop. Trauma 2020, 34, 8–17. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Morgenstern, M.; Senneville, E.; Borens, O.; Govaert, G.A.M.; Onsea, J.; Depypere, M.; Richards, R.G.; Trampuz, A.; Verhofstad, M.H.; et al. General treatment principles for fracture-related infection: Recommendations from an international expert group. Arch. Orthop. Trauma Surg. 2020, 140, 1013–1027. [Google Scholar] [CrossRef]
- May, J.W.; Jupiter, J.B.; Weiland, A.J.; Byrd, H.S. Clinical classification of post-traumatic tibial osteomyelitis. J. Bone Joint Surg. Am. 1989, 71, 1422–1428. [Google Scholar] [CrossRef]
- Cierny, G.; Mader, J.T.; Penninck, J.J. A clinical staging system for adult osteomyelitis. Clin. Orthop. Relat. Res. 2003, 10, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.; Marais, L.C. Management of tibial non-unions according to a novel treatment algorithm. Injury 2015, 46, 2422–2427. [Google Scholar] [CrossRef]
- Donati, D.; Biscaglia, R. The use of antibiotic-impregnated cement in infected reconstructions after resection for bone tumours. J. Bone Jt. Surg. Br. 1998, 80, 1045–1050. [Google Scholar] [CrossRef]
- Rauschmann, M.A.; Wichelhaus, T.A.; Stirnal, V.; Dingeldein, E.; Zichner, L.; Schnettler, R.; Alt, V. Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials 2005, 26, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Visani, J.; Staals, E.L.; Donati, D. Treatment of chronic osteomyelitis with antibiotic-loaded bone void filler systems: An experience with hydroxyapatites calcium-sulfate biomaterials. Acta Orthop. Belg. 2018, 84, 25–29. [Google Scholar] [PubMed]
- Hong, J.P.; Shin, H.W.; Kim, J.J.; Wei, F.C.; Chung, Y.K. The use of anterolateral thigh perforator flaps in chronic osteomyelitis of the lower extremity. Plast. Reconstr. Surg. 2005, 115, 142–147. [Google Scholar] [CrossRef]
- Zweifel-Schlatter, M.; Haug, M.; Schaefer, D.J.; Wolfinger, E.; Ochsner, P.; Pierer, G. Free fasciocutaneous flaps in the treatment of chronic osteomyelitis of the tibia: A retrospective study. J. Reconstr. Microsurg. 2006, 22, 41–47. [Google Scholar] [CrossRef]
- Sambri, A.; Dalla Rosa, M.; Scorianz, M.; Guido, D.; Donati, D.M.; Campanacci, D.A.; De Paolis, M. Different reconstructive techniques for tumours of the distal tibia. Bone Jt. J. 2020, 102-B, 1567–1573. [Google Scholar] [CrossRef]
- Sambri, A.; Spinnato, P.; Tedeschi, S.; Zamparini, E.; Fiore, M.; Zucchini, R.; Giannini, C.; Caldari, E.; Crombé, A.; Viale, P.; et al. Bone and Joint Infections: The Role of Imaging in Tailoring Diagnosis to Improve Patients’ Care. J. Pers. Med. 2021, 11, 1317. [Google Scholar] [CrossRef]
- Drampalos, E.; Mohammad, H.R.; Pillai, A. Augmented debridement for implant related chronic osteomyelitis with an absorbable, gentamycin loaded calcium sulfate/hydroxyapatite biocomposite. J. Orthop. 2020, 17, 173–179. [Google Scholar] [CrossRef]
- Ferguson, J.Y.; Dudareva, M.; Riley, N.D.; Stubbs, D.; Atkins, B.L.; McNally, M.A. The use of a biodegradable antibiotic-loaded calcium sulphate carrier containing tobramycin for the treatment of chronic osteomyelitis: A series of 195 cases. Bone Jt. J. 2014, 96-B, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Fleiter, N.; Walter, G.; Bösebeck, H.; Vogt, S.; Büchner, H.; Hirschberger, W.; Hoffmann, R. Clinical use and safety of a novel gentamicin-releasing resorbable bone graft substitute in the treatment of osteomyelitis/osteitis. Bone Jt. Res. 2014, 3, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Colangeli, M.; Colangeli, S.; Di Bella, C.; Gozzi, E.; Donati, D. Adult osteomyelitis: Debridement versus debridement plus Osteoset T pellets. Acta Orthop. Belg. 2007, 73, 238–243. [Google Scholar] [PubMed]
- Sambri, A.; Zucchini, R.; Giannini, C.; Zamparini, E.; Viale, P.; Donati, D.M.; De Paolis, M. Correction to: Silver-coated (PorAg®) endoprosthesis can be protective against reinfection in the treatment of tumor prostheses infection. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 1355. [Google Scholar] [CrossRef]
- Fiore, M.; Sambri, A.; Zucchini, R.; Giannini, C.; Donati, D.M.; De Paolis, M. Silver-coated megaprosthesis in prevention and treatment of peri-prosthetic infections: A systematic review and meta-analysis about efficacy and toxicity in primary and revision surgery. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 201–220. [Google Scholar] [CrossRef]
- Gosain, A.; Chang, N.; Mathes, S.; Hunt, T.K.; Vasconez, L. A study of the relationship between blood flow and bacterial inoculation in musculocutaneous and fasciocutaneous flaps. Plast. Reconstr. Surg. 1990, 86, 1152–1162; discussion 1163. [Google Scholar] [CrossRef]
- Richards, R.R.; McKee, M.D.; Paitich, C.B.; Anderson, G.I.; Bertoia, J.T. A comparison of the effects of skin coverage and muscle flap coverage on the early strength of union at the site of osteotomy after devascularization of a segment of canine tibia. J. Bone Jt. Surg. Am. 1991, 73, 1323–1330. [Google Scholar] [CrossRef]
- Moore, J.R.; Weiland, A.J. Vascularized tissue transfer in the treatment of osteomyelitis. Clin. Plast. Surg. 1986, 13, 657–662. [Google Scholar] [CrossRef]
- Salgado, C.J.; Mardini, S.; Jamali, A.A.; Ortiz, J.; Gonzales, R.; Chen, H.C. Muscle versus nonmuscle flaps in the reconstruction of chronic osteomyelitis defects. Plast. Reconstr. Surg. 2006, 118, 1401–1411. [Google Scholar] [CrossRef]
- Yazar, S.; Lin, C.H.; Lin, Y.T.; Ulusal, A.E.; Wei, F.C. Outcome comparison between free muscle and free fasciocutaneous flaps for reconstruction of distal third and ankle traumatic open tibial fractures. Plast. Reconstr. Surg. 2006, 117, 2468–2475; discussion 2476–2477. [Google Scholar] [CrossRef]
- Wyble, E.J.; Yakuboff, K.P.; Clark, R.G.; Neale, H.W. Use of free fasciocutaneous and muscle flaps for reconstruction of the foot. Ann. Plast. Surg. 1990, 24, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Musharafieh, R.; Atiyeh, B.; Macari, G.; Haidar, R. Radial forearm fasciocutaneous free-tissue transfer in ankle and foot reconstruction: Review of 17 cases. J. Reconstr. Microsurg. 2001, 17, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.P.J.; Goh, T.L.H.; Choi, D.H.; Kim, J.J.; Suh, H.S. The Efficacy of Perforator Flaps in the Treatment of Chronic Osteomyelitis. Plast. Reconstr. Surg. 2017, 140, 179–188. [Google Scholar] [CrossRef] [PubMed]
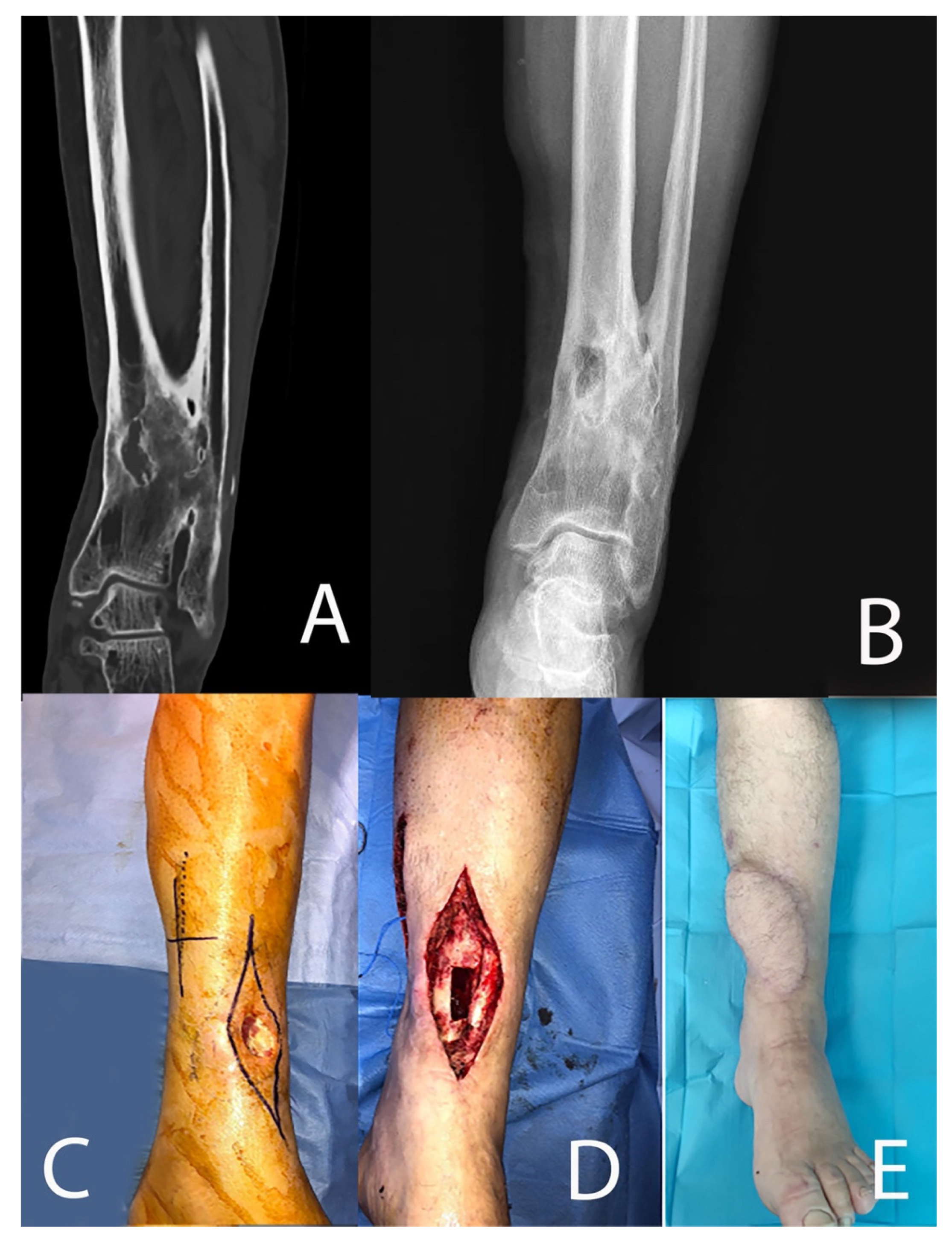
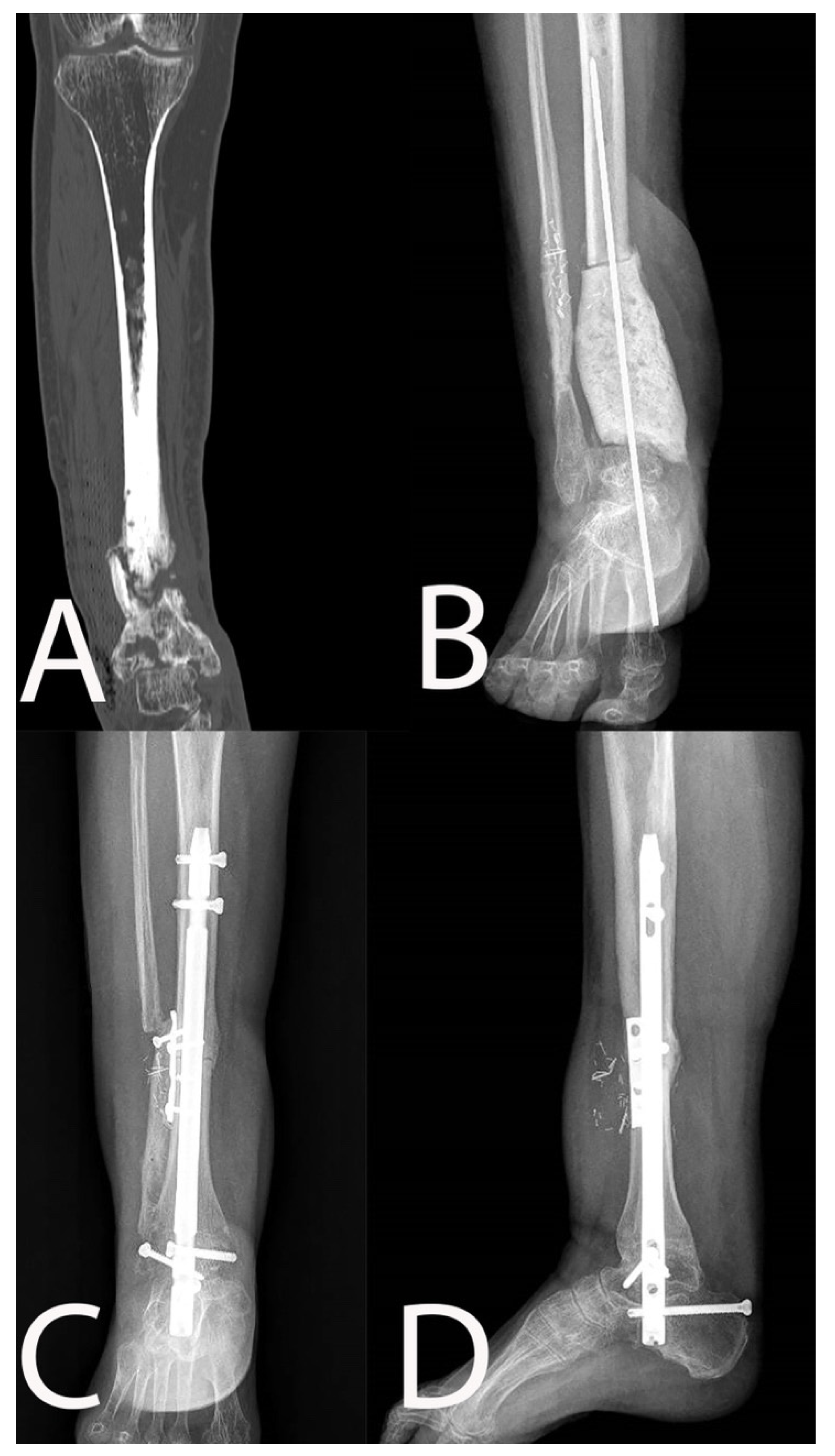

| Patient | Age at Surgery and Sex | ASA Score | Smoker | CCI | Pseudoarthrosis | MAY Classification | Cierny–Mader Classification |
|---|---|---|---|---|---|---|---|
| 1 | 63, M | 2 | No | 4 | Yes | Type 4 | Type 4 Bl |
| 2 | 62, M | 2 | No | 2 | No | Type 2 | Type 3 A |
| 3 | 50, M | 2 | Yes | 2 | Yes | Type 3 | Type 4 A |
| 4 | 45, M | 2 | Yes | 2 | Yes | Type 4 | Type 4 A |
| 5 | 57, M | 2 | No | 3 | No | Type 2 | Type 3 A |
| 6 | 33, M | 1 | Yes | 3 | Yes | Type 4 | Type 4 A |
| 7 | 64, F | 3 | Yes | 5 | Yes | Type 3 | Type 4 A |
| 8 | 33, M | 1 | Yes | 2 | No | Type 1 | Type 3 Bs |
| 9 | 59, M | 2 | No | 4 | Yes | Type 4 | Type 4 Bl |
| 10 | 58, M | 3 | No | 5 | Yes | Type 4 | Type 4 Bl |
| 11 | 18, F | 1 | No | 1 | No | Type 2 | Type 3 A |
| 12 | 46, F | 2 | Yes | 3 | No | Type 1 | Type 2 A |
| 13 | 30, M | 2 | Yes | 3 | No | Type 1 | Type 3 A |
| Patient # | Surgery (Resection vs. Debridement) | Hardware Removal | Length of Resection (mm) | Local Antibiotic | Bacteria | Free Flap | Follow-Up (Months) | Recurrence of Infection | Complications |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Resection | Yes | 120 | MRSA + E. faecalis + P. stutzeri | ALT | 37 | No | ||
| 2 | Debridement | Yes | Gentamicin | MRSA | ALT | 25 | No | ||
| 3 | Resection | No | 60 | P. aeruginosa | Latissimus dorsi | 22 | No | ||
| 4 | Resection | Yes | 75 | E. coli | ALT | 33 | No | Hematoma | |
| 5 | Debridement | Yes | Rifampicin | E. faecium + MRSA | ALT | 18 | No | ||
| 6 | Resection | No | 63 | MS CoNS | ALT | 16 | No | Persistence of infection, DVT | |
| 7 | Resection | Yes | 60 | MR CoNS | ALT | 19 | No | ||
| 8 | Debridement | Yes | Rifampicin | MSSA | Latissimus dorsi | 27 | No | ||
| 9 | Resection | Yes | 100 | MRSA + P. aeruginosa + E. faecalis | ALT | 30 | No | ||
| 10 | Resection | No | 100 | MSSA | ALT | 34 | No | ||
| 11 | Resection | No | 110 | MRSA + E. coli + E. faecium | Latissimus dorsi | 29 | No | ||
| 12 | Debridement | Yes | Gentamicin | MS CoNS | Latissimus dorsi | 15 | No | ||
| 13 | Debridement | Yes | Rifampicin | MSSA | Latissimus dorsi | 13 | No | Hematoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sambri, A.; Pignatti, M.; Tedeschi, S.; Lozano Miralles, M.E.; Giannini, C.; Fiore, M.; Filippini, M.; Cipriani, R.; Viale, P.; De Paolis, M. Combined Orthoplastic Approach in Fracture-Related Infections of the Distal Tibia. Microorganisms 2022, 10, 1640. https://doi.org/10.3390/microorganisms10081640
Sambri A, Pignatti M, Tedeschi S, Lozano Miralles ME, Giannini C, Fiore M, Filippini M, Cipriani R, Viale P, De Paolis M. Combined Orthoplastic Approach in Fracture-Related Infections of the Distal Tibia. Microorganisms. 2022; 10(8):1640. https://doi.org/10.3390/microorganisms10081640
Chicago/Turabian StyleSambri, Andrea, Marco Pignatti, Sara Tedeschi, Maria Elisa Lozano Miralles, Claudio Giannini, Michele Fiore, Matteo Filippini, Riccardo Cipriani, Pierluigi Viale, and Massimiliano De Paolis. 2022. "Combined Orthoplastic Approach in Fracture-Related Infections of the Distal Tibia" Microorganisms 10, no. 8: 1640. https://doi.org/10.3390/microorganisms10081640
APA StyleSambri, A., Pignatti, M., Tedeschi, S., Lozano Miralles, M. E., Giannini, C., Fiore, M., Filippini, M., Cipriani, R., Viale, P., & De Paolis, M. (2022). Combined Orthoplastic Approach in Fracture-Related Infections of the Distal Tibia. Microorganisms, 10(8), 1640. https://doi.org/10.3390/microorganisms10081640







