The Gene Expression Profile Differs in Growth Phases of the Bifidobacterium Longum Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Cell Growth
2.2. RNA Extraction, Library Preparation, and High-Throughput Sequencing
2.3. Data Processing and Analysis
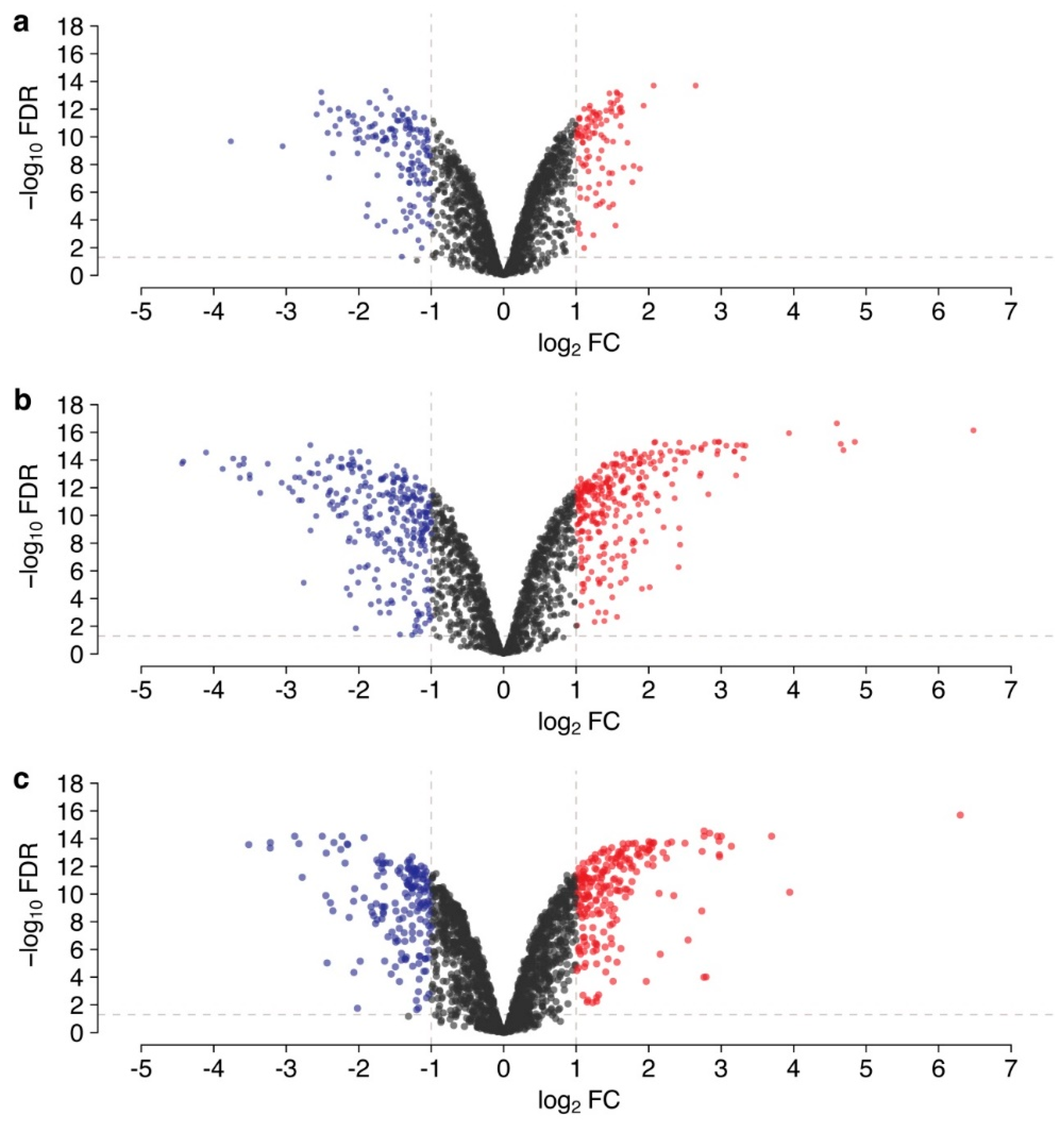
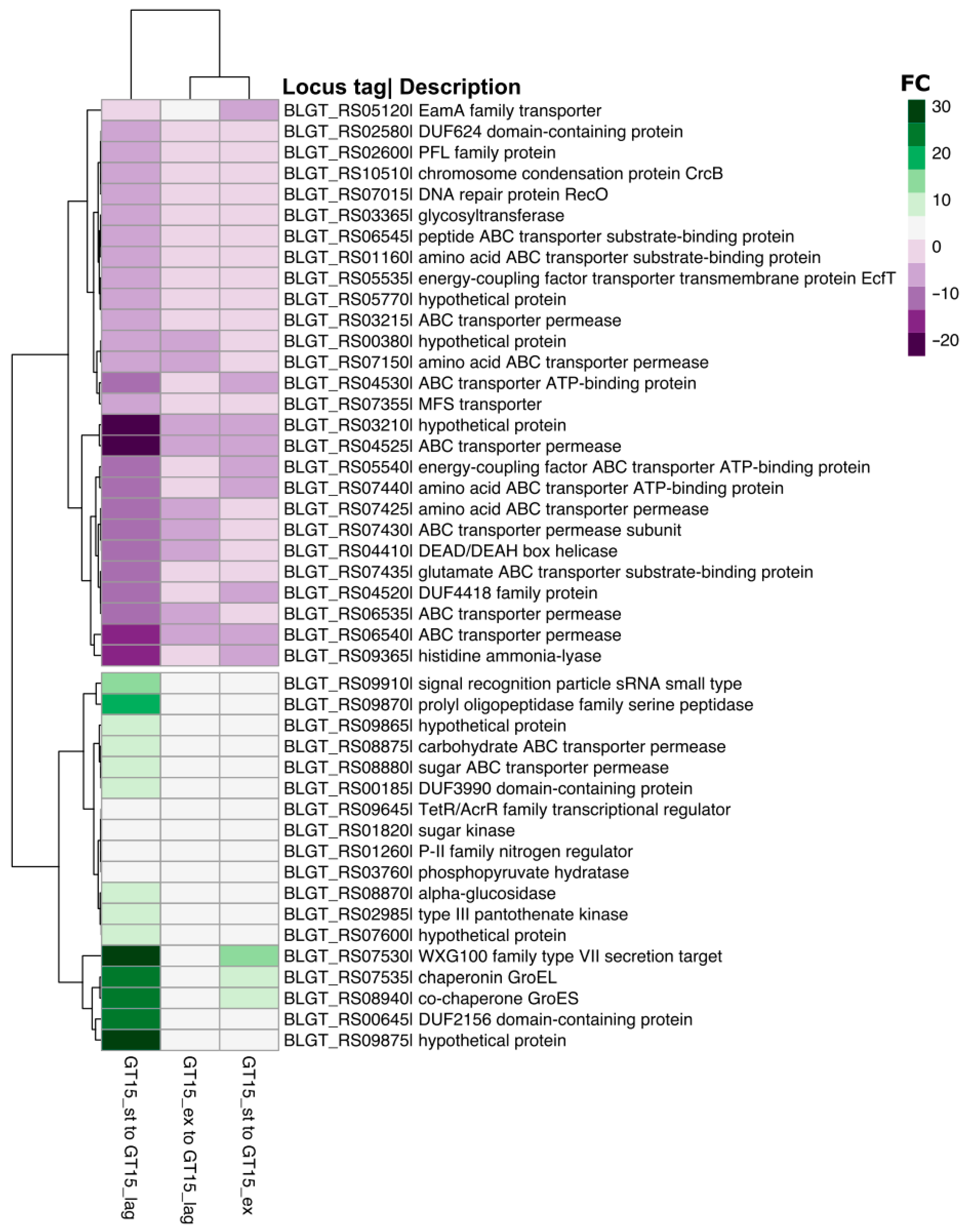
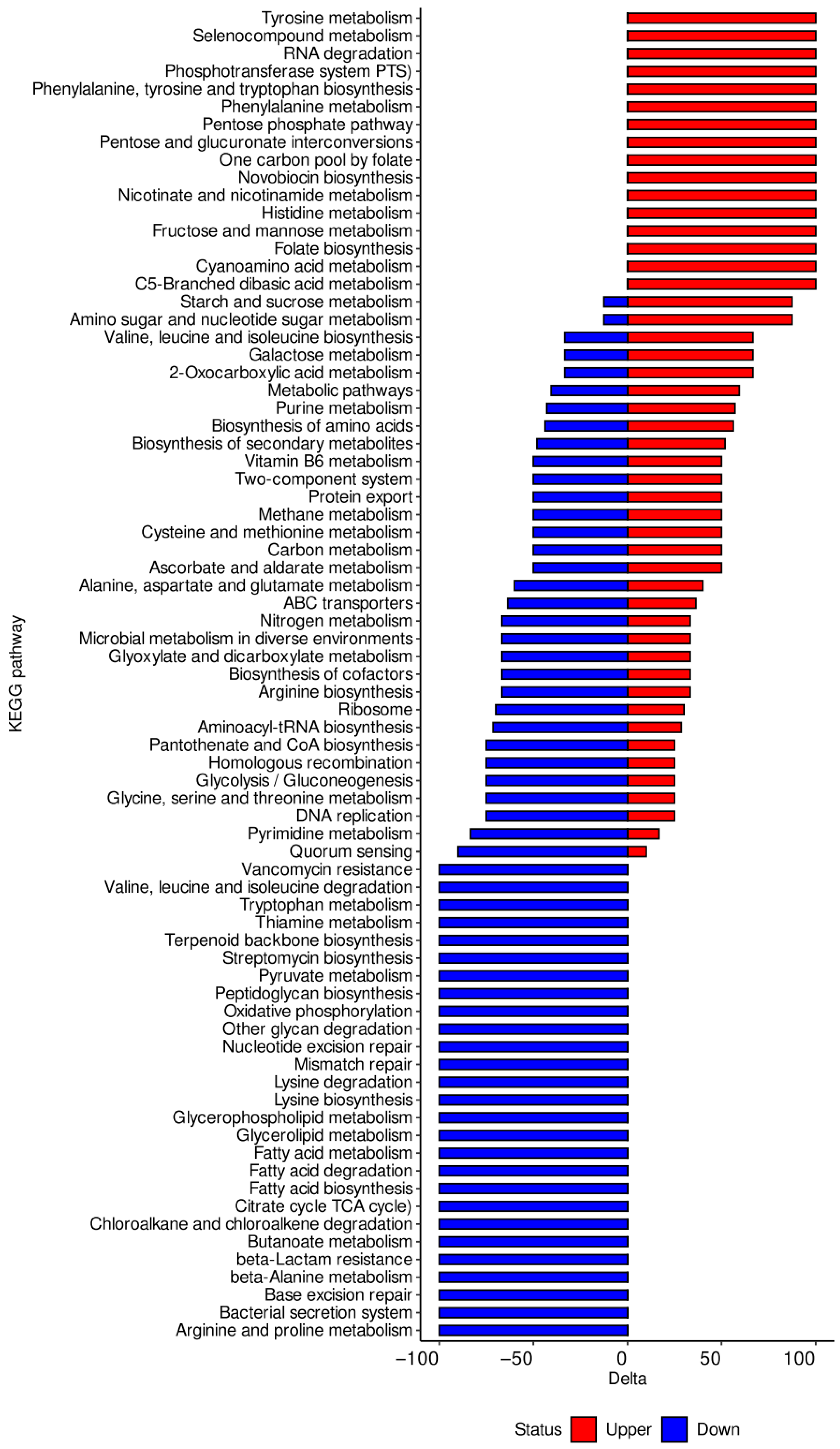
3. Results
3.1. Differences in Gene Transcription Profiles
3.2. KEGG Pathway and Functional Annotation of DEGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Navarro Llorens, J.M.; Tormo, A.; Martínez-García, E. Stationary Phase in Gram-Negative Bacteria. FEMS Microbiol. Rev. 2010, 34, 476–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishihama, A. Adaptation of Gene Expression in Stationary Phase Bacteria. Curr. Opin. Genet. Dev. 1997, 7, 582–588. [Google Scholar] [CrossRef]
- Pobre, V.; Barahona, S.; Dobrzanski, T.; Steffens, M.B.R.; Arraiano, C.M. Defining the Impact of Exoribonucleases in the Shift between Exponential and Stationary Phases. Sci. Rep. 2019, 9, 16271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, D.E.; Smalley, D.J.; Conway, T. Gene Expression Profiling of Escherichia Coli Growth Transitions: An Expanded Stringent Response Model. Mol. Microbiol. 2002, 45, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Blom, E.J.; Ridder, A.N.J.A.; Lulko, A.T.; Roerdink, J.B.T.M.; Kuipers, O.P. Time-Resolved Transcriptomics and Bioinformatic Analyses Reveal Intrinsic Stress Responses during Batch Culture of Bacillus Subtilis. PLoS ONE 2011, 6, e27160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bathke, J.; Konzer, A.; Remes, B.; McIntosh, M.; Klug, G. Comparative Analyses of the Variation of the Transcriptome and Proteome of Rhodobacter Sphaeroides throughout Growth. BMC Genom. 2019, 20, 358. [Google Scholar] [CrossRef] [Green Version]
- Arboleya, S.; Watkins, C.; Stanton, C.; Ross, R.P. Gut Bifidobacteria Populations in Human Health and Aging. Front. Microbiol. 2016, 7, 1204. [Google Scholar] [CrossRef] [Green Version]
- Collado, M.C.; Cernada, M.; Baüerl, C.; Vento, M.; Pérez-Martínez, G. Microbial Ecology and Host-Microbiota Interactions during Early Life Stages. Gut Microbes 2012, 3, 352. [Google Scholar] [CrossRef] [Green Version]
- Jaishankar, J.; Srivastava, P. Molecular Basis of Stationary Phase Survival and Applications. Front. Microbiol. 2017, 8, 2000. [Google Scholar] [CrossRef] [Green Version]
- Korem, T.; Zeevi, D.; Suez, J.; Weinberger, A.; Avnit-Sagi, T.; Pompan-Lotan, M.; Matot, E.; Jona, G.; Harmelin, A.; Cohen, N.; et al. Growth Dynamics of Gut Microbiota in Health and Disease Inferred from Single Metagenomic Samples. Science 2015, 349, 1101–1106. [Google Scholar] [CrossRef] [Green Version]
- Turroni, F.; Foroni, E.; Montanini, B.; Viappiani, A.; Strati, F.; Duranti, S.; Ferrarini, A.; Delledonne, M.; van Sinderen, D.; Ventura, M. Global Genome Transcription Profiling of Bifidobacterium Bifidum PRL2010 under In Vitro Conditions and Identification of Reference Genes for Quantitative Real-Time PCR. Appl. Environ. Microbiol. 2011, 77, 8578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laakso, K.; Koskenniemi, K.; Koponen, J.; Kankainen, M.; Surakka, A.; Salusjärvi, T.; Auvinen, P.; Savijoki, K.; Nyman, T.A.; Kalkkinen, N.; et al. Growth Phase-Associated Changes in the Proteome and Transcriptome of Lactobacillus Rhamnosus GG in Industrial-Type Whey Medium. Microb. Biotechnol. 2011, 4, 746–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhang, W.; Zhong, Z.; Wei, A.; Bao, Q.; Zhang, Y.; Sun, T.; Postnikoff, A.; Meng, H.; Zhang, H. Gene Expression Profile of Probiotic Lactobacillus Casei Zhang during the Late Stage of Milk Fermentation. Food Control 2012, 25, 321–327. [Google Scholar] [CrossRef]
- Wang, H.; An, J.; Fan, C.; Zhai, Z.; Zhang, H.; Hao, Y. Transcriptome Analysis Revealed Growth Phase-Associated Changes of a Centenarian-Originated Probiotic Bifidobacterium Animalis Subsp. Lactis A6. BMC Microbiol. 2022, 22, 61. [Google Scholar] [CrossRef]
- Zakharevich, N.V.; Averina, O.V.; Klimina, K.M.; Kudryavtseva, A.V.; Kasianov, A.S.; Makeev, V.J.; Danilenko, V.N. Complete Genome Sequence of Bifidobacterium Longum GT15: Unique Genes for Russian Strains. Genome Announc. 2014, 2, 1348–1362. [Google Scholar] [CrossRef] [Green Version]
- Veselovsky, V.A.; Dyachkova, M.S.; Menyaylo, E.A.; Polyaeva, P.S.; Olekhnovich, E.I.; Shitikov, E.A.; Bespiatykh, D.A.; Semashko, T.A.; Kasianov, A.S.; Ilina, E.N.; et al. Gene Networks Underlying the Resistance of Bifidobacterium Longum to Inflammatory Factors. Front. Immunol. 2020, 11, 595877. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, p.giab008. Available online: https://academic.oup.com/gigascience/article/10/2/giab008/6137722 (accessed on 25 June 2022). [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- Okonechnikov, K.; Conesa, A.; García-Alcalde, F. Qualimap 2: Advanced Multi-Sample Quality Control for High-Throughput Sequencing Data. Bioinformatics 2016, 32, 292–294. [Google Scholar] [CrossRef]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize Analysis Results for Multiple Tools and Samples in a Single Report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickham, H. Getting started with qplot. In ggplot2; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottacini, F.; Zomer, A.; Milani, C.; Ferrario, C.; Lugli, G.A.; Egan, M.; Ventura, M.; van Sinderen, D. Global Transcriptional Landscape and Promoter Mapping of the Gut Commensal Bifidobacterium Breve UCC2003. BMC Genom. 2017, 18, 991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senizza, A.; Callegari, M.L.; Senizza, B.; Minuti, A.; Rocchetti, G.; Morelli, L.; Patrone, V. Effects of Linoleic Acid on Gut-Derived Bifidobacterium Breve DSM 20213: A Transcriptomic Approach. Microorganisms 2019, 7, 710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Tang, Q.; Xu, L.; Li, Z.; Ma, Y.; Yao, D. Combining of Transcriptome and Metabolome Analyses for Understanding the Utilization and Metabolic Pathways of Xylo-Oligosaccharide in Bifidobacterium Adolescentis ATCC 15703. Food Sci. Nutr. 2019, 7, 3480–3493. [Google Scholar] [CrossRef] [Green Version]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The Role of Short Chain Fatty Acids in Appetite Regulation and Energy Homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef] [Green Version]
- Zuo, F.; Yu, R.; Xiao, M.; Khaskheli, G.B.; Sun, X.; Ma, H.; Ren, F.; Zhang, B.; Chen, S. Transcriptomic Analysis of Bifidobacterium Longum Subsp. Longum BBMN68 in Response to Oxidative Shock. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Regulski, K.; Courtin, P.; Meyrand, M.; Claes, I.J.J.; Lebeer, S.; Vanderleyden, J.; Hols, P.; Guillot, A.; Chapot-Chartier, M.P. Analysis of the Peptidoglycan Hydrolase Complement of Lactobacillus Casei and Characterization of the Major γ-D-Glutamyl-L-Lysyl-Endopeptidase. PLoS ONE 2012, 7, e32301. [Google Scholar] [CrossRef] [Green Version]
- Perčulija, V.; Ouyang, S. Diverse Roles of DEAD/DEAH-Box Helicases in Innate Immunity and Diseases. Helicases All Domains Life 2019, 141–171. [Google Scholar] [CrossRef]
- Pallen, M.J. The ESAT-6/WXG100 Superfamily—And a New Gram-Positive Secretion System? Trends Microbiol. 2002, 10, 209–212. [Google Scholar] [CrossRef]
- Nyström, T. Stationary-Phase Physiology. Annu. Rev. Microbiol. 2004, 58, 161–181. [Google Scholar] [CrossRef] [PubMed]

| Category | Growth Phase | Number of Raw Reads | GC, (%) | Average Length, bp | Number of Trimmed Reads | Number of Uniquely Mapped Reads | Percentage of Uniquely Mapped Reads (%) |
|---|---|---|---|---|---|---|---|
| Bl_K01_1 | lag phase | 4,114,356 | 60 | 101 | 4,104,837 | 3,684,561 | 89.80 |
| Bl_K01_2 | lag phase | 4,084,494 | 60 | 101 | 4,072,394 | 3,639,926 | 89.40 |
| Bl_K01_3 | lag phase | 3,795,673 | 60 | 101 | 3,786,859 | 3,380,989 | 89.30 |
| Bl_K05_1 | exponential phase | 4,182,552 | 60 | 101 | 4,171,537 | 3,754,019 | 90.00 |
| Bl_K05_2 | exponential phase | 3,983,507 | 60 | 101 | 3,972,238 | 3,566,746 | 89.80 |
| Bl_K05_3 | exponential phase | 3,879,511 | 60 | 101 | 3,870,191 | 3,473,633 | 89.80 |
| Bl_K09_1 | stationary phase | 4,470,198 | 59 | 101 | 4,463,213 | 4,007,847 | 89.80 |
| Bl_K09_2 | stationary phase | 4,161,821 | 59 | 101 | 4,148,602 | 3,668,915 | 88.40 |
| Bl_K09_3 | stationary phase | 3,881,059 | 59 | 101 | 3,867,621 | 3,419,213 | 88.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veselovsky, V.A.; Dyachkova, M.S.; Bespiatykh, D.A.; Yunes, R.A.; Shitikov, E.A.; Polyaeva, P.S.; Danilenko, V.N.; Olekhnovich, E.I.; Klimina, K.M. The Gene Expression Profile Differs in Growth Phases of the Bifidobacterium Longum Culture. Microorganisms 2022, 10, 1683. https://doi.org/10.3390/microorganisms10081683
Veselovsky VA, Dyachkova MS, Bespiatykh DA, Yunes RA, Shitikov EA, Polyaeva PS, Danilenko VN, Olekhnovich EI, Klimina KM. The Gene Expression Profile Differs in Growth Phases of the Bifidobacterium Longum Culture. Microorganisms. 2022; 10(8):1683. https://doi.org/10.3390/microorganisms10081683
Chicago/Turabian StyleVeselovsky, Vladimir A., Marina S. Dyachkova, Dmitry A. Bespiatykh, Roman A. Yunes, Egor A. Shitikov, Polina S. Polyaeva, Valeriy N. Danilenko, Evgenii I. Olekhnovich, and Ksenia M. Klimina. 2022. "The Gene Expression Profile Differs in Growth Phases of the Bifidobacterium Longum Culture" Microorganisms 10, no. 8: 1683. https://doi.org/10.3390/microorganisms10081683






