Antarctic Salt-Cones: An Oasis of Microbial Life? The Example of Boulder Clay Glacier (Northern Victoria Land)
Abstract
:1. Introduction
2. Materials and Methods
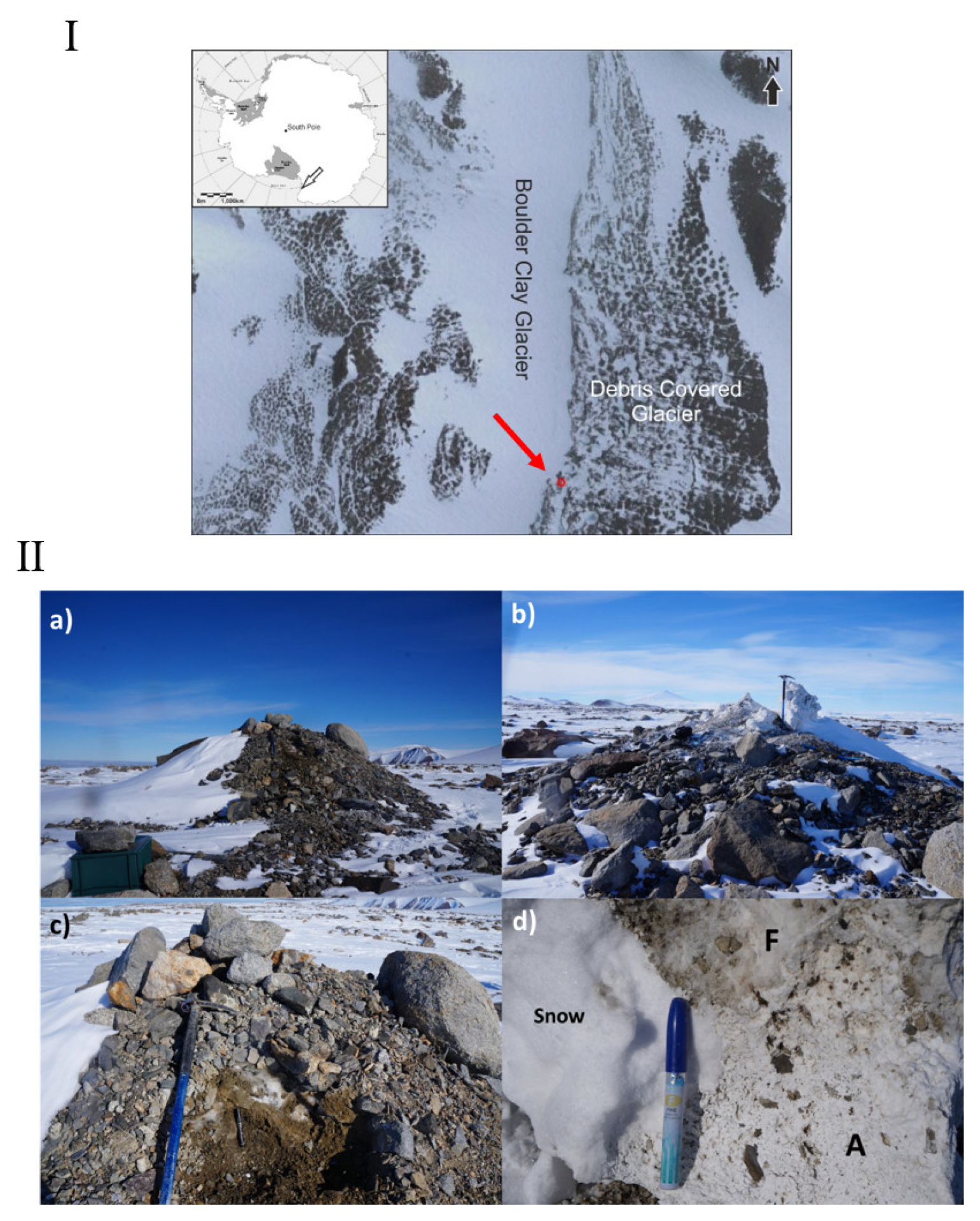
2.1. Sampling Area
2.2. Sample Collection
2.3. Mineralogical Analysis
2.4. Prokaryotic Community Composition
2.4.1. DNA Extraction and NGS Sequencing
2.4.2. Bioinformatic Analysis
2.4.3. Statistical Analyses
3. Results
3.1. Mineralogical Analyses
3.2. Prokaryotic Community Composition
3.3. Statistical Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peterson, R.C.; Nelson, W.; Madu, B.; Shurvell, H.F. Meridianiite: A new mineral species observed on Earth and predicted to exist on Mars. Am. Mineral. 2007, 92, 1756–1759. [Google Scholar] [CrossRef]
- Gulick, V.C. Origin of the valley networks on Mars: A hydrological perspective. Geomorphology 2001, 37, 241–268. [Google Scholar] [CrossRef]
- Oehler, D.Z.; Allen, C.C. Evidence for pervasive mud volcanism in Acidalia Planitia, Mars. Icarus 2010, 208, 636–657. [Google Scholar] [CrossRef]
- Burr, D.M.; Enga, M.-T.; Williams, R.M.E.; Zimbelman, J.R.; Howard, A.D.; Brennand, T.A. Pervasive aqueous paleoflow features in the Aeolis/Zephyria Plana region, Mars. Icarus 2009, 200, 52–76. [Google Scholar] [CrossRef]
- Stark, S.C.; O’Grady, B.V.; Burton, H.R.; Carpenter, P.D. Frigidly concentrated seawater and the evolution of Antarctic saline lakes. Australian J. Chem. 2003, 56, 181–186. [Google Scholar] [CrossRef]
- Babel, M.; Schreiber, B.C. Geochemistry of evaporites and evolution of seawater. In Treatise on Geochemistry, 2nd ed.; Turekian, K., Holland, H., Eds.; Elsevier: Oxford, UK, 2014. [Google Scholar]
- Liu, T.; Bish, D.; Socki, R.; Harvey, R.; Tonui, E. Mineralogy and formation of evaporite deposits from the Lewis Cliff ice tongue, Antarctica. Antarc. Sci. 2015, 27, 73–84. [Google Scholar] [CrossRef]
- Rivard, N.R.; Pewe, T.H. Origin and distribution of mirabilite, McMurdo Sound Region, Antarctica. Geol. Soc. America Abs. For 1961 Spec. Rept. 1962, 68, 119. [Google Scholar]
- Black, R.F.; Jackson, M.L.; Berg, T.E. Saline discharge from Taylor Glacier, Victoria Land, Antarctica. J. Geol. 1965, 73, 175–181. [Google Scholar] [CrossRef]
- Bowser, C.; Rafter, T.; Black, R. Geochemical evidence for the origin of mirabilite deposits near Hobbs Glacier, Victoria Land, Antarctica. Mineral. Soc. Am. Spec. Pap. 1970, 3, 261–272. [Google Scholar]
- Orombelli, G.; Baroni, C.; Denton, G.H. Late Cenozoic glacial history of the Terra Nova Bay region, northern Victoria Land, Antarctica. Geograf. Fis. Dinam. Quater. 1990, 13, 139–163. [Google Scholar]
- Guglielmin, M.; Dramis, F. Permafrost as a climatic indicator in northern Victoria Land, Antarctica. Ann. Glaciol. 1999, 29, 131–135. [Google Scholar] [CrossRef]
- Guglielmin, M.; Dalle Fratte, M.; Cannone, N. Permafrost warming and vegetation changes in continental Antarctica. Environ. Res. Lett. 2014, 9, 045001. [Google Scholar] [CrossRef]
- Guglielmin, M.; Cannone, N. A permafrost warming in a cooling Antarctica? Clim. Change 2012, 111, 177–195. [Google Scholar] [CrossRef]
- Chinn, T.J.H. Polar glacier margin and debris features. Mem. Soc. Geol. It. 1991, 46, 25–44. [Google Scholar]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Pirrung, M.; McCue, L.A. FQC Dashboard: Integrates FastQC results into a web-based, interactive, and extensible FASTQ quality control tool. Bioinformatics 2017, 33, 3137–3139. [Google Scholar] [CrossRef] [PubMed]
- Weißbecker, C.; Schnabel, B.; Heintz-Buschart, A. Dadasnake, a Snakemake implementation of DADA2 to process amplicon sequencing data for microbial ecology. GigaScience 2020, 9, giaa135. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.J.; Escavy, J.I.; Schreiber, B.C. Thenardite after mirabilite deposits as a cool climate indicator in the geological record: Lower Miocene of central Spain. Clim. Past 2015, 11, 1–13. [Google Scholar] [CrossRef]
- Zheng, M.; Zhao, Y.; Liu, J. Palaeoclimatic Indicators of China’s quaternary saline lake sediments and hydrochemistry. Acta Geol. Sin. 2000, 74, 259–265. [Google Scholar]
- Ortí, F.; Gündogan, I.; Helvaci, C. Sodium sulphate deposits of Neogene age: The Kirmir Formation, Beypazari Basin, Turkey. Sediment. Geol. 2002, 146, 305–333. [Google Scholar] [CrossRef]
- Lyons, W.B.; Mikucki, J.A.; German, L.A.; Welch, K.A.; Welch, S.A.; Gardner, C.B.; Tulaczyk, S.M.; Pettit, E.C.; Kowalski, J.; Dachwald, B. The geochemistry of englacial brine from Taylor Glacier, Antarctica. J. Geophys. Res. Biogeosci. 2019, 124, 633–648. [Google Scholar] [CrossRef]
- Cesur, R.M.; Ansari, I.M.; Chen, F.; Clark, B.C.; Schneegurt, M. A bacterial growth in brines formed by the deliquescence of salts relevant to cold arid worlds. Astrobiology 2022, 22, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Guglielmin, M. Ground surface temperature (GST), active layer and permafrost monitoring in continental Antarctica. Permafr. Periglac. Process. 2006, 17, 133–143. [Google Scholar] [CrossRef]
- Guglielmin, M.; French, H. Ground ice in the Northern Foothills, Northern Victoria Land, Antarctica. Ann. Glaciol. 2004, 39, 495–500. [Google Scholar] [CrossRef]
- Stivaletta, N.; Barbieri, R. Endolithic microorganisms from spring mound evaporite deposits (southern Tunisia). J. Arid Environ. 2009, 73, 33–39. [Google Scholar] [CrossRef]
- Stivaletta, N.; Barbieri, R.; Billi, D. Microbial Colonization of the Salt Deposits in the Driest Place of the Atacama Desert (Chile). Orig. Life Evol. Biosph. 2012, 42, 187–200. [Google Scholar] [CrossRef]
- Lee, Y.M.; Jin, Y.K.; Shin, S.C. Complete genome sequence of Antarcticibacterium flavum JB01H24T from an Antarctic marine sediment. Mar. Genom. 2020, 50, 100695. [Google Scholar] [CrossRef]
- Mikucki, J.A.; Priscu, J.C. Bacterial diversity associated with blood falls, a subglacial outflow from the Taylor Glacier, Antarctica. Appl. Environ. Microbiol. 2007, 73, 4029–4039. [Google Scholar] [CrossRef]
- Murray, A.E.; Kenig, F.; Fritsen, C.H.; McKay, C.P.; Cawley, K.M.; Edwards, R.; Kuhn, E.; McKnight, D.M.; Ostrom, N.E.; Peng, V.; et al. Microbial life at −13 °C in the brine of an ice-sealed Antarctic lake. Proc. Natl. Acad. Sci. USA 2012, 109, 20626–20631. [Google Scholar] [CrossRef]
- Papale, M.; Rizzo, C.; Caruso, G.; La Ferla, R.; Maimone, G.; Lo Giudice, A.; Azzaro, M.; Guglielmin, M. First insights into the microbiology of three antarctic briny systems of the Northern Victoria Land. Diversity 2021, 13, 323. [Google Scholar] [CrossRef]
- Lo Giudice, A.; Conte, A.; Papale, M.; Rizzo, C.; Azzaro, M.; Guglielmin, M. Prokaryotic diversity and metabolically active communities in brines from two perennially ice-covered Antarctic lakes. Astrobiology 2021, 21, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Van Trappen, S.; Vandecandelaere, I.; Mergaert, J.; Swings, J. Gillisia limnaea gen. nov., sp. nov., a new member of the family Flavobacteriaceae isolated from a microbial mat in Lake Fryxell, Antarctica. Int. J. Syst. Evol. Microbiol. 2004, 54, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Maida, I.; Fondi, M.; Papaleo, M.C.; Perrin, E.; Orlandini, V.; Emiliani, G.; de Pascale, D.; Parrilli, E.; Tutino, M.L.; Michaud, L.; et al. Phenotypic and genomic characterization of the Antarctic bacterium Gillisia sp. CAL575, a producer of antimicrobial compounds. Extremophiles 2014, 18, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.P.; Nichols, D.S. Novel members of the family Flavobacteriaceae from Antarctic maritime habitats including Subsaximicrobium wynnwilliamsii gen. nov., sp. nov., Subsaximicrobium saxinquilinus sp. nov., Subsaxibacter broadyi gen. nov., sp. nov., Lacinutrix copepodicola gen. nov., sp. nov., and novel species of the genera Bizionia, Gelidibacter and Gillisia. Int. J. Syst. Evol. Microbiol. 2005, 55, 1471–1486. [Google Scholar]
- Ying, J.Y.; Wang, B.J.; Yang, S.S.; Liu, S.J. Cyclobacterium lianum sp. nov., a marine bacterium isolated from sediment of an oilfield in the South China Sea, and emended description of the genus Cyclobacterium. Int. J. Syst. Evol. Microbiol. 2006, 56, 2927–2930. [Google Scholar] [CrossRef]
- Nedashkovskaya, O.I.; Kim, S.B.; Lee, M.S.; Park, M.S.; Lee, K.H.; Lysenko, A.M.; Oh, H.W.; Mikhailov, V.V.; Bae, K.S. Cyclobacterium amurskyense sp. nov., a novel marine bacterium isolated from sea water. Int. J. Syst. Evol. Microbiol. 2005, 55, 2391–2394. [Google Scholar] [CrossRef]
- Shivaji, S.; Reddy, P.V.; Rao, S.S.; Begum, Z.; Manasa, P.; Srinivas, T.N. Cyclobacterium qasimii sp. nov., a psychrotolerant bacterium isolated from Arctic marine sediment. Int. J. Syst. Evol. Microbiol. 2012, 62, 2133–2139. [Google Scholar] [CrossRef]
- Shahinpei, A.; Amoozegar, M.A.; Mirfeizi, L.; Nikou, M.M.; Ventosa, A.; Sánchez-Porro, C. Taxogenomics of the genus Cyclobacterium: Cyclobacterium xiamenense and Cyclobacterium halophilum as synonyms and description of Cyclobacterium plantarum sp. nov. Microorganisms 2020, 8, 610. [Google Scholar] [CrossRef]
- Van Trappen, S.; Mergaert, J.; Van Eygen, S.; Dawyndt, P.; Cnockaert, M.C.; Swings, J. Diversity of 746 heterotrophic bacteria isolated from microbial mats from ten Antarctic lakes. Syst. Appl. Microbiol. 2002, 25, 603–610. [Google Scholar] [CrossRef]
- Stougaard, P.; Jørgensen, F.; Johnsen, M.G.; Hansen, O.-C. Microbial diversity in ikaite tufa columns: An alkaline, cold ecological niche in Greenland. Environ. Microbiol. 2002, 4, 487–493. [Google Scholar] [CrossRef]
- Dong, C.; Liu, R.; Lai, Q.; Liu, Y.; Shao, Z. Thalassospira marina sp. nov., isolated from surface seawater. Int. J. Syst. Evol. Microbiol. 2018, 68, 2943–2948. [Google Scholar] [CrossRef] [PubMed]
- Gregson, B.H.; Metodieva, G.; Metodiev, M.V.; McKew, B.A. Differential protein expression during growth on linear versus branched alkanes in the obligate marine hydrocarbon-degrading bacterium Alcanivorax borkumensis SK2T. Environ. Microbiol. 2019, 21, 2347–2359. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, D.Y.; Roman, P.; Kolganova, T.V. Halo(natrono)archaea from hypersaline lakes can utilize sulfoxides other than DMSO as electron acceptors for anaerobic respiration. Extremophiles 2021, 25, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, D.Y.; Messina, E.; Smedile, F.; Roman, P.; Sinninghe Damste, J.S.; Ciordia, S.; del Carmen, M.M.; Ferrer, M.; Golyshin, P.N.; Kublanov, I.V.; et al. Discovery of the anaerobic lithoheterotrophic haloarchaea, ubiquitous in hypersaline habitats. ISME J. 2017, 11, 1245–1260. [Google Scholar] [CrossRef]
- Cao, L.; Cox, C.D.; He, Q. Patterns of syntrophic interactions in methanogenic conversion of propionate. Appl. Microbiol. Biotechnol. 2021, 105, 8937–8949. [Google Scholar] [CrossRef]
- Timmers, P.H.A.; Vavourakis, C.D.; Kleerebezem, R.; Damsté, J.S.S.; Muyzer, G.; Stams, A.J.M.; Sorokin, D.Y.; Plugge, C.M. Metabolism and occurrence of methanogenic and sulfate-reducing syntrophic acetate oxidizing communities in haloalkaline environments. Front. Microbiol. 2018, 9, 3039. [Google Scholar] [CrossRef] [Green Version]
- Papale, M.; Lo Giudice, A.; Conte, A.; Rizzo, C.; Rappazzo, A.C.; Maimone, G.; Caruso, G.; La Ferla, R.; Azzaro, M.; Gugliandolo, C.; et al. Microbial assemblages in pressurized antarctic brine pockets (Tarn Flat, Northern Victoria Land): A hotspot of biodiversity and activity. Microorganisms 2019, 7, 333. [Google Scholar] [CrossRef]
- Dahle, H.; Birkeland, N.-K. Thermovirga lienii gen. nov., sp. nov., a novel moderately thermophilic, anaerobic, amino-acid-degrading bacterium isolated from a North Sea oil well. Int. J. System. Evolut. Microbiol. 2006, 56, 1539–1545. [Google Scholar] [CrossRef] [Green Version]
- Miranda-Tello, E.; Fardeau, M.L.; Thomas, P.; Ramirez, F.; Casalot, L.; Cayol, J.L.; Garcia, J.L.; Ollivier, B. Petrotoga mexicana sp. nov., a novel thermophilic, anaerobic and xylanolytic bacterium isolated from an oil-producing well in the Gulf of Mexico. Int. J. Syst. Evol. Microbiol. 2004, 54, 169–174. [Google Scholar]
- L’Haridon, S.; Miroshnichenko, M.L.; Hippe, H.; Fardeau, M.L.; Bonch-Osmolovskaya, E.A.; Stackebrandt, E.; Jeanthon, C. Petrotoga olearia sp. nov. and Petrotoga sibirica sp. nov., two thermophilic bacteria isolated from a continental petroleum reservoir in Western Siberia. Int. J. Syst. Evol. Microbiol. 2004, 52, 1715–1722. [Google Scholar]









| Phylum | Genus | WSI | WSII | WSIII | BSI | BSII | BSIII | |
|---|---|---|---|---|---|---|---|---|
| Actinobacteriota | Aeromicrobium | 0 | 0 | 0 | 0.2 | 0 | 0.1 | |
| Arthrobacter | 1.7 | 2.1 | 1.9 | 0.7 | 28.1 | 25.2 | ||
| Cryobacterium | 0.4 | 0 | 0 | 0.4 | 0 | 0 | ||
| Cutibacterium | 0.1 | 0.1 | 0 | 0.2 | 0 | 0 | ||
| Demequina | 0.2 | 0.6 | 0.6 | 0.8 | 0.2 | 0.4 | ||
| Egicoccus | 0 | 0.3 | 0.2 | 0.4 | 0 | 0.1 | ||
| Ilumatobacter | 0.2 | 0 | 0.2 | 0 | 0.1 | 0.2 | ||
| IMCC26207 | 0.1 | 0 | 0.1 | 0.2 | 0.1 | 0 | ||
| Kocuria | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Lawsonella | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Luteococcus | 0.4 | 0.2 | 0.2 | 0.4 | 0.2 | 0.3 | ||
| Mycobacterium | 0.4 | 0.2 | 0.2 | 0.3 | 0 | 0.2 | ||
| Nocardioides | 1.8 | 3 | 3.4 | 12.6 | 2 | 2.9 | ||
| Quadrisphaera | 0 | 0 | 0 | 0 | 0.1 | 0.1 | ||
| Rhodoglobus | 4.2 | 18.6 | 14.4 | 16.4 | 1.2 | 3.4 | ||
| Sva0996 marine group | 0 | 0 | 0 | 0 | 0 | 0.2 | ||
| Tessaracoccus | 0.3 | 0 | 0.2 | 0.3 | 0.1 | 0.2 | ||
| Bacteroidota | Antarcticibacterium | 2 | 9.8 | 6.3 | 10.2 | 2.5 | 2.3 | |
| Belliella | 0 | 3.7 | 1.5 | 0 | 0 | 0 | ||
| Cryomorpha | 0 | 0 | 0 | 0 | 0 | 0.1 | ||
| Cyclobacterium | 0.4 | 1.1 | 1 | 0.8 | 0.4 | 0.7 | ||
| Flavobacterium | 0.2 | 0 | 0 | 0.1 | 0 | 0 | ||
| Gillisia | 25.4 | 17.8 | 20.9 | 10 | 8.1 | 8.1 | ||
| Lentimicrobium | 0.5 | 0.1 | 0.2 | 0.3 | 0 | 0.2 | ||
| Mesonia | 0 | 0 | 0 | 0.2 | 0 | 0 | ||
| Campylobacterota | Sulfurovum | 0.3 | 0 | 0 | 0.3 | 0 | 0 | |
| Deinococcota | Truepera | 0 | 0 | 0 | 0.1 | 0 | 0 | |
| Firmicutes | Alkalibacterium | 0.2 | 0 | 0 | 0.1 | 0 | 0 | |
| Anaerobacillus | 0.7 | 0.3 | 0.4 | 1 | 0.2 | 0.1 | ||
| Bacillus | 0.3 | 0 | 0.1 | 0.2 | 0 | 0 | ||
| Caminicella | 0.1 | 0 | 0.1 | 0 | 0 | 0 | ||
| Cellulosilyticum | 0.2 | 0 | 0.1 | 0 | 0 | 0 | ||
| Clostridium sensu stricto 13 | 0.3 | 0 | 0.2 | 0.3 | 0 | 0 | ||
| Dellaglioa | 0.2 | 0 | 0.1 | 0 | 0 | 0 | ||
| Desulfitibacter | 0.4 | 0 | 0 | 1.1 | 0 | 0.3 | ||
| Desulfosporosinus | 3.4 | 2.6 | 2.7 | 1.8 | 1.2 | 1.2 | ||
| Desulfuribacillus | 0.1 | 0 | 0.2 | 0.2 | 0 | 0 | ||
| Haloplasma | 0.4 | 0.2 | 0.2 | 0 | 0 | 0 | ||
| Herbinix | 0 | 0.1 | 0 | 0 | 0 | 0 | ||
| Hungateiclostridium | 0.1 | 0 | 0 | 0 | 0 | 0 | ||
| Lachnospiraceae NK4A136 group | 0 | 0 | 0 | 0.1 | 0 | 0 | ||
| Paenibacillus | 0.1 | 0 | 0 | 0.1 | 0 | 0 | ||
| Planococcus | 0.7 | 0.3 | 0.3 | 0 | 1.4 | 1 | ||
| Planomicrobium | 0.1 | 0 | 0.1 | 0 | 2.5 | 1.9 | ||
| Ruminiclostridium | 0 | 0 | 0.3 | 0.1 | 0 | 0 | ||
| Staphylococcus | 0 | 0 | 0.1 | 0 | 0 | 0 | ||
| Thermacetogenium | 0.2 | 0 | 0 | 0.4 | 0 | 0 | ||
| Tissierella | 0 | 0 | 0.1 | 0 | 0 | 0 | ||
| UCG-012 | 0.1 | 0 | 0 | 0 | 0 | 0 | ||
| Halanaerobiaeota | Orenia | 0.9 | 1.5 | 1.4 | 0.3 | 0.3 | 0.4 | |
| Halobacterota | Methanoculleus | 0 | 0 | 0 | 0.1 | 0 | 0 | |
| Myxococcota | Enhygromyxa | 0.4 | 0 | 0.3 | 0.3 | 0 | 0.1 | |
| Planctomycetota | Roseimaritima | 0 | 0.1 | 0 | 0 | 0.1 | 0 | |
| Rubinisphaera | 0.2 | 0 | 0 | 0.4 | 0 | 0 | ||
| Proteobacteria | Acinetobacter | 0.1 | 0.1 | 0.1 | 0 | 0 | 0 | |
| Afipia | 0 | 0 | 0 | 0.2 | 0 | 0 | ||
| Alcanivorax | 0.1 | 0 | 0 | 0 | 0 | 0 | ||
| Amaricoccus | 0.1 | 0 | 0 | 0 | 0 | 0 | ||
| Aureimonas | 0.2 | 0.2 | 0.1 | 0.9 | 0.2 | 0.1 | ||
| Blastomonas | 1.3 | 0.5 | 0.9 | 1.4 | 0.2 | 0.5 | ||
| Bradyrhizobium | 0 | 0 | 0 | 0.2 | 0 | 0 | ||
| Burkholderia-Caballeronia-Paraburkholderia | 0 | 0 | 0 | 0.1 | 0 | 0 | ||
| Hahella | 6.7 | 1 | 3.3 | 1.5 | 0.6 | 1.6 | ||
| Halomonas | 0.2 | 0 | 0 | 0.2 | 0 | 0 | ||
| Herbaspirillum | 0 | 0 | 0 | 0 | 0 | 0.1 | ||
| Jannaschia | 0 | 0.2 | 0.2 | 0 | 0.7 | 0.3 | ||
| Legionella | 0.8 | 0.1 | 0.4 | 0 | 0 | 0 | ||
| Luteimonas | 0.1 | 0 | 0 | 0 | 0 | 0 | ||
| Lysobacter | 0 | 0.1 | 0 | 0.3 | 0 | 0 | ||
| Maribius | 0 | 0 | 0 | 0.4 | 0 | 0.1 | ||
| Marinicella | 0.2 | 0.3 | 0.3 | 0.5 | 0.2 | 0.3 | ||
| Marinobacter | 10.4 | 15.3 | 14.1 | 1.6 | 1 | 1.4 | ||
| Marinomonas | 0 | 0 | 0 | 0.2 | 0 | 0 | ||
| Methylobacterium-Methylorubrum | 0.1 | 0 | 0 | 0 | 0 | 0 | ||
| Methyloceanibacter | 0.3 | 0 | 0.1 | 0.2 | 0 | 0 | ||
| Oceanobacter | 0 | 0 | 0 | 0.1 | 0 | 0 | ||
| Paracoccus | 0 | 0 | 0.1 | 0 | 0 | 0 | ||
| Pseudohongiella | 0.1 | 0 | 0 | 0.3 | 0 | 0.1 | ||
| Pseudomonas | 5.3 | 3.5 | 4 | 3.2 | 2.5 | 3.3 | ||
| Psychrobacter | 0.8 | 0.5 | 0.8 | 0.2 | 35.5 | 29.9 | ||
| Salinarimonas | 0.5 | 0.2 | 0.3 | 0.8 | 0.3 | 0.3 | ||
| Stenotrophomonas | 0.1 | 0 | 0 | 0 | 0 | 0 | ||
| Thalassospira | 0.6 | 0.1 | 0.2 | 0.7 | 0.2 | 0.3 | ||
| Thiobacillus | 0.2 | 0.1 | 0 | 0 | 0 | 0 | ||
| Thiomicrorhabdus | 0.5 | 0 | 0.1 | 0.5 | 0 | 0 | ||
| Synergistota | Acetomicrobium | 0.4 | 0.1 | 0.2 | 1.6 | 0 | 0.3 | |
| Thermovirga | 0.4 | 0 | 0 | 0.5 | 0 | 0 | ||
| Thermotogota | Petrotoga | 0.6 | 0 | 0.2 | 1.9 | 0 | 0 | |
| Verrucomicrobiota | Cand. Protochlamydia | 0.1 | 0 | 0 | 0 | 0 | 0 | |
| Luteolibacter | 0 | 0.1 | 0 | 0.2 | 0 | 0 | ||
| Not Assigned | 17.2 | 10 | 12 | 17.8 | 6 | 7.2 | ||
| n = 0 | 0.1 < n < 1 | 1.1 < n < 5 | 5.1 < n < 10 | 10.1 < n < 20 | n > 20 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzaro, M.; Papale, M.; Rizzo, C.; Forte, E.; Lenaz, D.; Guglielmin, M.; Lo Giudice, A. Antarctic Salt-Cones: An Oasis of Microbial Life? The Example of Boulder Clay Glacier (Northern Victoria Land). Microorganisms 2022, 10, 1753. https://doi.org/10.3390/microorganisms10091753
Azzaro M, Papale M, Rizzo C, Forte E, Lenaz D, Guglielmin M, Lo Giudice A. Antarctic Salt-Cones: An Oasis of Microbial Life? The Example of Boulder Clay Glacier (Northern Victoria Land). Microorganisms. 2022; 10(9):1753. https://doi.org/10.3390/microorganisms10091753
Chicago/Turabian StyleAzzaro, Maurizio, Maria Papale, Carmen Rizzo, Emanuele Forte, Davide Lenaz, Mauro Guglielmin, and Angelina Lo Giudice. 2022. "Antarctic Salt-Cones: An Oasis of Microbial Life? The Example of Boulder Clay Glacier (Northern Victoria Land)" Microorganisms 10, no. 9: 1753. https://doi.org/10.3390/microorganisms10091753
APA StyleAzzaro, M., Papale, M., Rizzo, C., Forte, E., Lenaz, D., Guglielmin, M., & Lo Giudice, A. (2022). Antarctic Salt-Cones: An Oasis of Microbial Life? The Example of Boulder Clay Glacier (Northern Victoria Land). Microorganisms, 10(9), 1753. https://doi.org/10.3390/microorganisms10091753












