Soil Microbial Co-Occurrence Patterns under Controlled-Release Urea and Fulvic Acid Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Materials
2.2. The Soil Microbial Incubation Experiment
2.3. The Soil Column Leaching Experiment
2.4. The Pot Experiment
2.5. Chemical Analyses
2.6. Molecular Sequence Analysis
2.7. Soil, Water, and Yield Data Analyses
2.8. Soil Microbial Co-Occurrence Network Analysis
3. Results
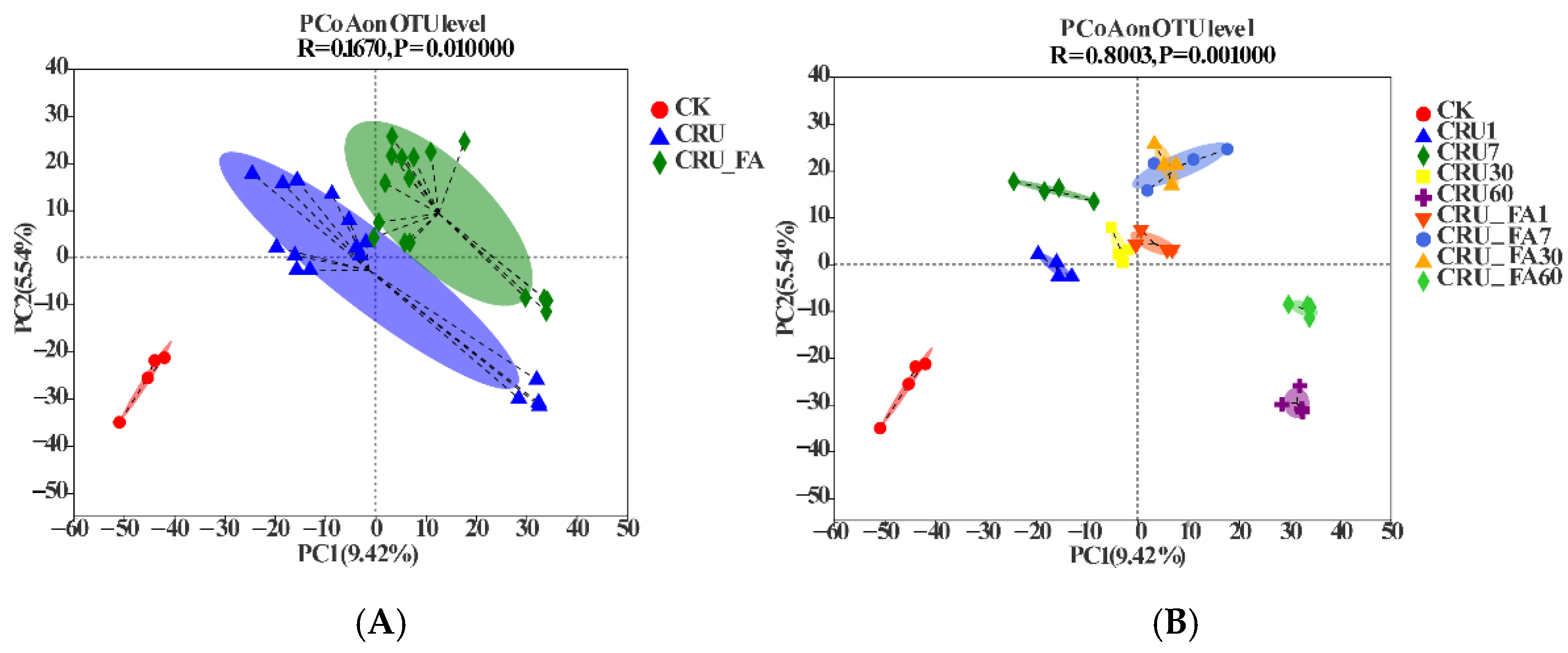
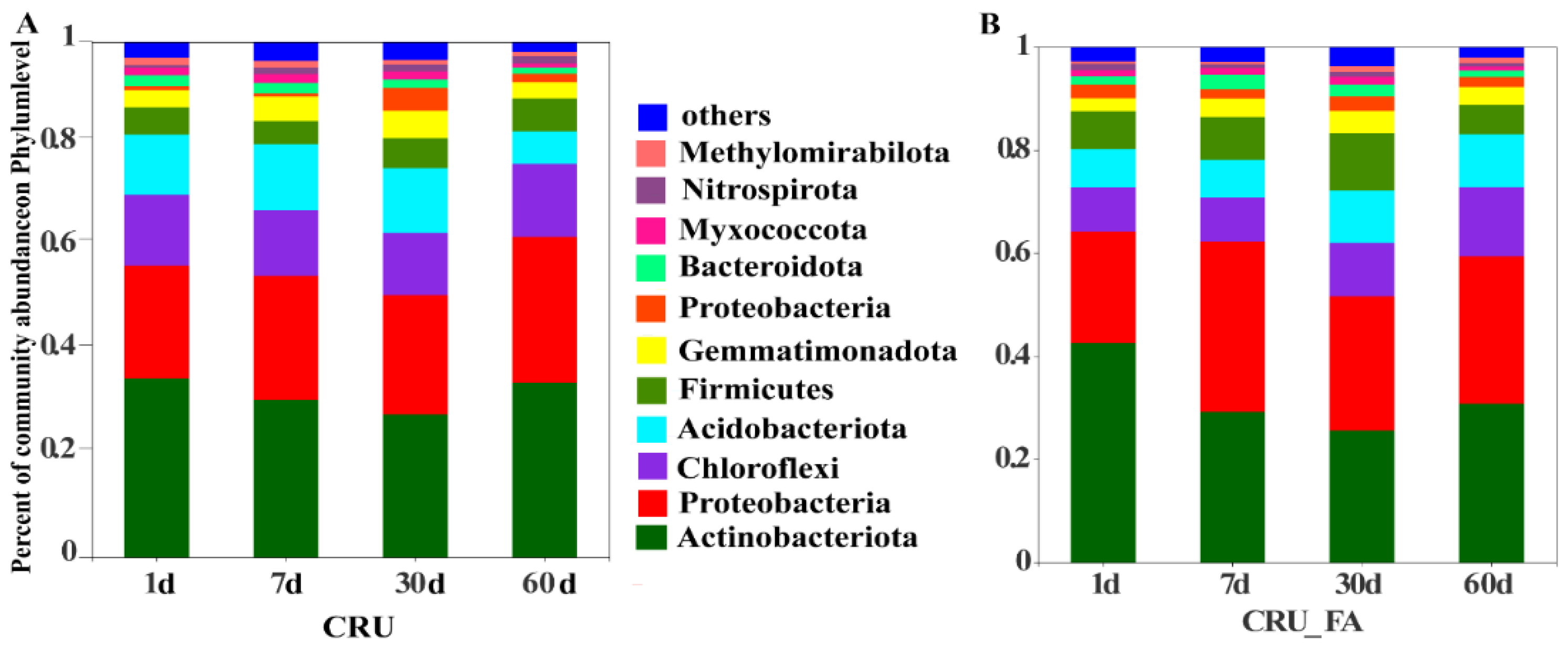
3.1. Effects of CRU with FA on Soil Bacterial Community Structure
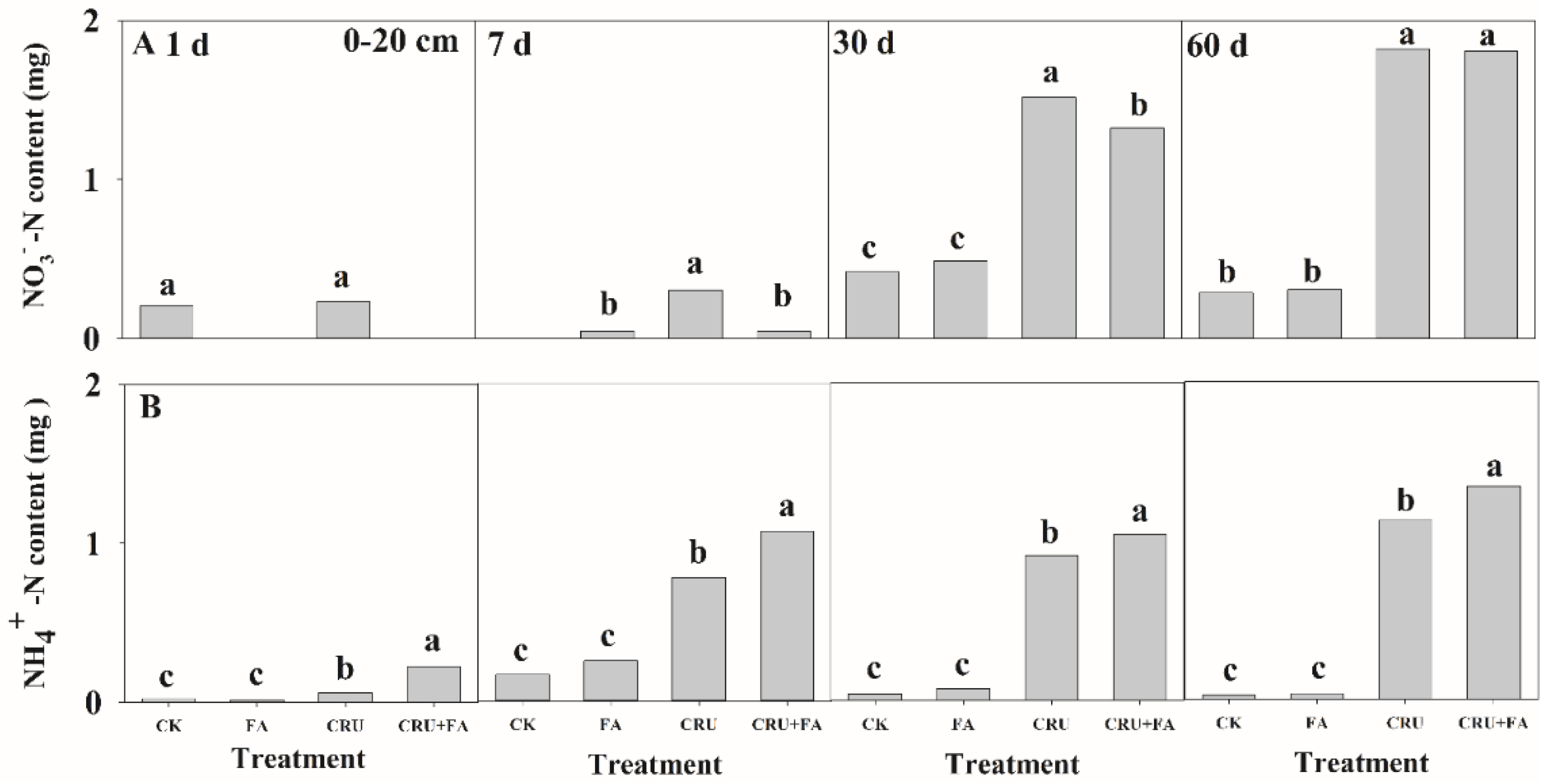
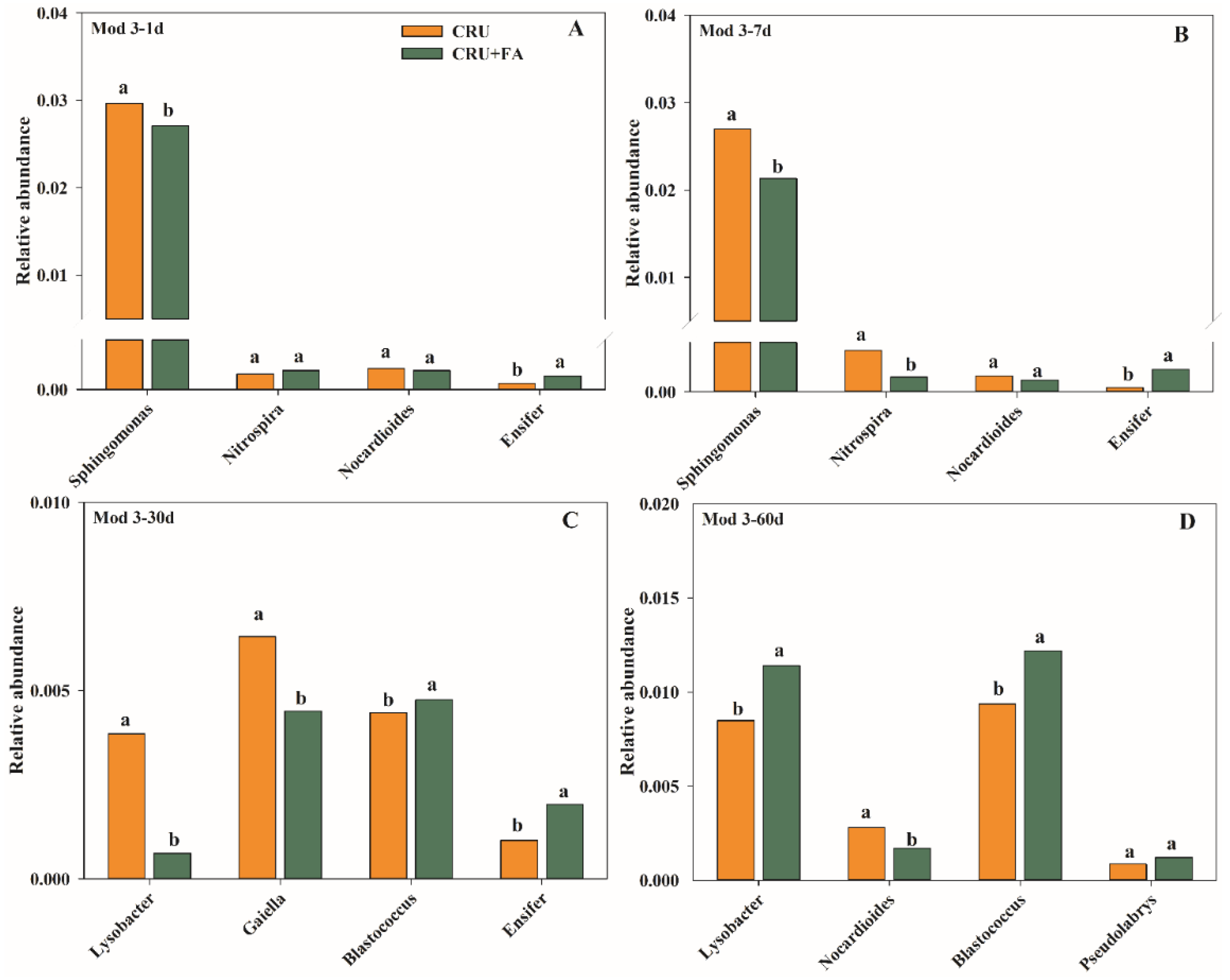
3.2. Microbial Taxa Associated with CRU Combined with FA Treatment on N Conversion
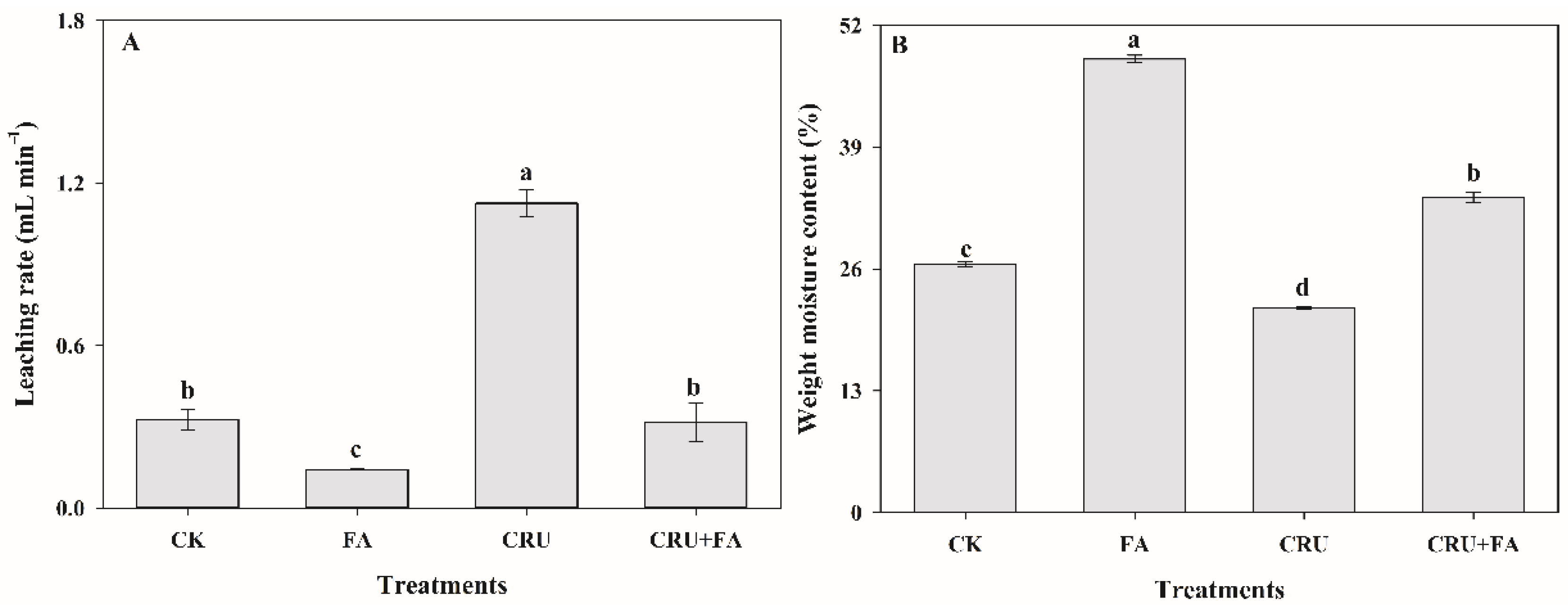
3.3. Application of Fulvic Acid Improved Soil Fertilizer Preserving
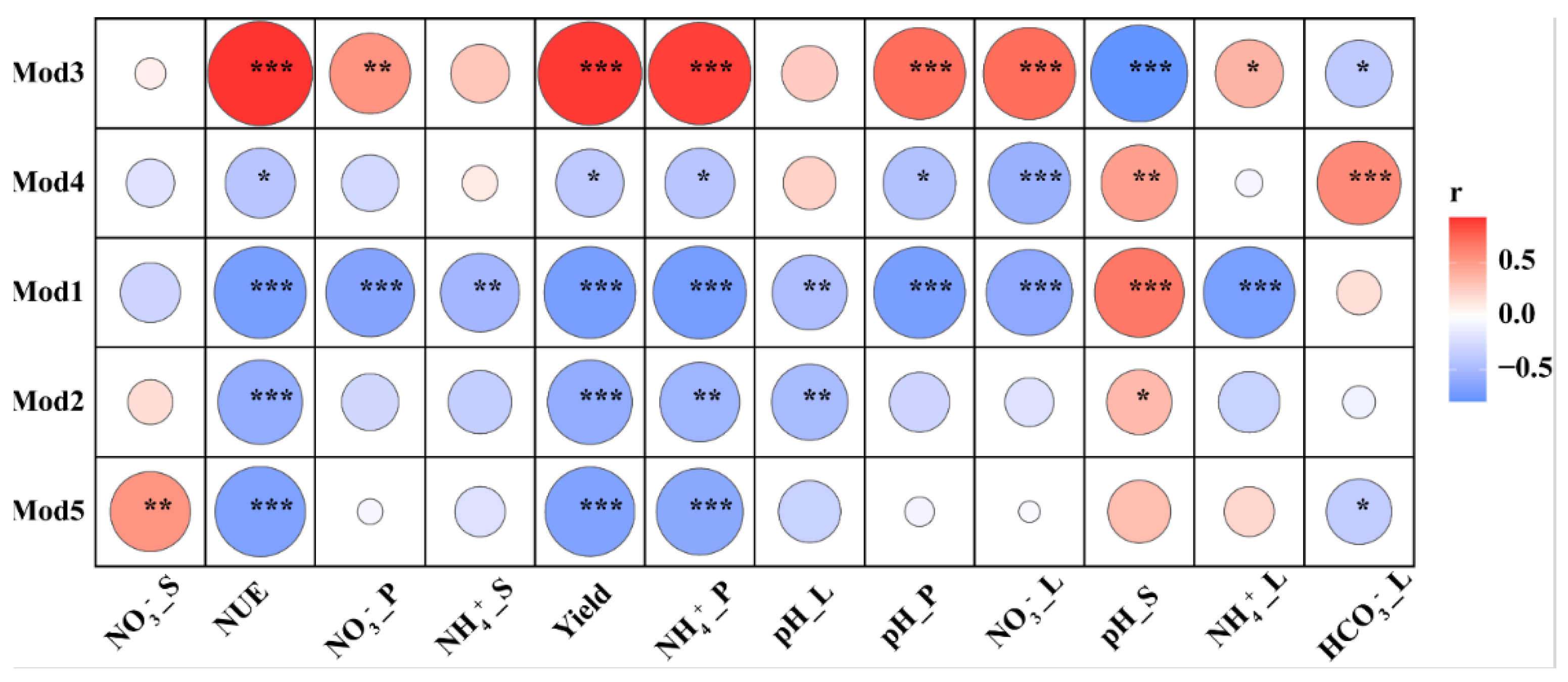
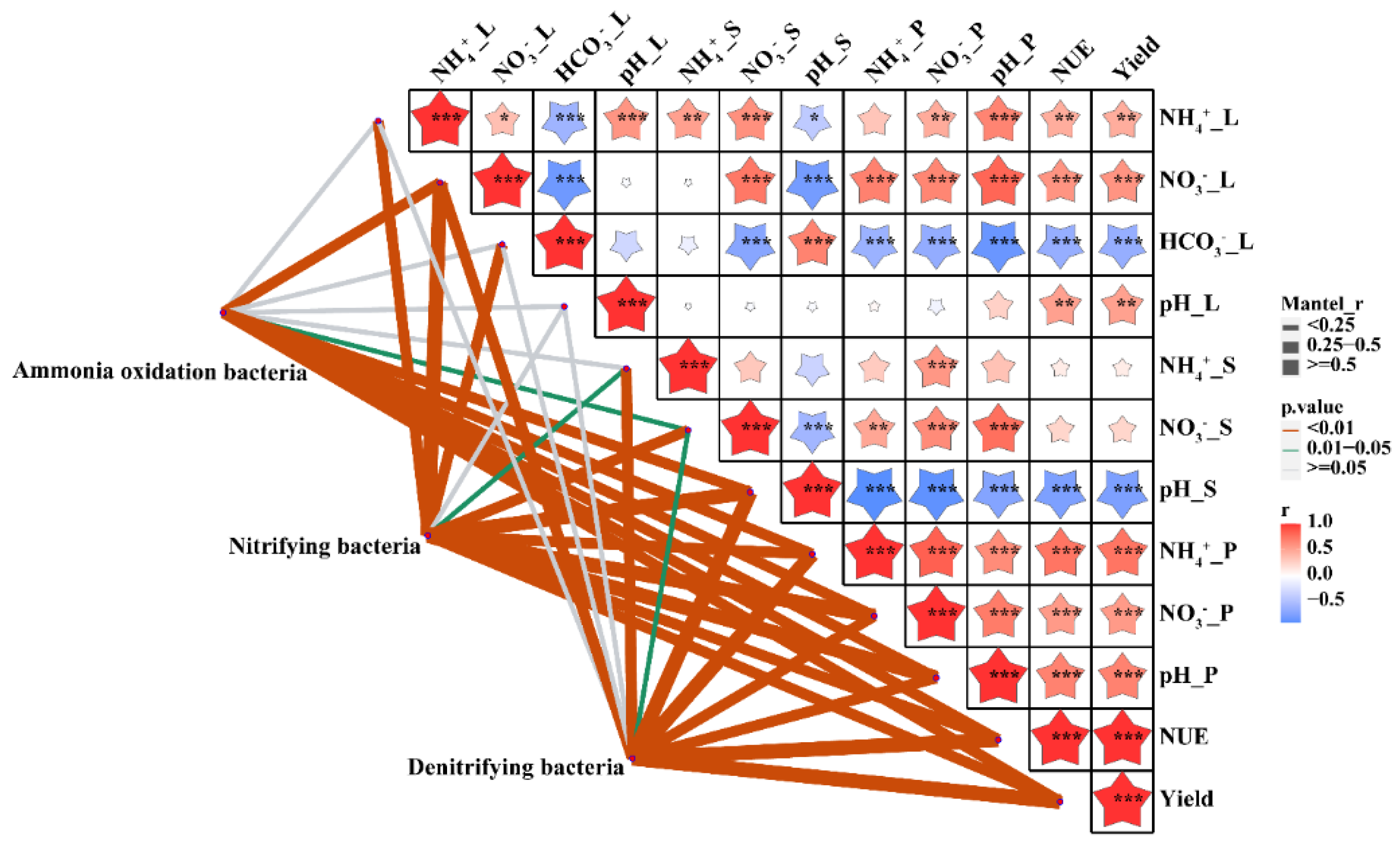
3.4. Environmental Drivers of Community Composition
3.5. Wheat Yield and Fertilizer Use Efficiency Affected by CRU Combined with FA
4. Discussion
4.1. Wheat Nitrogen Cycle Process Affected by CRU Combined with FA
4.2. The Soil Bacterial Communities Affected by CRU Combined with FA
4.3. Microbial Co-Occurrence Network Affected by CRU Combined with FA
4.4. The Soil Bacterial Community of Nitrogen Cycle Affected by CRU Combined with FA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gómez-Muñoz, B.; Magid, J.; Jensen, L.S. Nitrogen turnover, crop use efficiency and soil fertility in a long-term field experiment amended with different qualities of urban and agricultural waste. Agric. Ecosyst. Environ. 2017, 240, 300–313. [Google Scholar] [CrossRef]
- Cai, S.; Pittelkow, C.M.; Zhao, X.; Wang, S. Winter legume-rice rotations can reduce nitrogen pollution and carbon footprint while maintaining net ecosystem economic benefits. J. Clean. Prod. 2018, 195, 289–300. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Q.; Gao, F.; Meng, Q.; Li, M.; Zhang, Y.; Zhang, P.; Zhang, M.; Liu, Z. Controlled-release urea combined with fulvic acid enhanced carbon/nitrogen metabolic processes and maize growth. J. Sci. Food Agric. 2021, 102, 3644–3654. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Fan, X.; Tang, S.; Zhang, M.; Huang, X.; Yi, Q.; Pang, Y.; Huang, J. Seasonal differences in N release dynamic of controlled-released urea in paddy field and its impact on the growth of rice under double rice cropping system. Soil Tillage Res. 2019, 195, 104371. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, T.; Yang, Y.; Li, Y.C.; Wan, Y.; Zhang, M.; Tang, Y.; Allen, S.C. Controlled-release urea reduced nitrogen leaching and improved nitrogen use efficiency and yield of direct-seeded rice. J. Environ. Manag. 2018, 220, 191–197. [Google Scholar] [CrossRef]
- Zhao, C.; Tian, H.; Zhang, Q.; Liu, Z.; Zhang, M.; Wang, J. Preparation of urea-containing starch-castor oil superabsorbent polyurethane coated urea and investigation of controlled nitrogen release. Carbohydr. Polym. 2020, 253, 117240. [Google Scholar] [CrossRef]
- Farzadnia, S.; Nimmagadda, R.D.; McRae, C. A comparative structural study of nitrogen-rich fulvic acids from various Antarctic lakes. Environ. Chem. 2017, 14, 502–514. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Rocco, C.; Cenvinzo, V.; Agrelli, D.; Gioia, L.; Di Mola, I.; Adamo, P.; Pepe, O.; Fagnano, M. Giant reed growth and effects on soil biological fertility in assisted phytoremediation of an industrial polluted soil. Sci. Total Environ. 2017, 575, 1375–1383. [Google Scholar] [CrossRef]
- Piccolo, A. Humic Substances in Terrestrial Ecosystems; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Fang, Z.; Wang, X.; Zhang, X.; Zhao, D.; Tao, J. Effects of fulvic acid on the photosynthetic and physiological characteristics of Paeonia ostii under drought stress. Plant Signal. Behav. 2020, 15, 1774714. [Google Scholar] [CrossRef]
- Yang, W.; Li, P.; Guo, S.; Fan, B.; Song, R.; Zhang, J.; Yu, J. Compensating effect of fulvic acid and super-absorbent polymer on leaf gas exchange and water use efficiency of maize under moderate water deficit conditions. Plant Growth Regul. 2017, 83, 351–360. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Zheng, J.; Shen, Z.; Xu, X. Exogenous foliar application of fulvic acid alleviate cadmium toxicity in lettuce (Lactuca sativa L.). Ecotoxicol. Environ. Saf. 2018, 167, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Elmongy, M.S.; Zhou, H.; Cao, Y.; Liu, B.; Xia, Y. The effect of humic acid on endogenous hormone levels and antioxidant enzyme activity during in vitro rooting of evergreen azalea. Sci. Hortic. 2018, 227, 234–243. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, S.; Luo, C.; Zhu, X.; Kardol, P.; Zhang, Z.; Li, Y.; Wang, C.; Wang, Y.; Jones, D. Effects of warming and grazing on dissolved organic nitrogen in a Tibetan alpine meadow ecosystem. Soil Tillage Res. 2016, 158, 156–164. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Yang, Y.; Gao, B.; Wan, Y.; Li, Y.C.; Cheng, D. Activated-Lignite-Based Super Large Granular Slow-Release Fertilizers Improve Apple Tree Growth: Synthesis, Characterizations, and Laboratory and Field Evaluations. J. Agric. Food Chem. 2017, 65, 5879–5889. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Z.; Zhang, M.; Chen, Q.; Zheng, L.; Li, Y.C.; Sun, L. The combined application of controlled-release urea and fulvic acid improved the soil nutrient supply and maize yield. Arch. Agron. Soil Sci. 2020, 67, 633–646. [Google Scholar] [CrossRef]
- Guo, L.; Ning, T.; Nie, L.; Li, Z.; Lal, R. Interaction of deep placed controlled-release urea and water retention agent on nitrogen and water use and maize yield. Eur. J. Agron. 2016, 75, 118–129. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, Z.; Zhang, M.; Shi, Y.; Zhu, Q.; Sun, Y.; Zhou, H.; Li, C.; Yang, Y.; Geng, J. Improving crop yields, nitrogen use efficiencies, and profits by using mixtures of coated controlled-released and uncoated urea in a wheat-maize system. Field Crops Res. 2017, 205, 106–115. [Google Scholar] [CrossRef]
- Dai, M.; Chen, Q.; Hu, Z.; Li, X.; Zhang, J.; Wang, J.; Feng, H. Effects of Humic Acid and PASP Compound Fertilizer Application on Growth, Quality and Yield of Broccoli. J. Agric. Sci. Technol. 2016, 17, 535–538. (In Chinese) [Google Scholar] [CrossRef]
- Maji, D.; Misra, P.; Singh, S.; Kalra, A. Humic acid rich vermicompost promotes plant growth by improving microbial community structure of soil as well as root nodulation and mycorrhizal colonization in the roots of Pisum sativum. Appl. Soil Ecol. 2017, 110, 97–108. [Google Scholar] [CrossRef]
- Sorrenti, G.; Toselli, M. Soil leaching as affected by the amendment with biochar and compost. Agric. Ecosyst. Environ. 2016, 226, 56–64. [Google Scholar] [CrossRef]
- Liao, R.; Zhang, S.; Zhang, X.; Wang, M.; Wu, H.; Zhangzhong, L. Development of smart irrigation systems based on real-time soil moisture data in a greenhouse: Proof of concept. Agric. Water Manag. 2020, 245, 106632. [Google Scholar] [CrossRef]
- Douglas, L.A.; Riazi, A.; Smith, C.J. A Semi-Micro Method for Determining Total Nitrogen in Soils and Plant Material Containing Nitrite and Nitrate. Soil Sci. Soc. Am. J. 1980, 44, 431–433. [Google Scholar] [CrossRef]
- Devkota, M.; Martius, C.; Lamers, J.; Sayre, K.; Devkota, K.; Vlek, P. Tillage and nitrogen fertilization effects on yield and nitrogen use efficiency of irrigated cotton. Soil Tillage Res. 2013, 134, 72–82. [Google Scholar] [CrossRef]
- Javanmard, A.; Montanari, A. Online rules for control of false discovery rate and false discovery exceedance. Ann. Stat. 2018, 46, 526–554. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Zheng, M.; Jiang, L.; Luo, Y. Patterns and mechanisms of responses by soil microbial communities to nitrogen addition. Soil Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Yun, L.; Yu, Z.; Li, Y.; Luo, P.; Jiang, X.; Tian, Y.; Ding, X. Ammonia nitrogen and nitrite removal by a heterotrophic Sphingomonas sp. strain LPN080 and its potential application in aquaculture. Aquaculture 2018, 500, 477–484. [Google Scholar] [CrossRef]
- Huang, F.; Pan, L.; He, Z.; Zhang, M.; Zhang, M. Identification, interactions, nitrogen removal pathways and performances of culturable heterotrophic nitrification-aerobic denitrification bacteria from mariculture water by using cell culture and metagenomics. Sci. Total Environ. 2020, 732, 139268. [Google Scholar] [CrossRef]
- Ying, J.; Li, X.; Wang, N.; Lan, Z.; He, J.; Bai, Y. Contrasting effects of nitrogen forms and soil pH on ammonia oxidizing microorganisms and their responses to long-term nitrogen fertilization in a typical steppe ecosystem. Soil Biol. Biochem. 2017, 107, 10–18. [Google Scholar] [CrossRef]
- Mou, S.; Zhang, Y.; Li, G.; Li, H.; Liang, Y.; Tang, L.; Tao, J.; Xu, J.; Li, J.; Zhang, C.; et al. Effects of elevated CO2 and nitrogen supply on the growth and photosynthetic physiology of a marine cyanobacterium, Synechococcus sp. PCC7002. J. Appl. Phycol. 2017, 29, 1755–1763. [Google Scholar] [CrossRef]
- Baskaran, V.; Patil, P.K.; Antony, M.L.; Avunje, S.; Nagaraju, V.T.; Ghate, S.D.; Nathamuni, S.; Dineshkumar, N.; Alavandi, S.V.; Vijayan, K.K. Microbial community profiling of ammonia and nitrite oxidizing bacterial enrichments from brackishwater ecosystems for mitigating nitrogen species. Sci. Rep. 2020, 10, 5201–5211. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Peng, Y.; Liu, Y.; Peng, Y. Research progress and prospects of complete ammonia oxidizing bacteria in wastewater treatment. Front. Environ. Sci. Eng. 2022, 16, 123. [Google Scholar] [CrossRef]
- Swelum, A.A.; El-Saadony, M.T.; El-Hack, M.E.A.; Ghanima, M.M.A.; Shukry, M.; Alhotan, R.A.; Hussein, E.O.; Suliman, G.M.; Ba-Awadh, H.; Ammari, A.A.; et al. Ammonia emissions in poultry houses and microbial nitrification as a promising reduction strategy. Sci. Total Environ. 2021, 781, 146978. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Liang, W.; Liu, S. Rhizosphere Microbiome: The Emerging Barrier in Plant-Pathogen Interactions. Front. Microbiol. 2021, 12, 772420. [Google Scholar] [CrossRef] [PubMed]
- Siebielec, S.; Siebielec, G.; Klimkowicz-Pawlas, A.; Gałązka, A.; Grządziel, J.; Stuczyński, T. Impact of Water Stress on Microbial Community and Activity in Sandy and Loamy Soils. Agronomy 2020, 10, 1429. [Google Scholar] [CrossRef]
- Zhai, W.; Dai, Y.; Zhao, W.; Yuan, H.; Qiu, D.; Chen, J.; Gustave, W.; Maguffin, S.C.; Chen, Z.; Liu, X.; et al. Simultaneous immobilization of the cadmium, lead and arsenic in paddy soils amended with titanium gypsum. Environ. Pollut. 2019, 258, 113790. [Google Scholar] [CrossRef]
- Wang, Y.; Tabassum, S.; Li, J. Effects of fulvic acid on the denitrification performance of completely autotrophic nitrogen removal over nitrite (CANON) process for the treatment of landfill leachate. Clean. Eng. Technol. 2021, 4, 100173. [Google Scholar] [CrossRef]
- Lu, M.; Shi, X.; Feng, Q.; Li, X.; Lian, S.; Zhang, M.; Guo, R. Effects of humic acid modified oyster shell addition on lignocellulose degradation and nitrogen transformation during digestate composting. Bioresour. Technol. 2021, 329, 124834. [Google Scholar] [CrossRef]
- Rangel, F.; Rosso, P.; Zaghouani, W.; Charfi, A. Fine-grained analysis of language varieties and demographics. Nat. Lang. Eng. 2020, 26, 641–661. [Google Scholar] [CrossRef]
- Wongdee, J.; Boonkerd, N.; Teaumroong, N.; Tittabutr, P.; Giraud, E. Regulation of Nitrogen Fixation in Bradyrhizobium sp. Strain DOA9 Involves Two Distinct NifA Regulatory Proteins That Are Functionally Redundant During Symbiosis but Not During Free-Living Growth. Front. Microbiol. 2018, 9, 1644. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, S.; Zhao, X.; Long, H. Stable water and fertilizer supply by negative pressure irrigation improve tomato production and soil bacterial communities. SN Appl. Sci. 2019, 1, 718. [Google Scholar] [CrossRef]
- Zhao, Y.-X.; Guo, L.-L.; Sun, S.-L.; Guo, J.-J.; Dai, Y.-J. Bioconversion of indole-3-acetonitrile by the N2-fixing bacterium Ensifer meliloti CGMCC 7333 and its Escherichia coli-expressed nitrile hydratase. Int. Microbiol. 2019, 23, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Oh, J.-M.; Chun, S.; Park, H.Y.; Im, W.-T. Enzymatic Biotransformation of Ginsenoside Rb2 into Rd by Recombinant α-L-Arabinopyranosidase from Blastococcus saxobsidens. J. Microbiol. Biotechnol. 2020, 30, 391–397. [Google Scholar] [CrossRef] [PubMed]


| Treatment a | Grain Yield (g pot−1) b | Aboveground Biomass (g pot−1) b | 1000-Grain Weight (g) b | Seeds per Spike b | Spike No. per Plant b | Yield Increase (%) c |
|---|---|---|---|---|---|---|
| Control | 37.6 c | 82.6 c | 53.6 b | 21.7 b | 39.0 b | −53.9 |
| CRU | 81.6 b | 168.8 b | 47.1 c | 30.2 a | 71.5 a | - |
| CRU+FA | 99.6 a | 196.2 a | 59.3 a | 32.8 a | 65.0 a | 22.1 ** |
| Treatment a | NUE (%) b | Change (%) c | NAE (kg kg−1) b | NPE (kg kg−1) b |
|---|---|---|---|---|
| Control | - | - | - | - |
| CRU | 35.4 b | - | 7.0 b | 13. 0 b |
| CRU+FA | 50.0 a | 41.2 * | 9.9 a | 15.9 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhang, K.; Qiu, L.; Ding, S.; Wang, H.; Liu, Z.; Zhang, M.; Wei, Z. Soil Microbial Co-Occurrence Patterns under Controlled-Release Urea and Fulvic Acid Applications. Microorganisms 2022, 10, 1823. https://doi.org/10.3390/microorganisms10091823
Li Z, Zhang K, Qiu L, Ding S, Wang H, Liu Z, Zhang M, Wei Z. Soil Microbial Co-Occurrence Patterns under Controlled-Release Urea and Fulvic Acid Applications. Microorganisms. 2022; 10(9):1823. https://doi.org/10.3390/microorganisms10091823
Chicago/Turabian StyleLi, Zeli, Kexin Zhang, Lixue Qiu, Shaowu Ding, Huaili Wang, Zhiguang Liu, Min Zhang, and Zhanbo Wei. 2022. "Soil Microbial Co-Occurrence Patterns under Controlled-Release Urea and Fulvic Acid Applications" Microorganisms 10, no. 9: 1823. https://doi.org/10.3390/microorganisms10091823





