Characteristics of the Gut Bacterial Composition in People of Different Nationalities and Religions
Abstract
1. Introduction
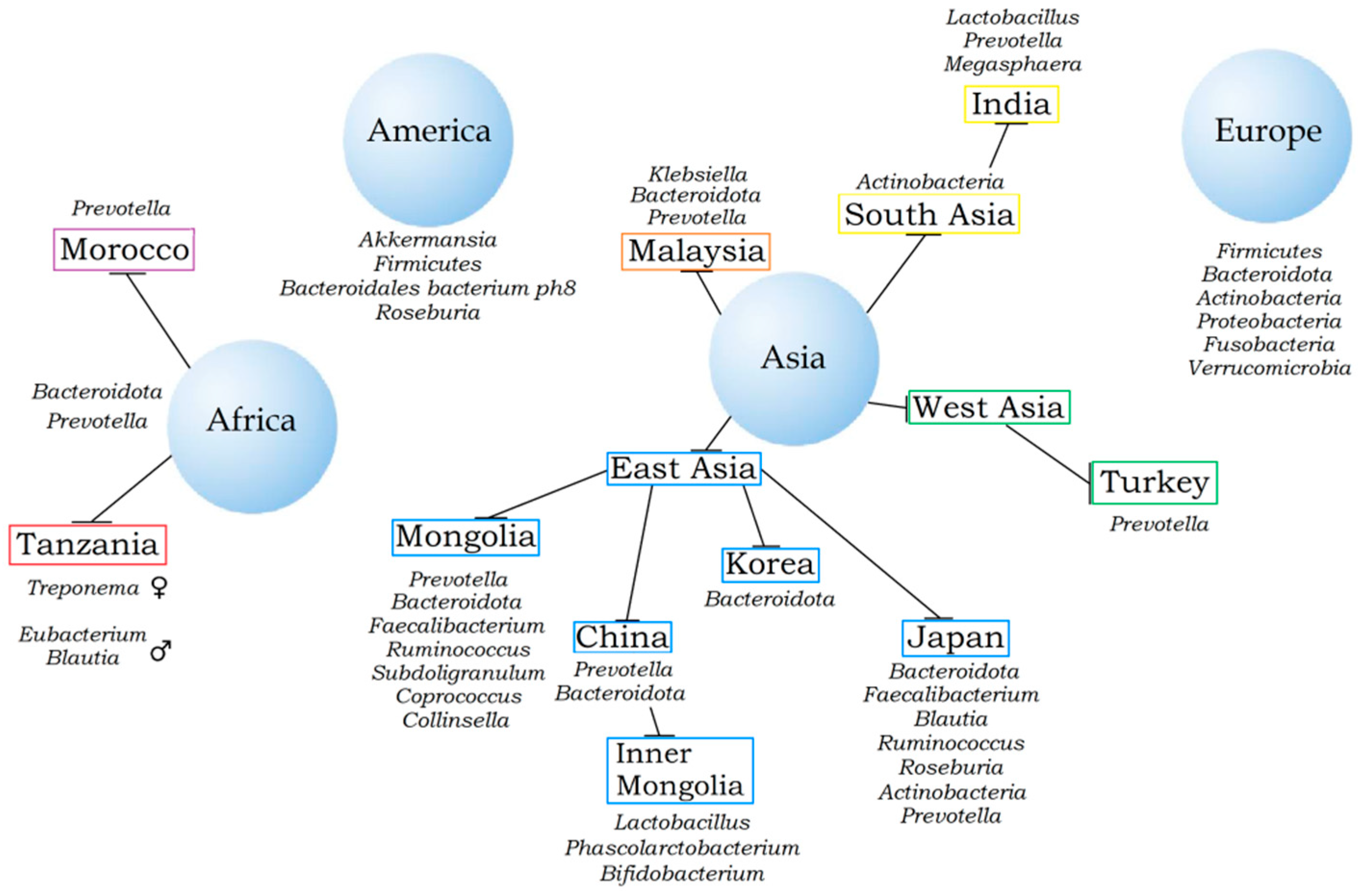
2. Nationality and Gut Microbiota
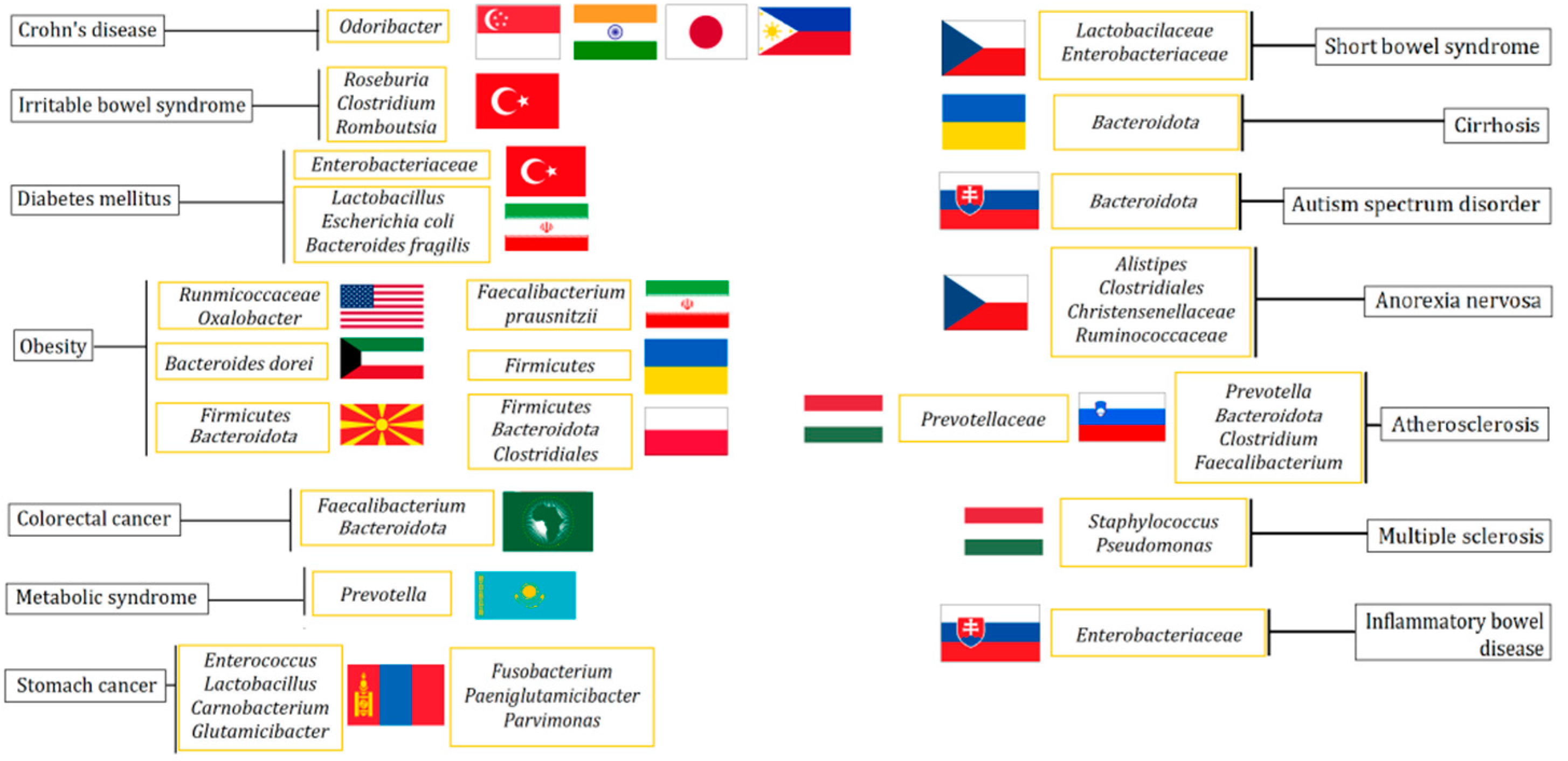
3. Gut Microbiota and Diseases of People of Different Nationalities
4. Religion and Gut Microbiota
5. Gut Microbiota of Slavic Peoples with and without Various Diseases
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, I.; Corwin, E.J.; Brennan, P.A.; Jordan, S.; Murphy, J.R.; Dunlop, A. The Infant Microbiome: Implications for Infant Health and Neurocognitive Development. Nurs. Res. 2016, 65, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Alvarez, A.-S.; de Vos, W.M. The Gut Microbiota in the First Decade of Life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Quin, C.; Gibson, D.L. Human behavior, not race or geography, is the strongest predictor of microbial succession in the gut bacteriome of infants. Gut Microbes 2020, 11, 1143–1171. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Milani, C.; Duranti, S.; Lugli, G.A.; Bernasconi, S.; Margolles, A.; Di Pierro, F.; van Sinderen, D.; Ventura, M. The infant gut microbiome as a microbial organ influencing host well-being. Ital. J. Pediatr. 2020, 46, 16. [Google Scholar] [CrossRef]
- Guinane, C.M.; Cotter, P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef]
- Kashtanova, D.A.; Tkacheva, O.N.; Popenko, A.S.; Tyakht, A.V.; Alekseev, D.G.; Kotovskaya, Y.V.; Boytsov, S.A. The composition of the intestinal microbiota and its relationship with risk factors for cardiovascular diseases among relatively healthy residents of Moscow and the Moscow region. Cardiovasc. Ther. Prev. 2017, 16, 56–61. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- Shanahan, F.; Ghosh, T.S.; O’Toole, P.W. The Healthy Microbiome-What Is the Definition of a Healthy Gut Microbiome? Gastroenterology 2021, 160, 483–494. [Google Scholar] [CrossRef]
- Glenny, E.M.; Bulik-Sullivan, E.C.; Tang, Q.; Bulik, C.M.; Carroll, I.M. Eating Disorders and the Intestinal Microbiota: Mechanisms of Energy Homeostasis and Behavioral Influence. Curr. Psychiatry Rep. 2017, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- Breton, J.; Déchelotte, P.; Ribet, D. Intestinal microbiota and Anorexia Nervosa. Clin. Nutr. Exp. 2019, 28, 11–21. [Google Scholar] [CrossRef]
- Kazemian, N.; Mahmoudi, M.; Halperin, F.; Wu, J.C.; Pakpour, S. Gut microbiota and cardiovascular disease: Opportunities and challenges. Microbiome 2020, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Ghenciulescu, A.; Park, R.J.; Burnet, P.W.J. The Gut Microbiome in Anorexia Nervosa: Friend or Foe? Front. Psychiatry 2021, 11, 611677. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, S.; Jiang, X.; Feng, C.; Gong, S.; Ma, J.; Fang, Z.; Yin, J.; Yin, Y. Gut Microbiota and Diarrhea: An Updated Review. Front. Cell. Infect. Microbiol. 2021, 11, 625210. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Gerner, R.R.; Moschen, A.R. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell 2018, 33, 954–964. [Google Scholar] [CrossRef]
- Royston, K.J.; Adedokun, B.; Olopade, O.I. Race, the microbiome and colorectal cancer. World J. Gastrointest. Oncol. 2019, 11, 773–787. [Google Scholar] [CrossRef]
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers 2019, 11, 38. [Google Scholar] [CrossRef]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef]
- Sampsell, K.; Hao, D.; Reimer, R.A. The Gut Microbiota: A Potential Gateway to Improved Health Outcomes in Breast Cancer Treatment and Survivorship. Int. J. Mol. Sci. 2020, 21, 9239. [Google Scholar] [CrossRef]
- Fujita, K.; Matsushita, M.; Banno, E.; De Velasco, M.A.; Hatano, K.; Nonomura, N.; Uemura, H. Gut microbiome and prostate cancer. Int. J. Urol. 2022, 6. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.D. The Gut Microbiome and Its Role in Obesity. Nutr. Today 2016, 51, 67–174. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A. Gut microbiota: A new path to treat obesity. Int. J. Obes. Suppl. 2019, 9, 10–19. [Google Scholar] [CrossRef]
- Aoun, A.; Darwish, F.; Hamod, N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev. Nutr. Food. Sci. 2020, 25, 113–123. [Google Scholar] [CrossRef]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef]
- Redondo-Useros, N.; Nova, E.; González-Zancada, N.; Díaz, L.E.; Gómez-Martínez, S.; Marcos, A. Microbiota and Lifestyle: A Special Focus on Diet. Nutrients 2020, 12, 1776. [Google Scholar] [CrossRef]
- Leeming, E.R.; Louca, P.; Gibson, R.; Menni, C.; Spector, T.D.; Le Roy, C.I. The complexities of the diet-microbiome relationship: Advances and perspectives. Genome Med. 2021, 13, 10. [Google Scholar] [CrossRef]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.N.; Baedke, J. Does the human microbiome tell us something about race? Humanit. Soc. Sci. Commun. 2021, 8, 97. [Google Scholar] [CrossRef]
- Byrd, D.A.; Carson, T.L.; Williams, F.; Vogtmann, E. Elucidating the role of the gastrointestinal microbiota in racial and ethnic health disparities. Genome Biol. 2020, 21, 192. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.W.; Priya, S.; Blekhman, R.; Bordenstein, S.R. Gut microbiota diversity across ethnicities in the United States. PLoS Biol. 2018, 16, e2006842. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.R.; Davenport, E.R.; Gautam, Y.; Bhandari, D.; Tandukar, S.; Ng, K.M.; Fragiadakis, G.K.; Holmes, S.; Gautam, G.P.; Leach, J.; et al. Gut microbiome transition across a lifestyle gradient in Himalaya. PLOS Biol. 2018, 16, e2005396. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, Z.; Yuan, F.; Chan, R.; Cooper, G.; Li, L. Race, Neighborhood Socioeconomic Status, and Gut Microbiome. Cancer Epidemiol. Biomark. Prev. 2020, 29, 695–696. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Selvaraju, V.; Chen, J.; Ayine, P.; Yang, L.; Babu, J.R.; Geetha, T.; Taneja, V. Ethnic variability associating gut and oral microbiome with obesity in children. Gut Microbes 2021, 13, 1882926. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Guo, Z.; Qiao, J.; Gesudu, Q.; Sun, Z.; Huo, D.; Huang, W.; Huo, Q.; Kwok, L.; et al. The diversity of intestinal microbiota of Mongolians living in Inner Mongolia, China. Benef. Microbes 2013, 4, 319–328. [Google Scholar] [CrossRef]
- Chong, C.W.; Ahmad, A.F.; Lim, Y.A.L.; Teh, C.S.J.; Yap, I.K.S.; Lee, S.C.; Chin, Y.T.; Loke, P.; Chua, K.H. Effect of ethnicity and socioeconomic variation to the gut microbiota composition among pre-adolescent in Malaysia. Sci. Rep. 2015, 5, 13338. [Google Scholar] [CrossRef]
- Dwiyanto, J.; Hussain, M.H.; Reidpath, D.; Ong, K.S.; Qasim, A.; Lee, S.W.H.; Lee, S.M.; Foo, S.C.; Chong, C.W.; Rahman, S. Ethnicity influences the gut microbiota of individuals sharing a geographical location: A cross-sectional study from a middle-income country. Sci. Rep. 2021, 11, 2618. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.H.; Deng, J.; Ang, D.S.W.; Hsiang, J.C.; Lee, L.S.; Aazmi, S.; Mohamed, E.H.M.; Yang, H.; Yap, S.Y.; Teh, L.; et al. Effects of proton pump inhibitor on the human gut microbiome profile in multi-ethnic groups in Singapore. Singap. Med. J. 2019, 60, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lawley, B.; Wong, G.; Otal, A.; Chen, L.; Ying, T.J.; Lin, X.; Pang, W.W.; Yap, F.; Chong, Y.S.; et al. Ethnic diversity in infant gut microbiota is apparent before the introduction of complementary diets. Gut Microbes 2020, 11, 1362–1373. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Inoue, R.; Oshima, A.; Sakazume, H.; Ogawa, K.; Tominaga, T.; Mihara, Y.; Sugaya, T.; Mizushima, K.; Uchiyama, K.; et al. Typing of the Gut Microbiota Community in Japanese Subjects. Microorganisms 2022, 10, 664. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Li, X.H.; Chen, W.N. Similarities and differences in gut microbiome composition correlate with dietary patterns of Indian and Chinese adults. AMB Express 2018, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Yamashiro, Y. Gut Microbiota Composition in Healthy Japanese Infants and Young Adults Born by C-Section. Ann. Nutr. Metab. 2018, 73, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zuo, T.; Cheung, C.P.; Gu, W.; Wan, Y.; Zhang, F.; Chen, N.; Zhan, H.; Yeoh, Y.K.; Niu, J.; et al. Population-Level Configurations of Gut Mycobiome Across 6 Ethnicities in Urban and Rural China. Gastroenterology 2021, 160, 272–286.e11. [Google Scholar] [CrossRef]
- Gaulke, C.A.; Sharpton, T.J. The influence of ethnicity and geography on human gut microbiome composition. Nat. Med. 2018, 24, 1495–1496. [Google Scholar] [CrossRef]
- Deschasaux, M.; Bouter, K.E.; Prodan, A.; Levin, E.; Groen, A.K.; Herrema, H.; Tremaroli, V.; Bakker, G.J.; Attaye, I.; Pinto-Sietsma, S.J.; et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 2018, 24, 1526–1531. [Google Scholar] [CrossRef]
- Ang, Q.Y.; Alba, D.L.; Upadhyay, V.; Bisanz, J.E.; Cai, J.; Lee, H.L.; Barajas, E.; Wei, G.; Noecker, C.; Patterson, A.D.; et al. The East Asian gut microbiome is distinct from colocalized White subjects and connected to metabolic health. eLife 2021, 10, e70349. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Morton, E.R.; Lynch, J.; Froment, A.; Lafosse, S.; Heyer, E.; Przeworski, M.; Blekhman, R.; Ségurel, L. Variation in rural African gut microbiota is strongly correlated with colonization by Entamoeba and subsistence. PLOS Genet. 2015, 1, e1005658. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Obregon-Tito, A.J.; Tito, R.Y.; Metcalf, J.; Sankaranarayanan, K.; Clemente, J.C.; Ursell, L.K.; Zech Xu, Z.; Van Treuren, W.; Knight, R.; Gaffney, P.M.; et al. Subsistence strategies in traditional societies distinguish gut microbiomes. Nat. Commun. 2015, 6, 6505. [Google Scholar] [CrossRef]
- Senghor, B.; Sokhna, C.; Ruimyc, R.; Lagier, J.-C. Gut microbiota diversity according to dietary habits and geographical provenance. Hum. Microbiome J. 2018, 7–8, 1–9. [Google Scholar] [CrossRef]
- Nam, Y.-D.; Jung, M.-J.; Roh, S.W.; Kim, M.-S.; Bae, J.-W. Comparative Analysis of Korean Human Gut Microbiota by Barcoded Pyrosequencing. PLoS ONE 2011, 6, e22109. [Google Scholar] [CrossRef]
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.W.; Hirose, Y.; Morita, H.; Hattori, M. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016, 23, 125–133. [Google Scholar] [CrossRef]
- Pareek, S.; Kurakawa, T.; Das, B.; Motooka, D.; Nakaya, S.; Rongsen-Chandola, T.; Goyal, N.; Kayama, H.; Dodd, D.; Okumura, R.; et al. Comparison of Japanese and Indian intestinal microbiota shows diet-dependent interaction between bacteria and fungi. npj Biofilms Microbiomes 2019, 5, 37. [Google Scholar] [CrossRef]
- Park, J.; Kato, K.; Murakami, H.; Hosomi, K.; Tanisawa, K.; Nakagata, T.; Ohno, H.; Konishi, K.; Kawashima, H.; Chen, Y.-A.; et al. Comprehensive Analysis of Gut Microbiota of a Healthy Population and Covariates Affecting Microbial Variation in Two Large Japanese Cohorts. Res. Sq. 2020, 20. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Z.; Lim, A.A.Q.; Zheng, Y.; Koh, E.Y.; Ho, D.; Qiao, J.; Huo, D.; Hou, Q.; Huang, W.; et al. Mongolians core gut microbiota and its correlation with seasonal dietary changes. Sci. Rep. 2014, 4, 5001. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, J.; Wu, C.; Cai, S.; Huang, W.; Chen, J.; Xi, X.; Liang, Z.; Hou, Q.; Zhou, B.; et al. Unique Features of Ethnic Mongolian Gut Microbiome revealed by metagenomic analysis. Sci. Rep. 2016, 6, 34826. [Google Scholar] [CrossRef]
- Li, J.; Xu, H.; Sun, Z.; Hou, Q.; Kwok, L.-Y.; Laga, W.; Wang, Y.; Ma, H.; Yu, Z.; Menghe, B.; et al. Effect of dietary interventions on the intestinal microbiota of Mongolian hosts. Sci. Bull. 2016, 61, 1605–1614. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Hou, Q.; Zhang, J.; Xu, H.; Sun, Z.; Menghe, B.; Zhang, H. Carbohydrate Staple Food Modulates Gut Microbiota of Mongolians in China. Front. Microbiol. 2017, 8, 484. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Y.H.; Huang, T.; Cai, Y.-D. Gene expression profiling gut microbiota in different races of humans. Sci. Rep. 2016, 6, 23075. [Google Scholar] [CrossRef] [PubMed]
- Dehingia, M.; Devi, K.T.; Talukdar, N.C.; Talukdar, R.; Reddy, N.; Mande, S.S.; Deka, M.; Khan, M.R. Gut bacterial diversity of the tribes of India and comparison with the worldwid data. Sci. Rep. 2015, 5, 18563. [Google Scholar] [CrossRef]
- Bhute, S.; Pande, P.; Shetty, S.A.; Shelar, R.; Mane, S.; Kumbhare, S.V.; Gawali, A.; Makhani, H.; Navandar, M.; Dhotre, D.; et al. Molecular characterization and meta-analysis of gut microbial communities illustrate enrichment of Prevotella and Megasphere in Indian subjects. Front. Microbiol. 2016, 7, 660. [Google Scholar] [CrossRef]
- Dhakan, D.B.; Maji, A.; Sharma, A.K.; Saxena, R.; Pulikkan, J.; Grace, T.; Gomez, A.; Scaria, J.; Amato, K.R.; Sharma, V.K. The unique composition of Indian gut microbiome, gene catalogue, and associated fecal metabolome deciphered using multi-omics approaches. GigaScienece 2019, 8, giz004. [Google Scholar] [CrossRef]
- Kaur, K.; Khatri, I.; Akhtar, A.; Subramanian, S.; Ramya, T.N.C. Metagenomics analysis reveals features unique to Indian distal gut microbiota. PLoS ONE 2020, 15, e0231197. [Google Scholar]
- Liu, K.; Zhang, Y.; Li, Q.; Li, H.; Long, D.; Yan, S.; Huang, W.; Long, R.; Huang, X. Ethnic Differences Shape the Alpha but Not Beta Diversity of Gut Microbiota from School Children in the Absence of Environmental Differences. Microorganisms 2020, 8, 254. [Google Scholar] [CrossRef]
- Goyal, S.; Farhana, L.; Antaki, F.; Judd, S.; Nangia-Makker, P.; Hadden, T.; Yu Yingjie, Y.; Levi, E.M.D.; Majumdar, A. Colorectal Cancer and Racial Disparity: Gut Microbiome as a Potential Regulator. Am. J. Gastroenterol. 2016, 111, S66. [Google Scholar] [CrossRef]
- Carson, T.L.; Wang, F.; Cui, X.; Jackson, B.E.; Van Der Pol, W.J.; Lefkowitz, E.J.; Morrow, C.; Baskin, M.L. Associations between Race, Perceived Psychological Stress, and the Gut Microbiota in a Sample of Generally Healthy Black and White Women: A Pilot Study on the Role of Race and Perceived Psychological Stress. Psychosom. Med. 2018, 80, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Pulikkan, J.; Maji, A.; Dhakan, D.B.; Saxena, R.; Mohan, B.; Anto, M.M.; Agarwal, N.; Grace, T.; Sharma, V.K. Gut microbial dysbiosis in Indian children with autism spectrum disorders. Microb. Ecol. 2018, 76, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, W.; Cai, Q.; Shrubsole, M.J.; Pei, Z.; Brucker, R.; Steinwandel, M.; Bordenstein, S.R.; Li, Z.; Blot, W.J.; et al. Racial Differences in the Oral Microbiome: Data from Low-Income Populations of African Ancestry and European Ancestry. mSystems 2019, 4, e00639-19. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Collard, D.; Prodan, A.; Levels, J.H.M.; Zwinderman, A.H.; Bäckhed, F.; Vogt, L.; Peters, M.J.L.; Muller, M.; Nieuwdorp, M.; et al. Associations between gut microbiota, faecal short-chain fatty acids, and blood pressure across ethnic groups: The HELIUS study. Eur. Heart J. 2020, 41, 4259–4267. [Google Scholar] [CrossRef]
- Kurt, O.; Munkhtsetseg, B.; Stensvold, C.R.; Özcan, O.; Andersen, L.O.; ÖktemOkullu, S.; Sezerman, O.U.; Nielsen, H.V.; Tözün, A.N. Irritable Bowel Syndrome and Microbiota: First Clinical Study on Turkish Patients for Correlations between Gut Bacteria, Dientamoeba fragilis, Blastocystis, and Eating Habits. IBS 2019. [Google Scholar]
- Cox, A.J.; Zhanga, P.; Bowden, D.W.; Devereaux, B.; Davoren, P.M.; Cripps, A.W.; West, N.P. Increased intestinal permeability as a risk factor for type 2 diabetes. Diabetes Metab. 2017, 43, 163–166. [Google Scholar] [CrossRef]
- Mønsted, M.Ø.; Falck, N.D.; Pedersen, K.; Buschard, K.; Holm, L.J.; Haupt-Jorgensen, M. Intestinal permeability in type 1 diabetes: An updated comprehensive overview. J. Autoimmun. 2021, 122, 102674. [Google Scholar] [CrossRef]
- Li, X.; Atkinson, M.A. The role for gut permeability in the pathogenesis of type 1 diabetes--a solid or leaky concept? Pediatr. Diabetes 2015, 16, 485–492. [Google Scholar] [CrossRef]
- Soyucen, E.; Gulcan, A.; Aktuglu-Zeybek, A.C.; Onal, H.; Kiykim, E.; Aydin, A. Differences in the gut microbiota of healthy children and those with type 1 diabetes. Pediatrics Int. 2014, 56, 336–343. [Google Scholar] [CrossRef]
- Ghaemi, F.; Fateh, A.; Sepahy, A.A.; Zangeneh, M.; Ghanei., M.; Siadat, S.D. Intestinal Microbiota Composition in Iranian Diabetic, Pre-diabetic and Healthy Individuals. J. Diabetes Metab. Disord. 2020, 19, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Balvers, M.; Deschasaux, M.; van den Born, B.-J.; Zwinderman, K.; Nieuwdorp, M.; Levin, E. Analyzing Type 2 Diabetes Associations with the Gut Microbiome in Individuals from Two Ethnic Backgrounds Living in the Same Geographic Area. Nutrients 2021, 13, 3289. [Google Scholar] [CrossRef] [PubMed]
- Tortora, S.C.; Bodiwala, V.M.; Quinn, A.; Martello, L.A.; Vignesh, S. Microbiome and colorectal carcinogenesis: Linked mechanisms and racial differences. World J. Gastrointest. Oncol. 2022, 14, 375–395. [Google Scholar] [CrossRef] [PubMed]
- Gantuya, B.; El Serag, H.B.; Matsumoto, T.; Ajami, N.J.; Uchida, T.; Oyuntsetseg, K.; Bolor, D.; Yamaoka, Y. Gastric mucosal microbiota in a Mongolian population with gastric cancer and precursor conditions. Aliment. Pharmacol. Ther. 2020, 51, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Kushugulova, A.; Forslund, S.K.; Costea, P.I.; Kozhakhmetovet, S.; Khassenbekova, Z.; Urazova, M.; Nurgozhin, T.; Zhumadilov, Z.; Benberin, V.; Driessen, M.; et al. Metagenomic analysis of gut microbial communities from a Central Asian population. BMJ Open 2018, 8, e021682. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Angelakis, E.; Bibi, F.; Azhar, E.I.; Bachar, D.; Lagier, J.C.; Gaborit, B.; Hassan, A.M.; Jiman-Fatani, A.A.; Alshali, K.Z.; et al. Comparison of the gut microbiota of people in France and Saudi Arabia. Nutr. Diabetes 2015, 5, e153. [Google Scholar] [CrossRef] [PubMed]
- Ettehad Marvasti, F.; Moshiri, A.; Taghavi, M.S.; Riazi, S.; Taati, M.; Sadati, S.F.; Ghaheri, A.; Masoomi, M.; Vaziri, F.; Fateh, A.; et al. The First Report of Differences in Gut Microbiota Composition between Obese and Normal Weight Iranian Subjects. Iran. Biomed. J. 2020, 24, 148–154. [Google Scholar] [CrossRef]
- Plummer, E.; Bulach, D.; Carter, G.; Albert, M.J. Gut microbiome of native Arab Kuwaitis. Gut Pathog. 2020, 12, 10. [Google Scholar] [CrossRef]
- Chauhan, N.S.; Pandey, R.; Mondal, A.K.; Gupta, S.; Verma, M.K.; Jain, S.; Ahmed, V.; Patil, R.; Agarwal, D.; Girase, B.; et al. Western Indian Rural Gut Microbial Diversity in Extreme Prakriti Endo-Phenotypes Reveals Signature Microbes. Front. Microbiol. 2018, 9, 118. [Google Scholar] [CrossRef]
- Li, J.; Fu, R.; Yang, Y.; Horz, H.P.; Guan, Y.; Lu, Y.; Lou, H.; Tian, L.; Zheng, S.; Liu, H.; et al. A metagenomic approach to dissect the genetic composition of enterotypes in Han Chinese and two Muslim groups. Syst. Appl. Microbiol. 2018, 41, 1–12. [Google Scholar] [CrossRef]
- Ozkul, C.; Yalınay, M.; Karakan, T. Islamic fasting leads to an increased abundance of Akkermansia muciniphila and Bacteroides fragilis group: A preliminary study on intermittent fasting. Turk. J. Gastroenterol. 2019, 30, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Karakan, T. Intermittent fasting and gut microbiota. Turk. J. Gastroenterol. 2019, 30, 1008. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, C.; Yalinay, M.; Karakan, T. Structural changes in gut microbiome after Ramadan fasting: A pilot study. Benef. Microbes 2020, 11, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Zhen, J.; Liu, A.; Yuan, J.; Wu, X.; Zhao, P.; Zhao, L.; Li, X.; Liu, Q.; Huang, G.; et al. Long-Term Vegan Meditation Improved Human Gut Microbiota. Evid.-Based Complement. Altern. Med. 2020, 5, 9517897. [Google Scholar]
- Wallace, R.K. The Microbiome in Health and Disease from the Perspective of Modern Medicine and Ayurveda. Medicina 2020, 56, 462. [Google Scholar] [CrossRef]
- Zimmer, J.; Lange, B.; Frick, J.S.; Sauer, H.; Zimmermann, K.; Schwiertz, A.; Rusch, K.; Klosterhalfen, S.; Enck, P. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur. J. Clin. Nutr. 2012, 66, 53–60. [Google Scholar] [CrossRef]
- Ruengsomwong, S.; La-ongkham, O.; Jiang, J.; Wannissorn, B.; Nakayama, J.; Nitisinprasert, S. Microbial Community of Healthy Thai Vegetarians and Non-Vegetarians, Their Core Gut Microbiota, and Pathogen Risk. J. Microbiol. Biotechnol. 2016, 26, 1723–1735. [Google Scholar] [CrossRef]
- Orlich, M.J.; Fraser, G.E. Vegetarian diets in the Adventist Health Study 2: A review of initial published findings. Am. J. Clin. Nutr. 2014, 100, 353S–358S. [Google Scholar] [CrossRef]
- Segovia-Siapco, G.; Sabaté, J. Health and sustainability outcomes of vegetarian dietary patterns: A revisit of the EPIC-Oxford and the Adventist Health Study-2 cohorts. Eur. J. Clin. Nutr. 2019, 72, 60–70. [Google Scholar] [CrossRef]
- Brathwaite, N.; Fraser, H.S.; Modeste, N.; Broome, H.; King, R. Obesity, diabetes, hypertension, and vegetarian status among Seventh-Day Adventists in Barbados: Preliminary results. Ethn. Dis. 2003, 13, 34–39. [Google Scholar]
- Le, L.T.; Sabaté, J. Beyond meatless, the health effects of vegan diets: Findings from the Adventist cohorts. Nutrients 2014, 27, 2131–2147. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, A.; Levy, J.; Berthezène, C.; Alpers, D.H.; Guéant, J.L. Health outcomes associated with vegetarian diets: An umbrella review of systematic reviews and meta-analyses. Clin. Nutr. 2020, 39, 3283–3307. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Liu, K.; Long, D.; Faisal, S.; Hilal, M.G.; Ali, I.; Huang, X.; Long, R. Ramadan Fasting Leads to Shifts in Human Gut Microbiota Structured by Dietary Composition. Front. Microbiol. 2021, 12, 642999. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, J.; Watanabe, K.; Jiang, J.; Matsuda, K.; Chao, S.H.; Haryono, P.; La-Ongkham, O.; Sarwoko, M.A.; Sujaya, I.N.; Zhao, L.; et al. Diversity in gut bacterial community of school-age children in Asia. Sci. Rep. 2015, 5, 8397. [Google Scholar] [CrossRef] [PubMed]
- Kirk, D. Europe’s Population in the Interwar Years. In Demographic Monographs; Gordon & Breach: London, UK, 1946; Volume 3, p. 309. [Google Scholar]
- Grąt, M.; Wronka, K.M.; Krasnodębski, M.; Masior, Ł.; Lewandowski, Z.; Kosińska, I.; Grąt, K.; Stypułkowski, J.; Rejowski, S.; Wasilewicz, M.; et al. Profile of Gut Microbiota Associated With the Presence of Hepatocellular Cancer in Patients With Liver Cirrhosis. Transplant. Proc. 2016, 48, 1687–1691. [Google Scholar] [CrossRef]
- Gózd-Barszczewska, A.; Kozioł-Montewka, M.; Barszczewski, P.; Młodzińska, A.; Humińska, K. Gut microbiome as a biomarker of cardiometabolic disorders. Ann. Agric. Environ. Med. 2017, 24, 416–422. [Google Scholar] [CrossRef]
- Budinska, E.; Gojda, J.; Heczkova, M.; Bratova, M.; Dankova, H.; Wohl, P.; Bastova, H.; Lanska, V.; Kostovcik, M.; Dastych, M.; et al. Microbiome and Metabolome Profiles Associated With Different Types of Short Bowel Syndrome: Implications for Treatment. JPEN J. Parenter. Enteral. Nutr. 2020, 44, 105–118. [Google Scholar] [CrossRef]
- Kulecka, M.; Fraczek, B.; Mikula, M.; Zeber-Lubecka, N.; Karczmarski, J.; Paziewska, A.; Ambrozkiewicz, F.; Jagusztyn-Krynicka, K.; Cieszczyk, P.; Ostrowski, J. The composition and richness of the gut microbiota differentiate the top Polish endurance athletes from sedentary controls. Gut Microbes 2020, 11, 1374–1384. [Google Scholar] [CrossRef]
- Mazur, O.; Plaksyvyi, O.; Pashkovska, N.; Bilooka, I. State of the Large Intestine Microbiota in Patients With Type 1 Diabetes Mellitus Depending on the Severity of Clinical Course. Int. J. Endocrinol. 2016, 5, 61–66. [Google Scholar]
- Stepanov, Y.M.; Chaliy, M.V.; Kononenko, I.S. Features of Bacterial Overgrowth Syndrome and Gut Flora Imbalance in Patients with Portal Hypertension. Gastroenterology 2016, 4, 32–36. [Google Scholar]
- Stepanov, N.M.; Driyanska, V.E.; Korol, L.V.; Mihal, L.Y.; Savchenko, V.S. The effects of gut indigenous microbiota on intensity of oxidative stress and the cytokine immunity in women with recurrent pyelonephritis. Med. Perspect. 2018, 23, 129–135. [Google Scholar] [CrossRef]
- Lohner, S.; Jakobik, V.; Mihályi, K.; Soldi, S.; Vasileiadis, S.; Theis, S.; Sailer, M.; Sieland, C.; Berényi, K.; Boehm, G.; et al. Inulin-Type Fructan Supplementation of 3- to 6-Year-Old Children Is Associated with Higher Fecal Bifidobacterium Concentrations and Fewer Febrile Episodes Requiring Medical Attention. J. Nutr. 2018, 148, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Szlachciński, R.; Szlachcińska, A.; Szlachciński, Ł.; Borycz-Stevens, I.; Almeer, F.; Tkaczyk, M. Intestinal microbiota in nephrotic children treated with immunosuppressive agents. Pediatr. Pol.-Pol. J. Paediatr. 2020, 95, 6–13. [Google Scholar] [CrossRef]
- Soltys, K.; Stuchlikova, M.; Hlavaty, T.; Gaalova, B.; Budis, J.; Gazdarica, J.; Krajcovicova, A.; Zelinkova, Z.; Szemes, T.; Kuba, D.; et al. Seasonal changes of circulating 25-hydroxyvitamin D correlate with the lower gut microbiome composition in inflammatory bowel disease patients. Sci. Rep. 2020, 10, 6024. [Google Scholar] [CrossRef] [PubMed]
- Skok, P.; Skok, K. Gut microbiota and the pathophysiology of cardiovascular disease. Arch. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Šikić Pogačar, M.; Mičetić-Turk, D. The influence of dietary compounds on gut microbiota. Slov. Med. J. 2021, 90, 178–192. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidota ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef]
- Koliada, A.; Moseiko, V.; Romanenko, M.; Piven, L.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Vaiserman, A. Seasonal variation in gut microbiota composition: Cross-sectional evidence from Ukrainian population. BMC Microbiol. 2020, 20, 100. [Google Scholar] [CrossRef]
- Mahnic, A.; Rupnik, M. Different host factors are associated with patterns in bacterial and fungal gut microbiota in Slovenian healthy cohort. PLoS ONE 2018, 13, e0209209. [Google Scholar]
- Mahnic, A.; Breskvar, M.; Dzeroski, S.; Skok, P.; Pintar, S.; Rupnik, M. Distinct Types of Gut Microbiota Dysbiosis in Hospitalized Gastroenterological Patients Are Disease Non-related and Characterized With the Predominance of Either Enterobacteriaceae or Enterococcus. Front. Microbiol. 2020, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Obermajer, T.; Grabnar, I.; Benedik, E.; Tušar, T.; Robič Pikel, T.; Fidler Mis, N.; Bogovič Matijašić, B.; Rogelj, I. Microbes in Infant Gut Development: Placing Abundance Within Environmental, Clinical and Growth Parameters. Sci. Rep. 2017, 7, 11230. [Google Scholar] [CrossRef] [PubMed]
- Barczynska, R.; Bandurska, K.; Slizewska, K.; Litwin, M.; Szalecki, M.; Libudzisz, Z.; Kapusniak, J. Intestinal Microbiota, Obesity and Prebiotics. Pol. J. Microbiol. 2015, 64, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Gózd-Barszczewska, A.; Kozioł-Montewka, M.; Barszczewski, P. Characteristics of gut microbiome of obese middle-aged men in the Lublin region—Preliminary report. J. Pre-Clin. Clin. Res. 2019, 13, 37–41. [Google Scholar] [CrossRef]
- Mitev, K.; Taleski, V. Association between the Gut Microbiota and Obesity. Open Access Maced. J. Med Sci. 2019, 7, 2050–2056. [Google Scholar] [CrossRef]
- Kurinna, O.H. Gut Microbiota Changes in Nonalcoholic Fatty Liver Disease and Concomitant Cardiovascular Diseases. bioRxiv 2020, 23. [Google Scholar] [CrossRef]
- Tomova, A.; Soltys, K.; Repiska, G.; Palkova, L.; Filcikova, D.; Minarik, G.; Turna, J.; Prochotska, K.; Babinska, K.; Ostatnikova, D. Specificity of gut microbiota in children with autism spectrum disorder in Slovakia and its correlation with astrocytes activity marker and specific behavioural patterns. Physiol. Behav. 2020, 214, 112745. [Google Scholar] [CrossRef]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef]
- Prochazkova, P.; Roubalova, R.; Dvorak, J.; Kreisinger, J.; Hill, M.; Tlaskalova-Hogenova, H.; Tomasova, P.; Pelantova, H.; Cermakova, M.; Kuzma, M.; et al. The intestinal microbiota and metabolites in patients with anorexia nervosa. Gut Microbes 2021, 13, 1902771. [Google Scholar] [CrossRef]
- Zakerska-Banaszak, O.; Tomczak, H.; Gabryel, M.; Baturo, A.; Wolko, L.; Michalak, M.; Malinska, N.; Mankowska-Wierzbicka, D.; Eder, P.; Dobrowolska, A.; et al. Dysbiosis of gut microbiota in Polish patients with ulcerative colitis: A pilot study. Sci. Rep. 2021, 11, 2166. [Google Scholar] [CrossRef]
- Bajer, L.; Kverka, M.; Kostovcik, M.; Macinga, P.; Dvorak, J.; Stehlikova, Z.; Brezina, J.; Wohl, P.; Spicak, J.; Drastich, P. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J. Gastroenterol. 2017, 23, 4548–4558. [Google Scholar] [CrossRef] [PubMed]
- Gryaznova, M.V.; Solodskikh, S.A.; Panevina, A.V.; Syromyatnikov, M.Y.; Dvoretskaya, Y.D.; Sviridova, T.N.; Popov, E.S.; Popov, V.N. Study of microbiome changes in patients with ulcerative colitis in the Central European part of Russia. Heliyon 2021, 7, e06432. [Google Scholar] [CrossRef] [PubMed]
- Szabo, H.; Hernyes, A.; Piroska, M.; Ligeti, B.; Fussy, P.; Zoldi, L.; Galyasz, S.; Makra, N.; Szabo, D.; Tarnoki, A.D.; et al. Association between Gut Microbial Diversity and Carotid Intima-Media Thickness. Medicina 2021, 57, 195. [Google Scholar] [CrossRef] [PubMed]
- Zwiernik, B.; Arłukowicz, T.; Marcin, M.; Zwiernik, J. Gut microbiota modification as an option in multiple sclerosis management. Pol. Ann. Med. 2020, 27, 238–243. [Google Scholar] [CrossRef]
- Madách, K.; Kristóf, K.; Tulassay, E.; Iványi, Z.; Erdei, A.; Király, A.; János, G.; Zsuzsa, B. Mucosal Immunity and the Intestinal Microbiome in the Development of Critical Illness. ISRN Immunol. 2011, 2011, 545729. [Google Scholar] [CrossRef]
- Tyakht, A.V.; Kostryukova, E.S.; Popenko, A.S.; Belenikin, M.S.; Pavlenko, A.V.; Larin, A.K.; Karpova, I.Y.; Selezneva, O.V.; Semashko, T.A.; Ospanova, E.A.; et al. Human gut microbiota community structures in urban and rural populations in Russia. Nat. Commun. 2013, 4, 2469. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef]
- Jahns, L.; Baturin, A.; Popkin, B.M. Obesity, diet, and poverty: Trends in the Russian transition to market economy. Eur. J. Clin. Nutr. 2003, 57, 1295–1302. [Google Scholar] [CrossRef][Green Version]
- Liefert, W. Food security in Russia: Economic growth and rising incomes are reducing insecurity. Food Secur. Assess. 2004, 15, 35–43. [Google Scholar]
- Tyakht, A.V.; Alexeev, D.G.; Popenko, A.S.; Kostryukova, E.S.; Govorun, V.M. Rural and urban microbiota: To be or not to be? Gut Microbes 2014, 5, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.E.; Lozupone, C.A.; Rey, F.E.; Wu, M.; Guruge, J.L.; Narra, A.; Goodfellow, J.; Zaneveld, J.R.; McDonald, D.T.; Goodrich, J.A.; et al. Pan-genome of the dominant human gut-associated archaeon, Methanobrevibacter smithii, studied in twins. Proc. Natl. Acad. Sci. USA 2011, 108, 4599–4606. [Google Scholar] [CrossRef]
- Zolnikova, O.Y.; Potskhverashvili, N.D.; Kudryavtseva, A.V.; Krasnov, G.S.; Guvatova, Z.G.; Truhmanov, A.S.; Kokina, N.I.; Ivashkin, V.T. Changes in gut microbiota with bronchial asthma. Ther. Arch. 2020, 92, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Puzanov, V.A.; Komissarova, O.G.; Nikonenko, B.V. Bacterial microbiota of lower gut and bronchi in tuberculosis patients. Tuberc. Lung Dis. 2020, 98, 37–43. [Google Scholar] [CrossRef]
- Kozhieva, M.; Naumova, N.; Alikina, T.; Boyko, A.; Vlassov, V.; Kabilov, M.R. Primary progressive multiple sclerosis in a Russian cohort: Relationship with gut bacterial diversity. BMC Microbiol. 2019, 19, 309. [Google Scholar] [CrossRef] [PubMed]
- Tikunov, A.; Morozov, V.; Shvalov, A.; Bardasheva, A.; Shrayner, E.V.; Maksimova, O.A.; Voloshina, I.O.; Morozova, V.V.; Vlasov, V.V.; Tikunova, N.V. Fecal microbiome change in patients with ulcerative colitis after fecal microbiota transplantation. Vavilov J. Genet. Breed. 2020, 24, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Simanenkov, V.I.; Suvorov, A.N.; Tikhonov, S.V.; Ermolenko, E.I.; Dekkanova, V.D.; Goncharov, A.E.; Tkachev, P.V.; Bakulina, N.V. Gallbladder microbiota in patients with gallstone disease. HERALD North-West. State Med. Univ. Named After I.I. Mechnikov. 2020, 12, 37–44. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syromyatnikov, M.; Nesterova, E.; Gladkikh, M.; Smirnova, Y.; Gryaznova, M.; Popov, V. Characteristics of the Gut Bacterial Composition in People of Different Nationalities and Religions. Microorganisms 2022, 10, 1866. https://doi.org/10.3390/microorganisms10091866
Syromyatnikov M, Nesterova E, Gladkikh M, Smirnova Y, Gryaznova M, Popov V. Characteristics of the Gut Bacterial Composition in People of Different Nationalities and Religions. Microorganisms. 2022; 10(9):1866. https://doi.org/10.3390/microorganisms10091866
Chicago/Turabian StyleSyromyatnikov, Mikhail, Ekaterina Nesterova, Maria Gladkikh, Yuliya Smirnova, Mariya Gryaznova, and Vasily Popov. 2022. "Characteristics of the Gut Bacterial Composition in People of Different Nationalities and Religions" Microorganisms 10, no. 9: 1866. https://doi.org/10.3390/microorganisms10091866
APA StyleSyromyatnikov, M., Nesterova, E., Gladkikh, M., Smirnova, Y., Gryaznova, M., & Popov, V. (2022). Characteristics of the Gut Bacterial Composition in People of Different Nationalities and Religions. Microorganisms, 10(9), 1866. https://doi.org/10.3390/microorganisms10091866






