Inhibitory Effect of Thymol on Tympanostomy Tube Biofilms of Methicillin-Resistant Staphylococcus aureus and Ciprofloxacin-Resistant Pseudomonas aeruginosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Drug
2.2. Determination of the Minimum Inhibitory Concentration of Thymol
2.3. Live and Dead Bacterial Cell Staining
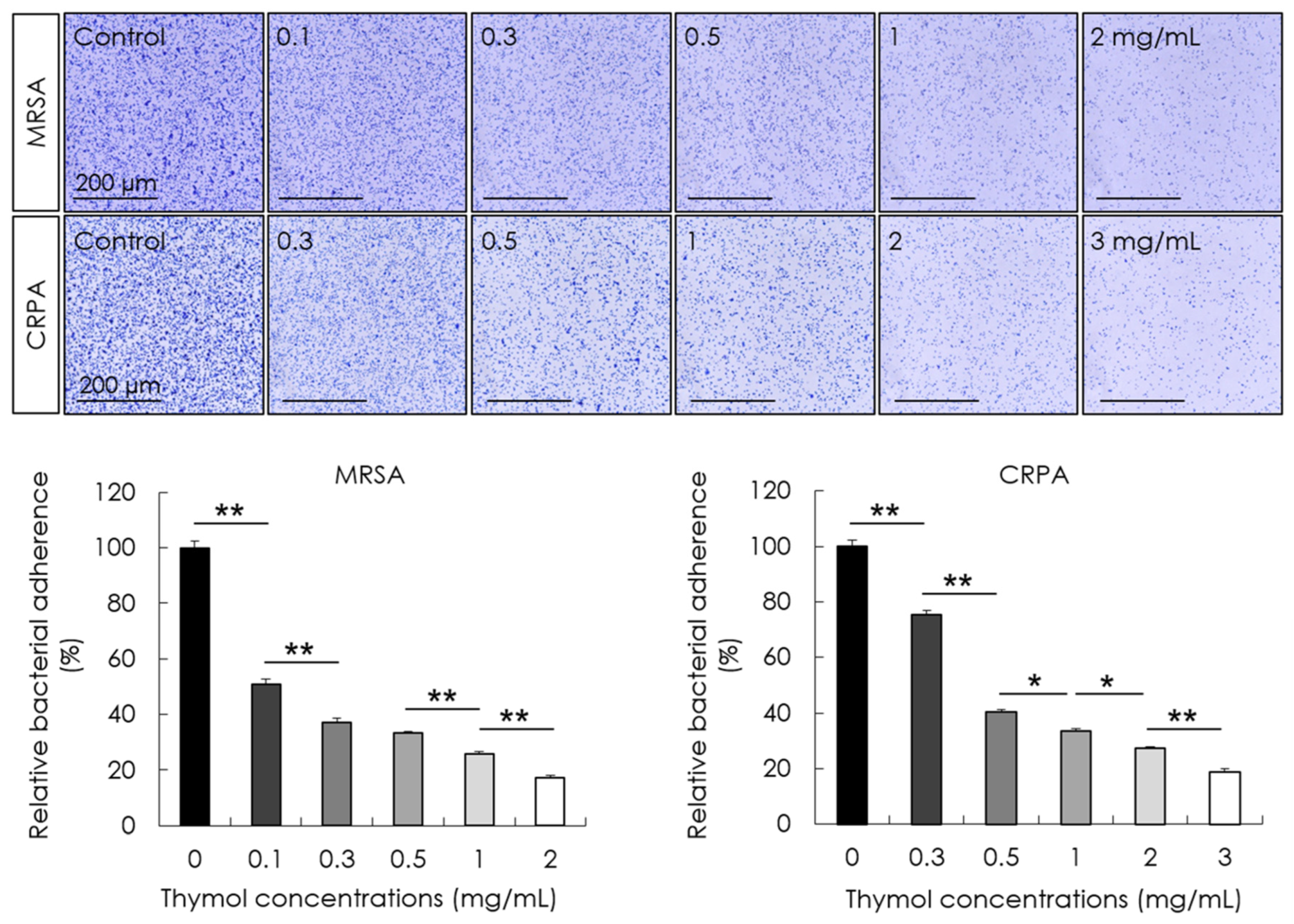
2.4. Adhesion Assay
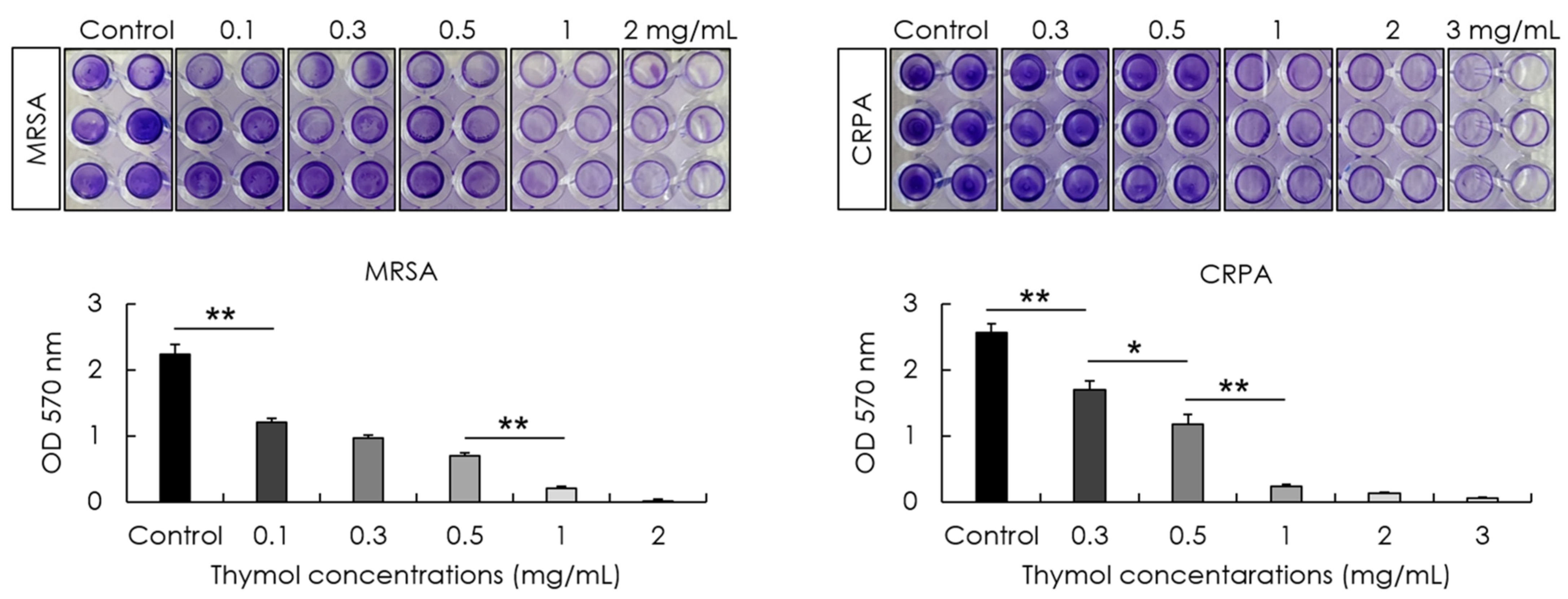
2.5. Biofilm Formation Assay
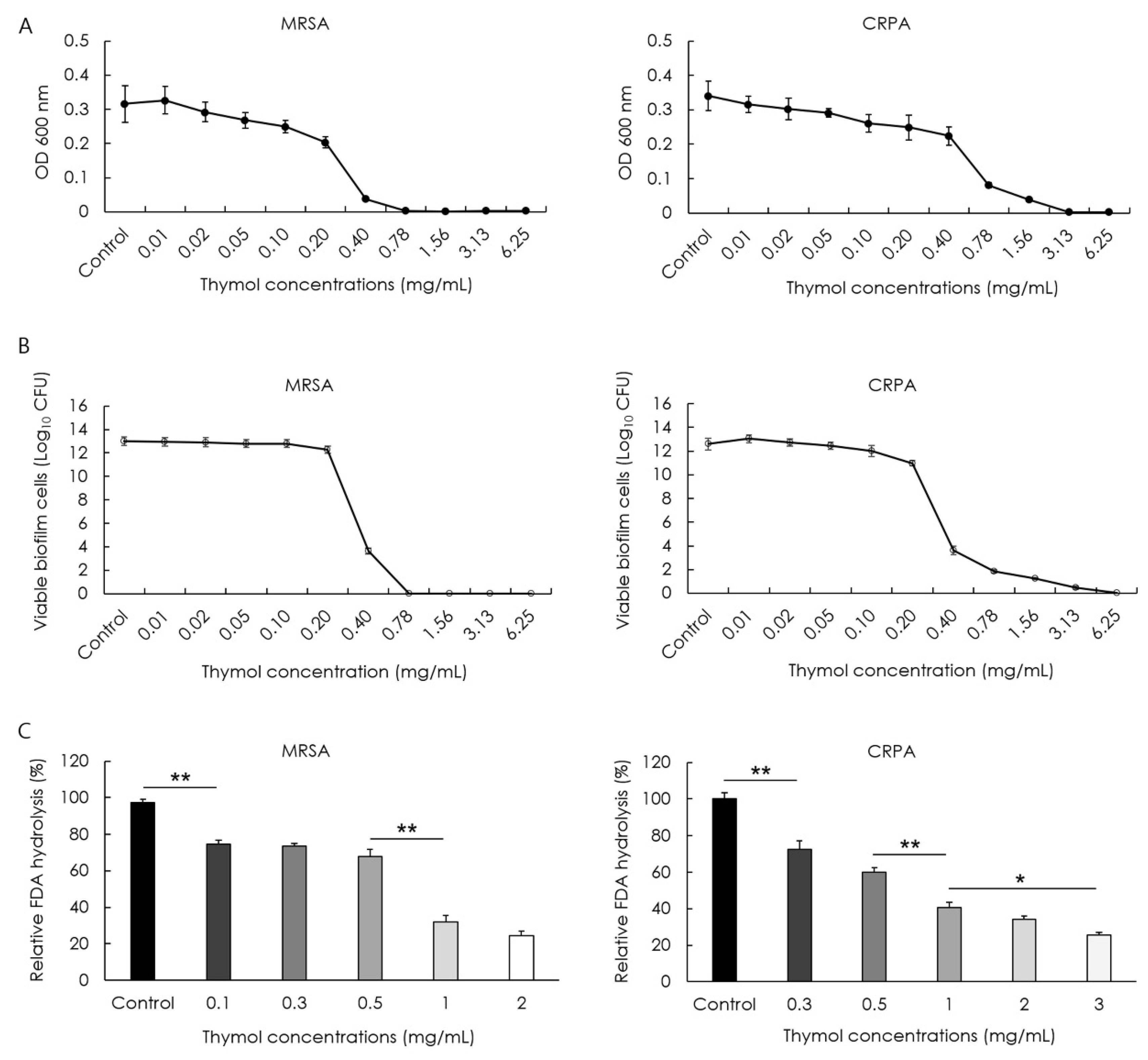
2.6. Determination of the Minimum Biofilm Eradication Concentration of Thymol
2.7. Biofilm Hydrolysis Assay
2.8. Scanning Electron Microscope Analysis of Bacterial Biofilms
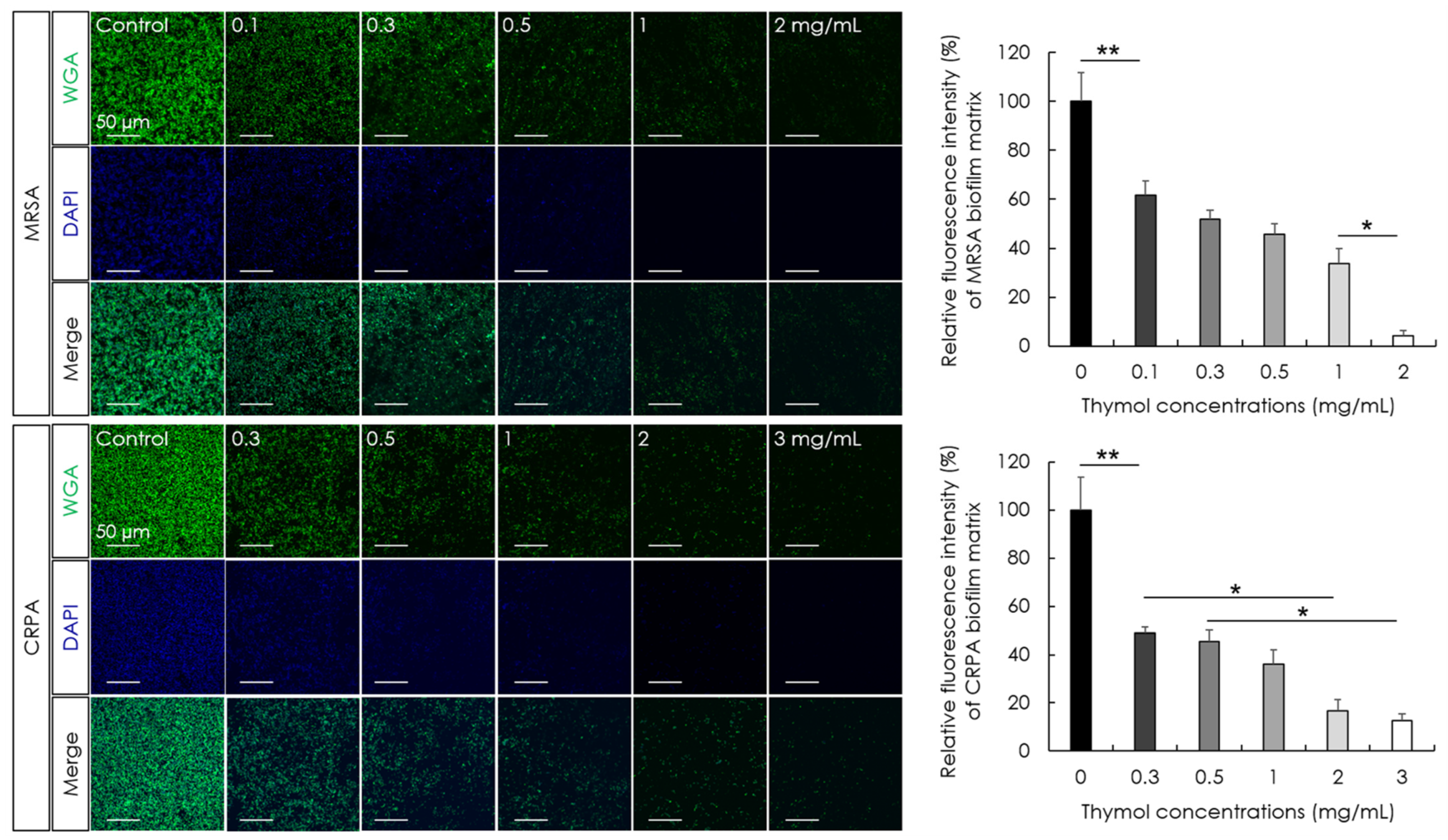
2.9. Fluorescence Microscopic Observation and Quantification of Biofilm Matrix
2.10. Statistical Analysis
3. Results
3.1. Inhibitory Effect of Thymol against MRSA and CRPA
3.2. Inhibitory Effect of Thymol against the Adhesion and Biofilm Formation of MRSA and CRPA
3.3. Eradication Effect of Thymol against MRSA and CRPA Biofilms
3.4. Biofilm-Destruction Activity of Thymol against Biofilm-Coated Tympanostomy Tubes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenfeld, R.M.; Shin, J.J.; Schwartz, S.R.; Coggins, R.; Gagnon, L.; Hackell, J.M.; Hoelting, D.; Hunter, L.L.; Kummer, A.W.; Payne, S.C.; et al. Clinical practice guideline: Otitis media with effusion (update). Otolaryngol. Head Neck Surg. 2016, 154, S1–S41. [Google Scholar] [CrossRef] [PubMed]
- Steele, D.W.; Adam, G.P.; Di, M.; Halladay, C.W.; Balk, E.M.; Trikalinos, T.A. Prevention and treatment of tympanostomy tube otorrhea: A meta-analysis. Pediatrics 2017, 139, e20170667. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, T.M.A.; Damoiseaux, R.A.M.J.; Schilder, A.G.M. Tympanostomy tube otorrhea in children: Prevention and treatment. Curr. Opin. Otolaryngol. Head Neck Surg. 2018, 26, 437–440. [Google Scholar] [CrossRef]
- Mandel, E.M.; Casselbrant, M.L.; Kurs-Lasky, M. Acute otorrhea: Bacteriology of a common complication of tympanostomy tubes. Ann. Otol. Rhinol. Laryngol. 1994, 103, 713–718. [Google Scholar] [CrossRef]
- van Dongen, T.M.; Venekamp, R.P.; Wensing, A.M.; Bogaert, D.; Sanders, E.A.; Schilder, A.G. Acute otorrhea in children with tympanostomy tubes: Prevalence of bacteria and viruses in the post-pneumococcal conjugate vaccine era. Pediatr. Infect. Dis. J. 2015, 34, 355–360. [Google Scholar] [CrossRef]
- Karlan, M.S.; Skobel, B.; Grizzard, M.; Cassisi, N.J.; Singleton, G.T.; Buscemi, P.; Goldberg, E.P. Myringotomy tube materials: Bacterial adhesion and infection. Otolaryngol. Head Neck Surg. 1980, 88, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, M.G.; Kim, S.S.; Cha, S.H.; Yeo, S.G. Change in detection rate of methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa and their antibiotic sensitivities in patients with chronic suppurative otitis media. J. Int. Adv. Otol. 2015, 11, 151–156. [Google Scholar] [CrossRef]
- Jotić, A.; Božić, D.D.; Milovanović, J.; Pavlović, B.; Ješić, S.; Pelemiš, M.; Novaković, M.; Ćirković, I. Biofilm formation on tympanostomy tubes depends on methicillin-resistant Staphylococcus aureus genetic lineage. Eur. Arch. Otorhinolaryngol. 2016, 273, 615–620. [Google Scholar] [CrossRef]
- Jang, C.H.; Cho, Y.B.; Choi, C.H. Effect of ion-bombarded silicone tympanostomy tube on ciprofloxacin-resistant Pseudomonas aeruginosa biofilm formation. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 1471–1473. [Google Scholar] [CrossRef]
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Jang, C.H.; Cho, Y.B.; Choi, C.H. Antibacterial effect of tea-tree oil on methicillin-resistant Staphylococcus aureus biofilm formation of the tympanostomy tube: An in vitro study. In Vivo 2007, 21, 1027–1030. [Google Scholar]
- Gómez-Sequeda, N.; Cáceres, M.; Stashenko, E.E.; Hidalgo, W.; Ortiz, C. Antimicrobial and antibiofilm activities of essential oils against Escherichia coli O157:H7 and methicillin-resistant Staphylococcus aureus (MRSA). Antibiotics 2020, 9, 730. [Google Scholar] [CrossRef]
- Kavanaugh, N.L.; Ribbeck, K. Selected antimicrobial essential oils eradicate Pseudomonas spp. and Staphylococcus aureus biofilms. Appl. Environ. Microbiol. 2012, 78, 4057–4061. [Google Scholar] [CrossRef]
- Yuan, Z.; Dai, Y.; Ouyang, P.; Rehman, T.; Hussain, S.; Zhang, T.; Yin, Z.; Fu, H.; Lin, J.; He, C.; et al. Thymol inhibits biofilm formation, eliminates pre-existing biofilms, and enhances clearance of methicillin-resistant Staphylococcus aureus (MRSA) in a mouse peritoneal implant infection model. Microorganisms 2020, 8, 99. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- de Castro, R.D.; de Souza, T.M.; Bezerra, L.M.; Ferreira, G.L.; Costa, E.M.; Cavalcanti, A.L. Antifungal activity and mode of action of thymol and its synergism with nystatin against Candida species involved with infections in the oral cavity: An in vitro study. BMC Complement. Altern. Med. 2015, 15, 417. [Google Scholar] [CrossRef]
- Sahoo, C.R.; Paidesetty, S.K.; Padhy, R.N. The recent development of thymol derivative as a promising pharmacological scaffold. Drug Dev. Res. 2021, 82, 1079–1095. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Khan, S.T.; Khan, M.; Ahmad, J.; Wahab, R.; Abd-Elkader, O.H.; Musarrat, J.; Alkhathlan, H.Z.; Al-Kedhairy, A.A. Thymol and carvacrol induce autolysis, stress, growth inhibition and reduce the biofilm formation by Streptococcus mutans. AMB Express 2017, 7, 49. [Google Scholar] [CrossRef]
- Silva, M.D.; Sillankorva, S. Otitis media pathogens—A life entrapped in biofilm communities. Crit. Rev. Microbiol. 2019, 45, 595–612. [Google Scholar] [CrossRef] [PubMed]
- Idicula, W.K.; Jurcisek, J.A.; Cass, N.D.; Ali, S.; Goodman, S.D.; Elmaraghy, C.A.; Jatana, K.R.; Bakaletz, L.O. Identification of biofilms in post-tympanostomy tube otorrhea. Laryngoscope 2016, 126, 1946–1951. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Dickschat, J.S. Quorum sensing and bacterial biofilms. Nat. Prod. Rep. 2010, 27, 343–369. [Google Scholar] [CrossRef]
- Marino, M.; Bersani, C.; Comi, G. Antimicrobial activity of the essential oils of Thymus vulgaris L. measured using a bioimpedometric method. J. Food Prot. 1999, 62, 1017–1023. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Saptami, K.; Arokia Balaya Rex, D.; Chandrasekaran, J.; Rekha, P.D. Competitive interaction of thymol with cviR inhibits quorum sensing and associated biofilm formation in Chromobacterium violaceum. Int. Microbiol. 2022, 25, 629–638. [Google Scholar] [CrossRef]
- Miranda-Cadena, K.; Marcos-Arias, C.; Mateo, E.; Aguirre-Urizar, J.M.; Quindós, G.; Eraso, E. In vitro activities of carvacrol, cinnamaldehyde and thymol against Candida biofilms. Biomed. Pharmacother. 2021, 143, 112218. [Google Scholar] [CrossRef]
- Walczak, M.; Michalska-Sionkowska, M.; Olkiewicz, D.; Tarnawska, P.; Warżyńska, O. Potential of carvacrol and thymol in reducing biofilm formation on technical surfaces. Molecules 2021, 26, 2723. [Google Scholar] [CrossRef]
- Valliammai, A.; Selvaraj, A.; Yuvashree, U.; Aravindraja, C.; Karutha Pandian, S. sarA-dependent antibiofilm activity of thymol enhances the antibacterial efficacy of rifampicin against Staphylococcus aureus. Front. Microbiol. 2020, 11, 1744. [Google Scholar] [CrossRef]
- Martínez, A.; Manrique-Moreno, M.; Klaiss-Luna, M.C.; Stashenko, E.; Zafra, G.; Ortiz, C. Effect of essential oils on growth inhibition, biofilm formation and membrane integrity of Escherichia coli and Staphylococcus aureus. Antibiotics 2021, 10, 1474. [Google Scholar] [CrossRef]
- Cendra, M.D.M.; Torrents, E. Pseudomonas aeruginosa biofilms and their partners in crime. Biotechnol Adv. 2021, 49, 107734. [Google Scholar] [CrossRef]
- Rosenfeld, R.M.; Schwartz, S.R.; Pynnonen, M.A.; Tunkel, D.E.; Hussey, H.M.; Fichera, J.S.; Grimes, A.M.; Hackell, J.M.; Harrison, M.F.; Haskell, H.; et al. Clinical practice guideline: Tympanostomy tubes in children. Otolaryngol. Head Neck Surg. 2013, 149, S1–S35. [Google Scholar] [CrossRef]
- Meeran, M.F.; Jagadeesh, G.S.; Selvaraj, P. Synthetic catecholamine triggers β1-adrenergic receptor activation and stimulates cardiotoxicity via oxidative stress mediated apoptotic cell death in rats: Abrogating action of thymol. Chem. Biol. Interact. 2016, 251, 17–25. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of Thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, E.-R.; Oh, J.; Cho, S.I. Inhibitory Effect of Thymol on Tympanostomy Tube Biofilms of Methicillin-Resistant Staphylococcus aureus and Ciprofloxacin-Resistant Pseudomonas aeruginosa. Microorganisms 2022, 10, 1867. https://doi.org/10.3390/microorganisms10091867
Jo E-R, Oh J, Cho SI. Inhibitory Effect of Thymol on Tympanostomy Tube Biofilms of Methicillin-Resistant Staphylococcus aureus and Ciprofloxacin-Resistant Pseudomonas aeruginosa. Microorganisms. 2022; 10(9):1867. https://doi.org/10.3390/microorganisms10091867
Chicago/Turabian StyleJo, Eu-Ri, Jeonghyun Oh, and Sung Il Cho. 2022. "Inhibitory Effect of Thymol on Tympanostomy Tube Biofilms of Methicillin-Resistant Staphylococcus aureus and Ciprofloxacin-Resistant Pseudomonas aeruginosa" Microorganisms 10, no. 9: 1867. https://doi.org/10.3390/microorganisms10091867




