Pep27 Mutant Immunization Inhibits Caspase-14 Expression to Alleviate Inflammatory Bowel Disease via Treg Upregulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Induction of Colitis and Immunization Protocol
2.3. Macroscopic Assessment of Colitis Severity
2.4. Histopathological Analysis
2.5. Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling Assay
2.6. Assessment of Myeloperoxidase (MPO) Activity and Malondialdehyde (MDA) Level
2.7. Permeability Assay
2.8. Gut Microbiome Analysis in Fecal Samples
2.9. Treg and Caspase-14 Neutralization
2.10. Protein Extraction and Western Blotting
2.11. cDNA Synthesis and Real-Time PCR
2.12. High-Throughput Sequencing and Transcriptomic Analysis
2.13. Statistical Analysis
2.14. Ethical Considerations
3. Results
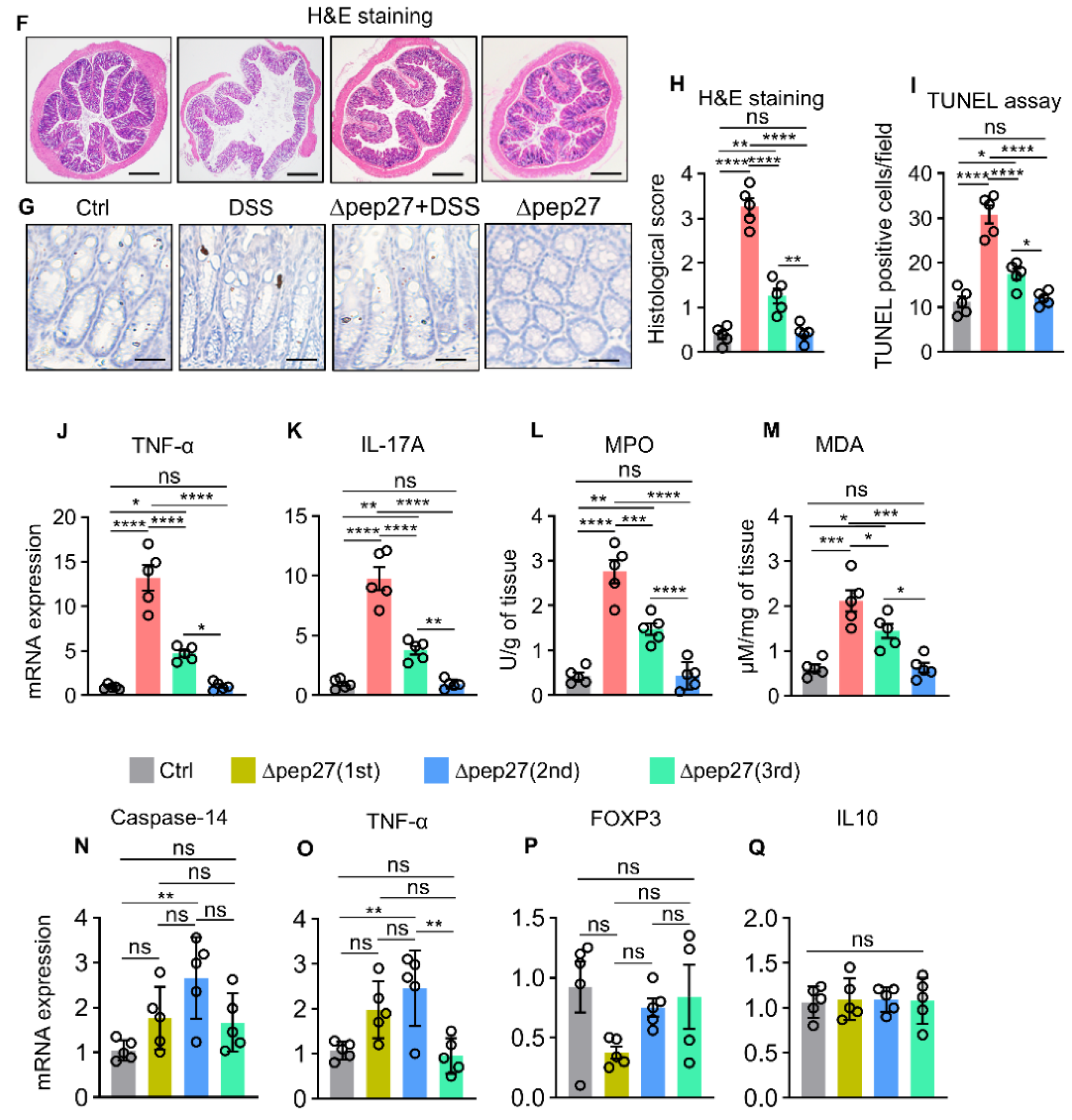
3.1. ∆Pep27 Immunization Attenuated Proinflammatory Cytokines and Oxidative Stress in the DIC
3.2. ∆Pep27 Immunization Diminished Proinflammatory Cytokines, and Caspase-14 Expression via Treg Upregulation to Prevent Barrier Disruption
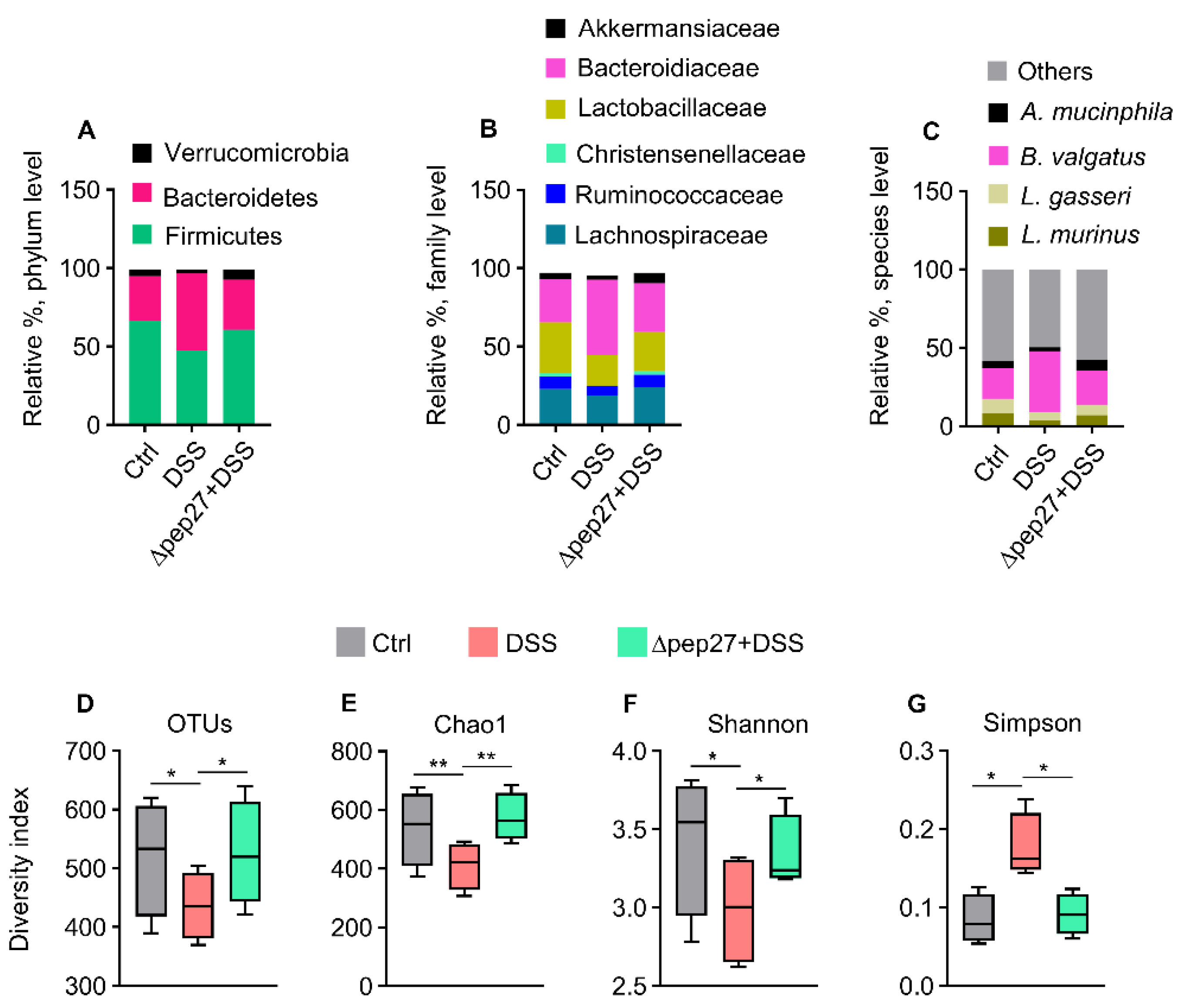
3.3. DIC-perturbed Biodiversity of Gut Microbiota Is Restored by ∆Pep27
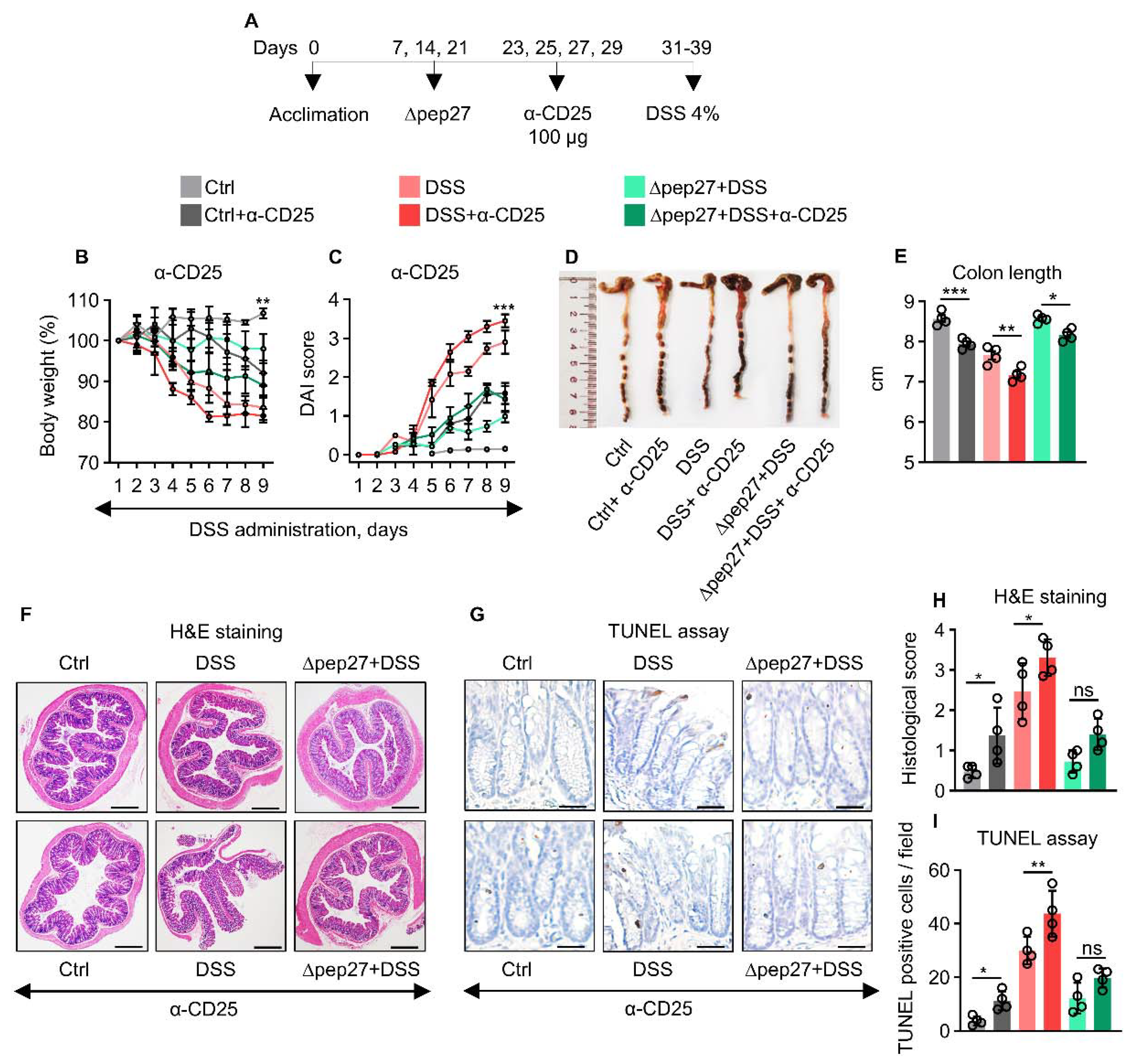
3.4. Treg Depletion Is Restored by ∆Pep27 to Prevent Inflammation and Apoptosis
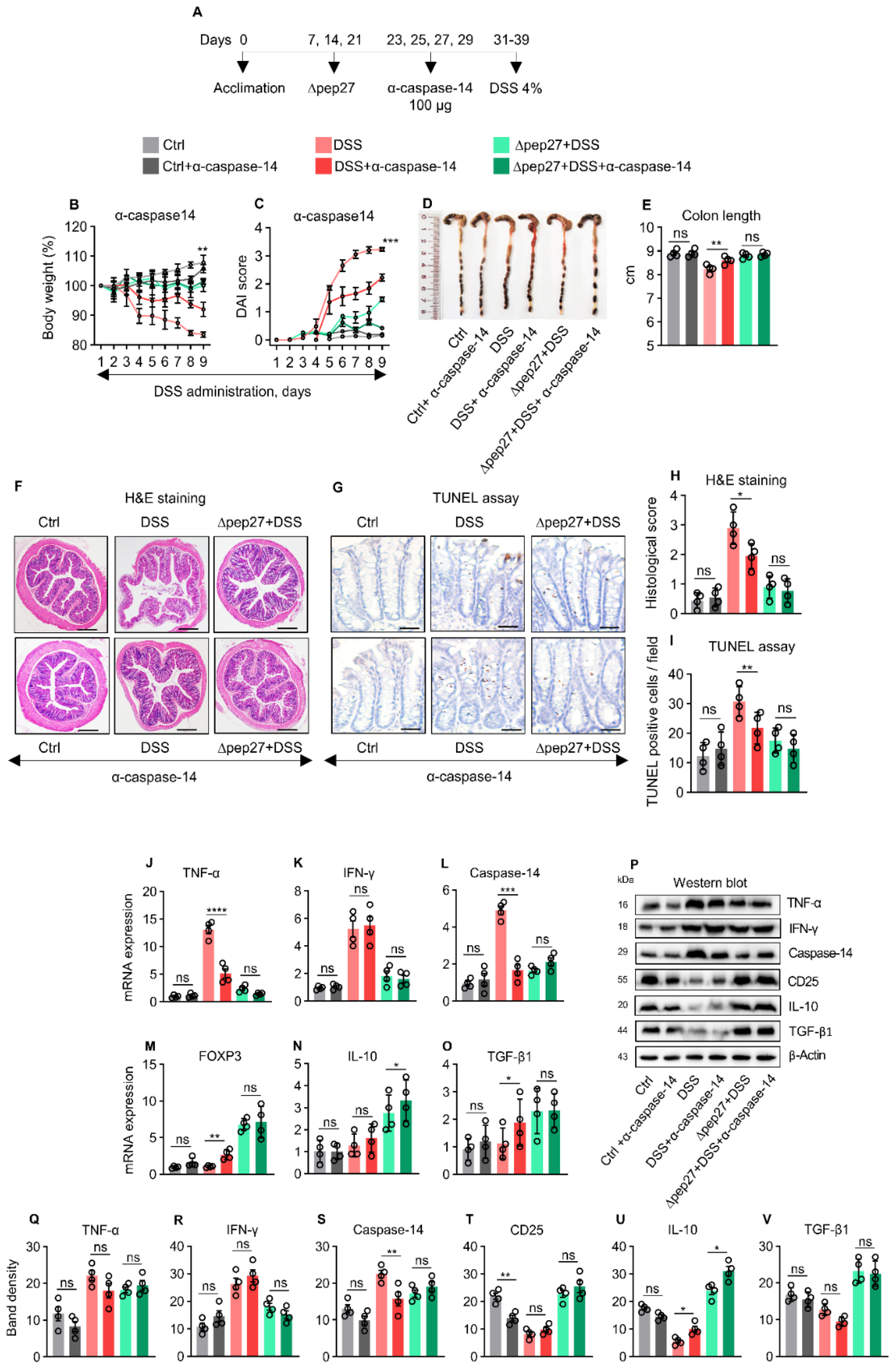
3.5. Caspase-14 Depletion Uncovers Proinflammatory and Apoptotic Nature of Caspase-14
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- de Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T. Pathophysiology of Inflammatory bowel diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Quetglas, E.G.; Armuzzi, A.; Wigge, S.; Fiorino, G.; Barnscheid, L.; Froelich, M.; Danese, S. The pharmacokinetics and pharmacodynamics of drugs used in inflammatory bowel disease treatment. Eur. J. Clin. Pharmacol. 2015, 71, 773–799. [Google Scholar] [CrossRef]
- Fujino, S.; Andoh, A.; Bamba, S.; Ogawa, A.; Hata, K.; Araki, Y.; Bamba, T.; Fujiyama, Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003, 52, 65–70. [Google Scholar] [CrossRef]
- Huber, S.; Gagliani, N.; Esplugues, E.; O’Connor, W., Jr.; Huber, F.J.; Chaudhry, A.; Kamanaka, M.; Kobayashi, Y.; Booth, C.J.; Rudensky, A.Y.; et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3− and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 2011, 34, 554–565. [Google Scholar] [CrossRef]
- Ueno, A.; Jeffery, L.; Kobayashi, T.; Hibi, T.; Ghosh, S.; Jijon, H. Th17 plasticity and its relevance to inflammatory bowel disease. J. Autoimmun. 2018, 87, 38–49. [Google Scholar] [CrossRef]
- Himmel, M.E.; Yao, Y.; Orban, P.C.; Steiner, T.S.; Levings, M.K. Regulatory T-cell therapy for inflammatory bowel disease: More questions than answers. Immunology 2012, 136, 115–122. [Google Scholar] [CrossRef]
- Caruso, R.; Lo, B.C.; Nunez, G. Host-microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 2020, 20, 411–426. [Google Scholar] [CrossRef]
- Basso, P.J.; Câmara, N.O.S.; Sales-Campos, H. Microbial-Based Therapies in the Treatment of Inflammatory Bowel Disease—An Overview of Human Studies. Front. Pharmacol. 2019, 9, 1571. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Ooijevaar, R.E.; Terveer, E.M.; Verspaget, H.W.; Kuijper, E.J.; Keller, J.J. Clinical Application and Potential of Fecal Microbiota Transplantation. Annu. Rev. Med. 2019, 70, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Kurashima, Y.; Kiyono, H. Mucosal ecological network of epithelium and immune cells for gut homeostasis and tissue healing. Annu. Rev. Immunol. 2017, 35, 119–147. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Tran, T.D.; Briles, D.E.; Rhee, D.K. Inactivated pep27 mutant as an effective mucosal vaccine against a secondary lethal pneumococcal challenge in mice. Clin. Exp. Vaccine Res. 2013, 2, 58–65. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, E.H.; Choi, S.Y.; Kwon, M.K.; Tran, T.D.; Park, S.S.; Lee, K.J.; Bae, S.M.; Briles, D.E.; Rhee, D.K. Streptococcus pneumoniae pep27 mutant as a live vaccine for serotype-independent protection in mice. Vaccine 2012, 30, 2008–2019. [Google Scholar] [CrossRef]
- Kim, G.L.; Choi, S.Y.; Seon, S.H.; Lee, S.; Park, S.S.; Song, J.Y.; Briles, D.E.; Rhee, D.K. Pneumococcal pep27 mutant immunization stimulates cytokine secretion and confers long-term immunity with a wide range of protection, including against non-typeable strains. Vaccine 2016, 34, 6481–6492. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.L.; Lee, S.; Kim, S.J.; Lee, S.O.; Pyo, S.; Rhee, D.K. Pulmonary Colonization Resistance to Pathogens via Noncanonical Wnt and Interleukin-17A by Intranasal pep27 Mutant Immunization. J. Infect. Dis. 2018, 217, 1977–1986. [Google Scholar] [CrossRef]
- Seon, S.H.; Choi, J.A.; Yang, E.; Pyo, S.; Song, M.K.; Rhee, D.K. Intranasal immunization with an attenuated pep27 mutant provides protection from Influenza virus and secondary pneumococcal infections. J. Infect. Dis. 2018, 217, 637–640. [Google Scholar] [CrossRef]
- Kim, B.G.; Ghosh, P.; Ahn, S.; Rhee, D.K. Pneumococcal pep27 mutant immunization suppresses allergic asthma in mice. Biochem. Biophys. Res. Commun. 2019, 514, 210–216. [Google Scholar] [CrossRef]
- Iqbal, H.; Kim, G.L.; Kim, J.H.; Ghosh, P.; Shah, M.; Lee, W.; Rhee, D.K. Pneumococcal pep27-mutant inhibits Wnt5a expression via the regulation of T helper cells to attenuate colitis. Int. Immunopharmacol. 2022, 109, 108927. [Google Scholar] [CrossRef]
- Geboes, K.; Riddell, R.; Ost, A.; Jensfelt, B.; Persson, T.; Lofberg, R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000, 47, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Yi, T.G.; Park, J.M.; Han, Y.M.; Kim, J.H.; Shin, D.H.; Tak, S.J.; Lee, K.; Lee, Y.S.; Jeon, M.S.; et al. Therapeutic effects of mouse bone marrow-derived clonal mesenchymal stem cells in a mouse model of inflammatory bowel disease. J. Clin. Biochem. Nutr. 2015, 57, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.; Kim, S.K.; Cha, K.M.; Jeong, M.S.; Ghosh, P.; Rhee, D.K. Korean Red Ginseng alleviates neuroinflammation and promotes cell survival in the intermittent heat stress-induced rat brain by suppressing oxidative stress via estrogen receptor beta and brain-derived neurotrophic factor upregulation. J. Ginseng Res. 2020, 44, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Radu, M.; Chernoff, J. An in vivo assay to test blood vessel permeability. J. Vis. Exp. 2013, 73, e50062. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Kim, K.Y.; Lee, J.H.; Choi, Y. The analysis of oral microbial communities of wild-type and toll-like receptor 2-deficient mice using a 454 GS FLX Titanium pyrosequencer. BMC Microbiol. 2010, 10, 101. [Google Scholar] [CrossRef]
- Ishikawa, D.; Okazawa, A.; Corridoni, D.; Jia, L.G.; Wang, X.M.; Guanzon, M.; Xin, W.; Arseneau, K.O.; Pizarro, T.T.; Cominelli, F. Tregs are dysfunctional in vivo in a spontaneous murine model of Crohn’s disease. Mucosal Immunol. 2013, 6, 267–275. [Google Scholar] [CrossRef]
- Lee, K.H.; Park, M.; Ji, K.Y.; Lee, H.Y.; Jang, J.H.; Yoon, I.J.; Oh, S.S.; Kim, S.M.; Jeong, Y.H.; Yun, C.H.; et al. Bacterial β-(1,3)-glucan prevents DSS-induced IBD by restoring the reduced population of regulatory T cells. Immunobiology 2014, 219, 802–812. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Green, D.R.; Llambi, F. Cell death signalling. Cold Spring Harb. Perspect Biol. 2015, 7, a006080. [Google Scholar] [CrossRef]
- Sahin, A.; Ilhan, A.; Derin, M.E.; Dogan, H.O.; Sahin, M. Low levels of pannexin-1 in Behcet’s syndrome. Int. J. Rheum. Dis. 2019, 22, 1474–1478. [Google Scholar] [CrossRef]
- Solomon, I.; Amann, M.; Goubier, A.; Vargas, F.A.; Zervas, D.; Qing, C.; Henry, J.Y.; Ghorani, E.; Akarca, A.U.; Marafioti, T.; et al. CD25-Treg-depleting antibodies preserving IL-2 signaling on effector T cells enhance effector activation and antitumor immunity. Nat. Cancer 2020, 1, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Nava, P.; Koch, S.; Laukoetter, M.G.; Lee, W.Y.; Kolegraff, K.; Capaldo, C.T.; Beeman, N.; Addis, C.; Gerner-Smidt, K.; Neumaier, I.; et al. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity 2010, 32, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ghosh, P.; Kwon, H.; Park, S.S.; Kim, G.L.; Choi, S.Y.; Kim, E.H.; Tran, T.D.; Seon, S.H.; Le, N.T.; et al. Induction of the pneumococcal vncRS operon by lactoferrin is essential for pneumonia. Virulence 2018, 9, 1562–1575. [Google Scholar] [CrossRef]
- Vermeij, W.P.; Florea, B.I.; Isenia, S.; Alia, A.; Brouwer, J.; Backendorf, C. Proteomic identification of in vivo interactors reveals novel function of skin cornification proteins. J. Proteome Res. 2012, 11, 3068–3076. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, W.P.; Backendorf, C. Skin cornification proteins provide global link between ROS detoxification and cell migration during wound healing. PLoS ONE 2010, 5, e11957. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Lee, S.; Kim, S.J.; Rhee, D.K. Korean Red Ginseng enhances pneumococcal Δpep27 vaccine efficacy by inhibiting reactive oxygen species production. J. Ginseng Res. 2019, 43, 218–225. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef]
- Lindenberg, F.; Krych, L.; Fielden, J.; Kot, W.; Frøkiær, H.; van Galen, G.; Nielsen, D.S.; Hansen, A.K. Expression of immune regulatory genes correlate with the abundance of specific Clostridiales and Verrucomicrobia species in the equine ileum and cecum. Sci. Rep. 2019, 9, 12674. [Google Scholar] [CrossRef]
- Dou, X.; Gao, N.; Yan, D.; Shan, A. Sodium Butyrate Alleviates Mouse Colitis by Regulating Gut Microbiota Dysbiosis. Animals 2020, 10, 1154. [Google Scholar] [CrossRef]
- Yan, S.; Yang, B.; Zhao, J.; Zhao, J.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, W. A ropy exopolysaccharide producing strain Bifidobacterium longum subsp. longum YS108R alleviates DSS-induced colitis by maintenance of the mucosal barrier and gut microbiota modulation. Food Funct. 2019, 10, 1595–1608. [Google Scholar] [CrossRef]
- Schwerk, C.; Schulze-Osthoff, K. Non-apoptotic functions of caspases in cellular proliferation and differentiation. Biochem. Pharmacol. 2003, 66, 1453–1458. [Google Scholar] [CrossRef]
- Denecker, G.; Ovaere, P.; Vandenabeele, P.; Declercq, W. Caspase-14 reveals its secrets. J. Cell Biol. 2008, 180, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lin, H.; Deng, X.; Li, S.; Zhang, J. MiR-1246 promotes anti-apoptotic effect of mini-αA in oxidative stress-induced apoptosis in retinal pigment epithelial cells. Clin. Exp. Ophthalmol. 2020, 48, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Suresh, P.K. Enhanced Induction of Apoptosis in HaCaT Cells by Luteolin Encapsulated in PEGylated Liposomes-Role of Caspase-3/Caspase-14. Appl. Biochem. Biotechnol. 2019, 188, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, A.; Liu, S.; He, Q.; Luo, Y.; He, Z.; Chen, Y.; Tao, A.; Yan, J. Inhibition of HtrA2 alleviated dextran sulfate sodium (DSS)-induced colitis by preventing necroptosis of intestinal epithelial cells. Cell Death Dis. 2019, 10, 344. [Google Scholar] [CrossRef]




| Gene Name | Sequence (5′ to 3′) | |
|---|---|---|
| Caspase-14 | F | GATGAGGTTGCTGTGCTCAA |
| R | TTGGTTTTCCTTCCTGCATC | |
| Claudin-1 | F | GATGTGGATGGCTGTCATTG |
| R | CCACTAATGTCGCCAGACCT | |
| FOXP3 | F | CCTGCCTTGGTACATTCGTG |
| R | TGTTGTGGGTGAGTGCTTTG | |
| IFN-γ | F | ACAGCTCTCCGTCCTCGTAT |
| R | CCTTAGAGTAGAAAGACCG | |
| IL-10 | F | AGCCACATGCTCCTAGAGC |
| R | GCCTGGGGCATCACTTCTAC | |
| IL-17A | F | TCCAGAAGGCCCTCAGACTA |
| R | AGCATCTTCTCGACCCTGAA | |
| TGF-β1 | F | ACCATGCCAACTTCTGTCTG |
| R | CGGGTTGTGTTGGTTGTAGA | |
| TNF-α | F | GCC TCTTCTCATTCCTGCTT |
| R | TGGGAACTTCTCATCCCT TTG | |
| GAPDH | F | TCAACAGCAACTCCCACTCTTCCA |
| R | ACCCTGTTGCTGTAGCCGTATTCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, H.; Kim, G.-L.; Kim, J.-H.; Ghosh, P.; Shah, M.; Lee, W.; Rhee, D.-K. Pep27 Mutant Immunization Inhibits Caspase-14 Expression to Alleviate Inflammatory Bowel Disease via Treg Upregulation. Microorganisms 2022, 10, 1871. https://doi.org/10.3390/microorganisms10091871
Iqbal H, Kim G-L, Kim J-H, Ghosh P, Shah M, Lee W, Rhee D-K. Pep27 Mutant Immunization Inhibits Caspase-14 Expression to Alleviate Inflammatory Bowel Disease via Treg Upregulation. Microorganisms. 2022; 10(9):1871. https://doi.org/10.3390/microorganisms10091871
Chicago/Turabian StyleIqbal, Hamid, Gyu-Lee Kim, Ji-Hoon Kim, Prachetash Ghosh, Masaud Shah, Wonsik Lee, and Dong-Kwon Rhee. 2022. "Pep27 Mutant Immunization Inhibits Caspase-14 Expression to Alleviate Inflammatory Bowel Disease via Treg Upregulation" Microorganisms 10, no. 9: 1871. https://doi.org/10.3390/microorganisms10091871
APA StyleIqbal, H., Kim, G.-L., Kim, J.-H., Ghosh, P., Shah, M., Lee, W., & Rhee, D.-K. (2022). Pep27 Mutant Immunization Inhibits Caspase-14 Expression to Alleviate Inflammatory Bowel Disease via Treg Upregulation. Microorganisms, 10(9), 1871. https://doi.org/10.3390/microorganisms10091871






