Abstract
Background: PJIs following total hip and knee arthroplasty represent severe complications with broad implications, and with significant disability, morbidity, and mortality. To be able to provide correct and effective management of these cases, an accurate diagnosis is needed. Classically, acute PJIs are characterized by a preponderance of virulent microorganisms, and chronic PJIs are characterized by a preponderance of less-virulent pathogens like coagulase-negative staphylococci or Cutibacterium species. This paper aims to analyze if there are any changes in the causative microorganisms isolated in the last years, as well as to provide a subanalysis of the types of PJIs. Methods: In this single-center study, we prospectively included all retrospectively consecutive collected data from patients aged over 18 years that were hospitalized from 2016 through 2022, and patients that underwent a joint arthroplasty revision surgery. A standardized diagnostic protocol was used in all cases, and the 2021 EBJIS definition criteria for PJIs was used. Results: 114 patients were included in our analysis; of them, 67 were diagnosed with PJIs, 12 were acute/acute hematogenous, and 55 were chronic PJIs. 49 strains of gram-positive aerobic or microaerophilic cocci and 35 gram-negative aerobic bacilli were isolated. Overall, Staphylococcus aureus was the most common isolated pathogen, followed by coagulase-negative staphylococci (CoNS). All cases of acute/acute hematogenous PJIs were caused by gram-positive aerobic or microaerophilic cocci pathogens. Both Staphylococcus epidermidis and methicillin-resistant S. aureus were involved in 91.66% of the acute/acute hematogenous PJIs cases. 21.8% of the chronic PJIs cases were caused by pathogens belonging to the Enterobacterales group of bacteria, followed by the gram-negative nonfermenting bacilli group of bacteria, which were involved in 18.4% of the cases. 12 chronic cases were polymicrobial. Conclusion: Based on our findings, empiric broad-spectrum antibiotic therapy in acute PJIs could be focused on the bacteria belonging to the gram-positive aerobic or microaerophilic cocci, but the results should be analyzed carefully, and the local resistance of the pathogens should be taken into consideration.
1. Introduction
There is a broad range of biofilm-related infections (BRI), from catheter-associated urinary tract infections (which still represent the most common BRIs) to central line-associated bloodstream infections, fracture-related infections, BRI associated with the use of fixed braces, and last but not least, periprosthetic joint infections [1,2,3].
Bacteria can be organized into two types: planktonic bacteria when they appear as single cell structures, and biofilms when they are structured in a sessile form and organized in multicellular aggregates [4,5]. To this day, we still do not have a consensus definition of a biofilm; but often, biofilms are defined as ‘a coherent cluster of bacterial cells imbedded in a biopolymer matrix, which, compared with planktonic cells, shows increased tolerance to antimicrobials and resists the antimicrobial properties of the host defence’ [5]. Bacteria are capable of a rapid transition between planktonic forms and biofilms [6,7,8]. One of the most important differences between planktonic bacteria and biofilm bacteria is represented by the antibiotic tolerance. Biofilm bacteria is known to present increased survival capabilities on exposure to multiple classes of antibiofilm antiobiotics. Characteristics that are related to many bacterial functions are the slow growth state, impaired expression of antimicrobial resistance mechanisms, and possible depletion aggregation [9,10,11,12,13]. Biofilms-related infections are recalcitrant to antibiotic strategies. It has been already published in the literature that the antimicrobial concentrations needed to eradicate biofilms are higher than the concentrations required to eradicate the same bacterial clones in a planktonica state [14].
Biofilms are commonly associated with a foreign body material, such as when a prosthetic is implanted; abiotic artificial surfaces that offer a perfect interface to which planktonic bacteria may attach and form a biofilm [15].
Staphylococcus aureus represents a frequent intra- and extracellular pathogen associated with orthopedic devices biofilm-related infections. In several publications in the literature, it is reported that biofilm-forming S. aureus is the most common pathogen in biofilm-related infections, as well as the main pathogen associated with reinfection, due to its high resistance to the immune response and antibiotic treatments, as well as to its ability to infect not only bone-forming cells (osteoblasts), but also the cells responsible for bone resorption (osteoclasts) [16]. The incidence of orthopedic methicillin-resistant Staphylococcus aureus infections has increased, and since it has also been proven effective against MRSA strains, vancomycin is recommended as the first-line antibiotic therapy choice for treatment of orthopedic MRSA infections [17].
Total joint arthroplasties are a very effective medical intervention. Unfortunately, complications may occur in some patients [18]. Periprosthetic joint infections (PJIs) are devastating complications following total joint arthroplasty, most commonly associated with total hip or knee arthroplasty (due to the increased number of this type of surgery), with broad implications, and with significant morbidity and mortality [18]. A total of 16.8% of all knee-revision surgeries and 14.8% of all hip-revision surgeries are due to failure caused by PJIs [19]. Prosthetic joint infections occur at a frequency of 1 to 3% and are still a major cause of healthcare expenditure [20]. Other authors report that periprosthetic joint infections of the hip and knee occur in approximately 1 to 2% of patients after total joint arthroplasties [21,22]. These are complications that lead to a prolonged hospital stay, multiple surgeries, and functional impairment [23]. Knowledge of the microbiological agent that causes the PJIs is one of the most important aspects, together with the antimicrobial susceptibility test (AST) results. Information that is also essential for guiding empiric antibiotic therapy, particularly in acute periprosthetic joint infections. Unfortunately, local data is not available. The aim of this paper is to analyze if there are any changes in the causative microorganisms isolated in the last years, as well as to provide a sub-analysis of the types of PJIs. We also analyzed the antimicrobial susceptibility test results especially to see if any changes in the frequency of antimicrobial-resistant organisms in PJIs have occurred in the last years.
2. Materials and Methods
2.1. Study Design
A single-center observational, cohort, ongoing study was conducted in the Emergency Clinical County Hospital, Romania. Before patients’ inclusion in the study, the study protocol was reviewed and approved by the institutional review board. A standardized diagnostic system was used to assess all patients who underwent surgical intervention for the revision of a joint prosthesis to determine implant failure. Our implemented diagnostic strategy included a sampling of intraoperative tissue specimens, sonication of the retreated implant and sonication fluid cultures, and cell counting of the synovial fluid. As a rapid method of bacteria detection from the sonication fluid, we used a bbFISH kit (hemoFISH Masterpanel, Miacom diagnostics GmbH Düsseldorf, Germany). All specimens were inoculated on aerobic and anaerobic culture media (Schaedler anaerobe broth, Sabouraud plate, MacConkey agar plate, glucose broth, lactose broth, and thioglycollate broth), and a 14-day period of incubation period was implemented as a standard.
2.2. Study Population
We prospectively included all consecutive patients, aged over 18 years, who were hospitalized from 2016 through October 2022 and underwent joint arthroplasty revision surgery for any reason. We excluded all cases with positive bacterial cultures from harvested specimens during the second stage of 2-stage revision surgery and all with positive sonication fluid cultures from spacer sonication. Detailed information was extracted from the medical records of the patients using a standardized electronic collection form. All data were available for all the enrolled patients.
2.3. Laboratory Studies
Our newly implemented diagnostic strategy included a standardized sampling of at least four intraoperative tissue samples (one of the samples used for the histopathological examination (the periprosthetic membrane) and the others were sent to the microbiological laboratory for bacterial cultures). For the sonication of the retrieved implants, in the operating theater, sterile Ringer’s or saline solution was added over the implants that were deposited in sterile containers. These containers were previously sterilized according to the manufacturer’s instructions and double-packed. The implants were processed within 30 min by sonication (1 min) using an ultrasound bath (BactoSonic14.2, Bandelin GmbH, Berlin, Germany) at a frequency of 42 kHz and a power density of 0.22 W/cm2. The resulting sonication fluid was vortexed, and 50 mL of sonication fluid was centrifuged at 2500 rpm for 5 min. The resulting precipitate was inoculated. If >50 CFU/mL were counted, sonication fluid cultures were considered positive. Ten milliliters of sonication fluid were incubated in blood culture bottles in a blood culture system (BD BACTEC™). Regarding the periprosthetic tissue cultures, tissue samples were collected in sterile vials and individually homogenized in 1 mL thioglycolate broth. Tissue homogenate samples (1 mL) were inoculated into the culture media. Synovial fluid was aspirated preoperatively in a native vial and inoculated into different media for culturing. All biological samples that required cultures were inoculated and incubated aerobically, anaerobically, and in a high concentration of CO2 (GENbag-GENbox Atmospheric generators bioMérieux, Marcy-l’Étoile, France) Schaedler anaerobe broth, Sabouraud plate, MacConkey agar plate, glucose broth, lactose broth, and thioglycollate broth, at 37 °C. The isolated bacteria were identified using a VITEK 2 Compact analyzer (bioMérieux, Marcy-l’Étoile, France). Minimum inhibitory concentrations were assessed according to the EUCAST (European Committee on Antimicrobial Susceptibility Testing) breakpoints. We were able to analyze cultures during working days and weekends. We previously published full details of the implemented protocol [24,25].
2.4. Study Definitions and Classification
A culture was marked as positive on the day that an isolate was identified by the VITEK 2 Compact analyzer (bioMérieux, Marcy-l’Étoile, France), the first day of growth. A periprosthetic joint infection was diagnosed using the 2021 European Bone and Joint Infection Society (EBJIS) definition for the diagnosis of periprosthetic joint infection (Table 1.) [26].

Table 1.
2021 European Bone and Joint Infection Society (EBJIS) definition for the diagnosis of periprosthetic joint infection [26].
We used the classification proposed by Zimmerli et al. to determine if there was an acute, late chronic, or acute late periprosthetic joint infection, a classification that defines the prosthetic joint infections as early (occurring within 3 months after surgery), delayed (3–24 months), or late (>24 months) [20]. Due to the small number of enrolled patients, we also used a much simpler classification, a classification from the Pocket Guide to Diagnosis & Treatment of Periprosthetic Joint Infection (PJI) of the PRO-IMPLANT Foundation, Berlin, Germany (coordinated by N. Renz and A. Trampuz), a guide that is in line with national and international recommendations and that defines periprosthetic infections as acute or chronic (Perioperative/Hematogenous or per continuitatem).
2.5. Statistical Analysis
We performed the statistical analysis using the IBM SPSS Statistics® version 28 software. Continuous variables were summarized as medians and interquartile ranges or mean and standard deviation and categorical variables as percentages of the total sample for that variable. Overall Percentages of culture positive PJIs were determined and estimated with a 95% CI. The Mantel-Haenszel chi-square test was used to determine if there was a statistically significant conditional dependence between the percentages of the identified PJI organisms and multidrug resistant bacteria over the studied period. A significance level of p ≤ 0.05 was used for all statistical tests.
3. Results
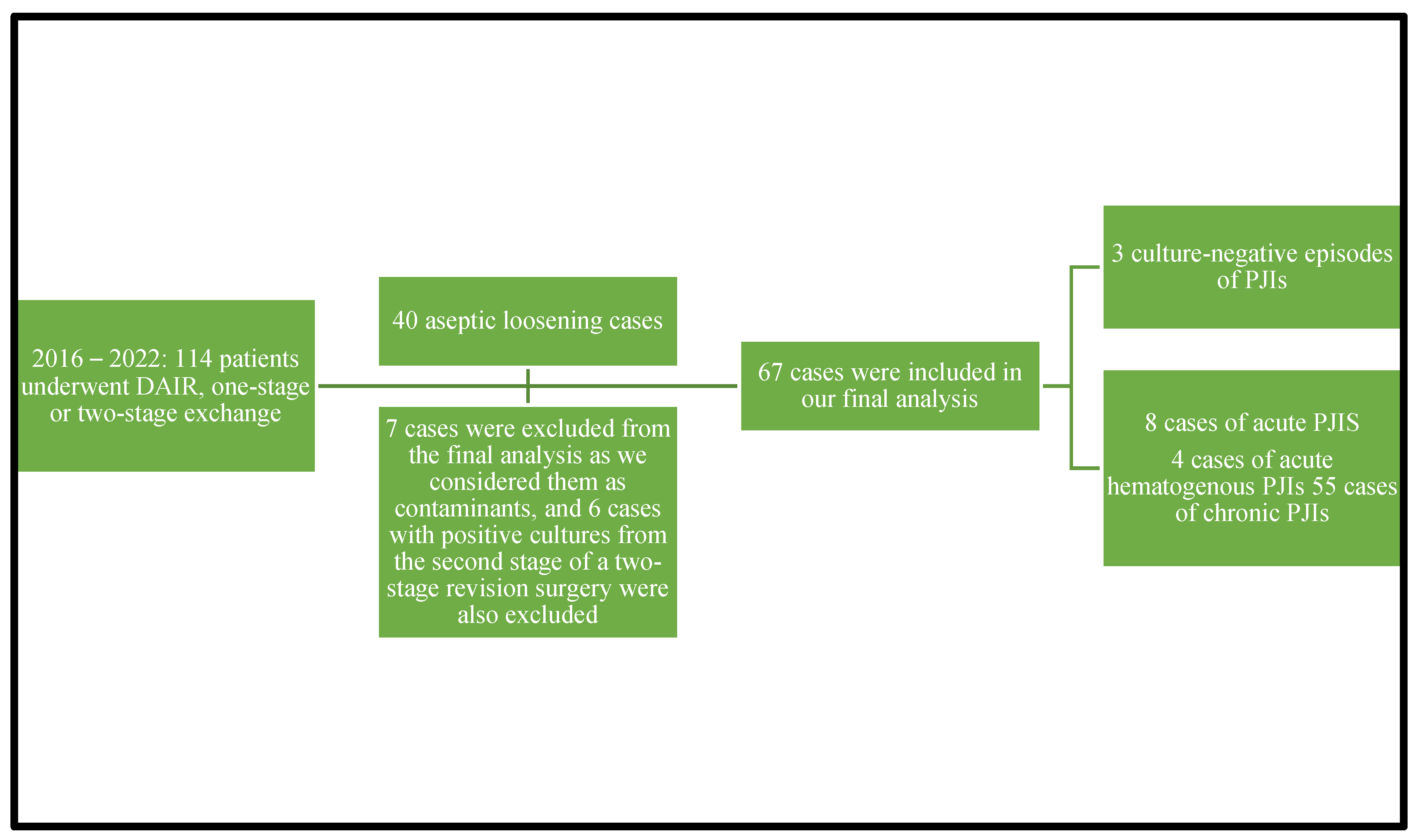
A total number of 114 patients underwent debridement, antibiotics, and implant retention procedures or one-stage or two-stage revision surgery from 2016 through 2022. A diagnosis of aseptic loosening of an endoprosthesis was established in 40 adult patients during the study period. A total number of 67 episodes of periprosthetic joint infections were diagnosed in the analyzed period from 67 cases, cases that were culture-positive ones. We excluded seven cultures from the final analysis that were considered contaminants and six cases with positive cultures from harvested specimens during the second stage of 2-stage revision surgery. Three patients had culture-negative PJIs. A total of 67 confirmed PJIs were included in our final analysis. Of the 67 cases analyzed in our study, 12 had acute/acute hematogenous PJIs and 55 had chronic PJIs. Eight cases were acute PJIs and four were acute hematogenous PJIs. Due to the small number of enrolled patients, we decided to analyze the acute and acute hematogenous PJIs together. The 67 cases of PJIs included 40 hip prosthesis and 27 knee prosthesis. We were able to isolate 12 microorganisms from the 12 acute PJI cultures. The 55 chronic PJI cultures yielded 72 isolated microorganisms. We were able to group the 67 patients diagnosed with a periprosthetic joint infection, using the classification proposed by Zimmerli et al., as follows: ten patients were diagnosed with early PJI, nine patients with delayed PJI, and forty-eight patients were diagnosed with a late PJI. Again, using the classification of the periprosthetic joint infections proposed in the Pocket Guide by the PRO-IMPLANT Foundation as we did in previously published articles [22], eight patients were diagnosed with an acute perioperative infection, four patients with acute hematogenous infection, and fifty-five patients with chronic PJI. We will report all our results using this classification. Figure 1 represents the flow diagram showing details of the enrolled patients.
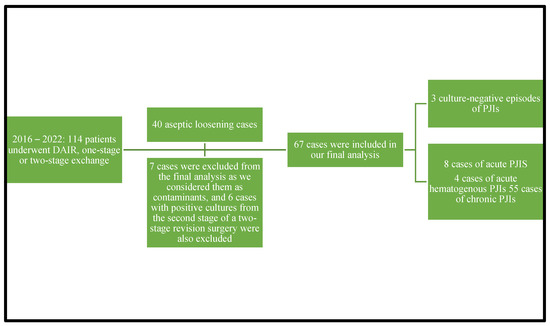
Figure 1.
Flow diagram showing details of the enrolled patients.
Characteristics of the enrolled patients in the study are outlined in Table 2. The mean age of the study population was 68.5 years old (±10.88 SD) and 34 (50.74%) were male patients. The median ASA score of the studied population was 2 and the median Charlson Comorbidity Index was 3. 92.53% of the enrolled patients had at least one comorbidity, the most common ones being arterial hypertension, heart failure, diabetes mellitus, chronic heart disease, peripheral vascular disease, and obesity. Osteoarthritis was the most common reason for primary prosthesis implantation (55.22%), followed by femoral neck fracture (10.44%), avascular necrosis (5.96%), and rheumatoid arthritis (2.98%). No trend changes in the baseline characteristics of the enrolled patients diagnosed with PJIs were found during the study period.

Table 2.
Baseline characteristics of the enrolled patients diagnosed with PJI from 2016 through 2022.
According to our results, 48 (55.2%; 95% CI 44.8–65.5) episodes of PJIs were caused by gram-positive aerobic or microaerophilic cocci and 35 (40.2%; 95% CI 29.9–50.6) by gram-negative aerobic bacilli. Overall, Staphylococcus aureus was the most common isolated pathogen, 21 (24.1%; 95% CI 14.9–33.3); followed by coagulase-negative staphylococci (CoNS), 19 (21.8%; 95% CI 13.8–31); Enterobacterales, 19 (21.8%; 95% CI13.8–31); and gram-negative nonfermenting bacilli, 16 (18.4%; 95% CI 10.3–26.4).
A microbiological diagnosis was obtained in 67 cases: 23 cases in 2016–2017, 33 in 2018–2019, and 11 in 2020–2022. A significant variation in the proportion of cases with a microbiological diagnosis using our diagnostic method was not observed during the study period. A total of 12 cases of PJIs were polymicrobial and all of them were chronic cases. Additionally, no significant trends over the study period in the proportion of polymicrobial PJIs were observed.
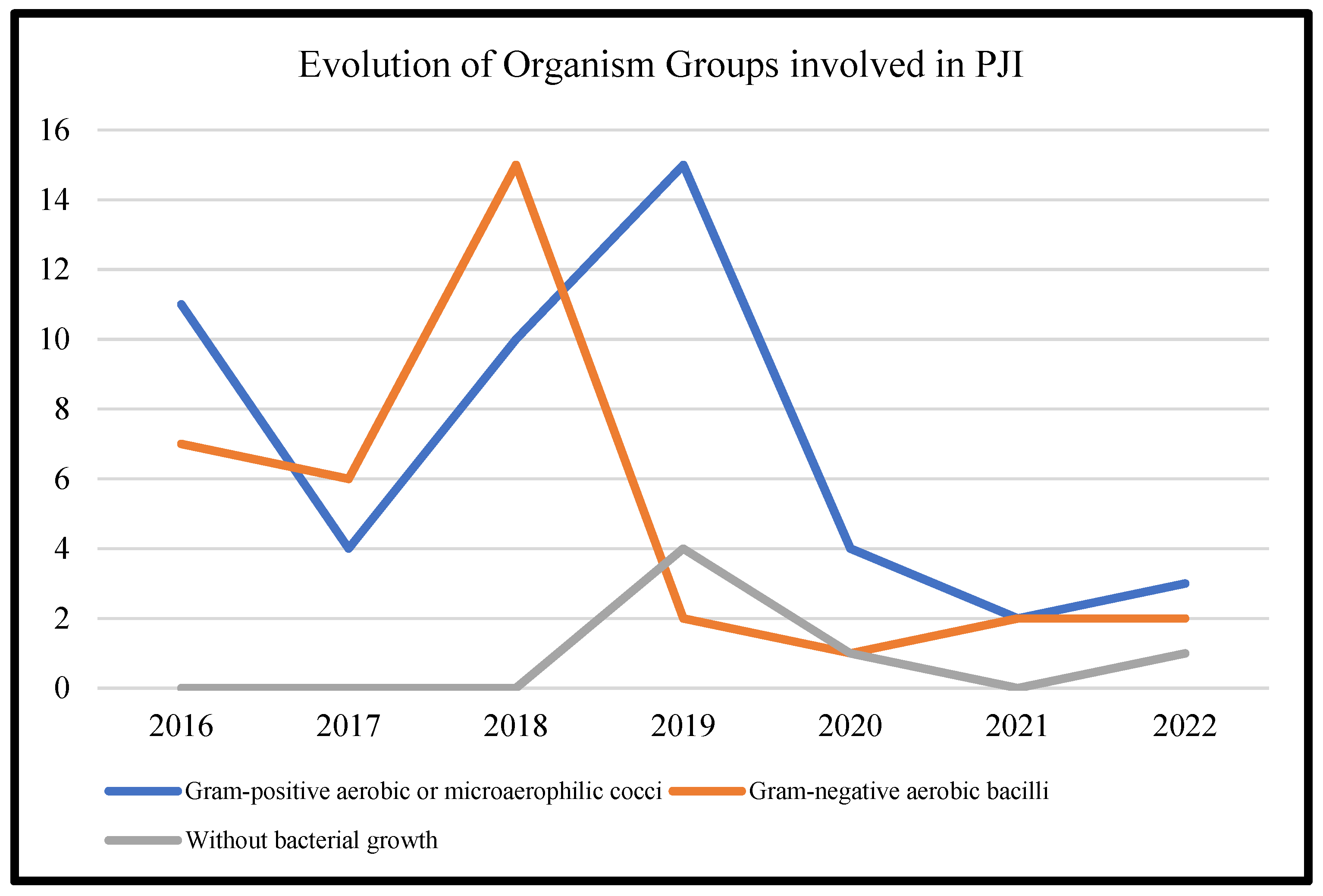
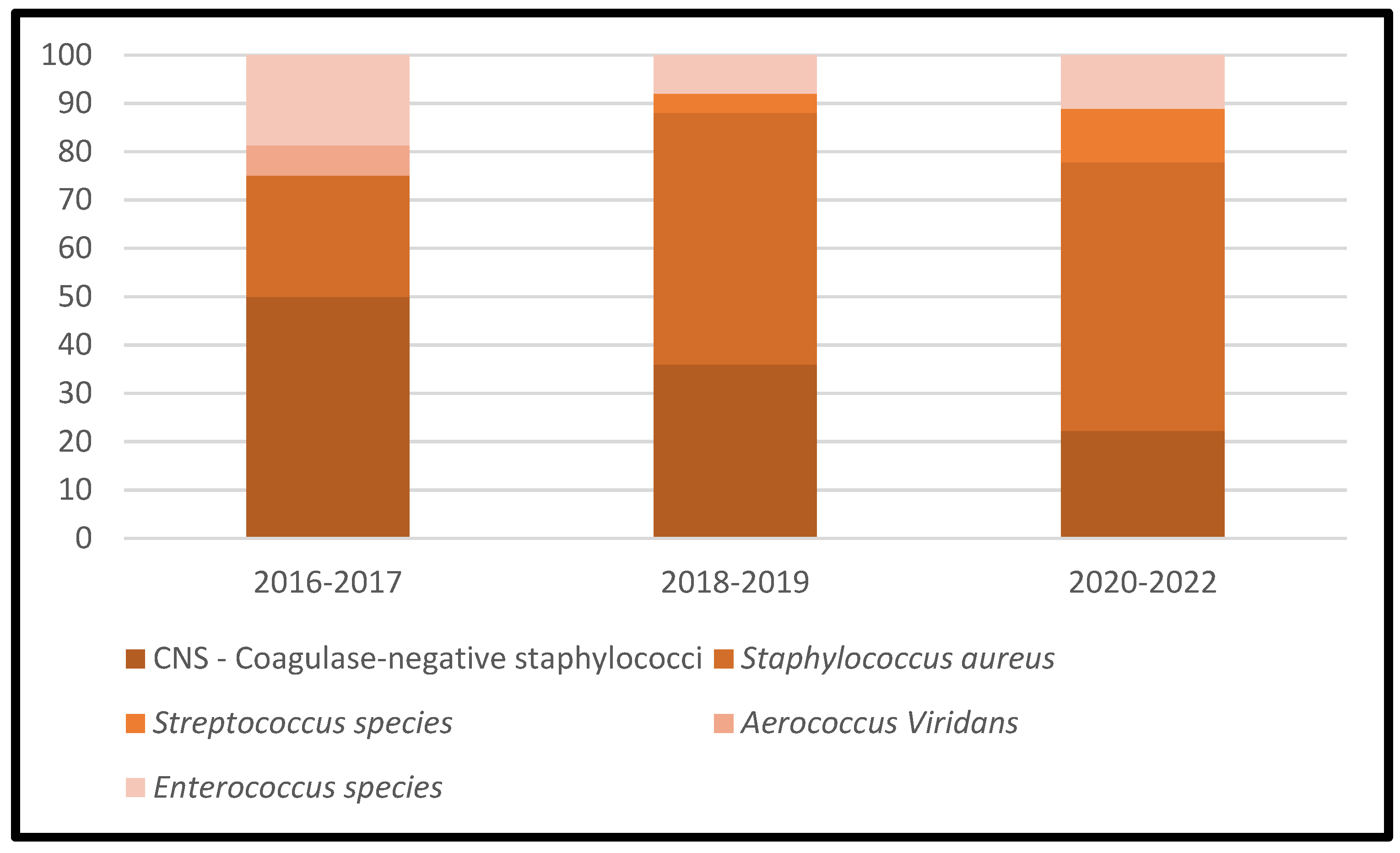
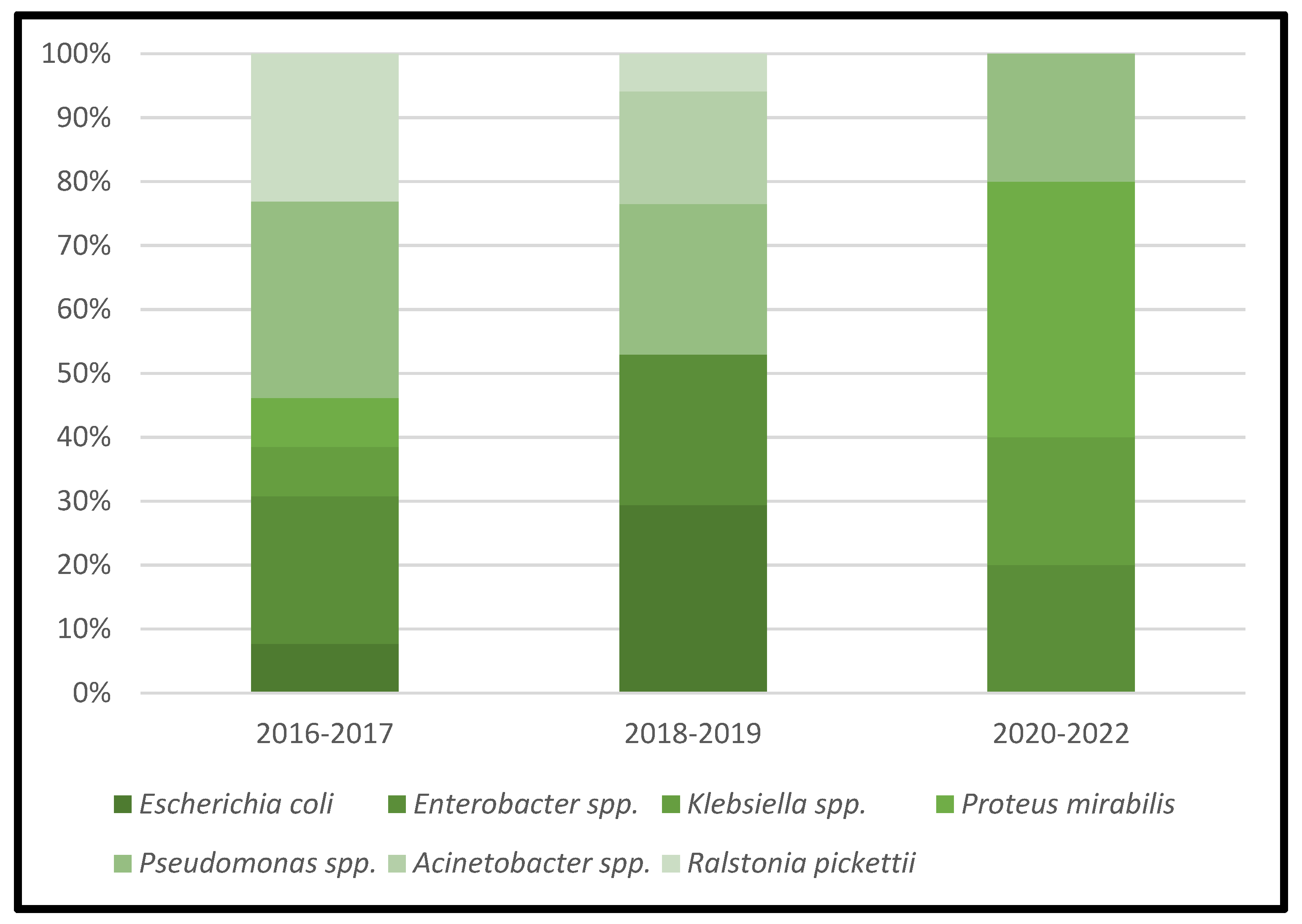
Table 3 represents the lists of causative microorganisms of PJIs during the study period (2016 through 2021). Gram-positive aerobic or microaerophilic cocci were the most common group of isolated organisms, followed by gram-negative aerobic bacilli. A biennial proportion analysis of the isolated microorganisms was performed. Unfortunately, no changes during the study period were observed, and no statistically significant rising or decreasing linear trends were observed for PJIs caused by gram-positive aerobic or microaerophilic cocci or by gram-negative aerobic bacilli (Figure 2, Figure 3 and Figure 4).

Table 3.
Lists of causative microorganisms of PJIs during the study period (2016 through 2021).
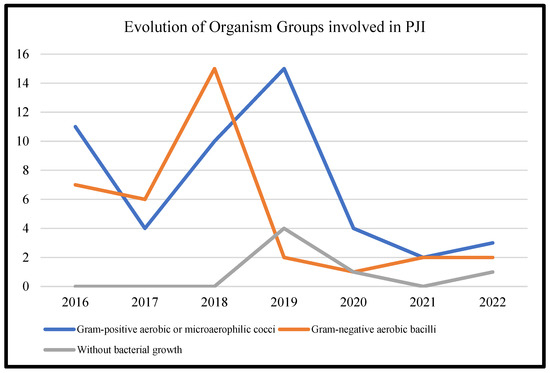
Figure 2.
Trends in the microbial etiology of PJIs.
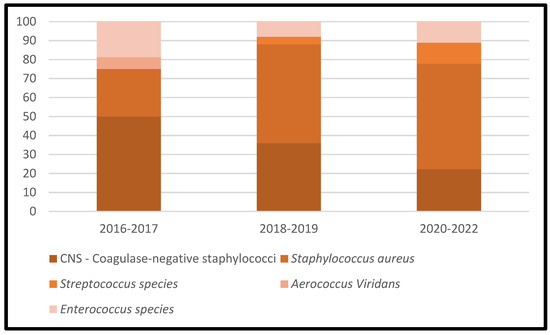
Figure 3.
Trends in the microbial etiology of PJIs: distribution of gram-positive aerobic or microaerophilic cocci.
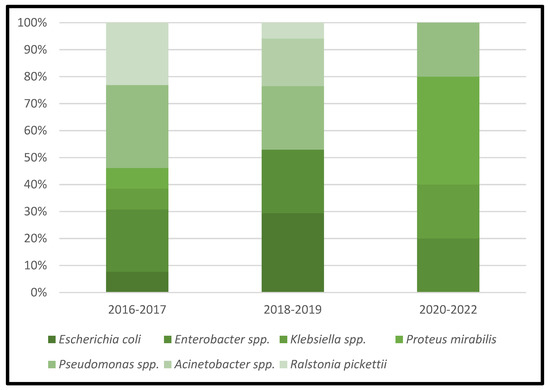
Figure 4.
Trends in the microbial etiology of PJIs: distribution of gram-negative aerobic bacilli.
All cases of acute/acute hematogenous PJIs were caused by gram-positive aerobic or microaerophilic cocci pathogens. Both Staphylococcus epidermidis and methicillin-resistant Staphylococcus aureus were involved in 91.66% of the acute/acute hematogenous PJIs cases. A total of 19 strains of pathogens from chronic PJIs cases belonged to the Enterobacterales group of bacteria, followed by the gram-negative nonfermenting bacilli group of bacteria, which were isolated in 16 samples.
48 strains of the isolated bacteria were multidrug-resistant bacterial strains (following the specified definition). Again, no statistically significant rising or decreasing linear trends were observed for multidrug-resistant bacteria.
3.1. Antimicrobial Susceptibility Test (AST) Results
We are reporting the AST based on the MICs (minimum inhibitory concentrations) of isolated bacterial strains that were evaluated following the European Committee on Antimicrobial Susceptibility Testing breakpoints (EUCAST) available at the time the strains were isolated. We will report the AST for the most isolated strains from our study. The AST results based on the MIC values are reported as follows: susceptible (S), intermediate (I), and resistant (R).
From the Coagulase-negative staphylococci (CoNS) species, eleven strains of Staphylococcus epidermidis were isolated, two strains of Staphylococcus lentus, three strains of Staphylococcus xylosus, one strain of Staphylococcus hominis, and two strains of Staphylococcus haemolyticus. Based on the MIC to oxacillin (4 mg/L–resistance), we can conclude that nine isolated strains were methicillin-resistant Staphylococcus epidermidis strains and two methicillin-susceptible Staphylococcus epidermidis strains. All strains of Staphylococcus lentus, Staphylococcus hominis, and Staphylococcus haemolyticus were also methicillin-resistant strains. All strains of Staphylococcus xylosus were methicillin-susceptible ones. A total of four CoNS strains were resistant to gentamycin and to quinolones/fluoroquinolones. All strains were susceptible to linezolid, teicoplanin, and vancomycin. All strains were also susceptible to trimethoprim/sulfamethoxazole, and just nine to rifampicin. According to EUCAST, it is known that vancomycin MIC values of 2 mg/L are on the border of the wild-type distribution and there may be an impaired clinical response. A MIC >2 mg/L for vancomycin measured using VITEK was not encountered, indicating that strains with reduced susceptibility to vancomycin were not isolated. We did not find an association between a MIC > 1 mg/L and co-resistance with rifampin: all rifampin-resistant strains (n = 2) had a vancomycin MIC <2 mg/L. Details regarding the AST results are reported in Table 4.

Table 4.
MICs (mg/L) of antimicrobials for Coagulase-negative staphylococci isolates.
Of the 21 strains of Staphylococcus aureus that were analyzed in this study based on the MIC to oxacillin, 14 strains were methicillin-resistant strains. All strains of Staphylococcus aureus maintained their susceptibility to fluoroquinolones, teicoplanin, linezolid, trimethoprim/sulfamethoxazole, and to rifampicin. A MIC >1 mg/L for teicoplanin measured using VITEK was not encountered. We did not find an association between a MIC >1 mg/L and co-resistance with rifampin, all isolated strains were susceptible to rifampin. Details regarding the AST results are reported in Table 5.

Table 5.
MICs (mg/L) of antimicrobials for Staphylococcus aureus isolates.
Six strains of Enterococcus faecalis were isolated in our study, of them, four strains were high-level resistant to gentamicin and other aminoglycosides except for streptomycin, and three strains were high-level resistant to streptomycin. All strains were susceptible to linezolid, teicoplanin, vancomycin, and tigecycline. A total of four out of the six Enterococcus faecalis-isolated strains were susceptible to trimethoprim/sulfamethoxazole. Details regarding the AST results are reported in Table 6.

Table 6.
MICs (mg/L) of antimicrobials for Enterococcus faecalis isolates.
Six strains of Escherichia coli were isolated in our study and AST was performed in all cases. Susceptibility was analyzed to beta-lactams-dibactams and in this case to ureidopenicillins such as piperacillin/ticarcillin in combination with a beta-lactamase inhibitor (piperacillin/tazobactam; ticarcillin/clavulanic acid)—the sensitivity being preserved for all strains. In five out of the six strains, resistance to gentamicin was recorded. As for susceptibility to carbapenems, it was preserved in all three tested antibiotics (ertapenem, imipenem, and meropenem). From the group of cephalosporins, the use of those of the 3rd or 4th generation is at least questionable, at least from the point of view of susceptibility, with four strains of Escherichia coli isolated being resistant to cefotaxime, ceftazidime, and cefepime. Thus, two of the most frequently used classes of antibiotics in the management of infections associated with orthopedic implants remain under discussion; quinolones/fluoroquinolones and sulfamides, five strains being sensitive to ciprofloxacin, five to trimethoprim/sulfamethoxazole, and all strains being intermediately sensitive to norfloxacin. Susceptibility to fosfomycin is preserved. Details regarding the AST results are reported in Table 7.

Table 7.
MICs (mg/L) of antimicrobials for Escherichia coli isolates.
From the Enterobacter species isolated strains, seven were identified as being Enterobacter cloacae complex and one was Enterobacter amnigenus 2. A total of five of the isolated strains were susceptible to Piperacillin/tazobactam, and four (50%) of the isolated strains were resistant to cephalosporins. 75% of the strains were resistant to gentamicin, three strains were resistant to ciprofloxacin, and susceptibility to fosfomycin was preserved for two strains. All strains maintained their susceptibility to trimethoprim/sulfamethoxazole. Details regarding the AST results are reported in Table 8.

Table 8.
MICs (mg/L) of antimicrobials for Enterobacter spp. isolates.
Of the two strains of Klebsiella spp. isolated, one strain was susceptible to most of the antibiotics used to treat bone and joint infections, and the other one was just intermediate to tigecycline.
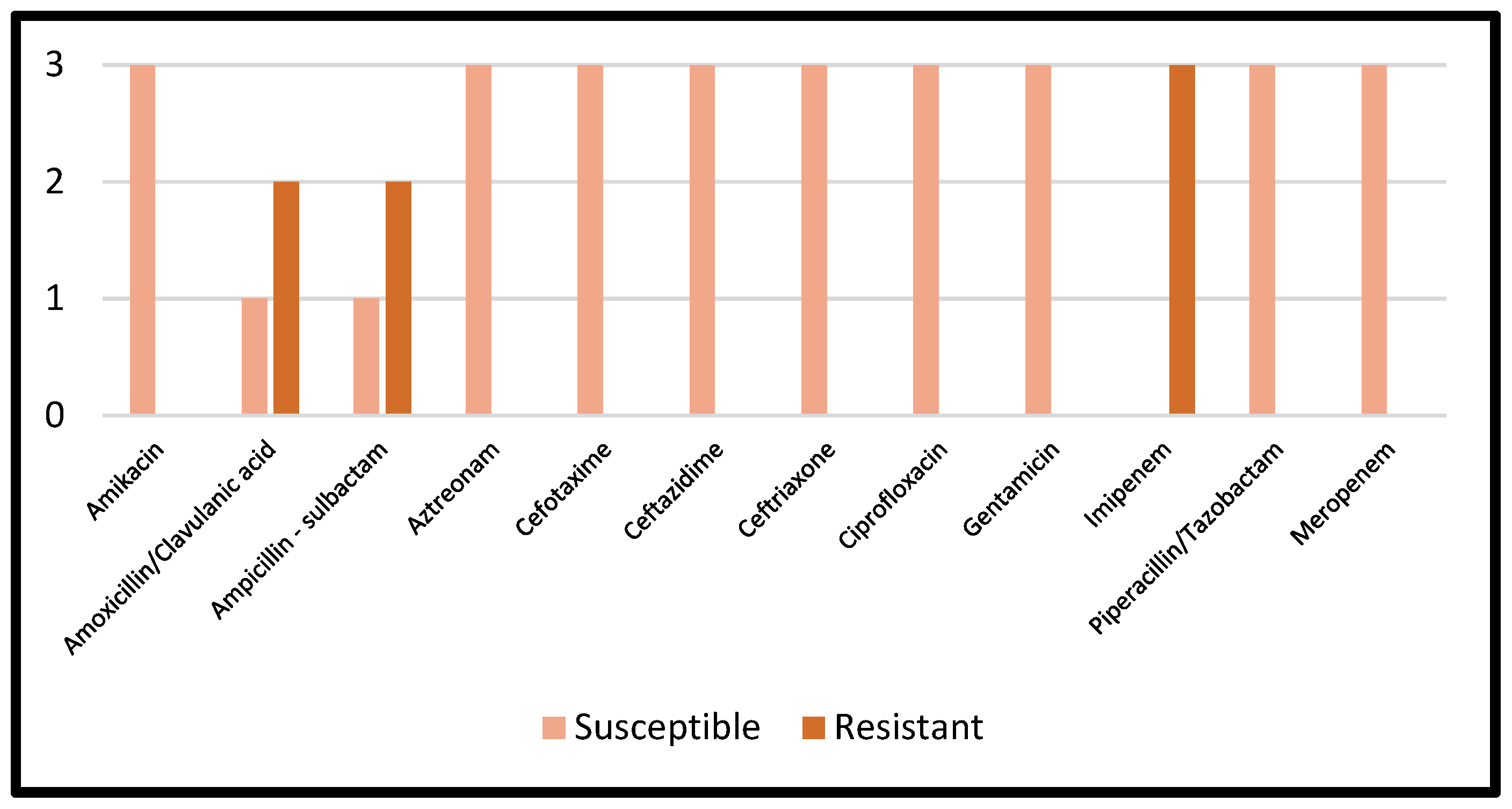
Details regarding the AST results of Proteus mirabilis are reported in Figure 5.
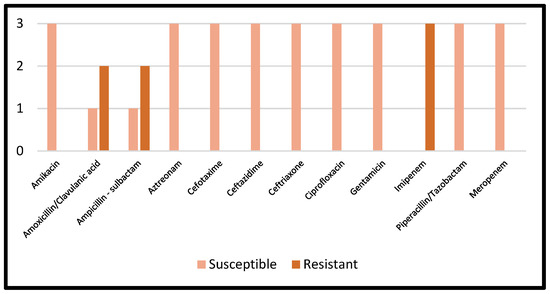
Figure 5.
AST results of Proteus mirabilis.
From the Pseudomonas species isolated strains, eight were identified as being Pseudomonas aeruginosa and one as Pseudomonas fluorescens. A total of five of the isolated strains were susceptible to ticarcillin/clavulanic acid, four to piperacillin/tazobactam, five to ceftazidime, six to imipenem, seven to meropenem, seven to ceftazidime/avibactam and ceftolozane/tazobactam, and five to ciprofloxacin. Details regarding the AST results are reported in Table 9.

Table 9.
MICs (mg/L) of antimicrobials for Pseudomonas spp. isolates.
A total of three strains of Acinetobacter species were identified in our study. Of them, all strains maintained their susceptibility to colistin and meropenem, and two strains maintained both to levofloxacin and trimethoprim/sulfamethoxazole. Details regarding the AST results are reported in Table 10.

Table 10.
MICs (mg/L) of antimicrobials for Acinetobacter spp. isolates.
A total of four strains of Ralstonia picketti species were identified in our study. Of them, all strains maintained their susceptibility to piperacillin, imipenem, meropenem, ciprofloxacin, pefloxacin, minocycline, and trimethoprim/sulfamethoxazole. All strains were resistant to tobramycin, and colistin. Details regarding the AST results are reported in Table 11.

Table 11.
MICs (mg/L) of antimicrobials for Ralstonia picketti isolates.
3.2. Multidrug-Resistant Periprosthetic Joint Infections
A total of 48 isolated strains were multidrug-resistant bacteria strains (following the specified definition) during the study period, including 14 methicillin-resistant S. aureus (MRSA) and 17 multidrug-resistant strains of gram-negative aerobic bacilli. MRSA and multidrug-resistant gram-negative aerobic bacilli were simultaneously involved in five cases of PJIs. As previously mentioned, no statistically significant rising or decreasing linear trend was observed for multidrug-resistant bacteria. The following species accounted for 100% of all isolated strains of multidrug-resistant gram-negative aerobic bacilli: Pseudomonas spp., four strains; Escherichia coli, five strains; Acinetobacter spp., two strains; Enterobacter cloacae complex, three strains; and Ralstonia pickettii, three strains. A total of 10 of the 17 multidrug-resistant gram-negative aerobic bacilli strains that were isolated in our study were also extended-spectrum β-lactamases-producing Enterobacterales strains. Concerning the resistance to specific antibiotics used in the management of PJIs, the most relevant is the resistance of some species at ciprofloxacin among gram-negative aerobic bacilli (11 strains).
4. Discussion
This is the first study from Romania that reported on causative microorganisms isolated in the last years from hip and knee periprosthetic joint infections. Most cases of periprosthetic joint infections in our study are monomicrobial (55 cases), and Staphylococcus aureus is also the most common cause of infection in this study, with similar data being previously reported in the literature [27,28,29,30,31]. No statistically significant rising or decreasing linear trends were observed for PJIs caused by gram-positive aerobic or microaerophilic cocci or by gram-negative aerobic bacilli, and also no statistically significant rising or decreasing linear trends were observed for multidrug-resistant bacteria, which is surprising compared to the data published by Benitio N. et al. in 2016, in which the authors report an increase of the proportion of PJIs caused by aerobic gram-negative aerobic bacilli [31]. Additionally, the same group of authors report an increase of the number of cases of PJIs caused by fungi; in our study, no cases of fungal PJIs were diagnosed. Our results should be analyzed according also to the limitations of our study. A total of 48 isolated strains were multidrug-resistant bacteria strains during the study period, including 14 methicillin-resistant S. aureus (MRSA) and 17 multidrug-resistant strains of gram-negative aerobic bacilli. MRSA and multidrug-resistant gram-negative aerobic bacilli were simultaneously involved in five cases of PJIs. No statistically significant rising or decreasing linear trends were observed for multidrug-resistant bacteria, although, in the literature, a rising trend of PJIs caused by MDR strains is reported [31].
To be able to decide on an appropriate empirical antimicrobial therapy, the common microbiological causes of periprosthetic joint infections should be known, most importantly, at a local/regional scale, but also at a national/global scale. In our study, all cases of acute/acute hematogenous PJIs were caused by gram-positive aerobic or microaerophilic cocci pathogens. Both Staphylococcus epidermidis and methicillin-resistant S. aureus were involved in 91.66% of the acute/acute hematogenous PJIs cases. In this context, empirical antimicrobial therapy for acute PJIs should be focused on gram-positive aerobic or microaerophilic cocci. Similar data are also reported in the literature were most of the acute PJIs are caused by Staphylococcus spp. infection [27,28,30,31]
In previous reports in the literature, gram-negative aerobic bacilli were involved in <10% of cases of PJI [20,29], while Benito N, et al. report that gram-negative aerobic bacilli, mainly Enterobacterales, were isolated in 28% of PJIs [31]. Other studies report the frequency of these pathogens in PJIs that range from 17% up to 42% [27,32,33]. In our study, 35 strains (40.2%; 95% CI 29.9–50.6) of gram-negative aerobic bacilli were isolated; of them, Enterobacteriaceae comprised 19 (21.8; 13.8–31) strains and gram-negative nonfermenting bacilli comprised 16 (18.4; 10.3–26.4) strains. Our data did not confirm a rise of multidrug-resistant gram-negative infections as others have [31]. Multidrug-resistant gram-negative bacilli infections in clinical settings have steadily increased in the last years and are becoming a public health care issue of importance in Europe [34]. In our study, the following species accounted for 100% of all isolated strains of multidrug-resistant gram-negative aerobic bacilli: Pseudomonas spp., four strains; Escherichia coli, five strains; Acinetobacter spp., two strains; Enterobacter cloacae complex, three strains; and Ralstonia pickettii, three strains. 10 of 17 multidrug-resistant gram-negative aerobic bacilli strains that were isolated in our study were also extended-spectrum β-lactamases producing Enterobacterales strains. Concerning the resistance to specific antibiotics used in the management of PJIs, the most relevant is the resistance of some species at ciprofloxacin among gram-negative aerobic bacilli (11 strains). Resistance to quinolones is of greatest concern because ciprofloxacin is widely used in the treatment of PJIs caused by gram-negative bacilli [35]. Benito N et al. showed that almost 18% of gram-negative aerobic bacilli strains are resistant to quinolones and that there is an increasing resistance trend [31]. Fourteen strains of methicillin-resistant S. aureus and nine strains of methicillin-resistant Staphylococcus epidermidis were isolated in our study, in the study published by Benito N et. al, the percentage of MRSA increased from 4.7% in 2003–2004 to 9.5% in 2009–2010, and decreased to 7.6% in 2011–2012, a total number 180 strains of methicillin-resistant S. aureus being isolated (7.9%; 95% CI 6.7–9) [31].
Zeller V. et al. reported, based on the epidemiology profile of bone and joint infections from a French center over the last 12 years, that Staphylococcus epidermidis strains isolated from had a methicillin resistance of 84%; similar data to our study, where nine out of the eleven strains (81.81%) are methicillin-resistant strains [36]. According to EUCAST, it is known that vancomycin MIC values of 2 mg/L are on the border of the wild-type distribution and there may be an impaired clinical response. A MIC >2 mg/L for vancomycin measured using VITEK was not encountered, indicating that Staphylococcus epidermidis strains with reduced susceptibility to vancomycin were not isolated. We did not find an association between a MIC > 1 mg/L and co-resistance with rifampin; all rifampin-resistant strains (n = 2) had a vancomycin MIC <2 mg/L. For Staphylococcus aureus strains, a MIC >1 mg/L for teicoplanin measured using VITEK was not encountered. We did not find an association between a MIC >1 mg/L and co-resistance with rifampin; all isolated strains were susceptible to rifampin. Casenaz A et al., report that methicillin resistance was found in 15.2% (19/125) of Staphylococcus aureus strains and 49.3% (35/71) of CoNS strains. The authors also report that 29.1% of the infections were polymicrobial; in our study, 17.91% of cases were polymicrobial infections [37].
The current study also has some limitations. The main limitation of our study is the sample size of enrolled patients, which prevents us from reaching firm conclusions regarding the epidemiological trends. The study assesses microbial etiology and trends in one hospital, based on prospectively collected data. Nevertheless, the number of enrolled patients was sufficiently high to have an overview of the local possible etiologies of prosthetic joint infection. It is important to keep in mind that differences in patient characteristics, as well as in hospital and health care systems, means that our results cannot be generalized to other countries, and maybe not even to other regions in Romania. This was a monocentric, observational, retrospective cohort study. The center where this study was conducted was not a dedicated center for the treatment of PJIs. As with any culture study, a possibility exists that the isolated strains were secondary to contamination. Larger studies are needed to confirm our results; nevertheless, they are very promising. The use of a standardized definition of multidrug-resistant microorganisms is a strength of our study [38].
5. Conclusions
Most cases of periprosthetic joint infections in our study are monomicrobial, and Staphylococcus aureus is also the most common cause of infection in this study. A good understanding of the local epidemiology is necessary to optimize the treatment strategies of PJIs, and in our opinion, each center that treats PJIs should conduct regular epidemiological studies to optimize their empiric broad-spectrum antibiotic therapy. Based on our findings, empiric broad-spectrum antibiotic therapy in acute PJIs could be focused on the bacteria belonging to the gram-positive aerobic or microaerophilic cocci, but the results should be analyzed carefully, and the local resistance of the pathogens should always be taken into consideration. A total of four CoNS strains were resistant to gentamycin and to quinolones/fluoroquinolones. All strains of CoNS were susceptible to vancomycin, and trimethoprim/sulfamethoxazole; and four out of nineteen were resistant to quinolones/fluoroquinolones. We did not find any association between a MIC > 1 mg/L and co-resistance with rifampin. All strains of Staphylococcus aureus maintained their susceptibility to fluoroquinolones, trimethoprim/sulfamethoxazole, and to rifampicin. A MIC >1 mg/L for teicoplanin was not encountered. We did not find any association between a MIC >1 mg/L and co-resistance with rifampin; all isolated strains were susceptible to rifampin. All strains of Enterococcus faecalis maintained their susceptibility to vancomycin, and four out of the six were susceptible to trimethoprim/sulfamethoxazole. Escherichia coli strains, in relationship with piperacillin/ticarcillin, in combination with a beta-lactamase inhibitor (piperacillin/tazobactam; ticarcillin/clavulanic acid), preserved the sensitivity all strains. As for susceptibility to carbapenems, it was preserved in all three tested antibiotics. Thus, two of the most frequently used classes of antibiotics in the management of infections associated with orthopedic implants remain under discussion; five strains being sensitive to ciprofloxacin, five to trimethoprim/sulfamethoxazole, and all strains being intermediately sensitive to norfloxacin. Susceptibility to fosfomycin of Escherichia coli is preserved. For the Enterobacter species isolated strains, five of the isolated strains were susceptible to Piperacillin/tazobactam, 50% were resistant to cephalosporins, 75% were resistant to gentamicin, and all strains maintained their susceptibility to trimethoprim/sulfamethoxazole. For the Pseudomonas-species isolated strains (n = 9), five of the isolated strains were susceptible to ticarcillin/clavulanic acid, four to piperacillin/tazobactam, five to ceftazidime, six to imipenem, seven to meropenem, seven to ceftazidime/avibactam and ceftolozane/tazobactam, and five to ciprofloxacin. All strains of Ralstonia picketti maintained their susceptibility to piperacillin, imipenem, meropenem, ciprofloxacin, pefloxacin, minocycline, and trimethoprim/sulfamethoxazole. Our data shows that it is also important to optimize and improve the antimicrobial treatment strategies based on the local AST data that involve antibiotics which have activity against biofilm-related infections. Multidisciplinary teams and accurate etiological diagnosis are necessary for the management of periprosthetic joint infection cases.
Author Contributions
Conceptualization, R.-M.B., S.R.F., M.D.R. and V.B.; methodology, R.-M.B., S.R.F., M.D.R., A.E.V., D.D. and V.B.; software, N.-I.-C.I., B.-A.B., A.E.V. and D.D.; validation, R.-M.B., S.R.F., M.D.R., N.-I.-C.I., B.-A.B., A.E.V., D.D. and V.B.; formal analysis, R.-M.B., N.-I.-C.I., B.-A.B., A.E.V. and D.D.; investigation, R.-M.B., S.R.F., M.D.R. and V.B.; resources, R.-M.B., S.R.F., M.D.R., N.-I.-C.I., B.-A.B., A.E.V., D.D., and V.B.; data curation, A.E.V. and D.D.; writing—original draft preparation, R.-M.B.; writing—review and editing, R.-M.B., S.R.F., M.D.R. and V.B.; visualization, R.-M.B., S.R.F., M.D.R. and V.B.; supervision, S.R.F., M.D.R. and V.B. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was received by the authors.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. The study was accepted by the Ethics Committee of the Emergency Clinical County Hospital Sibiu, Romania and they encouraged publishing the article (ID:15554/20 June 2022.). All methods were carried out following relevant guidelines and regulations.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Birlutiu, V.; Birlutiu, R.M. Endocarditis due to Abiotrophia defectiva, a biofilm-related infection associated with the presence of fixed braces: A case report. Medicine 2017, 96, e8756. [Google Scholar] [CrossRef] [PubMed]
- Birlutiu, V.; Birlutiu, R.M.; Costache, V.S. Viridans streptococcal infective endocarditis associated with fixed orthodontic appliance managed surgically by mitral valve plasty: A case report. Medicine 2018, 97, e11260. [Google Scholar] [CrossRef] [PubMed]
- Birlutiu, R.M.; Birlutiu, V.; Mihalache, M.; Mihalache, C.; Cismasiu, R.S. Diagnosis and management of orthopedic implant-associated infection: A comprehensive review of the literature. Biomed. Res. 2017, 28, 5063–5073. [Google Scholar]
- Kolpen, M.; Kragh, K.N.; Enciso, J.B.; Faurholt-Jepsen, D.; Lindegaard, B.; Egelund, G.B.; Jensen, A.V.; Ravn, P.; Mathiesen, I.H.M.; Gheorge, A.G.; et al. Bacterial biofilms predominate in both acute and chronic human lung infections. Thorax 2022, 77, 1015–1022. [Google Scholar] [CrossRef]
- Burmølle, M.; Thomsen, T.R.; Fazli, M.M.; Dige, I.; Christensen, L.; Homøe, P.; Tvede, M.; Nyvad, B.; Tolker-Nielsen, T.; Givskov, M.; et al. Biofilms in chronic infections–a matter of opportunity–monospecies biofilms in multispecies infections. FEMS Immunol. Med. Microbiol. 2010, 59, 324–336. [Google Scholar] [CrossRef]
- Sauer, K.; Camper, A.K.; Ehrlich, G.D.; Costerton, J.W.; Davies, D.G. Pseudomonas aeruginosa Displays Multiple Phenotypes during Development as a Biofilm. J. Bacteriol. 2002, 184, 1140–1154. [Google Scholar] [CrossRef]
- Pamp, S.J.; Sternberg, C.; Tolker-Nielsen, T. Insight into the microbial multicellular lifestyle via flow-cell technology and confocal microscopy. Cytom. A 2009, 75, 90–103. [Google Scholar] [CrossRef]
- Klausen, M.; Heydorn, A.; Ragas, P.; Lambertsen, L.; Aaes-Jørgensen, A.; Molin, S.; Tolker-Nielsen, T. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 2003, 48, 1511–1524. [Google Scholar] [CrossRef]
- Stewart, P.S. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob. Agents Chemother. 1996, 40, 2517–2522. [Google Scholar] [CrossRef]
- Kim, J.; Hahn, J.-S.; Franklin, M.J.; Stewart, P.; Yoon, J. Tolerance of dormant and active cells in Pseudomonas aeruginosa PA01 biofilm to antimicrobial agents. J. Antimicrob. Chemother. 2008, 63, 129–135. [Google Scholar] [CrossRef]
- Brochmann, R.P.; Toft, A.; Ciofu, O.; Briales, A.; Kolpen, M.; Hempel, C.; Bjarnsholt, T.; Høiby, N.; Jensen, P. Bactericidal effect of colistin on planktonic Pseudomonas aeruginosa is independent of hydroxyl radical formation. Int. J. Antimicrob. Agents 2014, 43, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Kolpen, M.; Kragh, K.N.; Bjarnsholt, T.; Line, L.; Hansen, C.R.; Dalbøge, C.S.; Hansen, N.; Kühl, M.; Høiby, N.; Jensen, P. Denitrification by cystic fibrosis pathogens–Stenotrophomonas maltophilia is dormant in sputum. Int. J. Med. Microbiol. 2015, 305, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Secor, P.R.; Michaels, L.A.; Ratjen, A.; Jennings, L.K.; Singh, P.K. Entropically driven aggregation of bacteria by host polymers promotes antibiotic tolerance in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2018, 115, 10780–10785. [Google Scholar] [CrossRef]
- Jensen, L.K.; Bjarnsholt, T.; Kragh, K.N.; Aalbæk, B.; Henriksen, N.L.; Blirup, S.A.; Pankoke, K.; Petersen, A.; Jensen, H.E. In Vivo Gentamicin Susceptibility Test for Prevention of Bacterial Biofilms in Bone Tissue and on Implants. Antimicrob. Agents Chemother. 2019, 63, e01889-18. [Google Scholar] [CrossRef] [PubMed]
- McConoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Calhoun, J.H.; Shirtliff, M.; Kathju, S.; Stoodley, P. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014, 9, 987–1007, Erratum in Future Microbiol. 2014, 9, 1234. [Google Scholar] [CrossRef]
- Stracquadanio, S.; Musso, N.; Costantino, A.; Lazzaro, L.M.; Stefani, S.; Bongiorno, D. Staphylococcus aureus Internalization in Osteoblast Cells: Mechanisms, Interactions and Biochemical Processes. What Did We Learn from Experimental Models? Pathogens 2021, 10, 239. [Google Scholar] [CrossRef]
- Bue, M.; Hanberg, P.; Koch, J.; Jensen, L.K.; Lundorff, M.; Aalbaek, B.; Jensen, H.E.; Søballe, K.; Tøttrup, M. Single-dose bone pharmacokinetics of vancomycin in a porcine implant-associated osteomyelitis model. J. Orthop. Res. 2018, 36, 1093–1098. [Google Scholar] [CrossRef]
- Klement, M.R.; Cunningham, D.J.; Wooster, B.M.; Wellman, S.S.; Bolognesi, M.P.; Green, C.L.; Garrigues, G.E. Comparing Standard Versus Extended Culture Duration in Acute Hip and Knee Periprosthetic Joint Infection. J. Am. Acad. Orthop. Surg. 2019, 27, e437–e443. [Google Scholar] [CrossRef]
- Ting, N.T.; Della Valle, C.J. Diagnosis of Periprosthetic Joint Infection—An Algorithm-Based Approach. J. Arthroplast. 2017, 32, 2047–2050. [Google Scholar] [CrossRef]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-Joint Infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef]
- Adeli, B.; Parvizi, J. Strategies for the prevention of periprosthetic joint infection. J. Bone Jt. Surg. 2012, 94 (Suppl. S11), 42–46. [Google Scholar] [CrossRef] [PubMed]
- Birlutiu, R.M.; Stoica, C.I.; Russu, O.; Cismasiu, R.S.; Birlutiu, V. Positivity Trends of Bacterial Cultures from Cases of Acute and Chronic Periprosthetic Joint Infections. J. Clin. Med. 2022, 11, 2238. [Google Scholar] [CrossRef] [PubMed]
- Wildeman, P.; Rolfson, O.; Söderquist, B.; Wretenberg, P.; Lindgren, V. What are the long-term outcomes of mortality, quality of life, and hip function after 243 prosthetic joint infection of the hip? A 10-year follow-up from Sweden. Clin. Orthop. Relat. Res. 2021, 479, 2203–2213. [Google Scholar] [CrossRef]
- Birlutiu, R.M.; Roman, M.D.; Cismasiu, R.S.; Fleaca, S.R.; Popa, C.M.; Mihalache, M.; Birlutiu, V.; Fleaca, S.R. Sonication contribution to identifying prosthetic joint infection with Ralstonia pickettii: A case report and review of the literature. BMC Musculoskelet. Disord. 2017, 18, 311. [Google Scholar] [CrossRef] [PubMed]
- Birlutiu, R.M.; Birlutiu, V.; Cismasiu, R.S.; Mihalache, M. bbFISH-ing in the sonication fluid. Medicine 2019, 98, e16501. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.; Sousa, R.; Wouthuyzen-Bakker, M.; Chen, A.F.; Soriano, A.; Vogely, H.C.; Clauss, M.; Higuera, C.A.; Trebše, R. The EBJIS definition of periprosthetic joint infection. Bone Jt. J. 2021, 103-B, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Peel, T.N.; Cheng, A.C.; Buising, K.L.; Choong, P.F.M. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: Are current antibiotic prophylaxis guidelines effective? Antimicrob. Agents Chemother. 2012, 56, 2386.e91. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.K.; Bakhshi, H.; Ecker, N.U.; Parvizi, J.; Gehrke, T.; Kendoff, D. Organism Profile in Periprosthetic Joint Infection: Pathogens Differ at Two Arthroplasty Infection Referral Centers in Europe and in the United States. J. Knee Surg. 2014, 27, 399.e406. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302.e45. [Google Scholar] [CrossRef]
- Moran, E.; Masters, S.; Berendt, A.R.; McLardy-Smith, P.; Byren, I.; Atkins, B.L. Guiding empirical antibiotic therapy in orthopaedics: The microbiology of prosthetic joint infection managed by debridement, irrigation and prosthesis retention. J. Infect. 2007, 55, 1.e7. [Google Scholar] [CrossRef]
- Benito, N.; Franco, M.; Ribera, A.; Soriano, A.; Rodriguez-Pardo, D.; Sorlí, L.; Fresco, G.; Fernández-Sampedro, M.; Dolores del Toro, M.; Guío, L.; et al. Time trends in the aetiology of prosthetic joint infections: A multicentre cohort study. Clin. Microbiol. Infect. 2016, 22, 732.e1. [Google Scholar] [CrossRef]
- Li, G.-Q.; Guo, F.-F.; Ou, Y.; Dong, G.-W.; Zhou, W. Epidemiology and outcomes of surgical site infections following orthopedic surgery. Am. J. Infect. Control. 2013, 41, 1268.e71. [Google Scholar] [CrossRef] [PubMed]
- Benito, N.; Franco, M.; Coll, P.; Gálvez, M.L.; Jordán, M.; López-Contreras, J.; Pomar, V.; Monllau, J.C.; Mirelis, B.; Gurguí, M. Etiology of surgical site infections after primary total joint arthroplasties. J. Orthop. Res. 2014, 32, 633–637. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Annual Epidemiological Report 2014. Antimicrobial Resistance and Healthcare-Associated Infections. Stockholm. 2015. Available online: https://ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-annual-epidemiological-report.pdf (accessed on 5 December 2022).
- Rodríguez-Pardo, D.; Pigrau, C.; Lora-Tamayo, J.; Soriano, A.; del Toro, M.; Cobo, J.; Palomino, J.; Euba, G.; Riera, M.; Sánchez-Somolinos, M.; et al. Gram-negative prosthetic joint infection: Outcome of a debridement, antibiotics and implant retention approach. A large multicentre study. Clin. Microbiol. Infect. 2014, 20, O911.e9. [Google Scholar] [CrossRef]
- Zeller, V.; Kerroumi, Y.; Meyssonnier, V.; Heym, B.; Metten, M.-A.; Desplaces, N.; Marmor, S. Analysis of postoperative and hematogenous 271 prosthetic joint-infection microbiological patterns in a large cohort. J. Infect. 2018, 76, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Casenaz, A.; Piroth, L.; Labattut, L.; Sixt, T.; Magallon, A.; Guilloteau, A.; Neuwirth, C.; Amoureux, L. Epidemiology and antibiotic resistance of prosthetic joint infections according to time of occurrence, a 10-year study. J. Infect. 2022, 85, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).