Current Trends in Metal Biomining with a Focus on Genomics Aspects and Attention to Arsenopyrite Leaching—A Review
Abstract
:1. Introduction
- Phylogeny of leaching microbiota and search for new leaching species/strains, which include:
- −
- Study on microbial biodiversity: search for new active species/strains capable of more active processes or capable of carrying out little known processes, or more resistant to toxicants, or stimulated by additives and changing process conditions;
- −
- Study on genomes of the leaching bacteria to identify genetically determined resistance to toxicants, the possibility of using stimulating additives, and the relationship to oxidative stress;
- −
- Attempts to create genetically engineered leaching strains (in fact, these developments are a logical continuation of two previous).
- Optimization of bioleaching processes via stimulation of microorganisms. This section presented some efforts which remain constant for decades, namely:
- −
- Experimental selection of stimulating additives and changes in cultivation/leaching conditions.
- The special attention is dedicated to investigations on genomics and bioleaching of arsenopyrite as problems of metal bioleaching from refractory arsenic-containing ores.
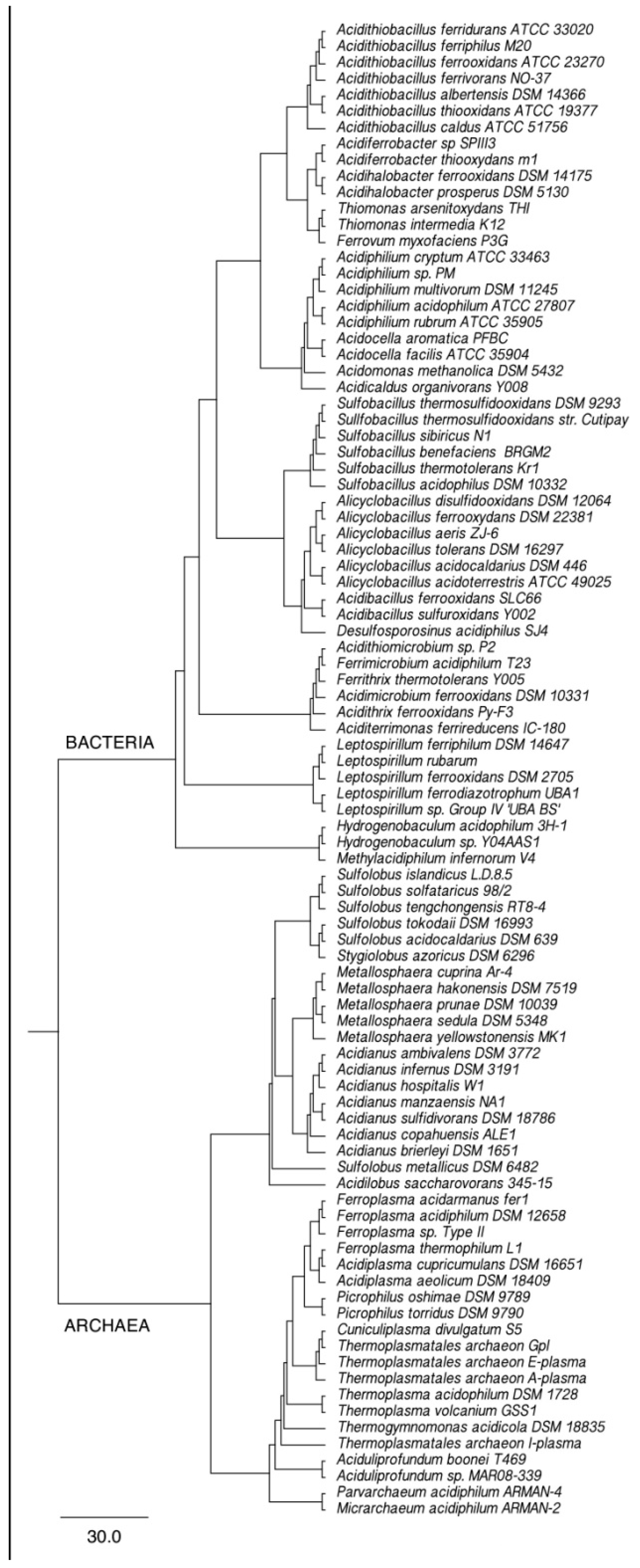
2. Phylogeny of Leaching Microbiota and Search for New Leaching Species/Strains
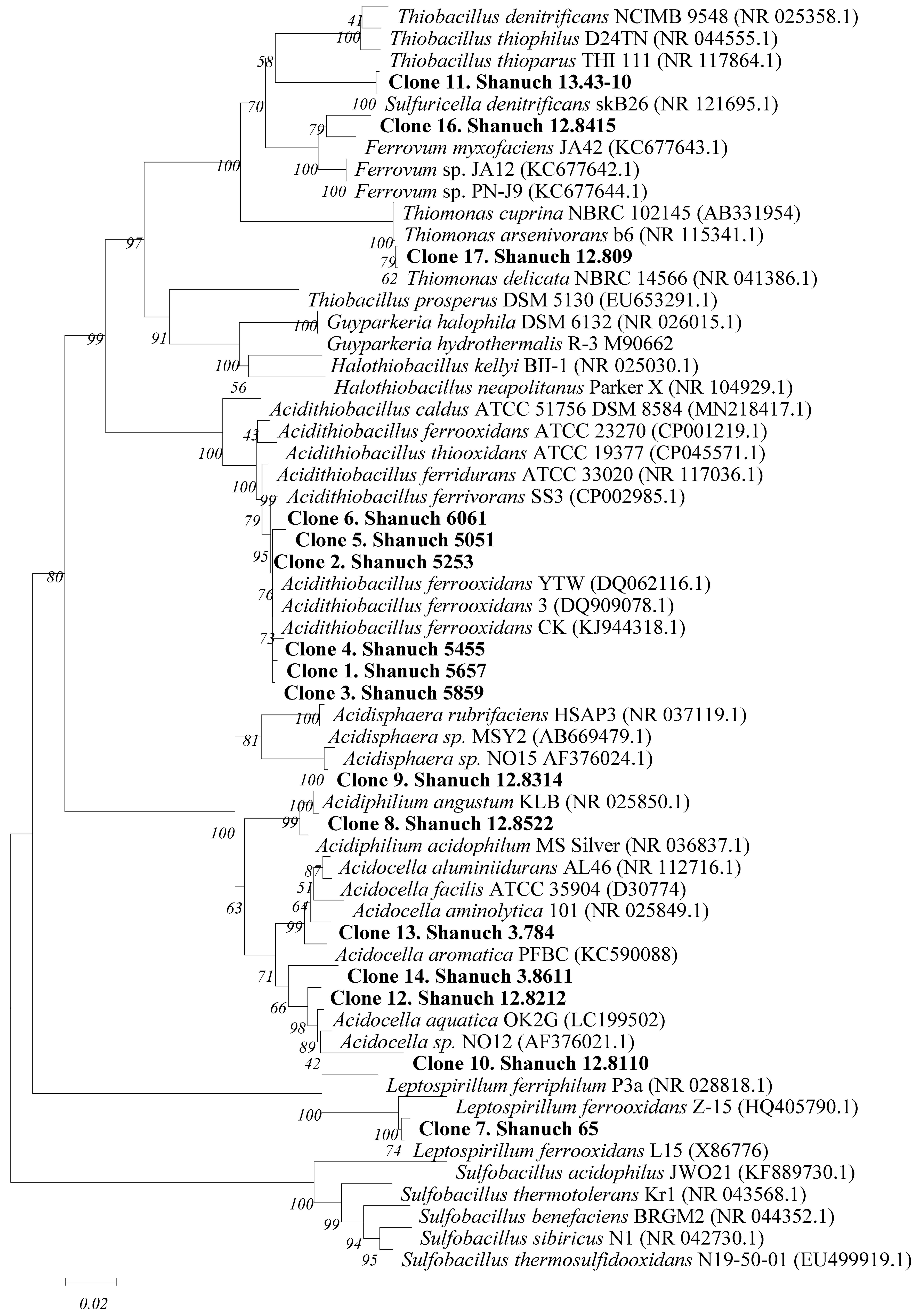
3. Optimization of Bioleaching Processes via Stimulation of Microorganisms
3.1. Selection of the Leaching Microorganisms
3.2. pH Selection
3.3. Effects of Redox and Temperature Conditions
3.4. Stimulation with Organic Supplements
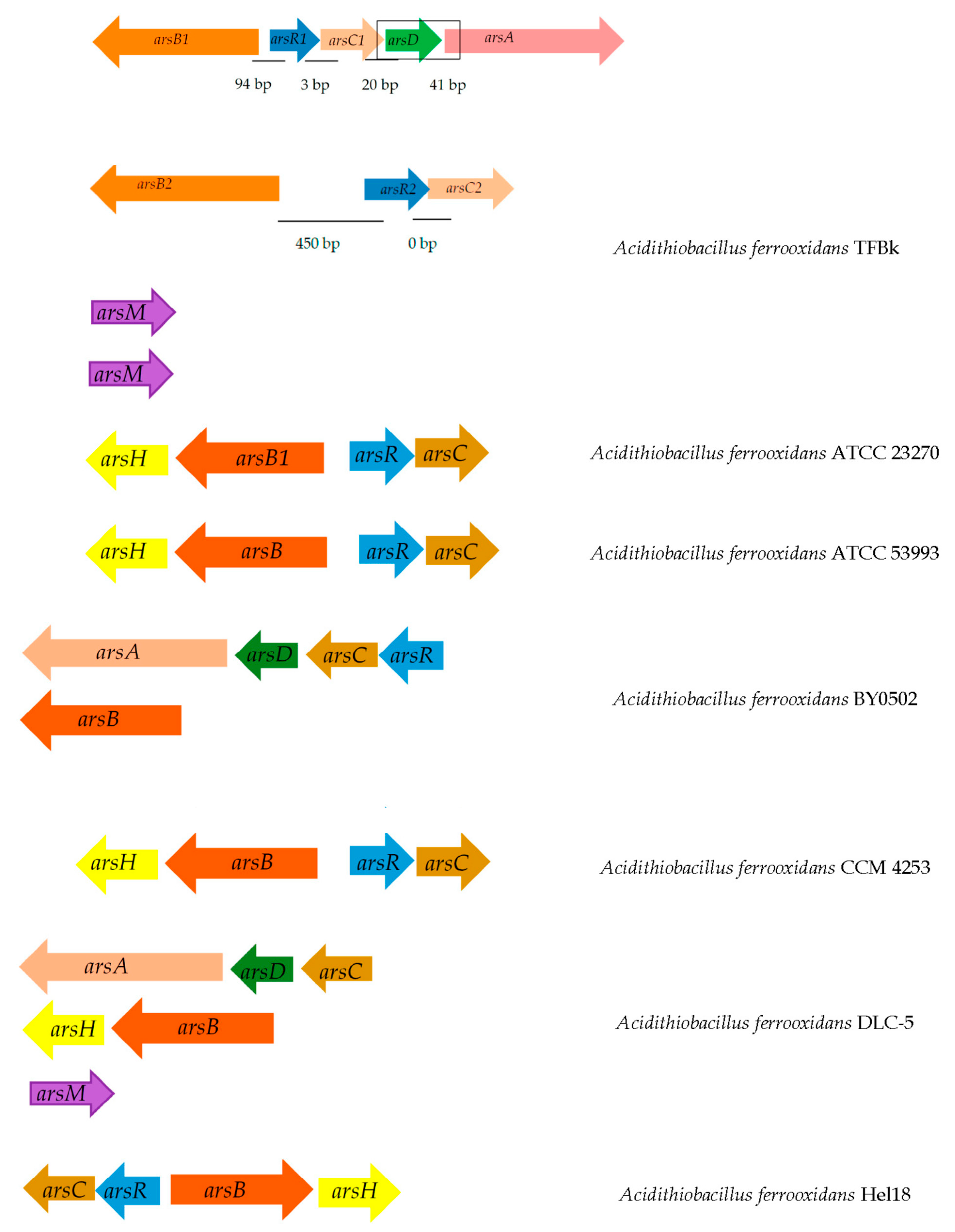
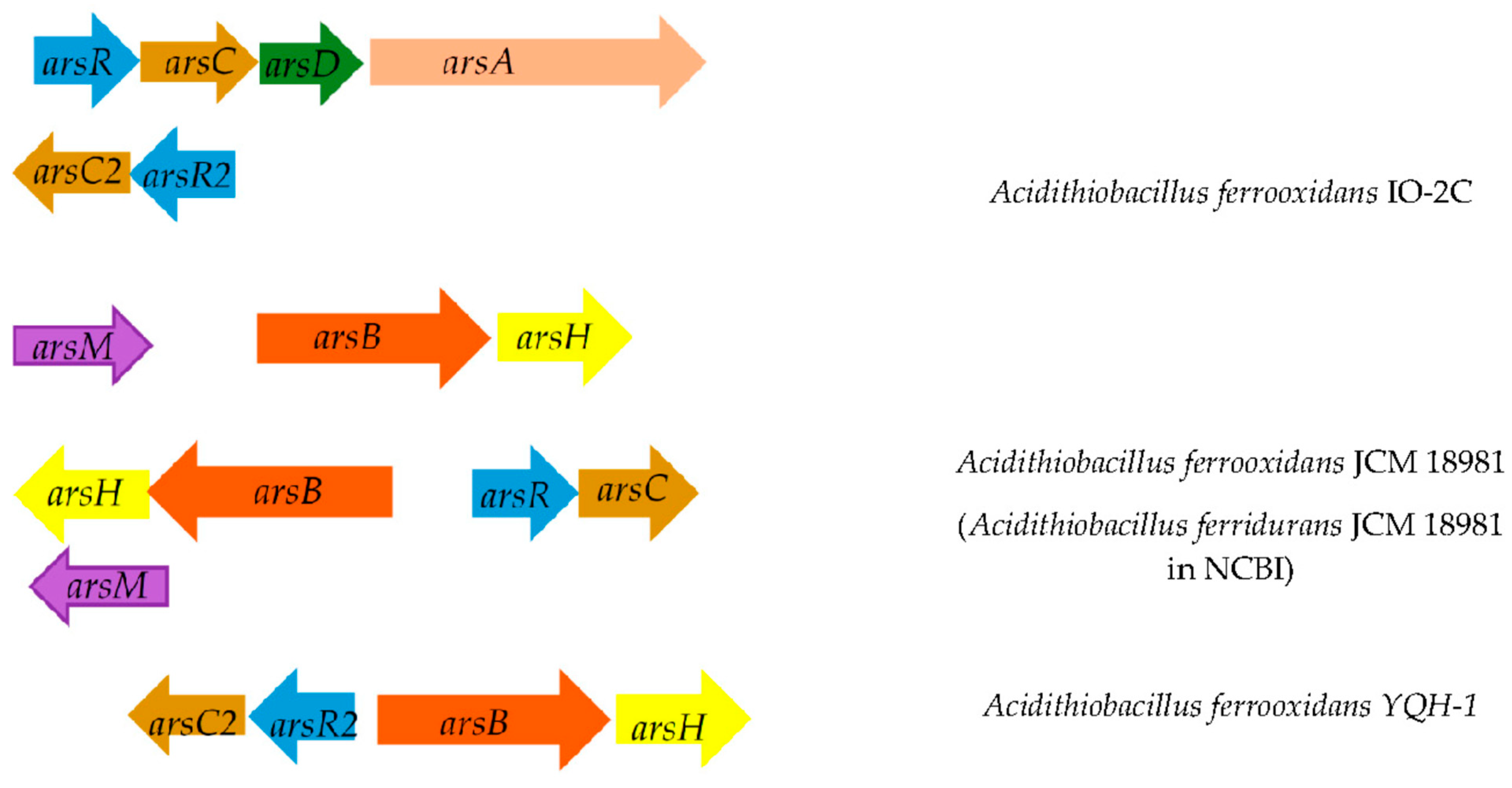
4. Problems of Bioleaching of Metals from Refractory Arsenic-Containing Ores
5. Genetic Modifications of Leaching Microorganisms
6. Concluding Remarks
- (1)
- Permanent and continuous ones:
- −
- studying the diversity of microorganisms to search new groups, species, strains capable of bioleaching processes in a wider range of conditions,
- −
- the development of bioleaching technologies, including a wide range of approaches: application of the species/strain associations, innovations in conditions and stages of the bioleaching process, use of stimulating additives;
- (2)
- New ones which expand with time:
- −
- studying genomes of the leaching microbiota to understand interactions inside of the leaching populations,
- −
- studying genomes of the leaching bacteria to identify their genetically determined resistance to toxicants, identifying some possible new properties and trying to create genetically engineered leaching strains.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaksonen, A.H.; Boxall, N.J.; Gumulya, Y.; Khaleque, H.N.; Morris, C.; Bohu, T.; Cheng, K.Y.; Usher, K.M.; Lakaniemi, A.-M. Recent progress in biohydrometallurgy and microbial characterization. Hydrometallurgy 2018, 180, 7–25. [Google Scholar] [CrossRef]
- New Mining Concept for Extraction Metals from Deep Ore Deposits Using Biotechnology. BIOMOre. 2018, 4652212–11/09/2018, 193917. Available online: https://cordis.europa.eu/project/id/642456 (accessed on 18 August 2022).
- Collet, A.; Regnault, O.; Ozhogin, A.; Imantayeva, A.; Garnier, L. Three-dimensional reactive transport simulation of uranium in situ recovery: Large-scale well field applications in Shu Saryssu Bassin, Tortkuduk deposit (Kazakhstan). Hydrometallurgy 2022, 211, 105873. [Google Scholar] [CrossRef]
- Zeng, S.; Song, J.; Sun, B.; Wang, F.; Ye, W.; Shen, Y.; Li, H. Seepage characteristics of the leaching solution during in situ leaching of uranium. Nucl. Eng. Technol. 2022, in press. [Google Scholar] [CrossRef]
- Zimmerley, S.; Wilson, D.; Prater, J. Cyclic Leaching Process Employing Iron-Oxidizing Bacteria. U.S. Patent 2829964, 8 April 1958. Available online: https://patents.google.com/patent/US2829964A/en (accessed on 15 November 2022).
- Yin, S.; Wang, L.; Kabwe, E.; Chen, X.; Yan, R.; An, K.; Zhang, L.; Wu, A. Copper Bioleaching in China: Review and Prospect. Minerals 2018, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Roberto, F.F.; Schippers, A. Progress in bioleaching: Part B; applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2022, 106, 5913–5928. [Google Scholar] [CrossRef]
- Ilyas, S.; Srivastava, R.R.; Kim, H.; Ilyas, N. Biotechnological recycling of hazardous waste PCBs using Sulfobacillus thermosulfidooxidans through pretreatment of toxicant metals: Process optimization and kinetic studies. Chemosphere 2022, 286, 131978. [Google Scholar] [CrossRef]
- Ilyas, S.; Srivastava, R.R.; Kim, H. Mobilization of platinum and palladium from exhausted catalytic converters using bio-cyanide and an ionic-liquid as mass transport carriers. Green Chem. 2022, 24, 5204–5218. [Google Scholar] [CrossRef]
- Işıldar, A.; van Hullebusch, E.D.; Lenz, M.; Laing, G.D.; Marra, A.; Cesaro, A.; Panda, S.; Akcil, A.; Kucuker, M.A.; Kuchta, K. Biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment (WEEE)—A review. J. Hazard. Mater. 2019, 362, 467–481. [Google Scholar] [CrossRef]
- Moosakazemi, F.; Ghassa, S.; Jafari, M.; Chelgani, S.C. Bioleaching for Recovery of Metals from Spent Batteries—A Review. Miner. Process. Extr. Metall. 2022. [Google Scholar] [CrossRef]
- Moazzam, P.; Boroumand, Y.; Rabiei, P.; Baghbaderani, S.S.; Mokarian, P.; Mohagheghian, F.; Mohammed, L.J.; Razmjou, A. Lithium bioleaching: An emerging approach for the recovery of Li from spent lithium ion batteries. Chemosphere 2021, 277, 130196. [Google Scholar] [CrossRef]
- Cruz, J.A.; Tubana, B.S.; Fultz, L.M.; Dalen, M.S.; Ham, J.H. Identification and profiling of silicate-solubilizing bacteria for plant growth-promoting traits and rhizosphere competence. Rhizosphere 2022, 23, 100566. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, A.; Bhardwaj, N.K.; Singh, A.; Dalal, S.; Sharma, J. A novel, simple, and quick plate assay to screen silicolytic bacteria and silicase production using different substrates. Biores. Technol. Rep. 2022, 17, 100971. [Google Scholar] [CrossRef]
- Abashina, T.N.; Vainshtein, M.B.; Khaustov, S.A. Bacterial Corrosion of Concrete and Bioleaching of Mining Wastes. Methodical Recommendation; Tula State University: Pushchino, Russia, 2015; 101p. (In Russian) [Google Scholar] [CrossRef]
- Zhang, R.; Schippers, A. Stirred-tank bioleaching of copper and cobalt from mine tailings in Chile. Miner. Eng. 2022, 180, 107514. [Google Scholar] [CrossRef]
- Gentina, J.C.; Acevedo, F. Copper bioleaching in Chile. Minerals. 2016, 6, 23. [Google Scholar] [CrossRef]
- Acevedo, F. The use of reactors in biomining processes. Electron. J. Biotechnol. 2000, 3, 184–194. [Google Scholar] [CrossRef]
- Johnson, D.B. The evolution, current status, and future prospects of using biotechnologies in the mineral extraction and metal recovery sectors. Minerals 2018, 8, 343. [Google Scholar] [CrossRef] [Green Version]
- Artykova, A.; Elkina, Y.; Nechaeva, A.; Melamud, V.; Boduen, A.; Bulaev, A. Options for increasing the rate of bioleaching of arsenic containing copper concentrate. Microbiol. Res. 2022, 13, 466–479. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, L.; Mao, Y.; Yao, B.; Jiang, P. Progress, challenges, and perspectives of bioleaching for recovering heavy metals from mine tailings. Adsorp. Sci. Technol. 2021, 2021, 9941979. [Google Scholar] [CrossRef]
- Sarkodie, E.K.; Jiang, L.; Li, K.; Yang, J.; Guo, Z.; Shi, J.; Deng, Y.; Liu, H.; Jiang, H.; Liang, Y.; et al. A review on the bioleaching of toxic metal(loid)s from contaminated soil: Insight into the mechanism of action and the role of influencing factors. Front. Microbiol. 2022, 13, 1049277. [Google Scholar] [CrossRef]
- Martins, F.L.; Leão, V.A. Chalcopyrite bioleaching in chloride media: A mini-review. Hydrometallurgy 2023, 216, 105995. [Google Scholar] [CrossRef]
- Quatrini, R.; Johnson, D.B. Microbiomes in extremely acidic environments: Functionalities and interactions that allow survival and growth of prokaryotes at low pH. Curr. Opin. Microbiol. 2018, 43, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, X.; Liang, Y.; Niu, J.; Zhang, X.; Ma, L.; Hao, X.; Gu, Y.; Yin, H. Insights into functional genes and taxonomical/phylogenetic diversity of microbial communities in biological heap leaching system and their correlation with functions. Appl. Microbiol. Biotechnol. 2016, 100, 9745–9756. [Google Scholar] [CrossRef]
- Norris, P.R.; Falagán, C.; Moya-Beltrán, A.; Castro, M.; Quatrini, R.; Johnson, D.B. Acidithiobacillus ferrianus sp. nov.: An ancestral extremely acidophilic and facultatively anaerobic chemolithoautotroph. Extremophiles 2020, 24, 329–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Hedrich, S.; Jin, D.; Breuker, A.; Schippers, A. Sulfobacillus harzensis sp. nov.; an acidophilic bacterium inhabiting mine tailings from a polymetallic mine. Int. J. Syst. Evol. Microbiol. 2021, 71, 004871. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Nakasato, M.; Ohmura, N.; Ando, A.; Saiki, H.; Ishii, M.; Igarashi, Y. Acidianus manzaensis sp. nov.; a novel thermoacidophilic archaeon growing autotrophically by the oxidation of H2 with the reduction of Fe3+. Curr Microbiol. 2006, 53, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yang, H.; Xin, Y.; Zhang, L.; Kang, W.; Wang, W. Isolation of an extremely acidophilic and highly efficient strain Acidithiobacillus sp. for chalcopyrite bioleaching. J. Ind. Microbiol. Biotechnol. 2012, 39, 1625–1635. [Google Scholar] [CrossRef]
- Issotta, F.; Moya-Beltran, A.; Mena, C.; Covarrubias, P.C.; Thyssen, C.; Bellenberg, S.; Sand, W.; Quatrini, R.; Vera, M. Insights into the biology of acidophilic members of the Acidiferrobacteraceae family derived from comparative genomic analyses. Res. Microbiol. 2018, 169, 608–617. [Google Scholar] [CrossRef]
- Govarthanan, M.; Lee, G.-W.; Park, J.-H.; Kim, J.-S.; Lim, S.-S.; Seo, S.-K.; Cho, M.; Myung, H.; Kamala-Kannan, S.; Oh, B.-T. Bioleaching characteristics; influencing factors of Cu solubilization and survival of Herbaspirillum sp. GW103 in Cu contaminated mine soil. Chemosphere 2014, 109, 42–48. [Google Scholar] [CrossRef]
- Qiu, M.; Xiong, S.; Zhang, W.; Wang, G. A comparison of bioleaching of chalcopyrite using pure culture or a mixed culture. Miner. Eng. 2005, 18, 987–990. [Google Scholar] [CrossRef]
- Vainshtein, M.B.; Vatsurina, A.V.; Sokolov, S.L.; Filonov, A.E.; Adamov, E.V.; Krylova, L.N. Compositions of microbial communities in sulfide nickel ore waste piles. Microbiology 2011, 80, 566–572. [Google Scholar] [CrossRef]
- Yin, H.; Qiu, G.; Wang, D.; Cao, L.; Dai, Z.; Wang, J.; Liu, X. Comparison of microbial communities in three different mine drainages and their bioleaching efficiencies to low grade of chalcopyrite. J. Cent. South Univ. Technol. 2007, 14, 460–466. [Google Scholar] [CrossRef]
- Gupta, A.; Sarkar, J.; Sar, P. Understanding the structure and function of extreme microbiome through genomics: Scope and challenges. In Microbial Diversity in the Genomic Era; Das, S., Dash, H.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; Chapter 33; pp. 581–610. [Google Scholar] [CrossRef]
- Abashina, T.N.; Rozova, O.N.; Vainshtein, M.B. Changes in bacterial community of mine waters under experimental conditions. Inland Water Biol. 2022, 15, 489–496. [Google Scholar] [CrossRef]
- Zeng, W.; Qiu, G.; Zhou, H.; Peng, J.; Chen, M.; Tan, S.N.; Chao, W.; Liu, X.; Zhang, Y. Community structure and dynamics of the free and attached microorganisms during moderately thermophilic bioleaching of chalcopyrite concentrate. Bioresour. Technol. 2010, 101, 7079–7086. [Google Scholar] [CrossRef] [PubMed]
- Smart, M.; Huddy, R.J.; Edward, C.J.; Fourie, C.; Shumba, T.; Iron, J.; Harrison, S.T.L. Linking microbial community dynamics in BIOX® leaching tanks to process conditions: Integrating lab and commercial experience. SSP 2017, 262, 38–42. [Google Scholar] [CrossRef]
- Xia, L.; Uribe, P.; Liu, X.; Yu, C.; Chai, L.; Liu, J.; Qiu, W.; Qiu, G. Comparison of chalcopyrite bioleaching after different microbial enrichment in shake flasks. World J. Microbiol. Biotechnol. 2013, 29, 275–280. [Google Scholar] [CrossRef]
- Ahmadi, A.; Schaffie, M.; Petersen, J.; Schippers, A.; Ranjbar, M. Conventional and electrochemical bioleaching of chalcopyrite concentrates by moderately thermophilic bacteria at high pulp density. Hydrometallurgy 2011, 106, 84–92. [Google Scholar] [CrossRef]
- Rastegar, S.O.; Mousavi, S.M.; Rezaei, M.; Shojaosadati, S.A. Statistical evaluation and optimization of effective parameters in bioleaching of metals from molybdenite concentrate using Acidianus brierleyi. J. Ind. Eng. Chem. 2014, 20, 3096–3101. [Google Scholar] [CrossRef]
- Gerayeli, F.; Ghojavand, F.; Mousavi, S.M.; Yaghmaei, S.; Amiri, F. Screening and optimization of effective parameters in biological extraction of heavy metals from refinery spent catalysts using a thermophilic bacterium. Sep. Purif. Technol. 2013, 118, 151–161. [Google Scholar] [CrossRef]
- Halinen, A.-K.; Rahunen, N.; Kaksonen, A.H.; Puhakka, J.A. Heap bioleaching of a complex sulfide ore: Part II. Effect of temperature on base metal extraction and bacterial compositions. Hydrometallurgy 2009, 98, 101–107. [Google Scholar] [CrossRef]
- He, Z.; Yin, Z.; Wang, X.; Zhong, H.; Sun, W. Microbial community changes during the process of pyrite bioleaching. Hydrometallurgy 2012, 125–126, 81–89. [Google Scholar] [CrossRef]
- Halinen, A.-K.; Rahunen, N.; Kaksonen, A.H.; Puhakka, J.A. Heap bioleaching of a complex sulfide ore: Part I: Effect of pH on metal extraction and microbial composition in pH controlled columns. Hydrometallurgy 2009, 98, 92–100. [Google Scholar] [CrossRef]
- Feng, S.; Yang, H.; Zhan, X.; Wang, W. Novel integration strategy for enhancing chalcopyrite bioleaching by Acidithiobacillus sp. in a 7-L fermenter. Bioresour. Technol. 2014, 161, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Third, K.A.; Cord-Ruwisch, R.; Watling, H.R. The role of iron-oxidizing bacteria in stimulation or inhibition of chalcopyrite bioleaching. Hydrometallurgy 2000, 57, 225–233. [Google Scholar] [CrossRef]
- Vilcáez, J.; Suto, K.; Inoue, C. Bioleaching of chalcopyrite with thermophiles: Temperature-pH-ORP dependence. Int. J. Mineral Process. 2008, 88, 37–44. [Google Scholar] [CrossRef]
- Khoshkhoo, M.; Dopson, M.; Engstrom, F.; Sandstrom, A. New insights into the influence of redox potential on chalcopyrite leaching behaviour. Miner. Eng. 2017, 100, 9–16. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Malverde, S.; Quezada, V. A Sustainable bioleaching of a low-grade chalcopyrite ore. Minerals. 2022, 12, 487. [Google Scholar] [CrossRef]
- Belyi, A.V.; Malashonok, A.P.; Leskiv, M.V.; Potylitsyn, N.V. Method for Bioleaching of Refractory Gold-Bearing Sulfide Flotation Concentrates. Patent RU 2637204, 30 November 2017. Available online: https://www.fips.ru/iiss/search.xhtml (accessed on 15 November 2022). (In Russian).
- Norris, P.R.; Burton, N.P.; Clark, D.A. Mineral sulfide concentrate leaching in high temperature bioreactors. Miner. Eng. 2013, 48, 10–19. [Google Scholar] [CrossRef]
- Xia, J.; Zhao, X.; Liang, C.; Yang, Y.; Nie, Z.; Tang, L.; Ma, C.; Zheng, L.; Zhao, Y.; Qiu, G. Sulfur speciation transformation during bioleaching of pyrite-containing sphalerite concentrate by thermophile Sulfolobus metallicus at 65°C. J. Cent. South Univ. 2012, 19, 1961–1966. [Google Scholar] [CrossRef]
- Fazzini, R.B.; Valdecantos, P.P.; Ohlbaum, R.B. Additive for Bioleaching That Is Substantially Made up of the Licanantase Lipoprotein; and Bioleaching Process to Which This Additive Is Added to Increase the Recovery of Copper. Patent US8728785B2, 20 May 2014. [Google Scholar]
- Zhang, X.; Liu, H.; Zhu, M.; Liu, D.; Cheng, G.; Tan, W. Method for Increasing Bioleaching Rate of Metal in Waste Lithium Batteries by Means of Glutathione. Patent CN 110669937B, 22 June 2021. [Google Scholar]
- Pronk, J.T.; Meijer, W.M.; Hazeu, W.; van Dijken, J.P.; Bos, P.; Kuenen, J.G. Growth of Thiobacillus ferrooxidans on formic acid. Appl. Environ. Microbiol. 1991, 57, 2057–2062. [Google Scholar] [CrossRef] [Green Version]
- Pronk, J.T.; van Dijken, J.P.; Bos, P.; Kuenen, J.G. High Yield Method of Growing Thiobacillus ferrooxidans on Formate. Patent ZA 923117B, 1992. Available online: https://patents.google.com/patent/ZA923117B/ru (accessed on 15 November 2022).
- Abashina, T.; Yachkula, A.; Kaparullina, E.; Vainshtein, M. Intensification of nickel bioleaching with neutrophilic bacteria Guyparkeria halophila as an approach to limitation of sulfuric acid pollution. Microorganisms 2021, 9, 2461. [Google Scholar] [CrossRef]
- Boden, R. Reclassification of Halothiobacillus hydrothermalis and Halothiobacillus halophilus to Guyparkeria gen. nov. in the Thioalkalibacteraceae fam. nov.; with emended descriptions of the genus Halothiobacillus and family Halothiobacillaceae. Int. J. Syst. Evol. Microbiol. 2017, 67, 3919–3928. [Google Scholar] [CrossRef] [PubMed]
- Yachkula, A.; Rozova, O.; Abashina, T.; Vainshtein, M.; Grouzdev, D.; Bulaev, A. Attempts to stimulate leaching activity of Acidithiobacillus ferrooxidans strain TFBk. Minerals 2022, 12, 1051. [Google Scholar] [CrossRef]
- Murciego, A.; Alvarez-Ayuso, E.; Pellitero, E.; Rodriguez, M.A.; Garcia-Sanchez, A.; Tamayo, A.; Rubio, J.; Rubio, F.; Rubin, J. Study of arsenopyrite weathering products in mine wastes from abandoned tungsten and tin exploitations. J. Hazard. Mater. 2011, 186, 590–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paikaray, S. Arsenic geochemistry of acid mine drainage. Mine Water Environ. 2015, 34, 181–196. [Google Scholar] [CrossRef]
- Merkulova, M.; Mathon, O.; Glatzel, P.; Rovezzi, M.; Batanova, V.; Marion, P.; Boiron, M.-C.; Manceau, A. Revealing the chemical form of “invisible” gold in natural arsenian pyrite and arsenopyrite with high energy-resolution X-ray absorption spectroscopy. ACS Earth Space Chem. 2019, 3, 1905–1914. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Zhao, H.; Sun, M.; Zhang, Y.; Zhang, Y.; Lv, X.; Kim, H.; Vainshtein, M.; Wang, S.; Qiu, G. The role of cupric ions in the oxidative dissolution process of marmatite: A dependence on Cu2+ concentration. Sci. Total Environ. 2019, 675, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, Y.; Sun, M.; Ou, P.; Zhang, Y.; Liao, R.; Qiu, G. Catalytic mechanism of silver in the oxidative dissolution process of chalcopyrite: Experiment and DFT calculation. Hydrometallurgy 2019, 187, 18–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H.; Meng, X.; Ou, P.; Lv, X.; Zhang, L.; Liu, L.; Chen, F.; Qiu, G. Mineralogical phase transformation of Fe containing sphalerite at acidic environments in the presence of Cu2+. J. Hazard. Mater. 2021, 403, 124058. [Google Scholar] [CrossRef]
- Johnson, D.B.; Dybowska, A.; Schofield, P.F.; Herrington, R.J.; Smith, S.L.; Santos, A.L. Bioleaching of arsenic-rich cobalt mineral resources, and evidence for concurrent biomineralisation of scorodite during oxidative bio-processing of skutterudite. Hydrometallurgy 2020, 195, 105395. [Google Scholar] [CrossRef]
- Pawlowska, A.; Sadowski, Z.; Winiarska, K. Effect of rhamnolipids and lipopolysaccharides on the bioleaching of arsenic-bearing waste. Minerals 2021, 11, 1303. [Google Scholar] [CrossRef]
- Brierley, C.L.; Brierley, J.A. Progress in bioleaching: Part B: Applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2013, 97, 7543–7552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, Y.-L.; Li, H.-R. Enhancement of bio-oxidation of refractory arsenopyritic gold ore by adding pyrolusite in bioleaching system. Trans. Nonferr. Met. Soc. China 2016, 26, 2479–2484. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, H.; Abashina, T.; Vainshtein, M.; Qiu, G. Review on arsenic removal from sulfide minerals: An emphasis on enargite and arsenopyrite. Miner. Eng. 2021, 172, 107133. [Google Scholar] [CrossRef]
- Antonio Diaz, J.; Serrano, J.; Leiva, E. Bioleaching of arsenic-bearing copper ores. Minerals 2018, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Lane, D.J.; Cook, N.J.; Grano, S.R.; Ehrig, K. Selective leaching of penalty elements from copper concentrates: A review. Miner. Eng. 2016, 98, 110–121. [Google Scholar] [CrossRef]
- Deng, T.; Liao, M. Gold recovery enhancement from a refractory flotation concentrate by sequential bioleaching and thiourea leach. Hydrometallurgy 2002, 63, 249–255. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, X.M.; Zhan, Y.; Lin, J.Q.; Yan, W.; Bian, J.; Liu, Y. Construction of an engineered Acidithiobacillus caldus with high-efficiency arsenic resistance. Acta Microbiol. Sin. 2005, 45, 675–679. [Google Scholar]
- Jiang, H.; Liang, Y.; Yin, H.; Xiao, Y.; Guo, X.; Xu, Y.; Hu, Q.; Liu, H.; Liu, X. Effects of arsenite resistance on the growth and functional gene expression of Leptospirillum ferriphilum and Acidithiobacillus thiooxidans in pure culture and coculture. BioMed Res. Int. 2015, 2015, 203197. [Google Scholar] [CrossRef] [Green Version]
- Fang, F.; Hong, Z.; Fangming, J.; Zhongyin, L.; Xiaowei, S.; Kaiyang, X. Domestication for arsenic-tolerant ability and bioleaching of Acidithiobacillus ferrooxidans. J. Cent. South Univ. Sci. Technol. 2013, 10, 3977–3983. [Google Scholar]
- Munoz, J.A.; Blazquez, M.L.; Gonzalez, F.; Ballester, A.; Acevedo, F.; Gentina, J.C.; Gonzalez, P. Electrochemical study of enargite bioleaching by mesophilic and thermophilic microorganisms. Hydrometallurgy 2006, 84, 175–186. [Google Scholar] [CrossRef]
- Escobar, B.; Huenupi, E.; Godoy, I.; Wiertz, J.V. Arsenic precipitation in the bioleaching of enargite by Sulfolobus BC at 70 °C. Biotechnol. Lett. 2000, 22, 205–209. [Google Scholar] [CrossRef]
- Song, J.; Lin, J.; Gao, L.; Lin, J.; Yinbo, Q.U. Modeling and simulation of enargite bioleaching. Chin. J. Chem. Eng. 2008, 16, 785–790. [Google Scholar] [CrossRef]
- Takatsugi, K.; Sasaki, K.; Hirajima, T. Mechanism of the enhancement of bioleaching of copper from enargite by thermophilic iron-oxidizing archaea with the concomitant precipitation of arsenic. Hydrometallurgy 2011, 109, 90–96. [Google Scholar] [CrossRef]
- Deng, S.; Gu, G.; He, G.; Li, L. Catalytic effect of pyrite on the leaching of arsenopyrite in sulfuric acid and acid culture medium. Electrochim. Acta 2018, 263, 8–16. [Google Scholar] [CrossRef]
- Deng, S.; Gu, G.; Xu, B.; Li, L.; Wu, B. Surface characterization of arsenopyrite during chemical and biological oxidation. Sci. Total Environ. 2018, 626, 349–356. [Google Scholar] [CrossRef]
- Corkhill, C.L.; Wincott, P.L.; Lloyd, J.R.; Vaughan, D.J. The oxidative dissolution of arsenopyrite (FeAsS) and enargite (Cu3AsS4) by Leptospirillum ferrooxidans. Geochim. Cosmochim. Acta 2008, 72, 5616–5633. [Google Scholar] [CrossRef]
- Zhang, D.-R.; Xia, J.-L.; Nie, Z.-Y.; Chen, H.-R.; Liu, H.-C.; Deng, Y.; Zhao, Y.-D.; Zhang, L.-L.; Wen, W.; Yang, H.-Y. Mechanism by which ferric iron promotes the bioleaching of arsenopyrite by the moderate thermophile Sulfobacillus thermosulfidooxidans. Process Biochem. 2019, 81, 11–21. [Google Scholar] [CrossRef]
- Sovmen, V.K.; Karavaiko, G.I.; Kondratieva, T.F.; Pivovarova, T.A.; Belyi, A.V.; Lipatova, T.V.; Gish, A.A. Association of Microorganisms Sulfobacillus olimpiadicus; Ferroplasma acidiphilum; Leptospirillum ferrooxidans for the Oxidation of Sulfide Gold-Bearing Concentrate. Patent RU 2332455, 20 February 2008. Available online: https://www.fips.ru/iiss/search.xhtml (accessed on 15 November 2022). (In Russian).
- Sovmen, V.K.; Karavaiko, G.I.; Kondratieva, T.F.; Pivovarova, T.A.; Belyi, A.V.; Lipatova, T.V.; Gish, A.A. Method of gold-bearing sulfide concentrates bacterial oxidation at receiving of gold. Patent RU 2346063, 2 October 2009. Available online: https://www.fips.ru/iiss/search.xhtml (accessed on 15 November 2022). (In Russian).
- Kolmakova, L.P.; Kovtun, O.N.; Kolmakova, A.A. 4-Stage Biooxidation with Different Air Supply Modes. Patent RU 2425898, 8 October 2011. Available online: https://www.fips.ru/iiss/search.xhtml (accessed on 15 November 2022). (In Russian).
- Krylova, L.N.; Sergeeva, I.A.; Krylov, N.V. A Method for Controlling the Process of Biooxidation of Sulfide Concentrates. Patent RU 2552207, 6 October 2015. Available online: https://www.fips.ru/iiss/search.xhtml (accessed on 15 November 2022). (In Russian).
- Belyi, A.V.; Solopova, N.V.; Bulaev, A.G.; Melamud, V.S. Thermithiobacillus tepidarius Strain for Post-Oxidation of Elemental Sulfur in the Residues of Biooxidation of Sulfide Gold-Arsenic Concentrate. Patent RU 2756647, 4 October 2021. Available online: https://www.fips.ru/iiss/search.xhtml (accessed on 15 November 2022). (In Russian).
- Belyi, A.V.; Bulaev, A.G.; Solopova, N.V.; Panyushkina, A.E.; Melamud, V.S. Association of Microorganisms Acidithiobacillus thiooxidans; Acidiphilum criptum; Leptospirillum ferriphillum; Ferroplasma acidiphilum for the Oxidation of Sulfide Gold-Bearing Concentrate. Patent RU 2758086, 26 October 2021. Available online: https://www.fips.ru/iiss/search.xhtml (accessed on 15 November 2022). (In Russian).
- Rhine, E.D.; Phelps, C.D.; Young, L.Y. Anaerobic arsenite oxidation by novel denitrifying isolates. Environ. Microbiol. 2006, 8, 899–908. [Google Scholar] [CrossRef]
- Handley, K.M.; Héry, M.; Lloyd, J.R. Redox cycling of arsenic by the hydrothermal marine bacterium Marinobacter santoriniensis. Environ. Microbiol. 2009, 11, 1601–1611. [Google Scholar] [CrossRef]
- Osborne, T.H.; Jamieson, H.E.; Hudson-Edwards, K.A.; Nordstrom, D.K.; Walker, S.R.; Ward, S.A.; Santini, J.M. Microbial oxidation of arsenite in a subartic environment: Diversity of arsenite oxidase genes and identification of a psychrotolerant arsenite oxidizer. BMC Microbiol. 2010, 10, 205. [Google Scholar] [CrossRef]
- Ben Fekih, I.; Zhang, C.; Li, Y.P.; Zhao, Y.; Alwathnani, H.A.; Saquib, Q.; Rensing, C.; Cervantes, C. Distribution of arsenic resistance genes in prokaryotes. Front. Microbiol. 2018, 23, 2473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Yang, J.; Liu, T.; Lu, D.; Zhang, S.; Yan, L.; Ni, Y. Complete genome sequence analysis of Acidithiobacillus ferrivorans XJFY6S-08 reveals environmental adaptation to Alpine acid mine drainage. Curr. Microbiol. 2021, 78, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Uhrynowski, W.; Radlinska, M.; Drewniak, L. Genomic Analysis of Shewanella sp. O23S—The natural host of the pSheB plasmid carrying genes for arsenic resistance and dissimilatory reduction. Int. J. Mol. Sci. 2019, 20, 1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhmetov, L.I.; Bykov, A.G.; Vainshtein, M.B.; Esikova, T.Z.; Filonov, A.E.; Krylova, L.N.; Mortazavi, S. Nickel toxicity for thionic bacteria. Proc. Tula State Univ. Nat. Sci. 2010, 1, 167–174. (In Russian) [Google Scholar]
- Cárdenas, J.P.; Valdés, J.; Quatrini, R.; Duarte, F.; Holmes, D.S. Lessons from the genomes of extremely acidophilic bacteria and archaea with special emphasis on bioleaching microorganisms. Appl. Microbiol. Biotechnol. 2010, 88, 605–620. [Google Scholar] [CrossRef]
- Cárdenas, J.P.; Quatrini, R.; Holmes, D.S. Genomic and metagenomic challenges and opportunities for bioleaching: A mini-review. Res. Microbiol. 2016, 167, 529–538. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, S.; Ai, C.; Blum, P. Enhancement of Metallosphaera sedula bioleaching by targeted recombination and adaptive laboratory evolution. Adv. Appl. Microbiol. 2018, 104, 135–165. [Google Scholar] [CrossRef]
- Peng, T.; Liao, W.; Wang, J.; Miao, J.; Peng, Y.; Gu, G.; Wu, X.; Qiu, G.; Zeng, W. Bioleaching and electrochemical behavior of chalcopyrite by a mixed culture at low temperature. Front. Microbiol. 2021, 12, 663757. [Google Scholar] [CrossRef]
- Kölbl, D.; Memic, A.; Schnideritsch, H.; Wohlmuth, D.; Klösch, G.; Albu, M.; Giester, G.; Bujdoš, M.; Milojevic, T. Thermoacidophilic bioleaching of industrial metallic steel waste product. Front. Microbiol. 2022, 13, 864411. [Google Scholar] [CrossRef]

| Modern Name | Former Name | Phylum | pH Optimum | Temperature Optimum, °C |
|---|---|---|---|---|
| Acidianus ambivalens | Desulfurolobus ambivalens | a | 2.5 | 80 |
| Acidanus brierley | Sulfolobus brierleyi | a | 1.5–2.5 | 70 |
| Acidianus infernus | a | 2.5 | 80 | |
| Acidianus sulfidivorans | a | 2.0 | 70 | |
| Idiferrobacter thiooxidans | b | 2.2 | 30 | |
| Acidiphilium acidophilum | Thiobacillus acidophilus | b | 4.5 | 30 |
| Acidiphilium angustum | b | 2.5–3.0 | 20 | |
| Acidiphilium cryptum | b | 3.0 | 28 | |
| Acidiphilium multivorum | b | 3.0 | 30 | |
| Acidiphilium organovorum | b | 3.5 | 37 | |
| Acidiphilium rubrum | b | 2.5–3.0 | 20 | |
| Acidithiobacillus albertensis | Thiobacillus albertis | b | 4.4 | 30 |
| Acidithiobacillus caldus | Thiobacillus caldus | b | 2.5 | 45 |
| Acidithiobacillus ferrianus | b | |||
| Acidithiobacillus ferridurans | b | 1.8 | 30 | |
| Acidithiobacillus ferriphilus | b | 2.0 | 30 | |
| Acidithiobacillus ferrivorans | b | 1.8 | 25 | |
| Acidithiobacillus ferrooxidans | Thiobacillus ferrooxidans | b | 1.8 | 25 |
| Acidithiobacillus sulfuriphilus | b | 3.0 | 25–28 | |
| Acidithiobacillus thiooxidans | Thiobacillus thiooxidans, Thiobacillus concretivorus | b | 4.4 | 25 |
| Acidocella aluminiidurans | b | 3.0 | 37 | |
| Acidocella aminolytica | Acidiphilium aminilyticum | b | 2.5–3.0 | 30 |
| Acidocella facilis | Acidiphilium facile | b | 3.0 | 20 |
| Ferroplasma acidiphilum | a | 1.6–1.8 | 35 | |
| Ferroplasma cupricumulans | a | 1.0–1.2 | 53 | |
| Guyparkeria halophila | Halothiobacillus halophilus | b | 7.3–7.5 | 30 |
| Guyparkeria hydrothermalis | Halothiobacillus hydrothermalis | b | 7.5 | 35 |
| Halothiobacillus kellyi | b | 6.6–7.0 | 37 | |
| Halothiobacillus neapolitanus | Thiobacillus neapolitanus | b | 6.6–7.0 | 25 |
| Leptospirillum ferriphilum | b | 1.8 | 37 | |
| Leptospirillum ferrooxidans | b | 1.8 | 30 | |
| Leptospirillum thermoferrooxidans | b | 3.5 | 65 | |
| Metallosphaera cuprina | a | 3.0 | 65 | |
| Metallosphaera hakonensis | Sulfolobus hakonensis | a | 3.0 | 75 |
| Metallosphaera prunae | a | 3.0 | 65 | |
| Metallosphaera sedula | a | 1.6–1.8 | 35 | |
| Sulfobacillus acidophilus | b | 2.0 | 45 | |
| Sulfobacillus benefaciens | b | 2.0 | 37 | |
| Sulfobacillus sibiricus | b | 1.9–2.4 | 50 | |
| Sulfobacillus thermosulfidooxidans | b | 1.9–2.4 | 50 | |
| Sulfobacillus thermotolerans | b | 1.9–2.4 | 40 | |
| Sulfolobus acidocaldarius | a | 2.0 | 70 | |
| Sulfolobus metallicus | a | 2.0 | 70 | |
| Sulfolobus shibatae | a | 3.0–4.0 | 75 | |
| Sulfolobus solfataricus | a | 4.0–4.2 | 70 | |
| Sulfolobus tokodaii | a | 2.0 | 75 | |
| Sulfolobus yangmingensis | a | 4.0 | 80 | |
| Thermithiobacillus tepidarius | Thiobacillus tepidarius | b | 6.9 | 43 |
| Thiobacillus aquaesulis | b | 7.6 | 42 | |
| Thiobacillus denitrificans | b | 7.0 | 30 | |
| Thiobacillus prosperus | b | 7.0 | 35 | |
| Thiobacillus thioparus | b | 6.6 | 26 | |
| Thiobacillus thiophilus | b | 7.0 | 25 |
| Genera | Number of Patents and Patent Applications | ||
|---|---|---|---|
| 1. Bioleaching | 2. Bioleaching AND Reactor | 2:1, % | |
| Thiobacillus (w/o Acidithiobacillus) | 131 | 81 | 61.8 |
| Sulfolobus | 83 | 53 | 63.8 |
| Leptospirillum | 77 | 52 | 67.5 |
| Pseudomonas | 67 | 43 | 54.2 |
| Bacillus | 66 | 36 | 54.5 |
| Sulfobacillus | 55 | 34 | 61.8 |
| Acidithiobacillus (w/o Thiobacillus) | 54 | 49 | 90.7 |
| Acidianus | 44 | 28 | 63.6 |
| Thiobacillus & Acidithiobacillus | 32 | 23 | 71.9 |
| Thiomicrospira | 5 | 5 | 100.0 |
| Halothiobacillus | 5 | 4 | 80.0 |
| Sulfurimonas | 1 | 1 | 100.0 |
| Genera | Number of Patents and Patent Applications | ||
|---|---|---|---|
| 1. Bioleaching | 2. Bioleaching AND Reactor | 2:1, % | |
| Thiobacillus (w/o Acidithiobacillus) | 59 | 21 | 35.6 |
| Sulfolobus | 16 | 11 | 68.8 |
| Leptospirillum | 28 | 16 | 57.1 |
| Pseudomonas | 41 | 13 | 31.7 |
| Bacillus | 40 | 11 | 27.5 |
| Sulfobacillus | 19 | 10 | 52.6 |
| Acidithiobacillus (w/o Thiobacillus) | 39 | 14 | 35.9 |
| Acidianus | 11 | 6 | 54.5 |
| Thiobacillus & Acidithiobacillus | 8 | 5 | 62.5 |
| Thiomicrospira | 1 | 1 | 100.0 |
| Halothiobacillus | 0 | 0 | - |
| Sulfurimonas | 0 | 0 | - |
| Genus | Ability of Pyrite Bioleaching | Ability of Sulfur Oxidation | Ability of Iron Oxidation | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Unclassified | 0.817 | 0.000 | 0.482 | 0.037 | 0.775 | 0.000 |
| Methylophilus | −0.664 | 0.002 | −0.493 | 0.032 | −0.408 | 0.083 |
| Methylotenera | −0.613 | 0.005 | −0.414 | 0.078 | −0.386 | 0.102 |
| Acidisoma | −0.309 | 0.198 | −0.031 | 0.901 | −0.340 | 0.154 |
| Thiomonas | 0.663 | 0.002 | 0.342 | 0.152 | 0.330 | 0.168 |
| Thermogymnomonas | 0.499 | 0.030 | 0.297 | 0.218 | 0.325 | 0.175 |
| Leptospirillum | −0.420 | 0.074 | −0.401 | 0.089 | −0.312 | 0.193 |
| Acidithiobacillus | −0.419 | 0.074 | −0.153 | 0.533 | −0.305 | 0.204 |
| Acidiphilium | −0.312 | 0.194 | 0.001 | 0.997 | −0.261 | 0.280 |
| Thiobacillus | 0.536 | 0.018 | 0.235 | 0.333 | 0.239 | 0.324 |
| Frateuria | 0.421 | 0.073 | 0.315 | 0.189 | 0.190 | 0.436 |
| Hydrotalea | 0.572 | 0.011 | 0.297 | 0.216 | 0.154 | 0.530 |
| Bacteria | Reached As-Resistance, g As/L | Method for Adaptation | References |
|---|---|---|---|
| Mixture of A. ferrooxidans and L. ferrooxidans | 18.0 | Culturing in the medium with increasing As-concentration | [74] |
| A. caldus | 3.375 | Transfer of the plasmida pSDRA4 coding As-resistance | [75] |
| L. ferriphilum YSK | 30.0 | Culturing in the As-concentration gradient | [76] |
| A. thiooxidans A01 | 60 | Culturing in the As-concentration gradient | [76] |
| A. ferrooxidans | 12 | Continuous replacing of dominants | [77] |
| Title | Main Content | References |
|---|---|---|
| Association of microorganisms Sulfobacillus olimpiadicus, Ferroplasma acidiphilum, Leptospirillum ferrooxidans for the oxidation of sulfide gold-bearing concentrate | Preselection of the most effective strains | [86] |
| Method of bacterial oxidation of gold-bearing sulfide concentrates in the production of gold | 3-stages of biooxidation under different conditions | [87] |
| Method for bacterial oxidation of sulfide gold-bearing concentrates for the gold recovery | 4-stages of biooxidation with the different regimes of aeration | [88] |
| Method for controlling the process of biooxidation of sulfide concentrates | Controlling via aeration for the biooxidation | [89] |
| Method for bioleaching of refractory gold-bearing sulfide flotation concentrates | Bioleaching is controlled by redox of medium | [51] |
| Thermithiobacillus tepidarius strain for post-oxidation of elemental sulfur in residues of biooxidation of sulfide gold-arsenic concentrate | New industrial strain Thermithiobacillus tepidarius OL2018-8. | [90] |
| Association of microorganisms Acidithiobacillus thiooxidans, Acidiphilum criptum, Leptospirillum ferriphillum, Ferroplasma acidiphilum for oxidation of sulfide gold-bearing concentrate | An effective association of the leaching strains | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abashina, T.; Vainshtein, M. Current Trends in Metal Biomining with a Focus on Genomics Aspects and Attention to Arsenopyrite Leaching—A Review. Microorganisms 2023, 11, 186. https://doi.org/10.3390/microorganisms11010186
Abashina T, Vainshtein M. Current Trends in Metal Biomining with a Focus on Genomics Aspects and Attention to Arsenopyrite Leaching—A Review. Microorganisms. 2023; 11(1):186. https://doi.org/10.3390/microorganisms11010186
Chicago/Turabian StyleAbashina, Tatiana, and Mikhail Vainshtein. 2023. "Current Trends in Metal Biomining with a Focus on Genomics Aspects and Attention to Arsenopyrite Leaching—A Review" Microorganisms 11, no. 1: 186. https://doi.org/10.3390/microorganisms11010186
APA StyleAbashina, T., & Vainshtein, M. (2023). Current Trends in Metal Biomining with a Focus on Genomics Aspects and Attention to Arsenopyrite Leaching—A Review. Microorganisms, 11(1), 186. https://doi.org/10.3390/microorganisms11010186






