Legionnaires’ Disease in China Caused by Legionella pneumophila Corby
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Information and Media
2.2. DNA Extraction and Identification of Legionella pneumophila Strain Corby
2.3. Antimicrobial Susceptibility Testing
2.4. Intracellular Growth Assay
2.5. Bioinformatics
2.6. Accession of the Genome Sequences
3. Results
3.1. Case Presentation
3.2. Antibiotic Susceptibility
3.3. Cell Infection Assays
3.4. General Features of the Legionella pneumophila Strain Corby (ICDC) Genome
3.5. Core–Pan Analysis and Phylogenetic Tree Construction
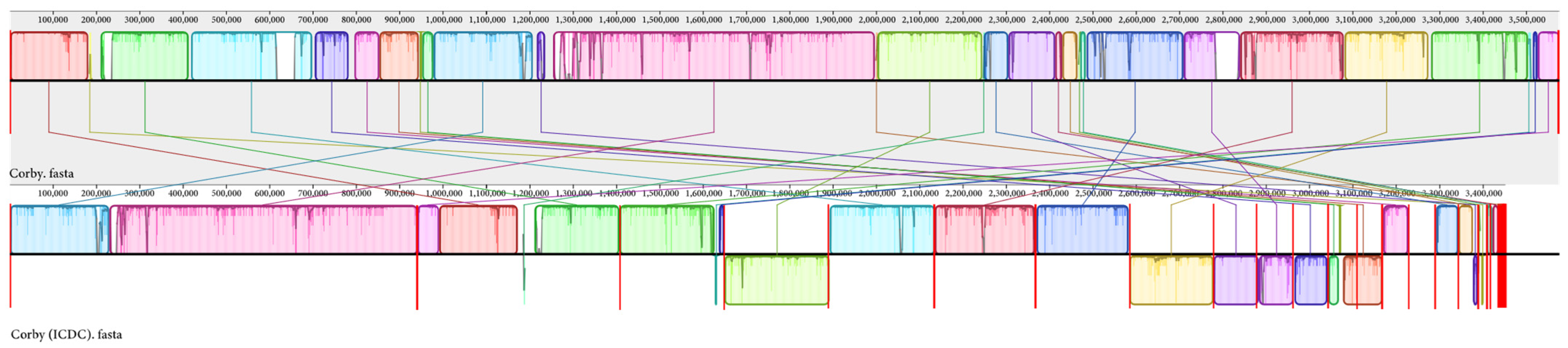
3.6. Multiple Genome Alignment
3.7. CRISPR-Cas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mondino, S.; Schmidt, S.; Rolando, M.; Escoll, P.; Gomez-Valero, L.; Buchrieser, C. Legionnaires’ Disease: State of the Art Knowledge of Pathogenesis Mechanisms of Legionella. Annu. Rev. Pathol. 2020, 15, 439–466. [Google Scholar] [CrossRef] [PubMed]
- Blatt, S.P.; Parkinson, M.D.; Pace, E.; Hoffman, P.; Dolan, D.; Lauderdale, P.; Zajac, R.A.; Melcher, G.P. Nosocomial Legionnaires’ disease: Aspiration as a primary mode of disease acquisition. Am. J. Med. 1993, 95, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Rowbotham, T.J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 1980, 33, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.W.; Tsai, T.R.; Orenstein, W.; Parkin, W.E.; Beecham, H.J.; Sharrar, R.G.; Harris, J.; Mallison, G.F.; Martin, S.M.; Mcdade, J.E.; et al. Legionnaires’ disease: Description of an epidemic of pneumonia. N. Engl. J. Med. 1977, 297, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Yu, V.L.; Plouffe, J.F.; Pastoris, M.C.; Stout, J.E.; Schousboe, M.; Widmer, A.; Summersgill, J.; File, T.; Heath, C.M.; Paterson, D.L.; et al. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: An international collaborative survey. J. Infect. Dis. 2002, 186, 127–128. [Google Scholar] [CrossRef]
- Doleans, A.; Aurell, H.; Reyrolle, M.; Lina, G.; Freney, J.; Vandenesch, F.; Etienne, J.; Jarraud, S. Clinical and environmental distributions of Legionella strains in France are different. J. Clin. Microbiol. 2004, 42, 458–460. [Google Scholar] [CrossRef]
- Jepras, R.I.; Fitzgeorge, R.B.; Baskerville, A. A comparison of virulence of two strains of Legionella pneumophila based on experimental aerosol infection of guinea-pigs. J. Hyg. (Lond.) 1985, 95, 29–38. [Google Scholar] [CrossRef]
- Schmeck, B.; N’Guessan, P.D.; Ollomang, M.; Lorenz, J.; Zahlten, J.; Opitz, B.; Flieger, A.; Suttorp, N.; Hippenstiel, S. Legionella pneumophila-induced NF-kappaB- and MAPK-dependent cytokine release by lung epithelial cells. Eur. Respir. J. 2007, 29, 25–33. [Google Scholar] [CrossRef]
- Palusinska-Szysz, M.; Luchowski, R.; Gruszecki, W.I.; Choma, A.; Szuster-Ciesielska, A.; Lück, C.; Petzold, M.; Sroka-Bartnicka, A.; Kowalczyk, B. The Role of Legionella pneumophila Serogroup 1 Lipopolysaccharide in Host-Pathogen Interaction. Front. Microbiol. 2019, 10, 2890. [Google Scholar] [CrossRef]
- Gomez-Valero, L.; Rusniok, C.; Jarraud, S.; Vacherie, B.; Rouy, Z.; Barbe, V.; Medigue, C.; Etienne, J.; Buchrieser, C. Extensive recombination events and horizontal gene transfer shaped the Legionella pneumophila genomes. BMC Genomics 2011, 12, 536. [Google Scholar] [CrossRef]
- Schunder, E.; Adam, P.; Higa, F.; Remer, K.A.; Lorenz, U.; Bender, J.; Schulz, T.; Flieger, A.; Steinert, M.; Heuner, K. Phospholipase PlaB is a new virulence factor of Legionella pneumophila. Int. J. Med. Microbiol. 2010, 300, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Glöckner, G.; Albert-Weissenberger, C.; Weinmann, E.; Jacobi, S.; Schunder, E.; Steinert, M.; Hacker, J.; Heuner, K. Identification and characterization of a new conjugation/type IVA secretion system (trb/tra) of Legionella pneumophila Corby localized on two mobile genomic islands. Int. J. Med. Microbiol. 2008, 298, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Eucast: Guidance Document on Antimicrobial Susceptibility Testing of Legionella pneumophila. Available online: https://www.eucast.org/eucastguidancedocuments/ (accessed on 1 November 2022).
- Bhaya, D.; Davison, M.; Barrangou, R. CRISPR-Cas systems in bacteria and archaea: Versatile small RNAs for adaptive defense and regulation. Annu. Rev. Genet. 2011, 45, 273–297. [Google Scholar] [CrossRef]
- Hille, F.; Richter, H.; Wong, S.P.; Bratovič, M.; Ressel, S.; Charpentier, E. The Biology of CRISPR-Cas: Backward and Forward. Cell 2018, 172, 1239–1259. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef]
- Pedro-Botet, M.L.; García-Cruz, A.; Tural, C.; Mateu, L.; Sopena, N.; Roure, S.; Rey-Joly, C.; Sabria, M. Severe Legionnaires’ disease successfully treated with levofloxacin and azithromycin. J. Chemother. 2006, 18, 559–561. [Google Scholar] [CrossRef]
- Woodhead, M.; Blasi, F.; Ewig, S.; Garau, J.; Huchon, G.; Ieven, M.; Ortqvist, A.; Schaberg, T.; Torres, A.; van der Heijden, G.; et al. Guidelines for the management of adult lower respiratory tract infections--full version. Clin. Microbiol. Infect. 2011, 17, E1–E59. [Google Scholar] [CrossRef]
- Jonas, D.; Engels, I.; Daschner, F.D.; Frank, U. The effect of azithromycin on intracellular Legionella pneumophila in the Mono Mac 6 cell line at serum concentrations attainable in vivo. J. Antimicrob. Chemother. 2000, 46, 385–390. [Google Scholar] [CrossRef][Green Version]
- Stout, J.E.; Arnold, B.; Yu, V.L. Activity of azithromycin, clarithromycin, roxithromycin, dirithromycin, quinupristin/dalfopristin and erythromycin against Legionella species by intracellular susceptibility testing in HL-60 cells. J. Antimicrob. Chemother. 1998, 41, 289–291. [Google Scholar] [CrossRef]
- Jia, X.; Ren, H.; Nie, X.; Li, Y.; Li, J.; Qin, T. Antibiotic Resistance and Azithromycin Resistance Mechanism of Legionella pneumophila Serogroup 1 in China. Antimicrob. Agents Chemother. 2019, 63, e00768-19. [Google Scholar] [CrossRef]
- Natås, O.B.; Brekken, A.L.; Bernhoff, E.; Hetland, M.; Löhr, I.H.; Lindemann, P.C. Susceptibility of Legionella pneumophila to antimicrobial agents and the presence of the efflux pump LpeAB. J. Antimicrob. Chemother. 2019, 74, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Zhang, W.; Liu, W.; Zhou, H.; Ren, H.; Shao, Z.; Lan, R.; Xu, J. Population structure and minimum core genome typing of Legionella pneumophila. Sci. Rep. 2016, 6, 21356. [Google Scholar] [CrossRef][Green Version]
- Coscollá, M.; Comas, I.; González-Candelas, F. Quantifying nonvertical inheritance in the evolution of Legionella pneumophila. Mol. Biol. Evol. 2011, 28, 985–1001. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coscollá, M.; González-Candelas, F. Population structure and recombination in environmental isolates of Legionella pneumophila. Environ. Microbiol. 2007, 9, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Cianciotto, N.P. Type II secretion and Legionella virulence. Curr. Top Microbiol. Immunol. 2013, 376, 81–102. [Google Scholar] [PubMed]
- Jeong, K.C.; Ghosal, D.; Chang, Y.W.; Jensen, G.J.; Vogel, J.P. Polar delivery of Legionella type IV secretion system substrates is essential for virulence. Proc. Natl. Acad. Sci. USA 2017, 114, 8077–8082. [Google Scholar] [CrossRef]
- Marra, A.; Blander, S.J.; Horwitz, M.A.; Shuman, H.A. Identification of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc. Natl. Acad. Sci. USA 1992, 89, 9607–9611. [Google Scholar] [CrossRef]
- Stone, B.J.; Kwaik, Y.A. Natural competence for DNA transformation by Legionella pneumophila and its association with expression of type IV pili. J. Bacteriol. 1999, 181, 1395–1402. [Google Scholar] [CrossRef]
- Sexton, J.A.; Vogel, J.P. Regulation of hypercompetence in Legionella pneumophila. J. Bacteriol. 2004, 186, 3814–3825. [Google Scholar] [CrossRef]
- Cazalet, C.; Rusniok, C.; Brüggemann, H.; Zidane, N.; Magnier, A.; Ma, L.; Tichit, M.; Jarraud, S.; Bouchier, C.; Vandenesch, F.; et al. Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat. Genet. 2004, 36, 1165–1173. [Google Scholar] [CrossRef]
- D’Auria, G.; Jiménez-Hernández, N.; Peris-Bondia, F.; Moya, A.; Latorre, A. Legionella pneumophila pangenome reveals strain-specific virulence factors. BMC Genom. 2010, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.; Guyard, C.; Pelaz, C.; Wasserscheid, J.; Bondy-Denomy, J.; Dewar, K.; Ensminger, A.W. Active and adaptive Legionella CRISPR-Cas reveals a recurrent challenge to the pathogen. Cell. Microbiol. 2016, 18, 1319–1338. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, F.F.; Cianciotto, N.P. The CRISPR-associated gene cas2 of Legionella pneumophila is required for intracellular infection of amoebae. MBio 2013, 4, e13–e74. [Google Scholar] [CrossRef] [PubMed]
- Faucher, S.P.; Shuman, H.A. Small Regulatory RNA and Legionella pneumophila. Front. Microbiol. 2011, 2, 98. [Google Scholar] [CrossRef] [PubMed]
- Mcginn, J.; Marraffini, L.A. Molecular mechanisms of CRISPR-Cas spacer acquisition. Nat. Rev. Microbiol. 2019, 17, 7–12. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, B.; Duan, G.; Wang, Y.; Hong, L.; Wang, L.; Guo, X.; Xi, Y.; Yang, H. Bioinformatics analyses of Shigella CRISPR structure and spacer classification. World J. Microbiol. Biotechnol. 2016, 32, 38. [Google Scholar] [CrossRef]





| Antibiotic Class. | Drug | MIC Range (mg/L) | ECOFF (mg/L) | ATCC 33152 | Corby (ICDC) |
|---|---|---|---|---|---|
| Quinolone | Ciprofloxacin | 0.25–2 | 1.0 | 0.38 a | 0.25 a |
| Quinolone | Levofloxacin | 0.064–1 | 0.50 | 0.125 a | 0.064 a |
| Quinolone | Moxifloxacin | 0.25–1 | 1.0 | 0.75 a | 0.5 a |
| Macrolide | Erythromycin | 0.032–2 | 1.0 | 0.50 a | 0.19 a |
| Macrolide | Azithromycin | 0.038–8 | 1.0 | 0.25 a | 0.047 a |
| Macrolide | Clarithromycin | 0.064–1 | 0.50 | 0.19 a | 0.064 a |
| Rifamycin | Rifampicin | 0.004–0.032 | 0.032 | <0.016 a | <0.016 a |
| Tetracycline | Tigecycline | 1–16 | 16 | 0.5 a | 0.75 a |
| Tetracycline | Doxycycline | 1–8 | 8 | 4 a | 1.5 a |
| Features | Corby (ICDC) | Corby |
|---|---|---|
| Genome length (bp) | 3,452,077 | 3,576,470 |
| Serotype | 1 | 1 |
| G + C content (%) | 38.91 | 38.48 |
| Number of CDS genes | 3201 | 3193 |
| tRNA | 42 | 44 |
| 16S/23S/5S | 1/1/3 | 3/3/3 |
| Average length of CDS (nt) | 952 | 984.35 |
| Number of plasmids | 0 | 0 |
| CRISPR1 | ||||
|---|---|---|---|---|
| Spacers | Coverage | Source of Proto-Spacers | Matching Category | Coding Product of Matching Genes |
| spacer12 | 27/32 | bacteria | Palaeococcus pacificus DY20341 chromosome | Cobaltochelatase subunit CobN |
| 27/32 | bacteria | Thermococcus sp. IOH2 chromosome | Cobaltochelatase subunit CobN | |
| 26/32 | bacteria | Methanobrevibacter smithii | NCR | |
| 26/32 | phage | Arcanobacterium phage vB-ApyS-JF1 | Hypothetical protein and NCR | |
| spacer26 | 27/32 | virus | Gokushovirus isolate SH-CHD11 | Putative VP1 |
| 27/32 | plasmid | Citrobacter sp. TSA-1 plasmid | Phage capsid protein | |
| 26/32 | virus | Microviridae sp. isolate 7408–1711 | Similar to VP1 | |
| spacer29 | 26/32 | bacteria | Methanonatronarchaeum thermophilum strain AMET1 AMET1_3 | Glycosyltransferase family 4 protein |
| spacer41 | 26/32 | plasmid | Listeria monocytogenes strain FDAARGOS_57 plasmid | Helix–turn–helix domain-containing protein |
| spacer43 | 26/32 | bacteria | Thermococcus radiotolerans strain EJ2 chromosome | Flippase |
| spacer52 | 26/32 | virus | Robinz microvirus RP_102 | Major capsid protein |
| 26/32 | virus | Gokushovirus WZ-2015a | VP1 | |
| spacer53 | 30/32 | virus | Microviridae sp. | Major capsid protein |
| 27/32 | virus | Gokushovirus WZ-2015a | VP1 | |
| 27/32 | virus | Flumine microvirus | NCR | |
| 27/32 | virus | Robinz microvirus RP_84 | Major capsid protein | |
| 27/32 | virus | Blackfly Microvirus SF02 isolate 174 | Major capsid protein | |
| 26/32 | virus | Capybara microvirus Cap1_SP_192 | Major capsid protein | |
| 26/32 | virus | Chimpanzee faeces-associated microphage 1 isolate CPNG_29298 | Gene: major CP | |
| spacer54 | 30/32 | plasmid | Legionella pneumophila plasmid | Repeat region |
| 27/32 | plasmid | Burkholderia pseudomallei strain 2008724860 plasmid p1 | Chromate efflux transporter | |
| NCR, non-coding region | ||||
| CRISPR2 | ||||
| spacers | Coverage | Source of proto-spacers | Matching category | Coding product of matching genes |
| spacer2 | 27/32 | plasmid | Legionella pneumophila subsp. pneumophila strain Allentown 1 (D-7475) plasmid | Repeat region |
| spacer17 | 26/32 | phage | Pseudomonas phage PSA11 | Hypothetical protein ORF028 |
| phage | Bacteriophage PA11 | NCR | ||
| spacer23 | 26/32 | phage | Aeromonas phage BUCT695 | Scaffolding protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.-X.; Ren, H.-Y.; Li, R.; Jin, X.-J.; Gao, Z.-C.; Qin, T. Legionnaires’ Disease in China Caused by Legionella pneumophila Corby. Microorganisms 2023, 11, 204. https://doi.org/10.3390/microorganisms11010204
Xu P-X, Ren H-Y, Li R, Jin X-J, Gao Z-C, Qin T. Legionnaires’ Disease in China Caused by Legionella pneumophila Corby. Microorganisms. 2023; 11(1):204. https://doi.org/10.3390/microorganisms11010204
Chicago/Turabian StyleXu, Pei-Xing, Hong-Yu Ren, Ran Li, Xiao-Jing Jin, Zhan-Cheng Gao, and Tian Qin. 2023. "Legionnaires’ Disease in China Caused by Legionella pneumophila Corby" Microorganisms 11, no. 1: 204. https://doi.org/10.3390/microorganisms11010204
APA StyleXu, P.-X., Ren, H.-Y., Li, R., Jin, X.-J., Gao, Z.-C., & Qin, T. (2023). Legionnaires’ Disease in China Caused by Legionella pneumophila Corby. Microorganisms, 11(1), 204. https://doi.org/10.3390/microorganisms11010204





