A Search for Tick-Associated, Bronnoya-like Virus Spillover into Sheep
Abstract
1. Introduction
2. Materials and Methods
2.1. Tick Collection and Preparation of Metatranscriptomics Libraries
2.1.1. Ticks Collected in France, 2010–2012
2.1.2. Ticks Collected in Romania, Iasi County, 2015
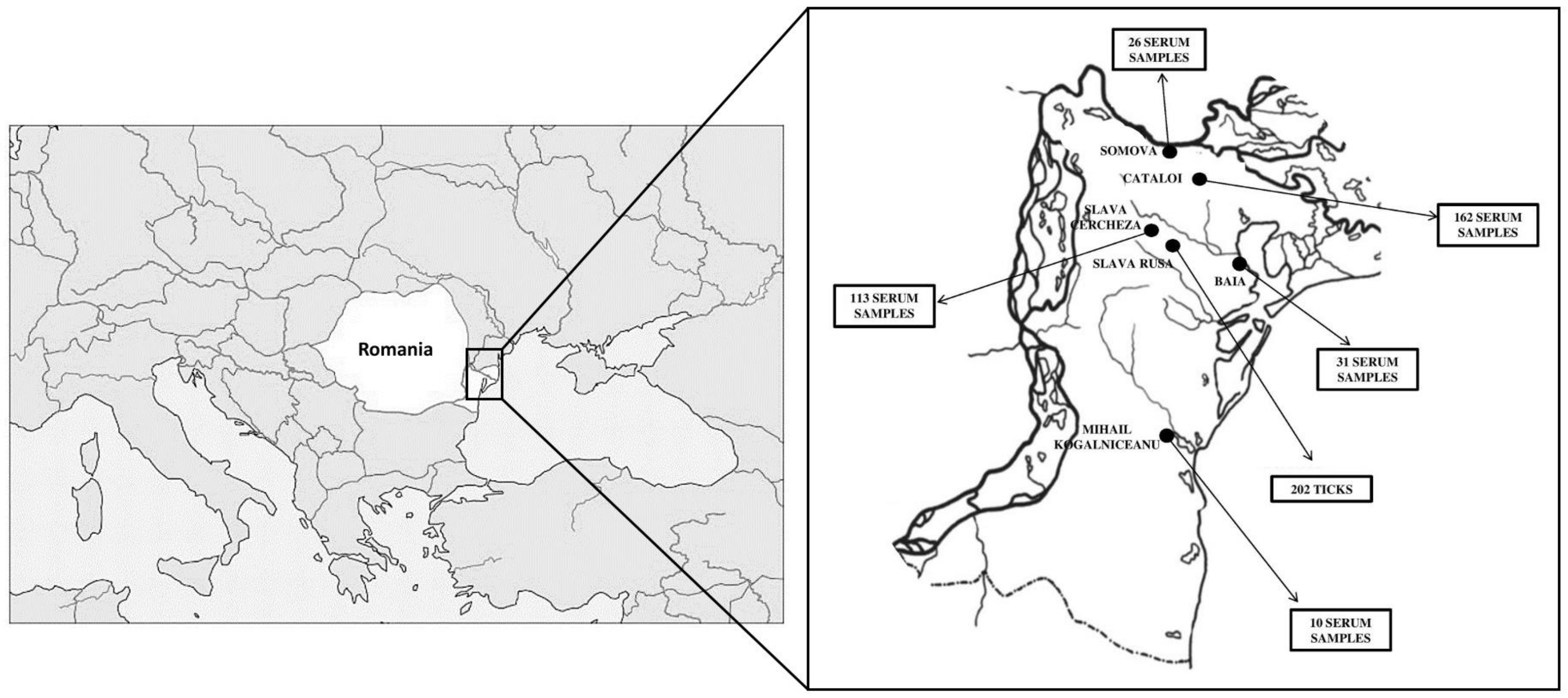
2.1.3. Ticks Collected in Romania, Danube Delta Reserve, 2020–2021
2.2. Determination of Tick Species
2.3. Virus Assignation
2.4. Phylogenetic Analyses
2.5. Sheep and Goats Sera Collection
2.6. Serological Screening of Small Ruminants
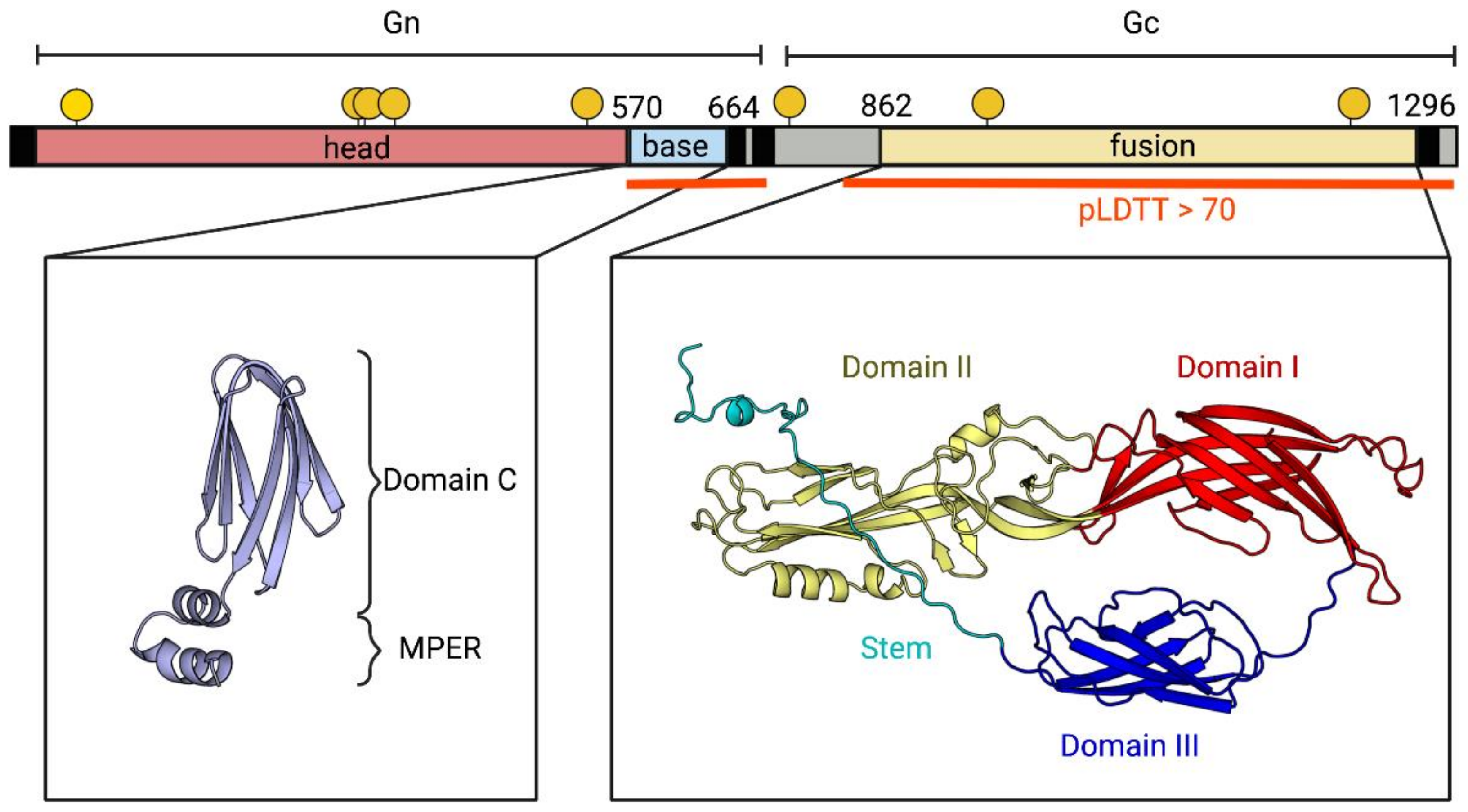
2.6.1. Antigen Design
2.6.2. Expression of Recombinant Proteins
2.6.3. LIPS Assay
2.7. Statistical Tests
3. Results
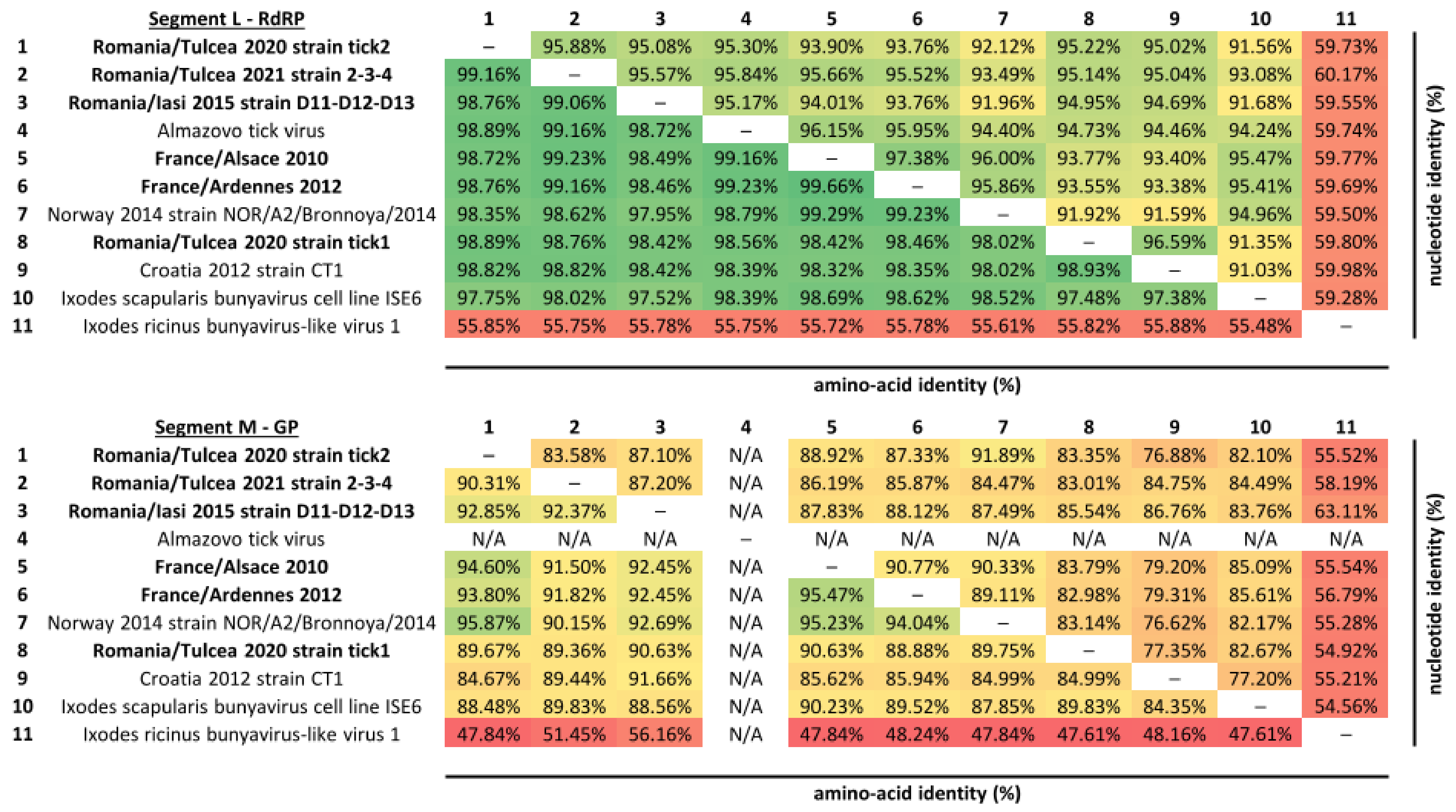
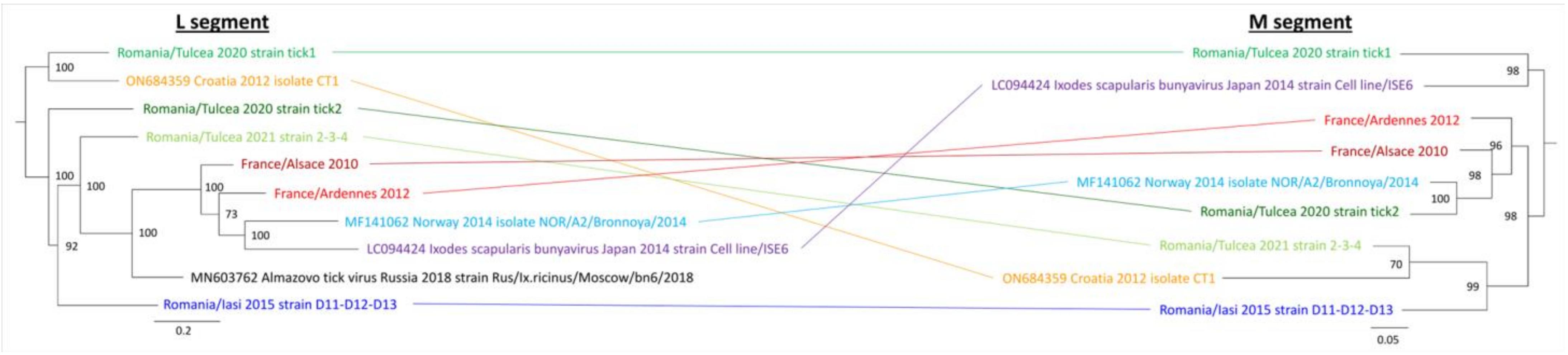
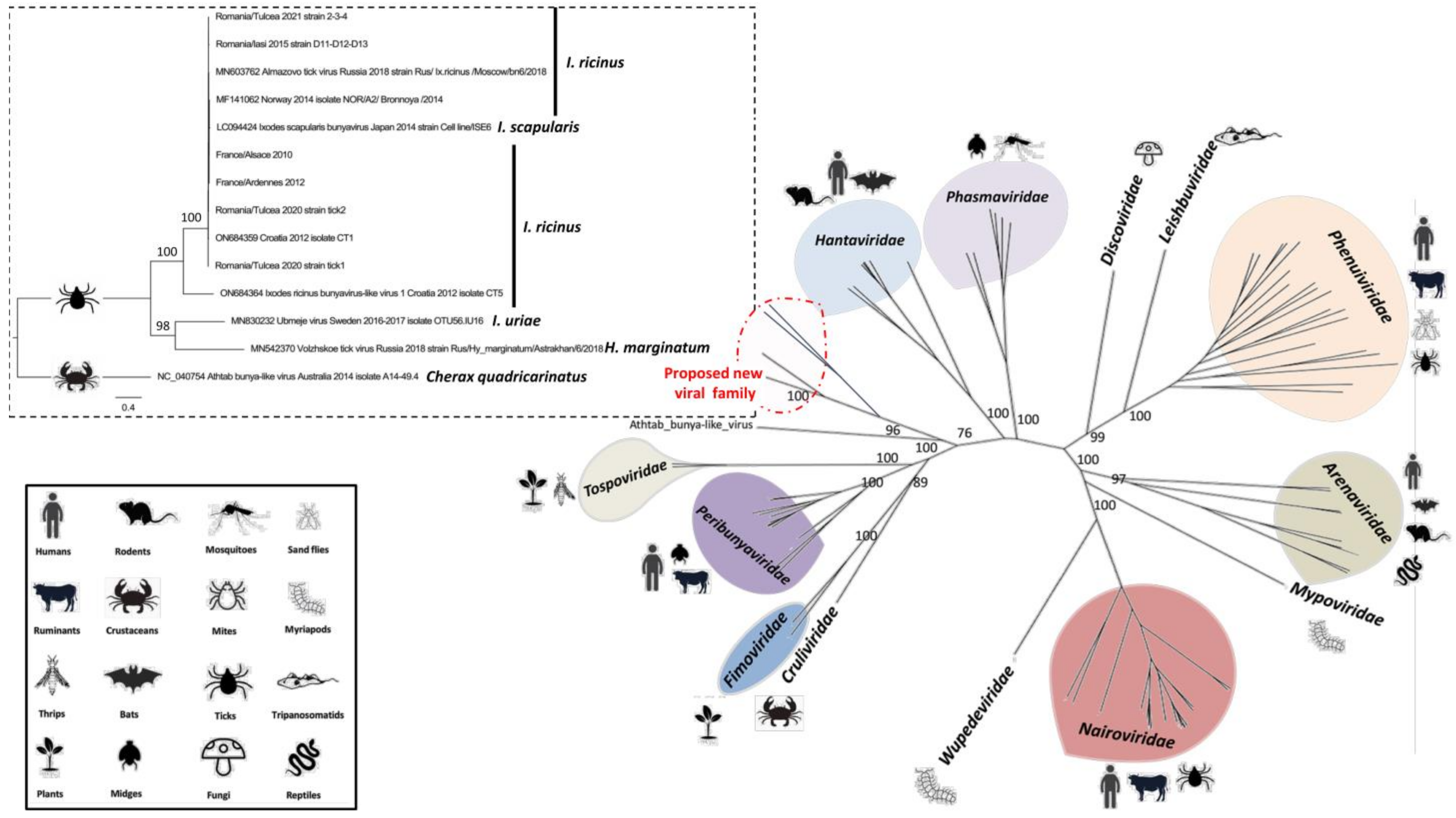
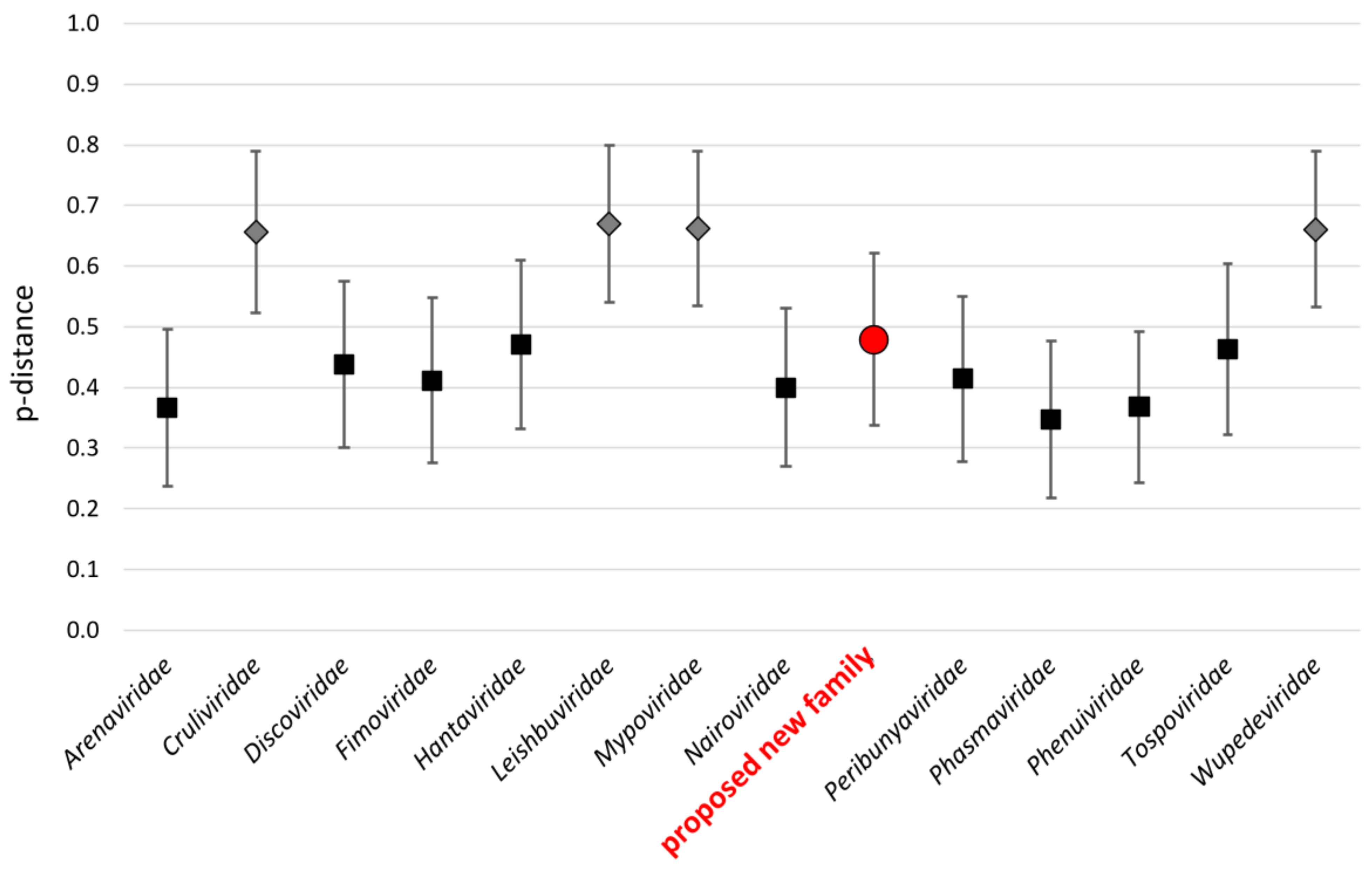
3.1. Identification and Evolution History of Bronnoya Viruses
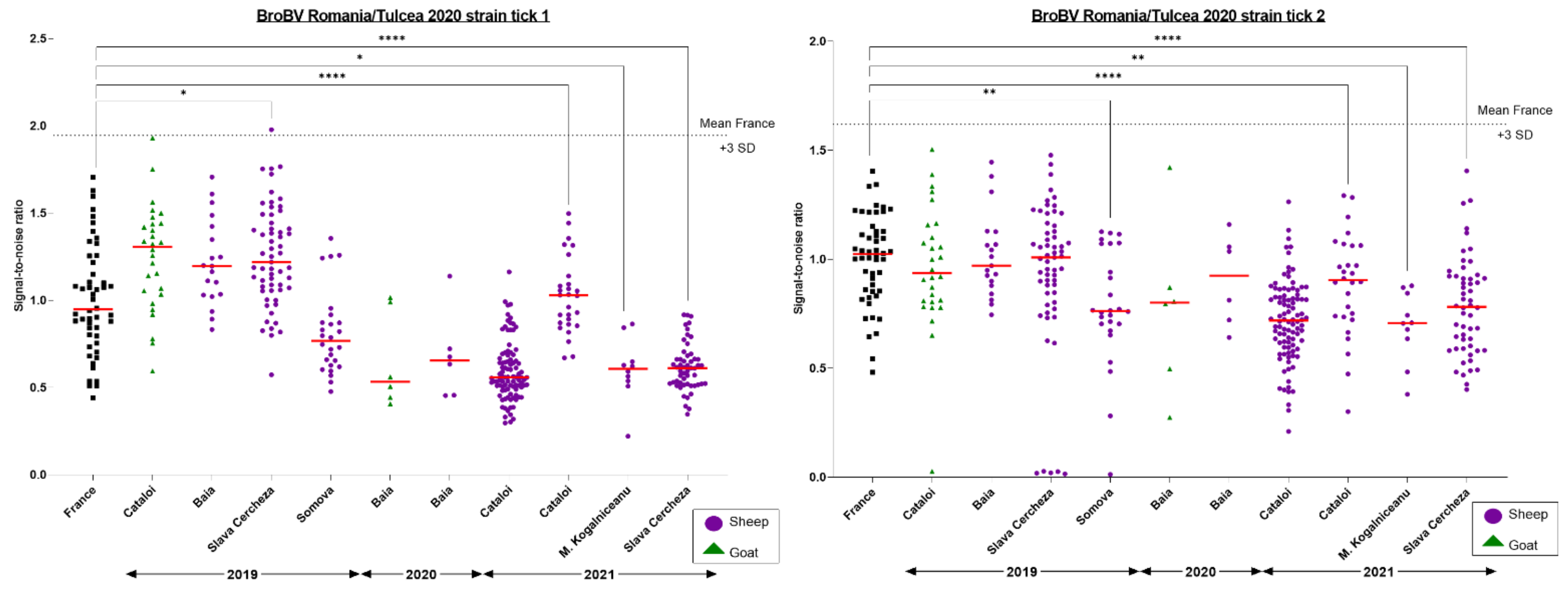
3.2. Transmission of Bronnoya Viruses to Small Ruminants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Charrel, R.N.; Attoui, H.; Butenko, A.M.; Clegg, J.C.; Deubel, V.; Frolova, T.V.; Gould, E.A.; Gritsun, T.S.; Heinz, F.X.; Labuda, M.; et al. Tick-borne virus diseases of human interest in Europe. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2004, 10, 1040–1055. [Google Scholar] [CrossRef] [PubMed]
- Jaaskelainen, A.; Tonteri, E.; Pieninkeroinen, I.; Sironen, T.; Voutilainen, L.; Kuusi, M.; Vaheri, A.; Vapalahti, O. Siberian subtype tick-borne encephalitis virus in Ixodes ricinus in a newly emerged focus, Finland. Ticks Tick-Borne Dis. 2016, 7, 216–223. [Google Scholar] [CrossRef]
- Pettersson, J.H.; Shi, M.; Bohlin, J.; Eldholm, V.; Brynildsrud, O.B.; Paulsen, K.M.; Andreassen, A.; Holmes, E.C. Characterizing the virome of Ixodes ricinus ticks from northern Europe. Sci. Rep. 2017, 7, 10870. [Google Scholar] [CrossRef] [PubMed]
- Moutailler, S.; Popovici, I.; Devillers, E.; Vayssier-Taussat, M.; Eloit, M. Diversity of viruses in Ixodes ricinus, and characterization of a neurotropic strain of Eyach virus. New Microbes New Infect. 2016, 11, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Abudurexiti, A.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Avsic-Zupanc, T.; Ballinger, M.J.; Bente, D.A.; Beer, M.; Bergeron, E.; Blair, C.D.; et al. Taxonomy of the order Bunyavirales: Update 2019. Arch. Virol. 2019, 164, 1949–1965. [Google Scholar] [CrossRef]
- Kuehnert, P.A.; Stefan, C.P.; Badger, C.V.; Ricks, K.M. Crimean-Congo Hemorrhagic Fever Virus (CCHFV): A Silent but Widespread Threat. Curr. Trop. Med. Rep. 2021, 8, 141–147. [Google Scholar] [CrossRef]
- Liu, Q.; He, B.; Huang, S.Y.; Wei, F.; Zhu, X.Q. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect. Dis. 2014, 14, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Brault, A.C.; Savage, H.M.; Duggal, N.K.; Eisen, R.J.; Staples, J.E. Heartland Virus Epidemiology, Vector Association, and Disease Potential. Viruses 2018, 10, 498. [Google Scholar] [CrossRef]
- Sameroff, S.; Tokarz, R.; Vucelja, M.; Jain, K.; Oleynik, A.; Boljfetic, M.; Bjedov, L.; Yates, R.A.; Margaletic, J.; Oura, C.A.L.; et al. Virome of Ixodes ricinus, Dermacentor reticulatus, and Haemaphysalis concinna Ticks from Croatia. Viruses 2022, 14, 929. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhang, G.; Jiang, L.; Wang, P.; Zhang, S.; Zheng, X.; Li, Y. Metatranscriptomics Reveals the Diversity of the Tick Virome in Northwest China. Microbiol. Spectr. 2022, 10, e0111522. [Google Scholar] [CrossRef] [PubMed]
- Nakao, R.; Matsuno, K.; Qiu, Y.; Maruyama, J.; Eguchi, N.; Nao, N.; Kajihara, M.; Yoshii, K.; Sawa, H.; Takada, A.; et al. Putative RNA viral sequences detected in an Ixodes scapularis-derived cell line. Ticks Tick-Borne Dis. 2017, 8, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Vayssier-Taussat, M.; Moutailler, S.; Michelet, L.; Devillers, E.; Bonnet, S.; Cheval, J.; Hebert, C.; Eloit, M. Next generation sequencing uncovers unexpected bacterial pathogens in ticks in western Europe. PLoS ONE 2013, 8, e81439. [Google Scholar] [CrossRef]
- Perez-Eid, C. Les Tiques: Identification, Biologie, Importance Medicale et Veterinaire; Medicales Internationales: Paris, France, 2007. [Google Scholar]
- Bratuleanu, B.E.; Temmam, S.; Chretien, D.; Regnault, B.; Perot, P.; Bouchier, C.; Bigot, T.; Savuta, G.; Eloit, M. The virome of Rhipicephalus, Dermacentor and Haemaphysalis ticks from Eastern Romania includes novel viruses with potential relevance for public health. Transbound. Emerg. Dis. 2022, 69, 1387–1403. [Google Scholar] [CrossRef]
- Bratuleanu, B.E.; Temmam, S.; Munier, S.; Chretien, D.; Bigot, T.; van der Werf, S.; Savuta, G.; Eloit, M. Detection of Phenuiviridae, Chuviridae Members, and a Novel Quaranjavirus in Hard Ticks From Danube Delta. Front. Vet. Sci. 2022, 9, 863814. [Google Scholar] [CrossRef]
- Perot, P.; Bigot, T.; Temmam, S.; Regnault, B.; Eloit, M. Microseek: A Protein-Based Metagenomic Pipeline for Virus Diagnostic and Discovery. Viruses 2022, 14, 1990. [Google Scholar] [CrossRef]
- Bigot, T.; Temmam, S.; Perot, P.; Eloit, M. RVDB-prot, a reference viral protein database and its HMM profiles. F1000Research 2019, 8, 530. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Lefort, V.; Longueville, J.E.; Gascuel, O. SMS: Smart Model Selection in PhyML. Mol. Biol. Evol. 2017, 34, 2422–2424. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Temmam, S.; Chretien, D.; Bigot, T.; Dufour, E.; Petres, S.; Desquesnes, M.; Devillers, E.; Dumarest, M.; Yousfi, L.; Jittapalapong, S.; et al. Monitoring Silent Spillovers Before Emergence: A Pilot Study at the Tick/Human Interface in Thailand. Front. Microbiol. 2019, 10, 2315. [Google Scholar] [CrossRef] [PubMed]
- Temmam, S.; Bigot, T.; Chretien, D.; Gondard, M.; Perot, P.; Pommelet, V.; Dufour, E.; Petres, S.; Devillers, E.; Hoem, T.; et al. Insights into the Host Range, Genetic Diversity, and Geographical Distribution of Jingmenviruses. mSphere 2019, 4, e00645-19. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, J.H.; Ellstrom, P.; Ling, J.; Nilsson, I.; Bergstrom, S.; Gonzalez-Acuna, D.; Olsen, B.; Holmes, E.C. Circumpolar diversification of the Ixodes uriae tick virome. PLoS Pathog. 2020, 16, e1008759. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Hu, S.; Liu, X.; Yang, J.; Liu, D.; Wu, L.; Wang, H.; Hu, Z.; Deng, F.; Shen, S. Migration, recombination, and reassortment are involved in the evolution of severe fever with thrombocytopenia syndrome bunyavirus. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2017, 47, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Guardado-Calvo, P.; Rey, F.A. The Viral Class II Membrane Fusion Machinery: Divergent Evolution from an Ancestral Heterodimer. Viruses 2021, 13, 2368. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Pena, A.; de la Fuente, J. Species interactions in occurrence data for a community of tick-transmitted pathogens. Sci. Data 2016, 3, 160056. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Hansford, K.M.; Bormane, A.; Derdakova, M.; Estrada-Pena, A.; George, J.C.; Golovljova, I.; Jaenson, T.G.; Jensen, J.K.; Jensen, P.M.; et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasites Vectors 2013, 6, 1. [Google Scholar] [CrossRef]
- Paulauskas, A.; Galdikas, M.; Galdikaite-Braziene, E.; Stanko, M.; Kahl, O.; Karbowiak, G.; Radzijevskaja, J. Microsatellite-based genetic diversity of Dermacentor reticulatus in Europe. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2018, 66, 200–209. [Google Scholar] [CrossRef]
- Foldvari, G.; Siroky, P.; Szekeres, S.; Majoros, G.; Sprong, H. Dermacentor reticulatus: A vector on the rise. Parasites Vectors 2016, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel, F.; Gonzalez, J.; Gonzalez, M.G.; Sanchez, M.; Tercero, J.M.; Elhachimi, L.; Carbonell, J.D.; Olmeda, A.S. Comparative Ecology of Hyalomma lusitanicum and Hyalomma marginatum Koch, 1844 (Acarina: Ixodidae). Insects 2020, 11, 303. [Google Scholar] [CrossRef] [PubMed]
| Year of Collection | Collection Site | Species of Small Ruminants | Number of Serum Samples |
|---|---|---|---|
| 2019 | Baia | Sheep | 19 |
| Cataloi | Goats | 28 | |
| Sheep | 28 | ||
| Slava Cercheză | Sheep | 59 | |
| Somova | Sheep | 26 | |
| 2020 | Baia | Sheep | 6 |
| Goats | 6 | ||
| 2021 | Cataloi | Sheep | 106 |
| Mihail Kogalniceanu | Sheep | 10 | |
| Slava Cercheză | Sheep | 54 | |
| TOTAL | 342 |
| Strain | Tick Species | Location | Year | Accession Number |
|---|---|---|---|---|
| Romania/Tulcea_2020_strain_tick1 | Ixodes ricinus | Slava Rusa, Romania | 2020 | OQ029680 (L) OQ029681(M) |
| Romania/Tulcea_2020_strain_tick2 | 2020 | OQ029682 (L) OQ029683 (M) | ||
| Romania/Tulcea_2021_strain_2-3-4 | 2021 | OQ029684 (L) OQ029685 (M) | ||
| Romania/Iasi_2015_strain_D11-D12-D13 | Iasi, Romania | 2015 | OQ029686 (L) OQ029687 (M) | |
| France/Alsace_2010 | Alsace, France | 2010 | OQ029688 (L) OQ029689 (M) | |
| France/Ardennes_2012 | Ardennes, France | 2012 | OQ029690 (L) OQ029691 (M) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bratuleanu, B.E.; Raileanu, C.; Chrétien, D.; Guardado-Calvo, P.; Bigot, T.; Savuta, G.; Temmam, S.; Eloit, M. A Search for Tick-Associated, Bronnoya-like Virus Spillover into Sheep. Microorganisms 2023, 11, 209. https://doi.org/10.3390/microorganisms11010209
Bratuleanu BE, Raileanu C, Chrétien D, Guardado-Calvo P, Bigot T, Savuta G, Temmam S, Eloit M. A Search for Tick-Associated, Bronnoya-like Virus Spillover into Sheep. Microorganisms. 2023; 11(1):209. https://doi.org/10.3390/microorganisms11010209
Chicago/Turabian StyleBratuleanu, Bianca Elena, Cristian Raileanu, Delphine Chrétien, Pablo Guardado-Calvo, Thomas Bigot, Gheorghe Savuta, Sarah Temmam, and Marc Eloit. 2023. "A Search for Tick-Associated, Bronnoya-like Virus Spillover into Sheep" Microorganisms 11, no. 1: 209. https://doi.org/10.3390/microorganisms11010209
APA StyleBratuleanu, B. E., Raileanu, C., Chrétien, D., Guardado-Calvo, P., Bigot, T., Savuta, G., Temmam, S., & Eloit, M. (2023). A Search for Tick-Associated, Bronnoya-like Virus Spillover into Sheep. Microorganisms, 11(1), 209. https://doi.org/10.3390/microorganisms11010209








