Evaluation of Serratia marcescens Adherence to Contact Lens Materials
Abstract
1. Introduction
2. Materials and Methods
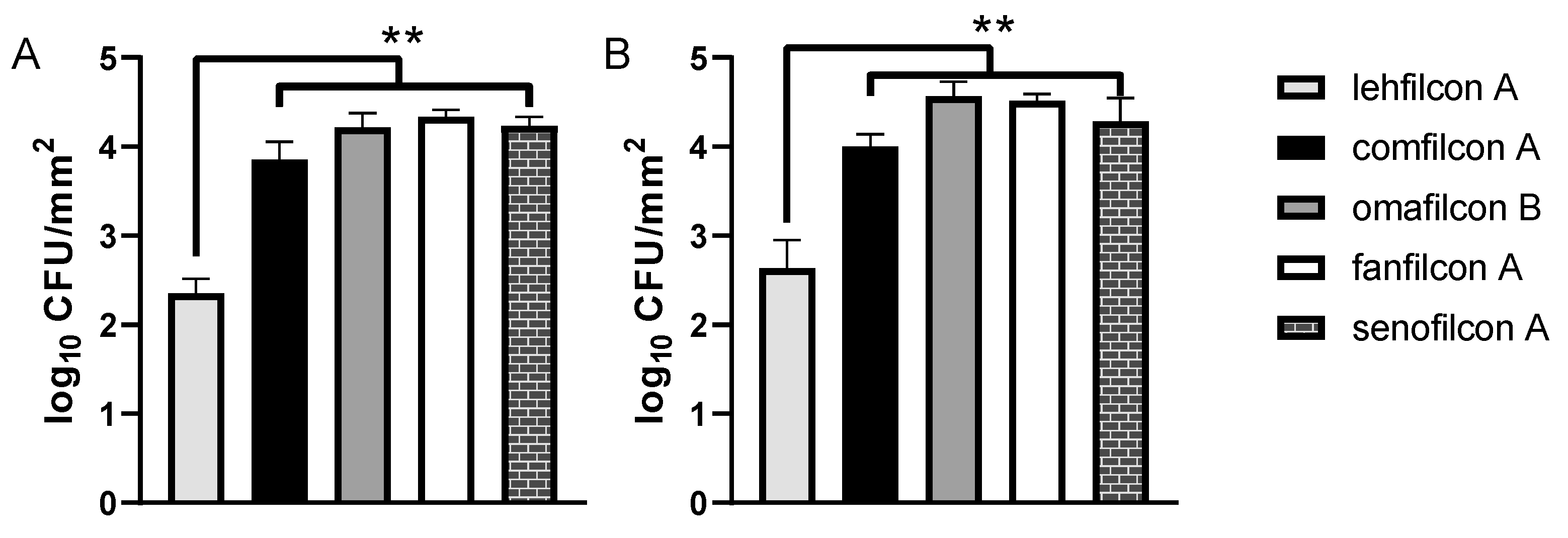
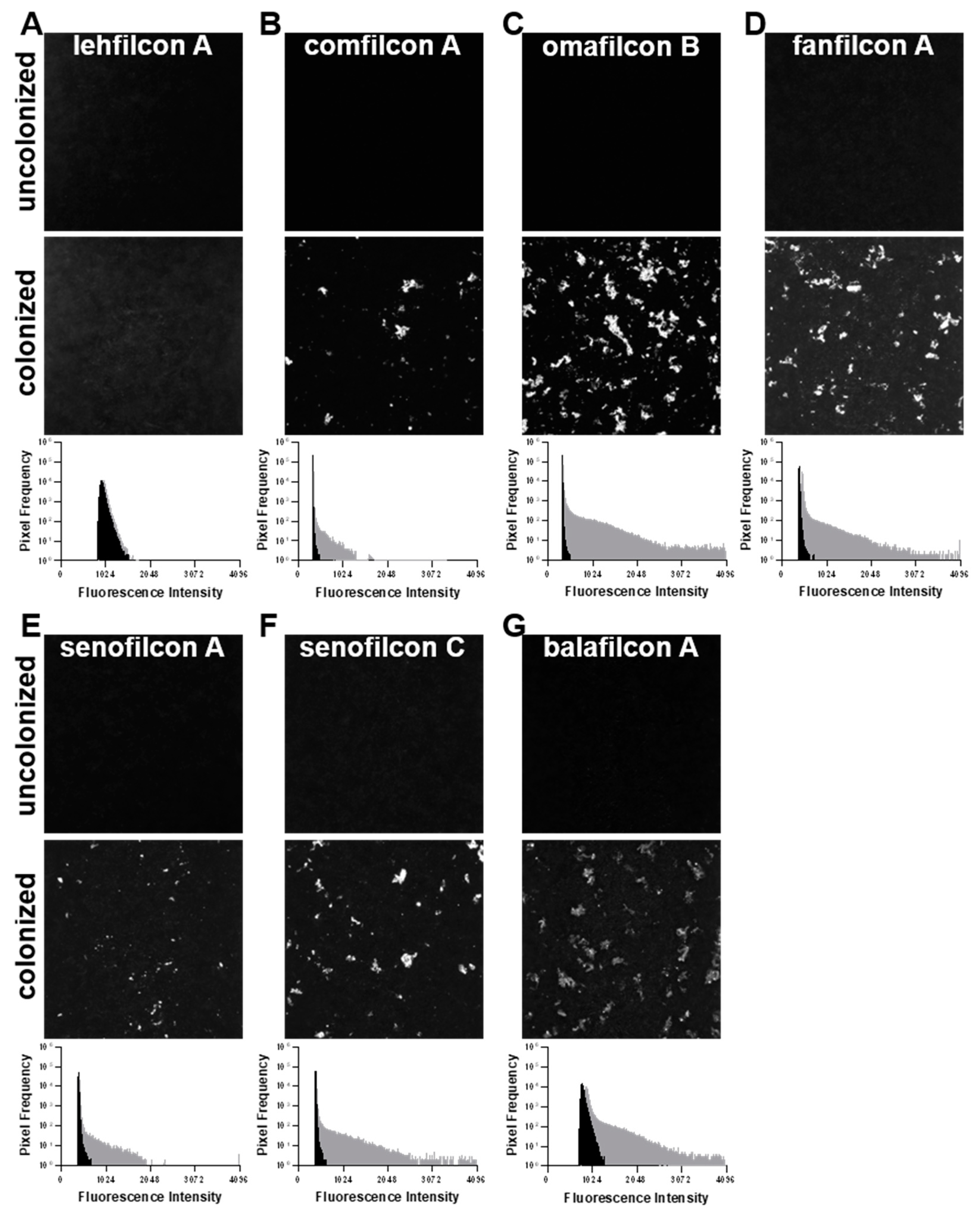
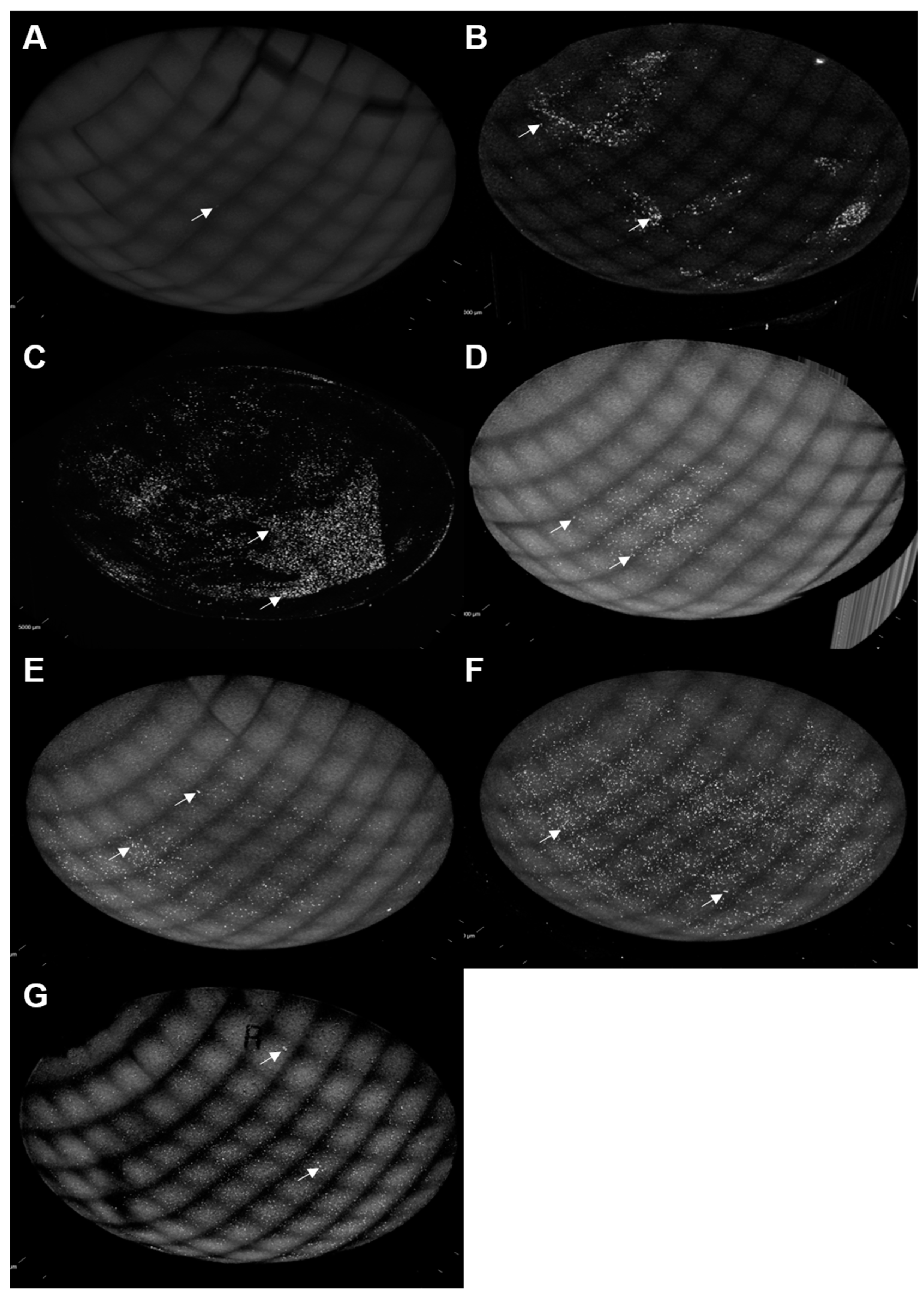
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleiszig, S.M.J.; Kroken, A.R.; Nieto, V.; Grosser, M.R.; Wan, S.J.; Metruccio, M.M.E.; Evans, D.J. Contact lens-related corneal infection: Intrinsic resistance and its compromise. Prog. Retin. Eye Res. 2020, 76, 100804. [Google Scholar] [CrossRef] [PubMed]
- Keay, L.; Edwards, K.; Naduvilath, T.; Taylor, H.R.; Snibson, G.R.; Forde, K.; Stapleton, F. Microbial keratitis predisposing factors and morbidity. Ophthalmology 2006, 113, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Bourcier, T.; Thomas, F.; Borderie, V.; Chaumeil, C.; Laroche, L. Bacterial keratitis: Predisposing factors, clinical and microbiological review of 300 cases. Br. J. Ophthalmol. 2003, 87, 834–838. [Google Scholar] [CrossRef]
- Mondino, B.J.; Weissman, B.A.; Farb, M.D.; Pettit, T.H. Corneal ulcers associated with daily-wear and extended-wear contact lenses. Am. J. Ophthalmol. 1986, 102, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Keay, L.; Edwards, K.; Naduvilath, T.; Dart, J.K.; Brian, G.; Holden, B.A. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology 2008, 115, 1655–1662. [Google Scholar] [CrossRef]
- Morgan, P.B.; Efron, N.; Hill, E.A.; Raynor, M.K.; Whiting, M.A.; Tullo, A.B. Incidence of keratitis of varying severity among contact lens wearers. Br. J. Ophthalmol. 2005, 89, 430–436. [Google Scholar] [CrossRef]
- Dart, J.K.; Radford, C.F.; Minassian, D.; Verma, S.; Stapleton, F. Risk factors for microbial keratitis with contemporary contact lenses: A case-control study. Ophthalmology 2008, 115, 1647–1654. [Google Scholar] [CrossRef]
- Kuzman, T.; Kutija, M.B.; Juri, J.; Jandrokovic, S.; Skegro, I.; Olujic, S.M.; Kordic, R.; Cerovski, B. Lens wearers non-compliance—Is there an association with lens case contamination? Contact Lens Anterior Eye 2014, 37, 99–105. [Google Scholar] [CrossRef]
- Stapleton, F.; Edwards, K.; Keay, L.; Naduvilath, T.; Dart, J.K.; Brian, G.; Holden, B. Risk factors for moderate and severe microbial keratitis in daily wear contact lens users. Ophthalmology 2012, 119, 1516–1521. [Google Scholar] [CrossRef]
- Cope, J.R.; Collier, S.A.; Rao, M.M.; Chalmers, R.; Mitchell, G.L.; Richdale, K.; Wagner, H.; Kinoshita, B.T.; Lam, D.Y.; Sorbara, L.; et al. Contact Lens Wearer Demographics and Risk Behaviors for Contact Lens-Related Eye Infections—United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 865–870. [Google Scholar] [CrossRef]
- Doan, T.; Akileswaran, L.; Andersen, D.; Johnson, B.; Ko, N.; Shrestha, A.; Shestopalov, V.; Lee, C.S.; Lee, A.Y.; Van Gelder, R.N. Paucibacterial Microbiome and Resident DNA Virome of the Healthy Conjunctiva. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5116–5126. [Google Scholar] [CrossRef]
- Park, S.H.; Lim, J.A.; Choi, J.S.; Kim, K.A.; Joo, C.K. The resistance patterns of normal ocular bacterial flora to 4 fluoroquinolone antibiotics. Cornea 2009, 28, 68–72. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, Q.; Li, W.; Wu, X.; Dong, Y.; Huang, Y. Profiling of Diagnostic Information of and Latent Susceptibility to Bacterial Keratitis From the Perspective of Ocular Bacterial Microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 645907. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, B.; Li, W. Defining the normal core microbiome of conjunctival microbial communities. Clin. Microbiol. Infect. 2016, 22, 643.e7–643.e12. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, J.; Nielsen, S.; Diez-Vives, C.; Coroneo, M.; Thomas, T.; Willcox, M. Temporal Stability and Composition of the Ocular Surface Microbiome. Sci. Rep. 2017, 7, 9880. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, J.; Zhu, H.; Gabriel, M.; Holden, B.A.; Willcox, M.D. Effect of prophylactic antibiotic drops on ocular microbiota and physiology during silicone hydrogel lens wear. Optom. Vis. Sci. 2012, 89, 326–335. [Google Scholar] [CrossRef]
- Ren, Z.; Li, W.; Liu, Q.; Dong, Y.; Huang, Y. Profiling of the Conjunctival Bacterial Microbiota Reveals the Feasibility of Utilizing a Microbiome-Based Machine Learning Model to Differentially Diagnose Microbial Keratitis and the Core Components of the Conjunctival Bacterial Interaction Network. Front. Cell. Infect. Microbiol. 2022, 12, 860370. [Google Scholar] [CrossRef]
- Shin, H.; Price, K.; Albert, L.; Dodick, J.; Park, L.; Dominguez-Bello, M.G. Changes in the Eye Microbiota Associated with Contact Lens Wearing. mBio 2016, 7, e00198. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sheorey, H.; Taylor, H.R.; Vajpayee, R.B. Association between cultures of contact lens and corneal scraping in contact lens related microbial keratitis. Arch. Ophthalmol. 2007, 125, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Lorente Pascua, J.; Garcia Bernal, A.; Garcia Sanchez, E.; Almeida Gonzalez, C.V. Microorganisms and Antibiotic Resistance of Bacterial Keratitis at a Rural County Hospital in Seville. Eye Contact Lens 2022, 48, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Mah-Sadorra, J.H.; Najjar, D.M.; Rapuano, C.J.; Laibson, P.R.; Cohen, E.J. Serratia corneal ulcers: A retrospective clinical study. Cornea 2005, 24, 793–800. [Google Scholar] [CrossRef]
- Atta, S.; Perera, C.; Nayyar, S.; Kowalski, R.P.; Jhanji, V. An 18-Year Overview of Serratia marcescens Ocular Infection. Eye Contact Lens 2021, 47, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Pinna, A.; Usai, D.; Sechi, L.A.; Carta, A.; Zanetti, S. Detection of virulence factors in Serratia strains isolated from contact lens-associated corneal ulcers. Acta Ophthalmol. 2011, 89, 382–387. [Google Scholar] [CrossRef]
- Shanks, R.M.; Stella, N.A.; Hunt, K.M.; Brothers, K.M.; Zhang, L.; Thibodeau, P.H. Identification of SlpB, a Cytotoxic Protease from Serratia marcescens. Infect. Immun. 2015, 83, 2907–2916. [Google Scholar] [CrossRef] [PubMed]
- Hume, E.B.; Zhu, H.; Cole, N.; Huynh, C.; Lam, S.; Willcox, M.D. Efficacy of contact lens multipurpose solutions against serratia marcescens. Optom. Vis. Sci. 2007, 84, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Kalaiselvan, P.; Dutta, D.; Bhombal, F.; Konda, N.; Vaddavalli, P.K.; Sharma, S.; Stapleton, F.; Willcox, M.D.P. Ocular microbiota and lens contamination following Mel4 peptide-coated antimicrobial contact lens (MACL) extended wear. Contact Lens Anterior Eye 2022, 45, 101431. [Google Scholar] [CrossRef]
- Kalaiselvan, P.; Konda, N.; Pampi, N.; Vaddavalli, P.K.; Sharma, S.; Stapleton, F.; Kumar, N.; Willcox, M.D.P.; Dutta, D. Effect of Antimicrobial Contact Lenses on Corneal Infiltrative Events: A Randomized Clinical Trial. Transl. Vis. Sci. Technol. 2021, 10, 32. [Google Scholar] [CrossRef]
- Dutta, D.; Cole, N.; Kumar, N.; Willcox, M.D. Broad spectrum antimicrobial activity of melimine covalently bound to contact lenses. Investig. Ophthalmol. Vis. Sci. 2013, 54, 175–182. [Google Scholar] [CrossRef]
- Cole, N.; Hume, E.B.; Vijay, A.K.; Sankaridurg, P.; Kumar, N.; Willcox, M.D. In vivo performance of melimine as an antimicrobial coating for contact lenses in models of CLARE and CLPU. Investig. Ophthalmol. Vis. Sci. 2010, 51, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D.; Hume, E.B.; Aliwarga, Y.; Kumar, N.; Cole, N. A novel cationic-peptide coating for the prevention of microbial colonization on contact lenses. J. Appl. Microbiol. 2008, 105, 1817–1825. [Google Scholar] [CrossRef]
- Datta, A.; Willcox, M.D.P.; Stapleton, F. In vivo efficacy of silver-impregnated barrel contact lens storage cases. Contact Lens Anterior Eye 2021, 44, 101357. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D.P.; Hume, E.B.H.; Vijay, A.K.; Petcavich, R. Ability of silver-impregnated contact lenses to control microbial growth and colonisation. J. Optom. 2010, 3, 143–148. [Google Scholar] [CrossRef]
- Zhu, H.; Kumar, A.; Ozkan, J.; Bandara, R.; Ding, A.; Perera, I.; Steinberg, P.; Kumar, N.; Lao, W.; Griesser, S.S.; et al. Fimbrolide-coated antimicrobial lenses: Their in vitro and in vivo effects. Optom. Vis. Sci. 2008, 85, 292–300. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Pierce, G.; Gabriel, M.; Morris, C.; Ahearn, D. Effects of quorum sensing molecules of Pseudomonas aeruginosa on organism growth, elastase B production, and primary adhesion to hydrogel contact lenses. Eye Contact Lens 2005, 31, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Cantu-Crouch, D.; Sharma, V.; Pruitt, J.; Yao, G.; Fukazawa, K.; Wu, J.Y.; Ishihara, K. Surface characterization of a silicone hydrogel contact lens having bioinspired 2-methacryloyloxyethyl phosphorylcholine polymer layer in hydrated state. Colloids Surf. B Biointerfaces 2021, 199, 111539. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Fukazawa, K.; Sharma, V.; Liang, S.; Shows, A.; Dunbar, D.C.; Zheng, Y.; Ge, J.; Zhang, S.; Hong, Y.; et al. Antifouling Silicone Hydrogel Contact Lenses with a Bioinspired 2-Methacryloyloxyethyl Phosphorylcholine Polymer Surface. ACS Omega 2021, 6, 7058–7067. [Google Scholar] [CrossRef]
- Huang, X.D.; Yao, K.; Zhang, H.; Huang, X.J.; Xu, Z.K. Surface modification of silicone intraocular lens by 2-methacryloyloxyethyl phosphoryl-choline binding to reduce Staphylococcus epidermidis adherence. Clin. Exp. Ophthalmol. 2007, 35, 462–467. [Google Scholar] [CrossRef]
- Fujii, K.; Matsumoto, H.N.; Koyama, Y.; Iwasaki, Y.; Ishihara, K.; Takakuda, K. Prevention of biofilm formation with a coating of 2-methacryloyloxyethyl phosphorylcholine polymer. J. Vet. Med. Sci. 2008, 70, 167–173. [Google Scholar] [CrossRef]
- Kunimatsu, R.; Tsuka, Y.; Nakajima, K.; Sumi, K.; Yoshimi, Y.; Kado, I.; Inada, A.; Kiritoshi, Y.; Tanimoto, K. The Influence of 2-Methacryloyloxyethyl Phosphorylcholine Polymer Materials on Orthodontic Friction and Attachment of Oral Bacteria. Materials 2022, 15, 5770. [Google Scholar] [CrossRef]
- Fujiwara, N.; Murakami, K.; Yoshida, K.; Sakurai, S.; Kudo, Y.; Ozaki, K.; Hirota, K.; Fujii, H.; Suzuki, M.; Miyake, Y.; et al. Suppressive effects of 2-methacryloyloxyethyl phosphorylcholine (MPC)-polymer on the adherence of Candida species and MRSA to acrylic denture resin. Heliyon 2020, 6, e04211. [Google Scholar] [CrossRef]
- Choi, A.; Yoo, K.H.; Yoon, S.Y.; Park, S.B.; Choi, Y.K.; Kim, Y.I. Enhanced antimicrobial and remineralizing properties of self-adhesive orthodontic resin containing mesoporous bioactive glass and zwitterionic material. J. Dent. Sci. 2022, 17, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Bajunaid, S.O.; Baras, B.H.; Weir, M.D.; Xu, H.H.K. Denture Acrylic Resin Material with Antibacterial and Protein-Repelling Properties for the Prevention of Denture Stomatitis. Polymers 2022, 14, 230. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Saito, T.; Shobuike, T.; Miyamoto, H.; Matsuda, J.; Fukazawa, K.; Ishihara, K.; Tanaka, S.; Moro, T. 2-Methacryloyloxyethyl Phosphorylcholine Polymer Coating Inhibits Bacterial Adhesion and Biofilm Formation on a Suture: An In Vitro and In Vivo Study. Biomed Res. Int. 2020, 2020, 5639651. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.; Alpaugh, M.; Krause, K.L.; Benedik, M.J. Differential secretion of isoforms of Serratia marcescens extracellular nuclease. Appl. Environ. Microbiol. 1995, 61, 4083–4088. [Google Scholar] [CrossRef]
- Neidhardt, F.C.; Bloch, P.L.; Smith, D.F. Culture medium for enterobacteria. J. Bacteriol. 1974, 119, 736–747. [Google Scholar] [CrossRef]
- Babaei Omali, N.; Subbaraman, L.N.; Heynen, M.; Fadli, Z.; Coles-Brennan, C.; Jones, L.W. In Vitro Effect of Lysozyme on Albumin Deposition to Hydrogel Contact Lens Materials. Optom. Vis. Sci. 2017, 94, 1047–1051. [Google Scholar] [CrossRef]
- Himpsl, S.D.; Pearson, M.M.; Arewang, C.J.; Nusca, T.D.; Sherman, D.H.; Mobley, H.L. Proteobactin and a yersiniabactin-related siderophore mediate iron acquisition in Proteus mirabilis. Mol. Microbiol. 2010, 78, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.J.; Sturge, C.R.; Moustafa, D.A.; Daly, S.M.; Marshall-Batty, K.R.; Felder, C.F.; Zamora, D.; Yabe-Gill, M.; Labandeira-Rey, M.; Bailey, S.M.; et al. Inhibition of Pseudomonas aeruginosa by Peptide-Conjugated Phosphorodiamidate Morpholino Oligomers. Antimicrob. Agents Chemother. 2017, 61, e01938-16. [Google Scholar] [CrossRef]
- Perry, R.D.; Brubaker, R.R. Accumulation of iron by yersiniae. J. Bacteriol. 1979, 137, 1290–1298. [Google Scholar] [CrossRef]
- Konne, N.M.; Collier, S.A.; Spangler, J.; Cope, J.R. Healthy Contact Lens Behaviors Communicated by Eye Care Providers and Recalled by Patients—United States, 2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 693–697. [Google Scholar] [CrossRef]
- Ramamoorthy, P.; Nichols, J.J. Compliance factors associated with contact lens-related dry eye. Eye Contact Lens 2014, 40, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Fleiszig, S.M.; Efron, N. Microbial flora in eyes of current and former contact lens wearers. J. Clin. Microbiol. 1992, 30, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.T.; Fang, P.C.; Chen, J.L.; Hsu, S.L.; Chao, T.L.; Yu, H.J.; Lai, Y.H.; Huang, Y.T.; Kuo, M.T. Molecular Bioburden of the Lens Storage Case for Contact Lens-Related Keratitis. Cornea 2018, 37, 1542–1550. [Google Scholar] [CrossRef]
- Dutta, D.; Willcox, M.D. Antimicrobial contact lenses and lens cases: A review. Eye Contact Lens 2014, 40, 312–324. [Google Scholar] [CrossRef]
- Dantam, J.; Zhu, H.; Willcox, M.; Ozkan, J.; Naduvilath, T.; Thomas, V.; Stapleton, F. In vivo assessment of antimicrobial efficacy of silver-impregnated contact lens storage cases. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.E.; Streger, S.H.; Rothmel, R.K.; Mailloux, B.J.; Hall, J.A.; Onstott, T.C.; Fredrickson, J.K.; Balkwill, D.L.; DeFlaun, M.F. Development of a vital fluorescent staining method for monitoring bacterial transport in subsurface environments. Appl. Environ. Microbiol. 2000, 66, 4486–4496. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pifer, R.; Harris, V.; Sanders, D.; Crary, M.; Shannon, P. Evaluation of Serratia marcescens Adherence to Contact Lens Materials. Microorganisms 2023, 11, 217. https://doi.org/10.3390/microorganisms11010217
Pifer R, Harris V, Sanders D, Crary M, Shannon P. Evaluation of Serratia marcescens Adherence to Contact Lens Materials. Microorganisms. 2023; 11(1):217. https://doi.org/10.3390/microorganisms11010217
Chicago/Turabian StylePifer, Reed, Valerie Harris, Deaja Sanders, Monica Crary, and Paul Shannon. 2023. "Evaluation of Serratia marcescens Adherence to Contact Lens Materials" Microorganisms 11, no. 1: 217. https://doi.org/10.3390/microorganisms11010217
APA StylePifer, R., Harris, V., Sanders, D., Crary, M., & Shannon, P. (2023). Evaluation of Serratia marcescens Adherence to Contact Lens Materials. Microorganisms, 11(1), 217. https://doi.org/10.3390/microorganisms11010217






