Comparative Genomic Analysis of the Hydrocarbon-Oxidizing Dibenzothiophene-Desulfurizing Gordonia Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phenotypic Characterization of Gordonia amicalis Strain 6-1
2.2. DNA Isolation, Genome Sequencing and Assembly
2.3. Genome Analysis
2.4. Genome Information
2.5. Nucleotide Sequence Accession Numbers
3. Results and Discussion
3.1. General Features of the Genomes of Strain G. amicalis 6-1 and Other Dibenzothiophene-Degrading Gordonia Strains
3.2. Taxonomic Assignment of G. amicalis 6-1 Based on Phylogenomic Analysis
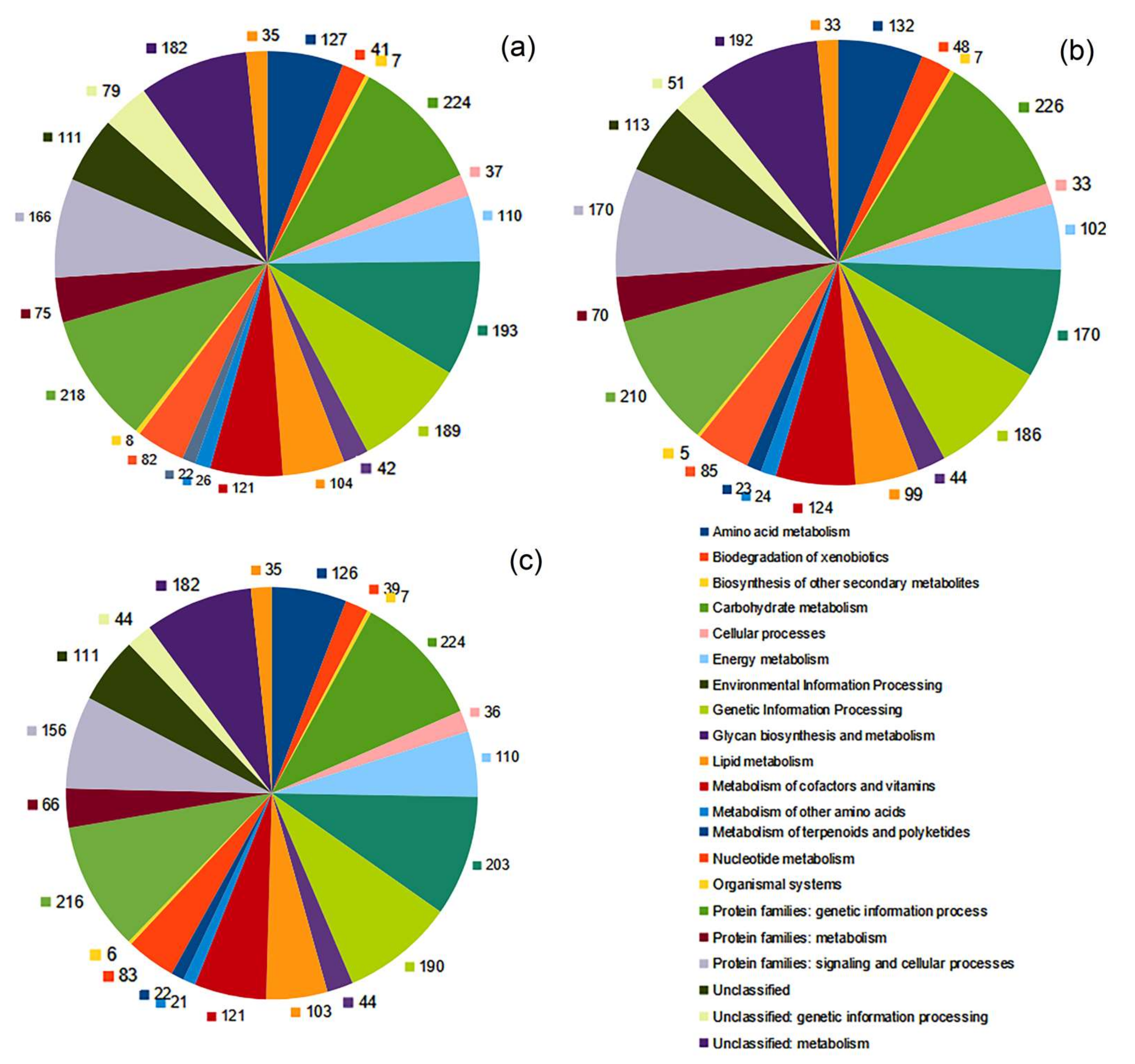
3.3. Functional Characterization of the Genomes of G. amicalis 6-1 and Other Gordonia Strains
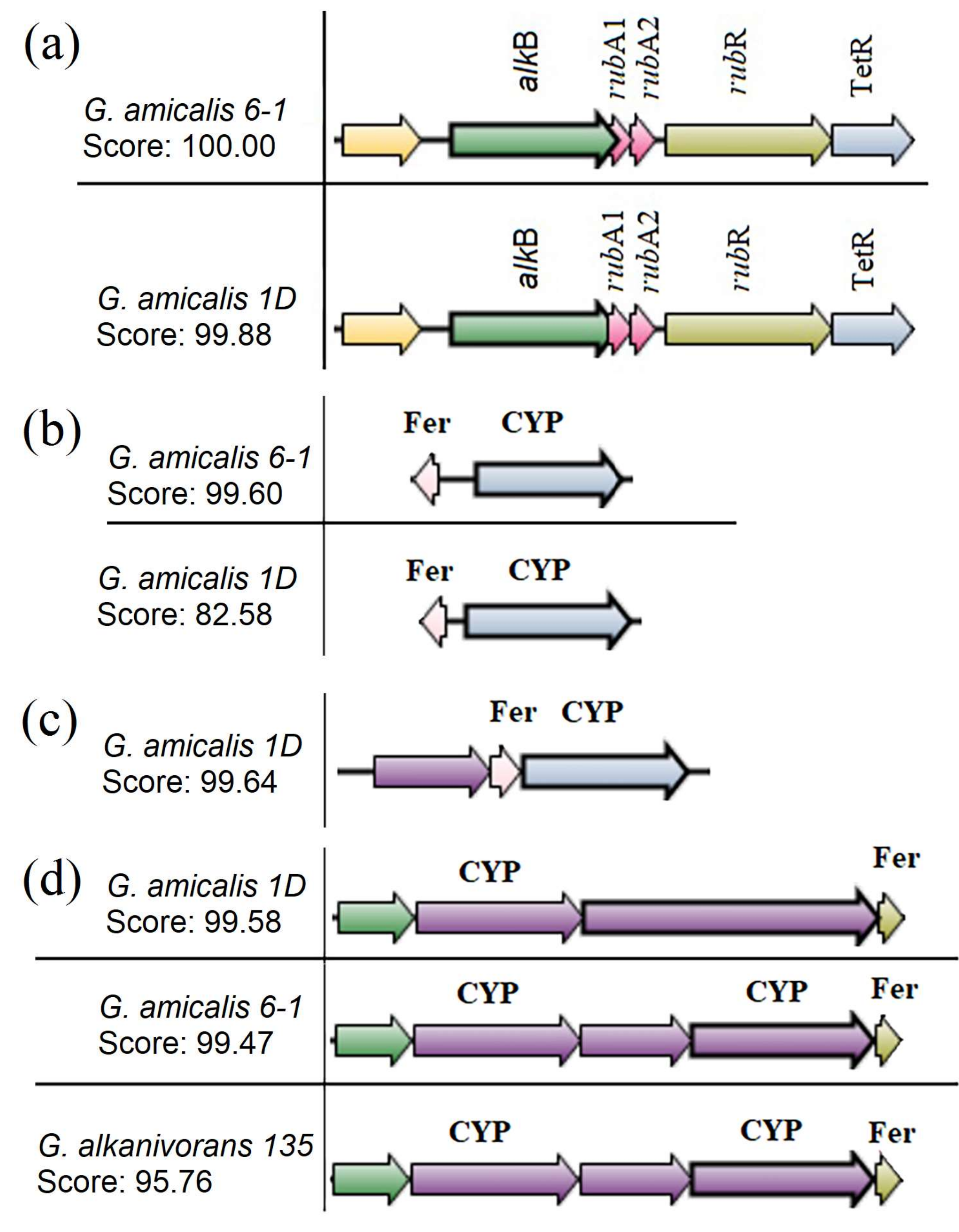
3.4. Genetic Systems for Alkane Oxidation
3.5. Genes of Benzoate Metabolism and Degradation of Organosulfur Compound (DBT)
3.6. Genes of Osmoprotectant Metabolism
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martín-Cabello, G.; Terrón-González, L.; Santero, E. Characterization of a dszEABC operon providing fast growth on dibenzothiophene and construction of broad-host-range biodesulfurization catalysts. Environ. Microbiol. 2022, 24, 1946–1963. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, G.; Ballb, A.S.; Rasekha, B.; Kaytasha, A. Biodesulfurization potential of a newly isolated bacterium Gordonia alkanivorans RIPI90A. Enz. Microbiol. Technol. 2008, 40, 578–584. [Google Scholar] [CrossRef]
- Ardakani, M.R.; Aminsefat, A.; Rasekh, B.; Yazdiyan, F.; Zargar, B.; Zarei, M.; Najafzadeh, H. Biodesulfurization of dibenzotiophene by a newly isolated Stenotrophomonas maltophilia strain Kho1. World Appl. Sci. J. 2010, 10, 272–278. [Google Scholar]
- Ma, T. The desulfurization pathway in Rhodococcus. In Biology of Rhodococcus; Alvarez, H.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 16, pp. 207–230. [Google Scholar] [CrossRef]
- Bhanjadeo, M.M.; Rath, K.; Gupta, D.; Pradhan, N.; Biswal, S.K.; Mishra, B.K.; Subudhi, U. Differential desulfurization of dibenzothiophene by newly identified MTCC strains: Influence of Operon Array. PLoS ONE 2018, 13, e0192536. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T. Degradation of thianthrene by dibenzothiophene desulfurizing bacteria Rhodococcus erythropolis KA2-5-1. J. Jpn. Pet. Inst. 2022, 65, 171–174. [Google Scholar] [CrossRef]
- Gilbert, S.C.; Morton, J.; Buchanan, S.; Oldfield, C.; McRoberts, A. Isolation of a unique benzothiophene-desulphurizing bacterium, Gordonia sp. Strain 213E (NCIMB 40816), and characterization of the desulphurization pathway. Microbiology 1998, 144, 2545–2553. [Google Scholar] [CrossRef] [Green Version]
- Kalita, M.; Chutia, M.; Jha, D.K.; Subrahmanyam, G. Mechanistic understanding of Gordonia sp. in biodesulfurization of organosulfur compounds. Curr. Microbiol. 2022, 79, 82. [Google Scholar] [CrossRef]
- Buzanello, E.B.; Rezende, R.P.; Sousa, F.M.O.; Marques, E.L.S.; Loguercio, L.L. A novel Bacillus pumilus-related strain from tropical landfarm soil is capable of rapid dibenzothiophene degradation and biodesulfurization. BMC Microbiol. 2014, 14, 257. [Google Scholar] [CrossRef] [Green Version]
- Ansari, F.; Prayuenyong, P.; Tothill, I.E. Biodesulfurization of dibenzothiophene by Shewanella putrefaciens NCIMB 8768. J. Biol. Phys. Chem. 2007, 7, 75–77. [Google Scholar] [CrossRef] [Green Version]
- Mohebali, G.; Ball, A.S. Biodesulfurization of diesel fuels—Past, present and future perspectives. Int. Biodeterior. Biodegrad. 2016, 110, 163–180. [Google Scholar] [CrossRef]
- Zhi, X.Y.; Li, W.J.; Stackebrandt, E. An update of the structure and 16S rRNA gene sequence-based definition of higher ranks of the class Actinobacteria, with the proposal of two new suborders and four new families and emended descriptions of the existing higher taxa. Int. J. Syst. Evol. Microbiol. 2009, 59, 589–608. [Google Scholar] [CrossRef]
- Euzéby, J.P. List of bacterial names with standing in nomenclature: A folder available on the Internet. Int. J. Syst. Bacteriol. 1997, 47, 590–592. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.B.; Brown, R.; Oldfield, C.; Gilbert, S.C.; Iliarionov, S.; Goodfellow, M. Gordonia amicalis sp. nov., a novel dibenzothiophene-desulphurizing actinomycete. Int. J. Syst. Evol. Microbiol. 2000, 50, 2031–2036. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Wang, M.-D.; Chen, H.; Chen, J.-M.; Shi, Y. Biodesulfurization of dibenzothiophene by growing cells of Gordonia sp. in batch cultures. Biotechnol. Lett. 2006, 28, 1175–1179. [Google Scholar] [CrossRef]
- Shen, F.T.; Young, L.S.; Hsieh, M.F.; Lin, S.Y.; Young, C.C. Molecular detection and phylogenetic analysis of the alkane 1-monooxygenase gene from Gordonia spp. Syst. Appl. Microbiol. 2010, 33, 53–55. [Google Scholar] [CrossRef]
- Wu, X.; Liang, R.; Dai, Q.; Jin, D.; Wang, Y.; Chao, W. Complete degradation of di-n-octyl phthalate by biochemical cooperation between Gordonia sp. strain JDC-2 and Arthrobacter sp. strain JDC-32 isolated from activated sludge. J. Hazard. Mater. 2010, 176, 262–268. [Google Scholar] [CrossRef]
- Lo Piccolo, L.; De Pasquale, C.; Fodale, R.; Puglia, A.-M.; Quatrini, P. Involvement of an alkane hydroxylase system of Gordonia sp. strain SoCg in degradation of solid n–alkanes. Appl. Environ. Microbiol. 2011, 77, 1204–1213. [Google Scholar] [CrossRef] [Green Version]
- Drzyzga, O. The strengths and weaknesses of Gordonia: A review of an emerging genus with increasing biotechnological potential. Crit. Rev. Microbiol. 2012, 38, 300–316. [Google Scholar] [CrossRef]
- Sowani, H.; Kulkarni, M.; Zinjarde, S. An insight into the ecology, diversity and adaptations of Gordonia species. Crit. Rev. Microbiol. 2018, 44, 393–413. [Google Scholar] [CrossRef]
- Hu, T.; Yang, C.; Hou, Z.; Liu, T.; Mei, X.; Zheng, L.; Zhong, W. Phthalate esters metabolic strain Gordonia sp. GZ-YC7, a potential soil degrader for high concentration di-(2-ethylhexyl) phthalate. Microorganisms 2022, 10, 641. [Google Scholar] [CrossRef]
- Singh, P.; Srivastava, P. An improved protocol for electroporation in members of the genus Gordonia. J. Microbiol. Methods 2013, 95, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, D.V.; Ahmad, A.; Khan, M.I.; Baothman, O.A.; Khan, M.J. Desulfurization of benzothiophene by an isolated Gordonia sp. IITR100. J. Microbiol. Biotechnol. Food Sci. 2021, 10, e2787. [Google Scholar] [CrossRef]
- Adlakha, J.; Singh, P.; Ram, S.K.; Kumar, M.; Singh, M.P.; Singh, D.; Sahai, V.; Srivastava, P. Optimization of conditions for deep desulfurization of heavy crude oil and hydrodesulfurized diesel by Gordonia sp. IITR100. Fuel 2016, 184, 761–769. [Google Scholar] [CrossRef]
- Zargar, A.N.; Mishra, S.; Kumar, M.; Srivastava, P. Isolation and chemical characterization of the biosurfactant produced by Gordonia sp. IITR100. PLoS ONE 2022, 17, e0264202. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Akhtar, K.; Ghauri, M.A. Biodesulfurization of thiophenic compounds by a 2-hydroxybiphenyl-resistant Gordonia sp. HS126-4N carrying dszABC genes. Curr. Microbiol. 2018, 75, 597–603. [Google Scholar] [CrossRef]
- Shavandi, M.; Sadeghizadeh, M.; Zomorodipour, A.; Khajeh, K. Biodesulfurization of dibenzothiophene by recombinant Gordonia alkanivorans RIPI90A. Bioresour. Technol. 2009, 100, 475–479. [Google Scholar] [CrossRef]
- Shavandi, M.; Sadeghizadeh, M.; Khajeh, K.; Mohebali, G.; Zomorodipour, A. Genomic structure and promoter analysis of the dsz operon for dibenzothiophene biodesulfurization from Gordonia alkanivorans RIPI90A. Appl. Microbiol. Biotechnol. 2010, 87, 1455–1461. [Google Scholar] [CrossRef]
- Wang, W.; Ma, T.; Lian, K.; Zhang, Y.; Tian, H.; Ji, K.; Li, G. Genetic analysis of benzothiophene biodesulfurization pathway of Gordonia terrae strain C-6. PLoS ONE 2013, 8, e84386. [Google Scholar] [CrossRef] [Green Version]
- Finkel’shtein, Z.I.; Baskunov, B.P.; Golovlev, E.L.; Golovleva, L.A. Desulfurization of 4,6-dimethyldibenzothiophene and dibenzothiophene by Gordona aichiensis 51. Microbiology 1999, 68, 154–157. [Google Scholar]
- Kim, S.B.; Brown, R.; Oldfield, C.; Gilbert, S.C.; Goodfellow, M. Gordonia desulfuricans sp. nov., a benzothiophene-desulphurizing actinomycete. Int. J. Syst. Bacteriol. 1999, 49, 1845–1851. [Google Scholar] [CrossRef] [Green Version]
- Aminsefat, A.; Rasekh, B.; Ardakani, M.R. Biodesulfurization of dibenzothiophene by Gordonia sp. AHV-01 and optimization by using of response surface design procedure. Microbiology 2012, 81, 154–159. [Google Scholar] [CrossRef]
- Santos, S.C.C.; Alviano, D.S.; Alviano, C.S.; Pádula, M.; Leitão, A.C.; Martins, O.B.; Ribeiro, C.M.S.; Sassaki, M.Y.M.; Matta, C.P.S.; Bevilaqua, J.; et al. Characterization of Gordonia sp. strain F.5.25.8 capable of dibenzothiophene desulfurization and carbazole utilization. Appl. Microbiol. Biotechnol. 2006, 71, 355–362. [Google Scholar] [CrossRef]
- Gupta, N.; Roychoudhury, P.K.; Deb, J.K. Biotechnology of desulfurization of diesel: Prospects and challenges. Appl. Microbiol. Biotechnol. 2005, 66, 356–366. [Google Scholar] [CrossRef]
- Denome, S.A.; Olson, E.S.; Young, K.D. Identification and cloning of genes involved in specific desulfurization of dibenzothiophene by Rhodococcus sp. strain IGTS8. Appl. Environ. Microbiol. 1993, 9, 2837–2843. [Google Scholar] [CrossRef] [Green Version]
- Kodama, K.; Nakatani, S.; Umehara, K.; Shimizu, K.; Minoda, Y.; Yamada, K. Microbial conversion of petro-sulfur compounds: Part III. Isolation and identification of products from dibenzothiophene. J. Agric. Food Chem. 1970, 34, 1320–1324. [Google Scholar] [CrossRef]
- Delegan, Y.A.; Valentovich, L.N.; Shafieva, S.M.; Ganbarov, K.G.; Filonov, A.E.; Vainstein, M.B. Characterization and genomic analysis of highly efficient thermotolerant oil-degrading bacterium Gordonia sp. 1D. Folia. Microbiol. 2019, 64, 41–48. [Google Scholar] [CrossRef]
- Delegan, Y.; Valentovich, L.; Vetrova, A.; Frantsuzova, E.; Kocharovskaya, Y. Complete genome sequence of Gordonia sp. 135, a promising dibenzothiophene- and hydrocarbon-degrading strain. Microbiol. Resour. Announc. 2020, 9, e01450-19. [Google Scholar] [CrossRef] [Green Version]
- Delegan, Y.; Kocharovskaya, Y.; Frantsuzova, E.; Streletskii, R.; Vetrova, A. Characterization and genomic analysis of Gordonia alkanivorans 135, a promising dibenzothiophene-degrading strain. Biotechnol. Rep. 2021, 29, e00591. [Google Scholar] [CrossRef]
- Nazina, T.N.; Sokolova, D.S.; Babich, T.L.; Semenova, E.M.; Ershov, A.P.; Bidzhieva, S.K.; Borzenkov, I.A.; Tourova, T.P.; Poltaraus, A.B.; Khisametdinov, M.R. Microorganisms of low-temperature heavy oil reservoirs (Russia) and their possible application for enhanced oil recovery. Microbiology 2017, 86, 773–785. [Google Scholar] [CrossRef]
- Borzenkov, I.A.; Sokolova, D.S.; Nazina, T.N.; Babich, T.L.; Semenova, E.M.; Ershov, A.P.; Khisametdinov, M.R. Gordonia Amicalis Strain with Ability of Generation Directly in Oil Reservoir of Oil-Displacing Agent—BioPAV and Decreasing Content of Organosulfur Compounds of Oil. RU Patent No. 2673747 C1, 29 November 2018. Available online: http://www1.fips.ru/fips_servl/fips_servlet?DB=RUPAT&DocNumber=2673747&TypeFile=html (accessed on 29 November 2018). (In Russian).
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P. Assembly of long, error-prone reads using repeat graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.-H.; Ha, S.-M.; Lim, J.; Kwon, S.; Chun, J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 2017, 110, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Disz, T.; Edwards, R.; Formsma, K.; Gerdes, S.; Glass, E.; et al. RAST server: Rapid annotations using subsystems technology. BMC Genom. 2008, 8, 75. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Oberto, J. SyntTax: A web server linking synteny to prokaryotic taxonomy. BMC Bioinform. 2013, 14, 4. [Google Scholar] [CrossRef] [Green Version]
- Darling, A.C.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef] [Green Version]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.W.; De Meyer, S.; et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 2013, 14, 60. [Google Scholar] [CrossRef]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef] [Green Version]
- Whyte, L.G.; Smits, T.H.; Labbé, D.; Witholt, B.; Greer, C.W.; van Beilen, J.B. Gene cloning and characterization of multiple alkane hydroxylase systems in Rhodococcus strains Q15 and NRRL B-16531. Appl. Environ. Microbiol. 2002, 68, 5933–5942. [Google Scholar] [CrossRef] [Green Version]
- van Beilen, J.B.; Funhoff, E.G.; van Loon, A.; Just, A.; Kaysser, L.; Bouza, M.; Holtackers, R.; Röthlisberger, M.; Li, Z.; Witholt, B. Cytochrome P450 alkane hydroxylases of the CYP153 family are common in alkane-degrading eubacteria lacking integral membrane alkane hydroxylases. Appl. Environ. Microbiol. 2006, 72, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Funhoff, E.G.; Bauer, U.; García-Rubio, I.; Witholt, B.; van Beilen, J.B. CYP153A6, a soluble P450 oxygenase catalyzing terminal-alkane hydroxylation. J. Bacteriol. 2006, 188, 5220–5227. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, W.; Lai, Q.; Shao, Z. Gene diversity of CYP153A and AlkB alkane hydroxylases in oil-degrading bacteria isolated from the Atlantic Ocean. Environ. Microbiol. 2010, 12, 1230–1242. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Wu, Y.; Zhou, Z.; Lai, Q.; Shao, Z. Multiple alkane hydroxylase systems in a marine alkane degrader, Alcanivorax dieselolei B-5. Environ. Microbiol. 2011, 13, 1168–1178. [Google Scholar] [CrossRef]
- Viggor, S.; Jõesaar, M.; Vedler, E.; Kiiker, R.; Pärnpuu, L.; Heinaru, A. Occurrence of diverse alkane hydroxylase alkB genes in indigenous oil-degrading bacteria of Baltic Sea surface water. Mar. Pollut. Bull. 2015, 101, 507–516. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Liu, Y.; Wu, X. Biological process of alkane degradation by Gordonia sihwaniensis. ACS Omega 2022, 7, 55–63. [Google Scholar] [CrossRef]
- Blum, M.; Chang, H.-Y.; Chuguransky, S.; Grego, T.; Kandasaamy, S.; Mitchell, A.; Nuka, G.; Paysan-Lafosse, T.; Qureshi, M.; Raj, S.; et al. The InterPro protein families and domains database: 20 years on. Nucleic Acids Res. 2021, 49, D344–D354. [Google Scholar] [CrossRef]
- Cuthbertson, L.; Nodwell, J.R. The TetR family of regulators. Microbiol. Mol. Biol. Rev. 2013, 77, 440–475. [Google Scholar] [CrossRef] [PubMed]
- Murarka, P.; Bagga, T.; Singh, P.; Rangra, S.; Srivastava, P. Isolation and identification of a TetR family protein that regulates the biodesulfurization operon. AMB Express 2019, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Keshav, A.; Murarka, P.; Srivastava, P. Bending is required for activation of dsz operon by the TetR family protein (DszGR). Gene 2022, 810, 146061. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium, UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [CrossRef] [PubMed] [Green Version]
- Wicht, D.K. The reduced flavin-dependent monooxygenase SfnG converts dimethylsulfone to methanesulfinate. Arch. Biochem. Biophys. 2016, 604, 159–166. [Google Scholar] [CrossRef]
- Imhoff, J.F.; Rahn, T.; Künzel, S.; Keller, A.; Neulinger, S.C. Osmotic adaptation and compatible solute biosynthesis of phototrophic bacteria as revealed from genome analyses. Microorganisms 2021, 9, 46. [Google Scholar] [CrossRef]
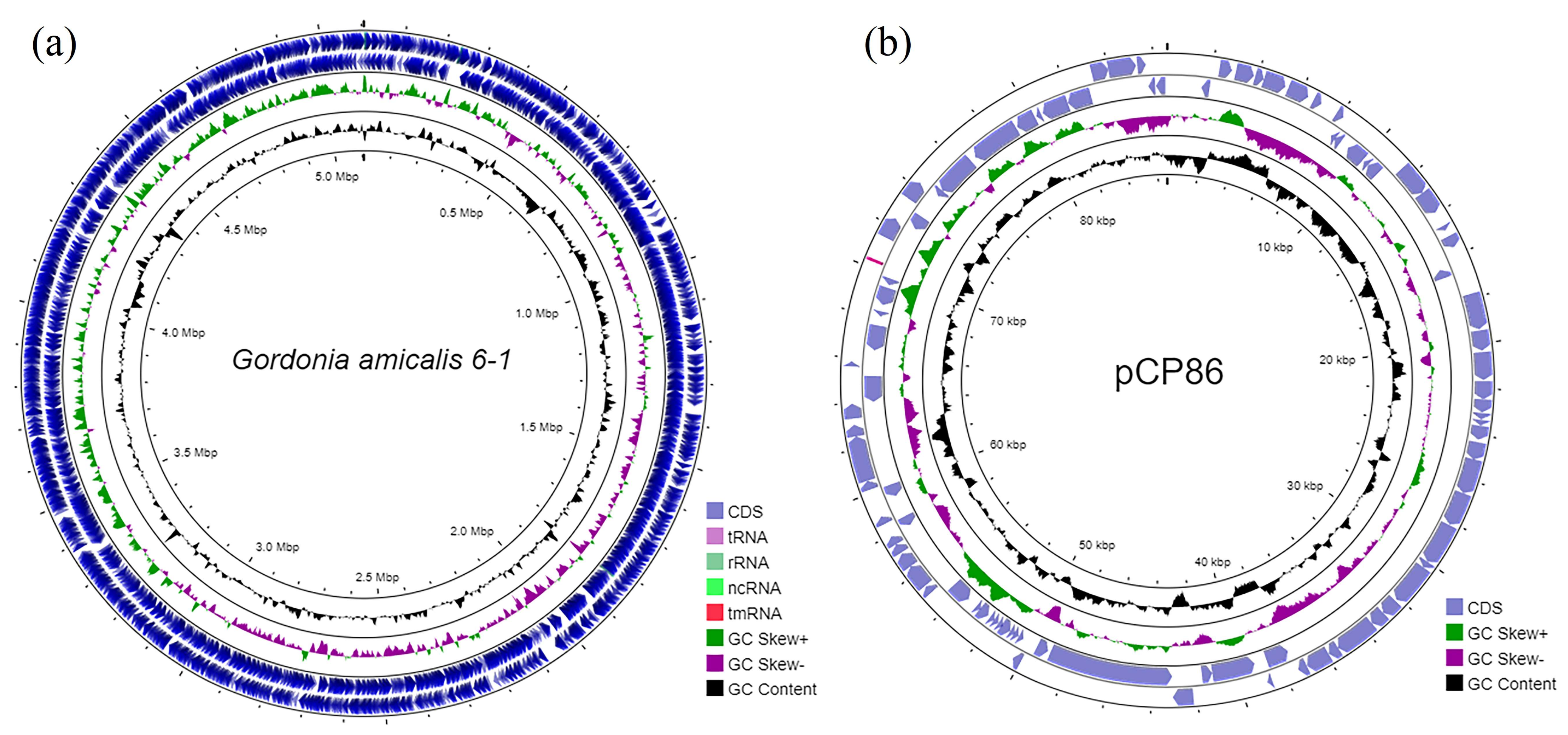



| Property | G. amicalis 6-1 | G. amicalis 1D | G. alkanivorans 135 |
|---|---|---|---|
| Chromosome | |||
| Assembly Accession no. | GCA_023238565.1 | GCA_002327125.1 | GCA_009720185.1 |
| Chromosome size (bp) | 5,105,798 | 5,151,623 | 5,039,827 |
| G + C content (%) | 67.4 | 67.3 | 67.4 |
| Genes (Total) | 4739 | 4677 | 4704 |
| Protein-coding genes | 4429 | 4448 | 4492 |
| Total RNAs | 59 | 59 | 66 |
| tRNAs | 47 | 47 | 51 |
| ncRNAs | 3 | 3 | 3 |
| Pseudo Genes | 251 | 170 | 146 |
| Closest Placement | G. amicalis NBRC 100051 | G. amicalis NBRC 100051 | G. alkanivorans NBRC 16433 |
| ANI/dDDH | 97.7%/87.9% | 98.1%/88.6% | 98.4%/86.8% |
| Plasmid | pCP86 | pG135 | |
| Accession no. | CP096597.1 | CP046258.1 | |
| Total length | 86,621 | 164,963 | |
| G + C content (%) | 65.0 | 64.5 |
| Strain | Encoded Product | GeneBank no. |
|---|---|---|
| G. amicalis 6-1 | SfnB | UPW13782.1 |
| oxidoreductase | UPW14477.1 | |
| acyl-CoA dehydrogenase | UPW14265.1 | |
| acyl-CoA dehydrogenase | UPW15449.1 | |
| G. alkanivorans 135 | SfnB | QGP87489.1 |
| acyl-CoA dehydrogenase | QGP87979.1 | |
| acyl-CoA dehydrogenase | QGP89059.1 | |
| G. amicalis 1D | SfnB | ATD70150.1 |
| oxidoreductase | ATD71050.1 | |
| acyl-CoA dehydrogenase | ATD71233.1 | |
| acyl-CoA dehydrogenase | ATD71690.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frantsuzova, E.; Delegan, Y.; Bogun, A.; Sokolova, D.; Nazina, T. Comparative Genomic Analysis of the Hydrocarbon-Oxidizing Dibenzothiophene-Desulfurizing Gordonia Strains. Microorganisms 2023, 11, 4. https://doi.org/10.3390/microorganisms11010004
Frantsuzova E, Delegan Y, Bogun A, Sokolova D, Nazina T. Comparative Genomic Analysis of the Hydrocarbon-Oxidizing Dibenzothiophene-Desulfurizing Gordonia Strains. Microorganisms. 2023; 11(1):4. https://doi.org/10.3390/microorganisms11010004
Chicago/Turabian StyleFrantsuzova, Ekaterina, Yanina Delegan, Alexander Bogun, Diyana Sokolova, and Tamara Nazina. 2023. "Comparative Genomic Analysis of the Hydrocarbon-Oxidizing Dibenzothiophene-Desulfurizing Gordonia Strains" Microorganisms 11, no. 1: 4. https://doi.org/10.3390/microorganisms11010004






