Fighting Tuberculosis: In Search of a BCG Replacement
Abstract
:1. Introduction
2. Immune Response to Mycobacterium Tuberculosis
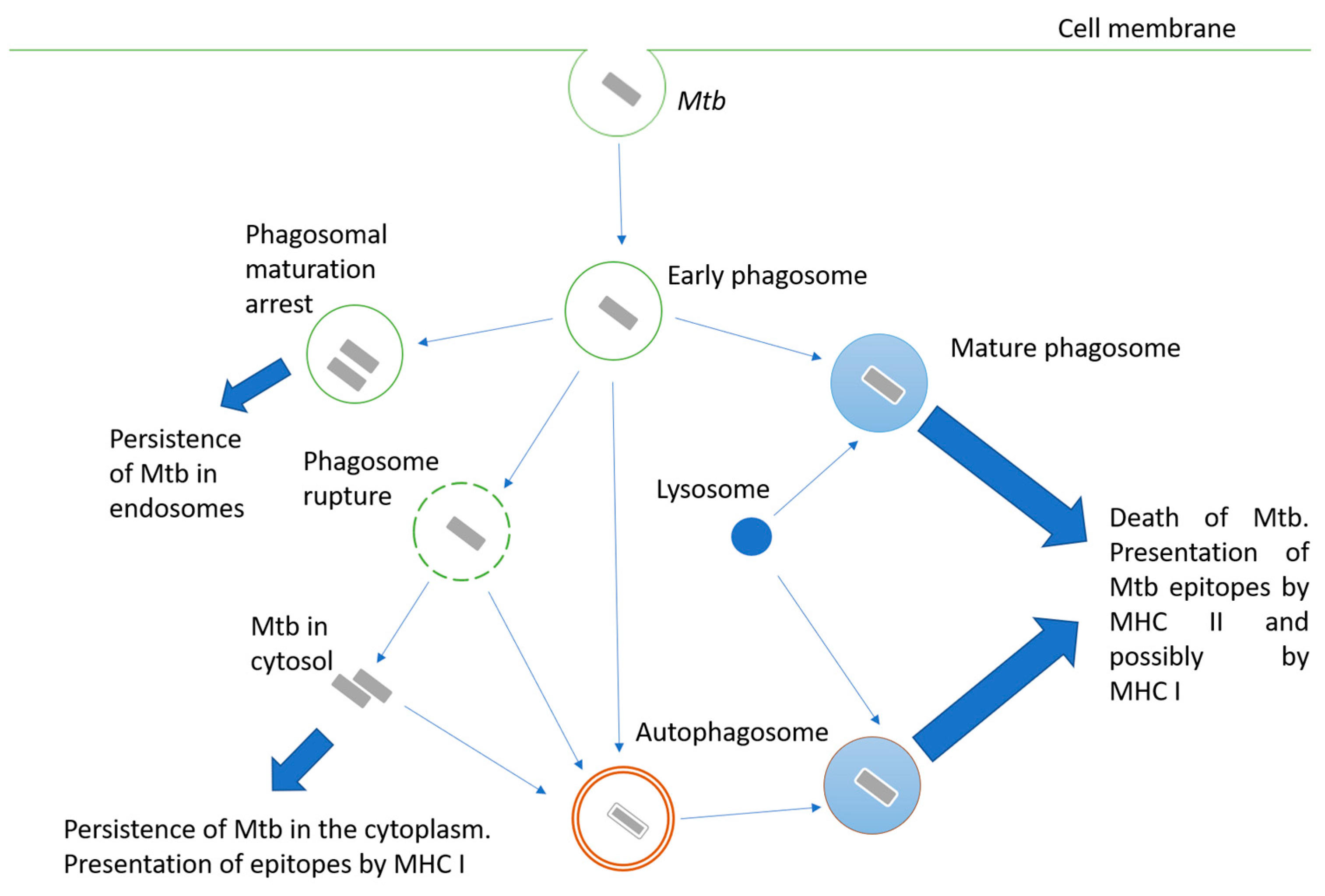
2.1. Interaction of Mycobacterium Tuberculosis with A Host Cell
2.2. Adaptive Immunity to Mtb
2.3. Immune Response and Protection Induced by BCG Vaccination
3. Strategies to Advance BCG
3.1. ESX-1 Secretion System
3.2. Ag85 Complex
3.3. Protein HspX
3.4. Cyclic di-AMP
3.5. Mycobacterial Superoxide Dismutase A (SodA) and SecA2
3.6. Genes Involved in Phagosome Maturation Delay
3.7. Exit of Phagosome
4. The Most Promising Recombinant Live Vaccines under Clinical Evaluation
4.1. AERAS 422
4.2. VPM1002
4.3. MTBVAC
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Foster, M.; Hill, P.C.; Setiabudiawan, T.P.; Koeken, V.A.C.M.; Alisjahbana, B.; Crevel, R. BCG-induced protection against Mycobacterium tuberculosis infection: Evidence, mechanisms, and implications for next-generation vaccines. Immunol. Rev. 2021, 301, 122–144. [Google Scholar] [CrossRef] [PubMed]
- Scriba, T.J.; Kaufmann, S.H.E.; Henri Lambert, P.; Sanicas, M.; Martin, C.; Neyrolles, O. Vaccination Against Tuberculosis with Whole-Cell Mycobacterial Vaccines. J. Infect. Dis. 2016, 214, 659–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welin, A.; Lerm, M. Inside or outside the phagosome? The controversy of the intracellular localization of Mycobacterium tuberculosis. Tuberculosis 2012, 92, 113–120. [Google Scholar] [CrossRef]
- Mantegazza, A.R.; Magalhaes, J.G.; Amigorena, S.; Marks, M.S. Presentation of Phagocytosed Antigens by MHC Class I and II. Traffic 2013, 14, 135–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colbert, J.D.; Cruz, F.M.; Rock, K.L. Cross-presentation of exogenous antigens on MHC I molecules. Curr. Opin. Immunol. 2020, 64, 1–8. [Google Scholar] [CrossRef]
- Welin, A.; Winberg, M.E.; Abdalla, H.; Särndahl, E.; Rasmusson, B.; Stendahl, O.; Lerm, M. Incorporation of Mycobacterium tuberculosis Lipoarabinomannan into Macrophage Membrane Rafts Is a Prerequisite for the Phagosomal Maturation Block. Infect. Immun. 2008, 76, 2882–2887. [Google Scholar] [CrossRef] [Green Version]
- Indrigo, J.; Hunter, R.L.; Actor, J.K. Cord factor trehalose 6,6′-dimycolate (TDM) mediates trafficking events during mycobacterial infection of murine macrophages. Microbiology 2003, 149, 2049–2059. [Google Scholar] [CrossRef] [Green Version]
- Walburger, A.; Koul, A.; Ferrari, G.; Nguyen, L.; Prescianotto-Baschong, C.; Huygen, K.; Klebl, B.; Thompson, C.; Bacher, G.; Pieters, J. Protein Kinase G from Pathogenic Mycobacteria Promotes Survival Within Macrophages. Science 2004, 304, 1800–1804. [Google Scholar] [CrossRef] [Green Version]
- Puri, R.V.; Reddy, P.V.; Tyagi, A.K. Secreted Acid Phosphatase (SapM) of Mycobacterium tuberculosis Is Indispensable for Arresting Phagosomal Maturation and Growth of the Pathogen in Guinea Pig Tissues. PLoS ONE 2013, 8, e70514. [Google Scholar] [CrossRef] [Green Version]
- Master, S.S.; Rampini, S.K.; Davis, A.S.; Keller, C.; Ehlers, S.; Springer, B.; Timmins, G.S.; Sander, P.; Deretic, V. Mycobacterium tuberculosis Prevents Inflammasome Activation. Cell Host Microbe 2008, 3, 224–232. [Google Scholar] [CrossRef]
- Lin, W.; Mathys, V.; Ang, E.L.Y.; Koh, V.H.Q.; Martínez Gómez, J.M.; Ang, M.L.T.; Zainul Rahim, S.Z.; Tan, M.P.; Pethe, K.; Alonso, S. Urease Activity Represents an Alternative Pathway for Mycobacterium tuberculosis Nitrogen Metabolism. Infect. Immun. 2012, 80, 2771–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Wel, N.; Hava, D.; Houben, D.; Fluitsma, D.; van Zon, M.; Pierson, J.; Brenner, M.; Peters, P.J.M. tuberculosis and M. leprae Translocate from the Phagolysosome to the Cytosol in Myeloid Cells. Cell 2007, 129, 1287–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grode, L. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J. Clin. Investig. 2005, 115, 2472–2479. [Google Scholar] [CrossRef] [Green Version]
- Deretic, V.; Levine, B. Autophagy, Immunity, and Microbial Adaptations. Cell Host Microbe 2009, 5, 527–549. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Jing, W.; Runpeng, Z.; Xuewei, X.; Min, M.; Ru, C.; Yingru, X.; Shengfa, N.; Rongbo, Z. ESAT6 inhibits autophagy flux and promotes BCG proliferation through MTOR. Biochem. Biophys. Res. Commun. 2016, 477, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Wu, J.; Wang, W.; Mu, M.; Zhao, R.; Xu, X.; Chen, Z.; Xiao, J.; Hu, F.; Yang, Y.; et al. Autophagy regulation revealed by SapM-induced block of autophagosome-lysosome fusion via binding RAB7. Biochem. Biophys. Res. Commun. 2015, 461, 401–407. [Google Scholar] [CrossRef]
- Ablasser, A.; Dorhoi. Inflammasome Activation and Function During Infection with Mycobacterium tuberculosis. In Inflammasome Signaling and Bacterial Infections; Current Topics in Microbiology and Immunology; Backert, S., Ed.; Springer: Cham, Switzerland, 2016; Volume 397, pp. 183–197. [Google Scholar] [CrossRef]
- Vanaja, S.K.; Rathinam, V.A.K.; Fitzgerald, K.A. Mechanisms of inflammasome activation: Recent advances and novel insights. Trends Cell Biol. 2015, 25, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Di Paolo, N.C.; Shafiani, S.; Day, T.; Papayannopoulou, T.; Russell, D.W.; Iwakura, Y.; Sherman, D.; Urdahl, K.; Shayakhmetov, D.M. Interdependence between Interleukin-1 and Tumor Necrosis Factor Regulates TNF-Dependent Control of Mycobacterium tuberculosis Infection. Immunity 2015, 43, 1125–1136. [Google Scholar] [CrossRef] [Green Version]
- Mayer-Barber, K.D.; Barber, D.L.; Shenderov, K.; White, S.D.; Wilson, M.S.; Cheever, A.; Kugler, D.; Hieny, S.; Caspar, P.; Núñez, G.; et al. Cutting Edge: Caspase-1 Independent IL-1β Production Is Critical for Host Resistance to Mycobacterium tuberculosis and Does Not Require TLR Signaling In Vivo. J. Immunol. 2010, 184, 3326–3330. [Google Scholar] [CrossRef]
- Philips, J.A.; Ernst, J.D. Tuberculosis Pathogenesis and Immunity. Annu. Rev. Pathol. Mech. Dis. 2012, 7, 353–384. [Google Scholar] [CrossRef]
- Behar, S.M.; Martin, C.J.; Booty, M.G.; Nishimura, T.; Zhao, X.; Gan, H.-X.; Divangahi, M.; Remold, H.G. Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunol. 2011, 4, 279–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, S.; Ernst, J.D. Cell-to-Cell Transfer of M. tuberculosis Antigens Optimizes CD4 T Cell Priming. Cell Host Microbe 2014, 15, 741–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogues, T.; Goodrich, M.E.; Ryan, L.; LaCourse, R.; North, R.J. The Relative Importance of T Cell Subsets in Immunity and Immunopathology of Airborne Mycobacterium tuberculosis Infection in Mice. J. Exp. Med. 2001, 193, 271–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geldmacher, C.; Zumla, A.; Hoelscher, M. Interaction between HIV and Mycobacterium tuberculosis. Curr. Opin. HIV AIDS 2012, 7, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, J.S.; Wu, Y.; Behar, S.M. Mycobacterium tuberculosis-Specific CD8 + T Cells Require Perforin to Kill Target Cells and Provide Protection In Vivo. J. Immunol. 2008, 181, 8595–8603. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.M.; Dalton, D.K.; Stewart, T.A.; Griffin, J.P.; Russell, D.G.; Orme, I.M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 1993, 178, 2243–2247. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, T.M.; Saunders, B.M.; Ryan, A.A.; Britton, W.J. Mycobacterium bovis BCG-Specific Th17 Cells Confer Partial Protection against Mycobacterium tuberculosis Infection in the Absence of Gamma Interferon. Infect. Immun. 2010, 78, 4187–4194. [Google Scholar] [CrossRef] [Green Version]
- Larson, R.P.; Shafiani, S.; Urdahl, K.B. Foxp3+ regulatory T cells in tuberculosis. In The New Paradigm of Immunity to Tuberculosis; Springer: New York, NY, USA, 2013; pp. 165–180. [Google Scholar]
- Glatman-Freedman, A. Advances in antibody-mediated immunity against Mycobacterium tuberculosis: Implications for a novel vaccine strategy. FEMS Immunol. Med. Microbiol. 2003, 39, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Dockrell, H.M.; Smith, S.G. What Have We Learnt about BCG Vaccination in the Last 20 Years? Front. Immunol. 2017, 8, 1134. [Google Scholar] [CrossRef]
- Trunz, B.B.; Fine, P.; Dye, C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: A meta-analysis and assessment of cost-effectiveness. Lancet 2006, 367, 1173–1180. [Google Scholar] [CrossRef]
- Moorlag, S.J.C.F.M.; Arts, R.J.W.; van Crevel, R.; Netea, M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019, 25, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Soares-Weiser, K.; López-López, J.A.; Kakourou, A.; Chaplin, K.; Christensen, H.; Martin, N.K.; Sterne, J.A.C.; Reingold, A.L. Association of BCG, DTP, and measles containing vaccines with childhood mortality: Systematic review. BMJ 2016, 355, i5170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aaby, P.; Kollmann, T.R.; Benn, C.S. Nonspecific effects of neonatal and infant vaccination: Public-health, immunological and conceptual challenges. Nat. Immunol. 2014, 15, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.B.; Jacobs, C.; van Loenhout, J.; Xavier, R.J.; Aaby, P.; van der Meer, J.W.M.; et al. Long-Lasting Effects of BCG Vaccination on Both Heterologous Th1/Th17 Responses and Innate Trained Immunity. J. Innate Immun. 2014, 6, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Mathurin, K.S.; Martens, G.W.; Kornfeld, H.; Welsh, R.M. CD4 T-Cell-Mediated Heterologous Immunity between Mycobacteria and Poxviruses. J. Virol. 2009, 83, 3528–3539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettenati, C.; Ingersoll, M.A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat. Rev. Urol. 2018, 15, 615–625. [Google Scholar] [CrossRef]
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-guerin in the Treatment of Superficial Bladder Tumors. J. Urol. 1976, 116, 180–182. [Google Scholar] [CrossRef]
- Suttmann, H.; Riemensberger, J.; Bentien, G.; Schmaltz, D.; Stöckle, M.; Jocham, D.; Böhle, A.; Brandau, S. Neutrophil Granulocytes Are Required for Effective Bacillus Calmette-Guérin Immunotherapy of Bladder Cancer and Orchestrate Local Immune Responses. Cancer Res. 2006, 66, 8250–8257. [Google Scholar] [CrossRef] [Green Version]
- van Puffelen, J.H.; Keating, S.T.; Oosterwijk, E.; van der Heijden, A.G.; Netea, M.G.; Joosten, L.A.B.; Vermeulen, S.H. Trained immunity as a molecular mechanism for BCG immunotherapy in bladder cancer. Nat. Rev. Urol. 2020, 17, 513–525. [Google Scholar] [CrossRef]
- Miller, A.; Reandelar, M.J.; Fasciglione, K.; Roumenova, V.; Li, Y.; Otazu, G. Correlation between universal BCG vaccination policy and reduced mortality for COVID-19. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Hanekom, W.A. The Immune Response to BCG Vaccination of Newborns. Ann. N. Y. Acad. Sci. 2005, 1062, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, E.; Nicol, M.P.; Zar, H.J.; Tena-Coki, N.G.; Kampmann, B. Age-related waning of immune responses to BCG in healthy children supports the need for a booster dose of BCG in TB endemic countries. Sci. Rep. 2018, 8, 15309. [Google Scholar] [CrossRef] [Green Version]
- Kagina, B.M.N.; Abel, B.; Scriba, T.J.; Hughes, E.J.; Keyser, A.; Soares, A.; Gamieldien, H.; Sidibana, M.; Hatherill, M.; Gelderbloem, S.; et al. Specific T Cell Frequency and Cytokine Expression Profile Do Not Correlate with Protection against Tuberculosis after Bacillus Calmette-Guérin Vaccination of Newborns. Am. J. Respir. Crit. Care Med. 2010, 182, 1073–1079. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Eisenhut, M.; Harris, R.J.; Rodrigues, L.C.; Sridhar, S.; Habermann, S.; Snell, L.; Mangtani, P.; Adetifa, I.; Lalvani, A.; et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: Systematic review and meta-analysis. BMJ 2014, 349, g4643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuan, R.; Muskat, K.; Peters, B.; Lindestam Arlehamn, C.S. Is mapping the BCG vaccine-induced immune responses the key to improving the efficacy against tuberculosis? J. Intern. Med. 2020, 288, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, A.L.; Nagai, S.; Houen, G.; Andersen, P.; Andersen, A.B. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 1995, 63, 1710–1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berthet, F.-X.; Rasmussen, P.B.; Rosenkrands, I.; Andersen, P.; Gicquel, B. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology 1998, 144, 3195–3203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodin, P.; Majlessi, L.; Marsollier, L.; de Jonge, M.I.; Bottai, D.; Demangel, C.; Hinds, J.; Neyrolles, O.; Butcher, P.D.; Leclerc, C.; et al. Dissection of ESAT-6 System 1 of Mycobacterium tuberculosis and Impact on Immunogenicity and Virulence. Infect. Immun. 2006, 74, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-M.; Shi, C.-H.; Fan, X.-L.; Xue, Y.; Bai, Y.-L.; Xu, Z.-K. Expression and immunogenicity of recombinant Mycobacterium bovis Bacillus Calmette-Guérin strains secreting the antigen ESAT-6 from Mycobacterium tuberculosis in mice. Chin. Med. J. 2007, 120, 1220–1225. [Google Scholar] [CrossRef]
- Namvarpour, M.; Tebianian, M.; Mansouri, R.; Ebrahimi, S.M.; Kashkooli, S. Comparison of different immunization routes on the immune responses induced by Mycobacterium tuberculosis ESAT-6/CFP-10 recombinant protein. Biologicals 2019, 59, 6–11. [Google Scholar] [CrossRef]
- Wang, C.; Lu, J.; Du, W.; Wang, G.; Li, X.; Shen, X.; Su, C.; Yang, L.; Chen, B.; Wang, J.; et al. Ag85b/ESAT6-CFP10 adjuvanted with aluminum/poly-IC effectively protects guinea pigs from latent Mycobacterium tuberculosis infection. Vaccine 2019, 37, 4477–4484. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xie, J. Roles and underlying mechanisms of ESAT-6 in the context of Mycobacterium tuberculosis-host interaction from a systems biology perspective. Cell. Signal. 2012, 24, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Balaji, K.N. The PE and PPE proteins of Mycobacterium tuberculosis. Tuberculosis 2011, 91, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Wiker, H.G.; Harboe, M. The antigen 85 complex: A major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 1992, 56, 648–661. [Google Scholar] [CrossRef]
- Huygen, K. The Immunodominant T-Cell Epitopes of the Mycolyl-Transferases of the Antigen 85 Complex of M. tuberculosis. Front. Immunol. 2014, 5, 321. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-J.; Ptak, C.P.; Hsieh, C.-L.; Akey, B.L.; Chang, Y.-F. Elastin, a Novel Extracellular Matrix Protein Adhering to Mycobacterial Antigen 85 Complex. J. Biol. Chem. 2013, 288, 3886–3896. [Google Scholar] [CrossRef] [Green Version]
- Kremer, L.; Maughan, W.N.; Wilson, R.A.; Dover, L.G.; Besra, G.S. The M. tuberculosis antigen 85 complex and mycolyltransferase activity. Lett. Appl. Microbiol. 2002, 34, 233–237. [Google Scholar] [CrossRef]
- Miller, B.K.; Zulauf, K.E.; Braunstein, M. The Sec Pathways and Exportomes of Mycobacterium tuberculosis. Microbiol. Spectr. 2017, 5, 2–5. [Google Scholar] [CrossRef]
- Nisa, A.; Counoupas, C.; Pinto, R.; Britton, W.J.; Triccas, J.A. Characterization of the Protective Immune Responses Conferred by Recombinant BCG Overexpressing Components of Mycobacterium tuberculosis Sec Protein Export System. Vaccines 2022, 10, 945. [Google Scholar] [CrossRef]
- Kadir, N.-A.; Sarmiento, M.E.; Acosta, A.; Norazmi, M.-N. Cellular and humoral immunogenicity of recombinant Mycobacterium smegmatis expressing Ag85B epitopes in mice. Int. J. Mycobacteriology 2016, 5, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Komine-Aizawa, S.; Jiang, J.; Mizuno, S.; Hayakawa, S.; Matsuo, K.; Boyd, L.F.; Margulies, D.H.; Honda, M. MHC-restricted Ag85B-specific CD8 + T cells are enhanced by recombinant BCG prime and DNA boost immunization in mice. Eur. J. Immunol. 2019, 49, 1399–1414. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.F.; Spreadbury, C.L. Mycobacterial Stationary Phase Induced by Low Oxygen Tension: Cell Wall Thickening and Localization of the 16-Kilodalton α-Crystallin Homolog. J. Bacteriol. 1998, 180, 801–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousefi-Avarvand, A.; Tafaghodi, M.; Soleimanpour, S.; Khademi, F. HspX protein as a candidate vaccine against Mycobacterium tuberculosis: An overview. Front. Biol. 2018, 13, 293–296. [Google Scholar] [CrossRef]
- Shi, C.; Chen, L.; Chen, Z.; Zhang, Y.; Zhou, Z.; Lu, J.; Fu, R.; Wang, C.; Fang, Z.; Fan, X. Enhanced protection against tuberculosis by vaccination with recombinant BCG over-expressing HspX protein. Vaccine 2010, 28, 5237–5244. [Google Scholar] [CrossRef]
- Vasilyev, K.; Shurygina, A.-P.; Zabolotnykh, N.; Sergeeva, M.; Romanovskaya-Romanko, E.; Pulkina, A.; Buzitskaya, J.; Dogonadze, M.Z.; Vinogradova, T.I.; Stukova, M.A. Enhancement of the Local CD8+ T-Cellular Immune Response to Mycobacterium tuberculosis in BCG-Primed Mice after Intranasal Administration of Influenza Vector Vaccine Carrying TB10.4 and HspX Antigens. Vaccines 2021, 9, 1273. [Google Scholar] [CrossRef]
- Yousefi Avarvand, A.; Meshkat, Z.; Khademi, F.; Tafaghodi, M. Immunogenicity of HspX/EsxS fusion protein of Mycobacterium tuberculosis along with ISCOMATRIX and PLUSCOM nano-adjuvants after subcutaneous administration in animal model. Microb. Pathog. 2021, 154, 104842. [Google Scholar] [CrossRef]
- Mansury, D.; Ghazvini, K.; Amel Jamehdar, S.; Badiee, A.; Tafaghodi, M.; Nikpoor, A.R.; Amini, Y.; Jaafari, M.R. Increasing Cellular Immune Response in Liposomal Formulations of DOTAP Encapsulated by Fusion Protein Hspx, PPE44, And Esxv, as a Potential Tuberculosis Vaccine Candidate. Rep. Biochem. Mol. Biol. 2019, 7, 156–166. [Google Scholar]
- Moradi, B.; Sankian, M.; Amini, Y.; Gholoobi, A.; Meshkat, Z. A new DNA vaccine expressing HspX-PPE44-EsxV fusion antigens of Mycobacterium tuberculosis induced strong immune responses. Iran. J. Basic Med. Sci. 2020, 23, 909–914. [Google Scholar] [CrossRef]
- Valizadeh, A.; Khosravi, F.; Sedighian, H.; Behzadi, E.; Parizad, E.; Imani Fooladi, A.A. Evaluation of Triple Fragment Vaccine HSPX (Rv2031c) + PPE44 (Rv2770c) + Mouse IgG1 (Fcγ2a) with Auxiliary Adjuncts IL-22 in Comparison with BCG Vaccine. Iran. J. Pathol. 2022, 17, 303–313. [Google Scholar] [CrossRef]
- Witte, G.; Hartung, S.; Büttner, K.; Hopfner, K.-P. Structural Biochemistry of a Bacterial Checkpoint Protein Reveals Diadenylate Cyclase Activity Regulated by DNA Recombination Intermediates. Mol. Cell 2008, 30, 167–178. [Google Scholar] [CrossRef]
- Yin, W.; Cai, X.; Ma, H.; Zhu, L.; Zhang, Y.; Chou, S.-H.; Galperin, M.Y.; He, J. A decade of research on the second messenger c-di-AMP. FEMS Microbiol. Rev. 2020, 44, 701–724. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Sater, A.A.; Tattoli, I.; Jin, L.; Grajkowski, A.; Levi, A.; Koller, B.H.; Allen, I.C.; Beaucage, S.L.; Fitzgerald, K.A.; Ting, J.P.-Y.; et al. Cyclic-di-GMP and cyclic-di-AMP activate the NLRP3 inflammasome. EMBO Rep. 2013, 14, 900–906. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Bai, Y.; Zhang, Y.; Gabrielle, V.D.; Jin, L.; Bai, G. Deletion of the cyclic di-AMP phosphodiesterase gene ( cnpB ) in Mycobacterium tuberculosis leads to reduced virulence in a mouse model of infection. Mol. Microbiol. 2014, 93, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Ning, H.; Wang, L.; Zhou, J.; Lu, Y.; Kang, J.; Ding, T.; Shen, L.; Xu, Z.; Bai, Y. Recombinant BCG With Bacterial Signaling Molecule Cyclic di-AMP as Endogenous Adjuvant Induces Elevated Immune Responses after Mycobacterium tuberculosis Infection. Front. Immunol. 2019, 10, 1519. [Google Scholar] [CrossRef] [Green Version]
- Ning, H.; Kang, J.; Lu, Y.; Liang, X.; Zhou, J.; Ren, R.; Zhou, S.; Zhao, Y.; Xie, Y.; Bai, L.; et al. Cyclic di-AMP as endogenous adjuvant enhanced BCG-induced trained immunity and protection against Mycobacterium tuberculosis in mice. Front. Immunol. 2022, 13, 943667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.; Bai, G. Cyclic di-AMP-mediated interaction between Mycobacterium tuberculosis ΔcnpB and macrophages implicates a novel strategy for improving BCG vaccination. Pathog. Dis. 2018, 76, fty008. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.J.; Dey, B.; Singh, A.K.; Praharaj, M.; Bishai, W. Bacillus Calmette-Guérin Overexpressing an Endogenous Stimulator of Interferon Genes Agonist Provides Enhanced Protection Against Pulmonary Tuberculosis. J. Infect. Dis. 2020, 221, 1048–1056. [Google Scholar] [CrossRef] [Green Version]
- Ning, H.; Liang, X.; Xie, Y.; Bai, L.; Zhang, W.; Wang, L.; Kang, J.; Lu, Y.; Ma, Y.; Bai, G.; et al. c-di-AMP Accumulation Regulates Growth, Metabolism, and Immunogenicity of Mycobacterium smegmatis. Front. Microbiol. 2022, 13, 865045. [Google Scholar] [CrossRef]
- Sadagopal, S.; Braunstein, M.; Hager, C.C.; Wei, J.; Daniel, A.K.; Bochan, M.R.; Crozier, I.; Smith, N.E.; Gates, H.O.; Barnett, L.; et al. Reducing the Activity and Secretion of Microbial Antioxidants Enhances the Immunogenicity of BCG. PLoS ONE 2009, 4, e5531. [Google Scholar] [CrossRef] [Green Version]
- Hinchey, J.; Lee, S.; Jeon, B.Y.; Basaraba, R.J.; Venkataswamy, M.M.; Chen, B.; Chan, J.; Braunstein, M.; Orme, I.M.; Derrick, S.C.; et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J. Clin. Investig. 2007, 117, 2279–2288. [Google Scholar] [CrossRef]
- Forman, M.T.H.J. Signaling by the Respiratory Burst in Macrophages. IUBMB Life (Int. Union Biochem. Mol. Biol. Life) 2001, 51, 365–371. [Google Scholar] [CrossRef]
- Kantengwa, S.; Jornot, L.; Devenoges, C.; Nicod, L.P. Superoxide Anions Induce the Maturation of Human Dendritic Cells. Am. J. Respir. Crit. Care Med. 2003, 167, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Vemula, M.H.; Medisetti, R.; Ganji, R.; Jakkala, K.; Sankati, S.; Chatti, K.; Banerjee, S. Mycobacterium tuberculosis Zinc Metalloprotease-1 Assists Mycobacterial Dissemination in Zebrafish. Front. Microbiol. 2016, 7, 1347. [Google Scholar] [CrossRef] [Green Version]
- Vemula, M.H.; Ganji, R.; Sivangala, R.; Jakkala, K.; Gaddam, S.; Penmetsa, S.; Banerjee, S. Mycobacterium tuberculosis Zinc Metalloprotease-1 Elicits Tuberculosis-Specific Humoral Immune Response Independent of Mycobacterial Load in Pulmonary and Extra-Pulmonary Tuberculosis Patients. Front. Microbiol. 2016, 7, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansen, P.; Fettelschoss, A.; Amstutz, B.; Selchow, P.; Waeckerle-Men, Y.; Keller, P.; Deretic, V.; Held, L.; Kündig, T.M.; Böttger, E.C.; et al. Relief from Zmp1-Mediated Arrest of Phagosome Maturation Is Associated with Facilitated Presentation and Enhanced Immunogenicity of Mycobacterial Antigens. Clin. Vaccine Immunol. 2011, 18, 907–913. [Google Scholar] [CrossRef]
- Sander, P.; Clark, S.; Petrera, A.; Vilaplana, C.; Meuli, M.; Selchow, P.; Zelmer, A.; Mohanan, D.; Andreu, N.; Rayner, E.; et al. Deletion of zmp1 improves Mycobacterium bovis BCG-mediated protection in a guinea pig model of tuberculosis. Vaccine 2015, 33, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Festjens, N.; Bogaert, P.; Batni, A.; Houthuys, E.; Plets, E.; Vanderschaeghe, D.; Laukens, B.; Asselbergh, B.; Parthoens, E.; De Rycke, R.; et al. Disruption of the SapM locus in Mycobacterium bovis BCG improves its protective efficacy as a vaccine against M. tuberculosis. EMBO Mol. Med. 2011, 3, 222–234. [Google Scholar] [CrossRef]
- Gröschel, M.I.; Sayes, F.; Shin, S.J.; Frigui, W.; Pawlik, A.; Orgeur, M.; Canetti, R.; Honoré, N.; Simeone, R.; van der Werf, T.S.; et al. Recombinant BCG Expressing ESX-1 of Mycobacterium marinum Combines Low Virulence with Cytosolic Immune Signaling and Improved TB Protection. Cell Rep. 2017, 18, 2752–2765. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.; Skeiky, Y.A.W.; Izzo, A.; Dheenadhayalan, V.; Imam, Z.; Penn, E.; Stagliano, K.; Haddock, S.; Mueller, S.; Fulkerson, J.; et al. Novel recombinant BCG expressing perfringolysin O and the over-expression of key immunodominant antigens; pre-clinical characterization, safety and protection against challenge with Mycobacterium tuberculosis. Vaccine 2009, 27, 4412–4423. [Google Scholar] [CrossRef]
- Gopalaswamy, R.; Subbian, S. An Update on Tuberculosis Vaccines. In Vaccine Design; Humana: New York, NY, USA, 2022; pp. 387–409. [Google Scholar]
- Arbues, A.; Aguilo, J.I.; Gonzalo-Asensio, J.; Marinova, D.; Uranga, S.; Puentes, E.; Fernandez, C.; Parra, A.; Cardona, P.J.; Vilaplana, C.; et al. Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine 2013, 31, 4867–4873. [Google Scholar] [CrossRef]
- Hoft, D.F.; Blazevic, A.; Selimovic, A.; Turan, A.; Tennant, J.; Abate, G.; Fulkerson, J.; Zak, D.E.; Walker, R.; McClain, B.; et al. Safety and Immunogenicity of the Recombinant BCG Vaccine AERAS-422 in Healthy BCG-naïve Adults: A Randomized, Active-controlled, First-in-human Phase 1 Trial. EBioMedicine 2016, 7, 278–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, J.; Miko, D.; Catic, A.; Lehmensiek, V.; Russell, D.G.; Kaufmann, S.H.E. Mycobacterium bovis bacille Calmette–Guérin strains secreting listeriolysin of Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 1998, 95, 5299–5304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufmann, S.H.E. Immunity to Intracellular Bacteria. Annu. Rev. Immunol. 1993, 11, 129–163. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.E. Vaccination Against Tuberculosis: Revamping BCG by Molecular Genetics Guided by Immunology. Front. Immunol. 2020, 11, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotton, M.F.; Madhi, S.A.; Luabeya, A.K.; Tameris, M.; Hesseling, A.C.; Shenje, J.; Schoeman, E.; Hatherill, M.; Desai, S.; Kapse, D.; et al. Safety and immunogenicity of VPM1002 versus BCG in South African newborn babies: A randomised, phase 2 non-inferiority double-blind controlled trial. Lancet Infect. Dis. 2022, 22, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Blossey, A.M.; Brückner, S.; May, M.; Parzmair, G.P.; Sharma, H.; Shaligram, U.; Grode, L.; Kaufmann, S.H.E.; Netea, M.G.; Schindler, C. VPM1002 as Prophylaxis Against Severe Respiratory Tract Infections Including Coronavirus Disease 2019 in the Elderly: A Phase 3 Randomized, Double-Blind, Placebo-Controlled, Multicenter Clinical Study. Clin. Infect. Dis. 2022, ciac881. [Google Scholar] [CrossRef]
- Gonzalo-Asensio, J.; Malaga, W.; Pawlik, A.; Astarie-Dequeker, C.; Passemar, C.; Moreau, F.; Laval, F.; Daffé, M.; Martin, C.; Brosch, R.; et al. Evolutionary history of tuberculosis shaped by conserved mutations in the PhoPR virulence regulator. Proc. Natl. Acad. Sci. USA 2014, 111, 11491–11496. [Google Scholar] [CrossRef] [Green Version]
- Gonzalo-Asensio, J.; Pérez, I.; Aguiló, N.; Uranga, S.; Picó, A.; Lampreave, C.; Cebollada, A.; Otal, I.; Samper, S.; Martín, C. New insights into the transposition mechanisms of IS6110 and its dynamic distribution between Mycobacterium tuberculosis Complex lineages. PLOS Genet. 2018, 14, e1007282. [Google Scholar] [CrossRef] [Green Version]
- Pérez, E.; Samper, S.; Bordas, Y.; Guilhot, C.; Gicquel, B.; Martín, C. An essential role for phoP in Mycobacterium tuberculosis virulence. Mol. Microbiol. 2001, 41, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Gonzalo-Asensio, J.; Mostowy, S.; Harders-Westerveen, J.; Huygen, K.; Hernández-Pando, R.; Thole, J.; Behr, M.; Gicquel, B.; Martín, C. PhoP: A Missing Piece in the Intricate Puzzle of Mycobacterium tuberculosis Virulence. PLoS ONE 2008, 3, e3496. [Google Scholar] [CrossRef]
- Frigui, W.; Bottai, D.; Majlessi, L.; Monot, M.; Josselin, E.; Brodin, P.; Garnier, T.; Gicquel, B.; Martin, C.; Leclerc, C.; et al. Control of M. tuberculosis ESAT-6 Secretion and Specific T Cell Recognition by PhoP. PLoS Pathog. 2008, 4, e33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asensio, J.G.; Maia, C.; Ferrer, N.L.; Barilone, N.; Laval, F.; Soto, C.Y.; Winter, N.; Daffé, M.; Gicquel, B.; Martín, C.; et al. The Virulence-associated Two-component PhoP-PhoR System Controls the Biosynthesis of Polyketide-derived Lipids in Mycobacterium tuberculosis. J. Biol. Chem. 2006, 281, 1313–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirksey, M.A.; Tischler, A.D.; Siméone, R.; Hisert, K.B.; Uplekar, S.; Guilhot, C.; McKinney, J.D. Spontaneous Phthiocerol Dimycocerosate-Deficient Variants of Mycobacterium tuberculosis Are Susceptible to Gamma Interferon-Mediated Immunity. Infect. Immun. 2011, 79, 2829–2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho, L.R.; Constant, P.; Raynaud, C.; Lanéelle, M.-A.; Triccas, J.A.; Gicquel, B.; Daffé, M.; Guilhot, C. Analysis of the Phthiocerol Dimycocerosate Locus of Mycobacterium tuberculosis. J. Biol. Chem. 2001, 276, 19845–19854. [Google Scholar] [CrossRef] [Green Version]
- Augenstreich, J.; Arbues, A.; Simeone, R.; Haanappel, E.; Wegener, A.; Sayes, F.; Le Chevalier, F.; Chalut, C.; Malaga, W.; Guilhot, C.; et al. ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell. Microbiol. 2017, 19, e12726. [Google Scholar] [CrossRef] [Green Version]
- Infante, E.; Aguilar, L.D.; Gicquel, B.; Pando, R.H. Immunogenicity and protective efficacy of the Mycobacterium tuberculosis fadD26 mutant. Clin. Exp. Immunol. 2005, 141, 21–28. [Google Scholar] [CrossRef]
- Clark, S.; Lanni, F.; Marinova, D.; Rayner, E.; Martin, C.; Williams, A. Revaccination of Guinea Pigs With the Live Attenuated Mycobacterium tuberculosis Vaccine MTBVAC Improves BCG’s Protection Against Tuberculosis. J. Infect. Dis. 2017, 216, 525–533. [Google Scholar] [CrossRef]
- Aguilo, N.; Gonzalo-Asensio, J.; Alvarez-Arguedas, S.; Marinova, D.; Gomez, A.B.; Uranga, S.; Spallek, R.; Singh, M.; Audran, R.; Spertini, F.; et al. Reactogenicity to major tuberculosis antigens absent in BCG is linked to improved protection against Mycobacterium tuberculosis. Nat. Commun. 2017, 8, 16085. [Google Scholar] [CrossRef] [Green Version]
- Aguilo, N.; Uranga, S.; Marinova, D.; Monzon, M.; Badiola, J.; Martin, C. MTBVAC vaccine is safe, immunogenic and confers protective efficacy against Mycobacterium tuberculosis in newborn mice. Tuberculosis 2016, 96, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Tameris, M.; Mearns, H.; Penn-Nicholson, A.; Gregg, Y.; Bilek, N.; Mabwe, S.; Geldenhuys, H.; Shenje, J.; Luabeya, A.K.K.; Murillo, I.; et al. Live-attenuated Mycobacterium tuberculosis vaccine MTBVAC versus BCG in adults and neonates: A randomised controlled, double-blind dose-escalation trial. Lancet Respir. Med. 2019, 7, 757–770. [Google Scholar] [CrossRef]
| Vaccine Name | Main Feature | Reference |
|---|---|---|
| AERAS 422 | Expression of perfringolysin from C. perfringens and antigens Ag85A and Ag85B | [91] |
| VPM1002 | Expression of listeriolysin from L. monocytogenes Deletion of the ureC gene | [92] |
| MTBVAC | Mtb with inactivated genes phoP and fadD26 | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadolinskaia, N.I.; Kotliarova, M.S.; Goncharenko, A.V. Fighting Tuberculosis: In Search of a BCG Replacement. Microorganisms 2023, 11, 51. https://doi.org/10.3390/microorganisms11010051
Nadolinskaia NI, Kotliarova MS, Goncharenko AV. Fighting Tuberculosis: In Search of a BCG Replacement. Microorganisms. 2023; 11(1):51. https://doi.org/10.3390/microorganisms11010051
Chicago/Turabian StyleNadolinskaia, Nonna I., Maria S. Kotliarova, and Anna V. Goncharenko. 2023. "Fighting Tuberculosis: In Search of a BCG Replacement" Microorganisms 11, no. 1: 51. https://doi.org/10.3390/microorganisms11010051






