Epidemiology and Drug Resistance Patterns of Mycobacterium tuberculosis in High-Burden Area in Western Siberia, Russia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. M. tuberculosis Culture and Species Identification
2.3. M. tuberculosis Drug Susceptibility Testing
2.4. Molecular Methods
2.5. Descriptive Epidemiology Methods
2.6. Definitions
3. Results
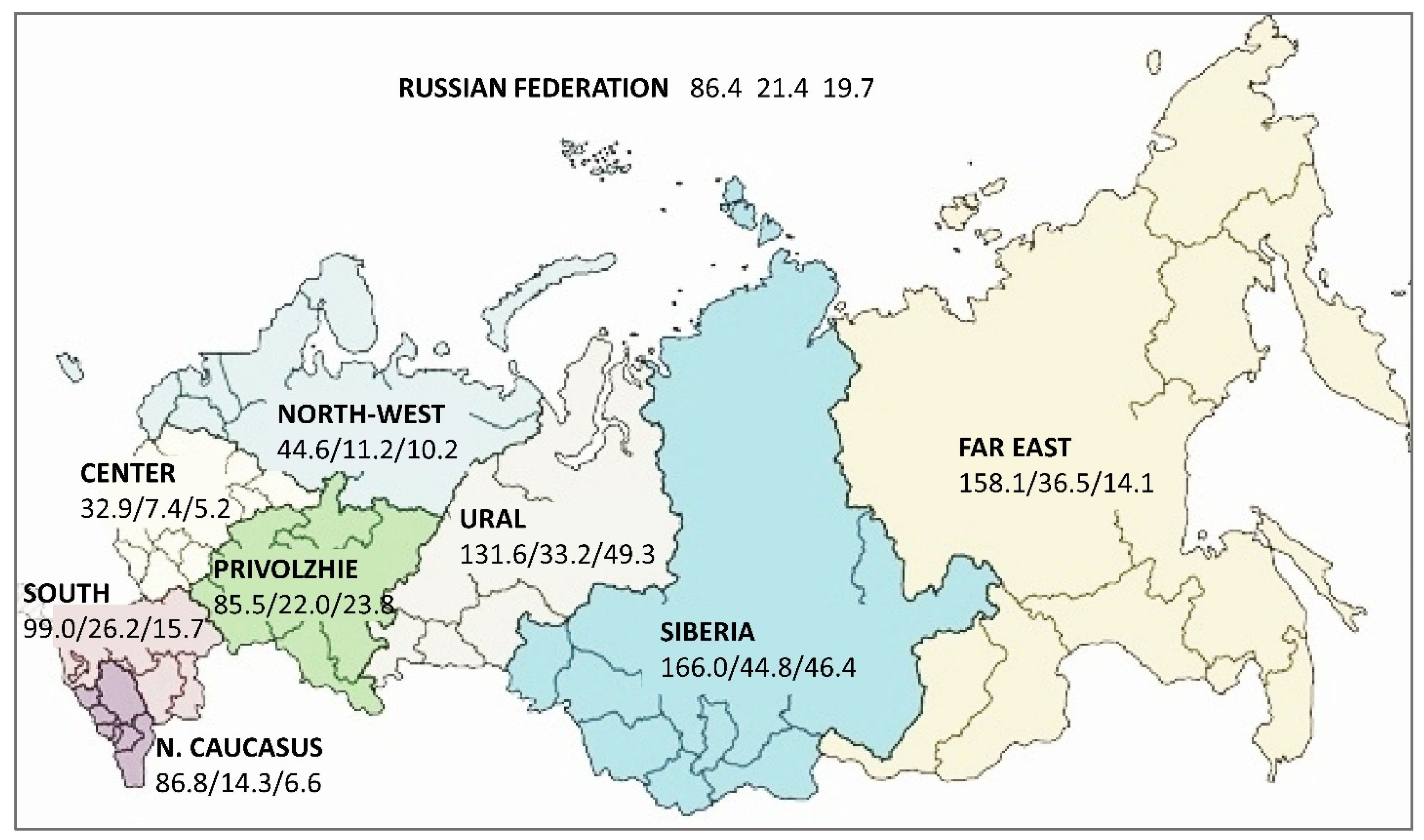
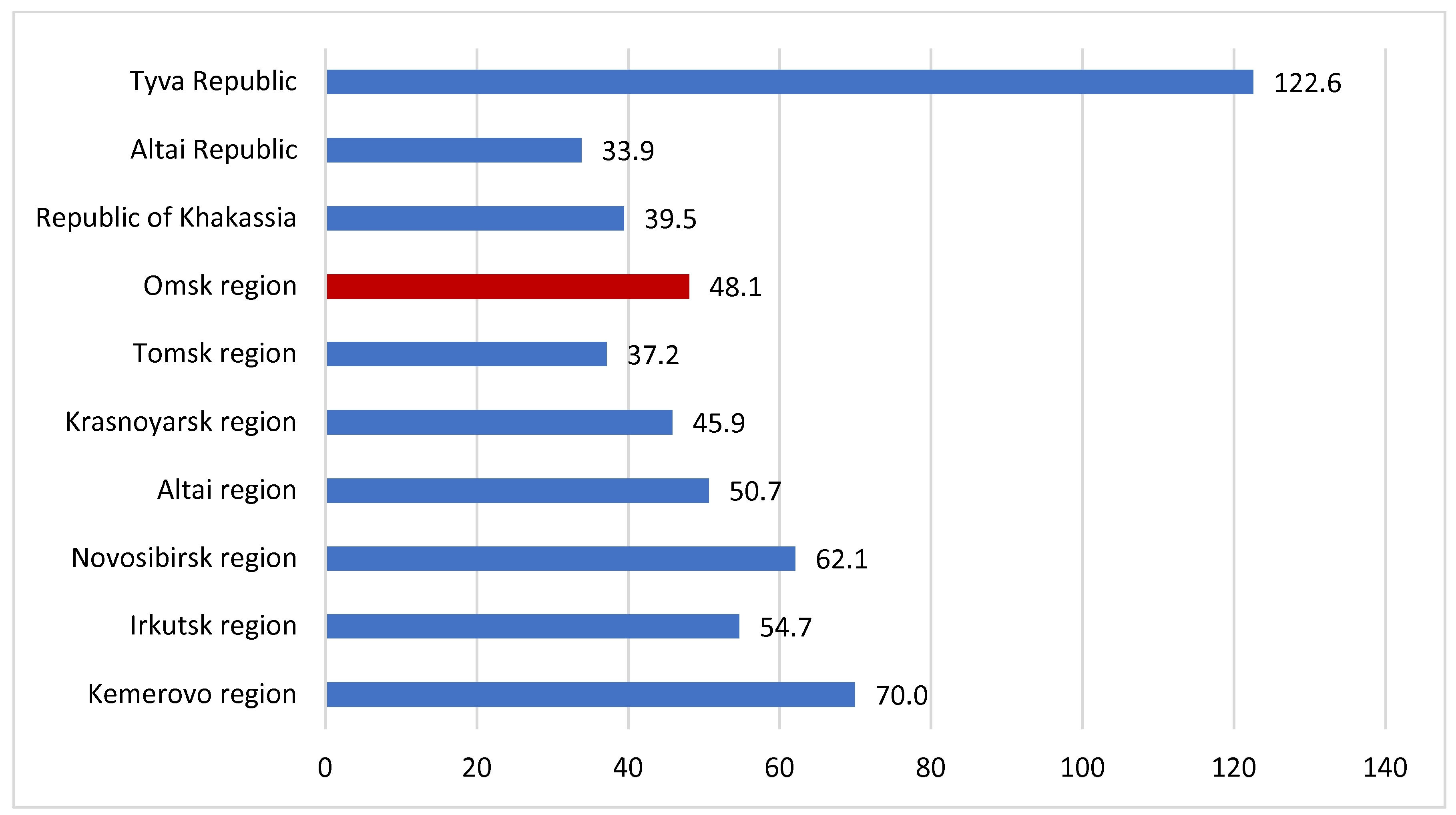
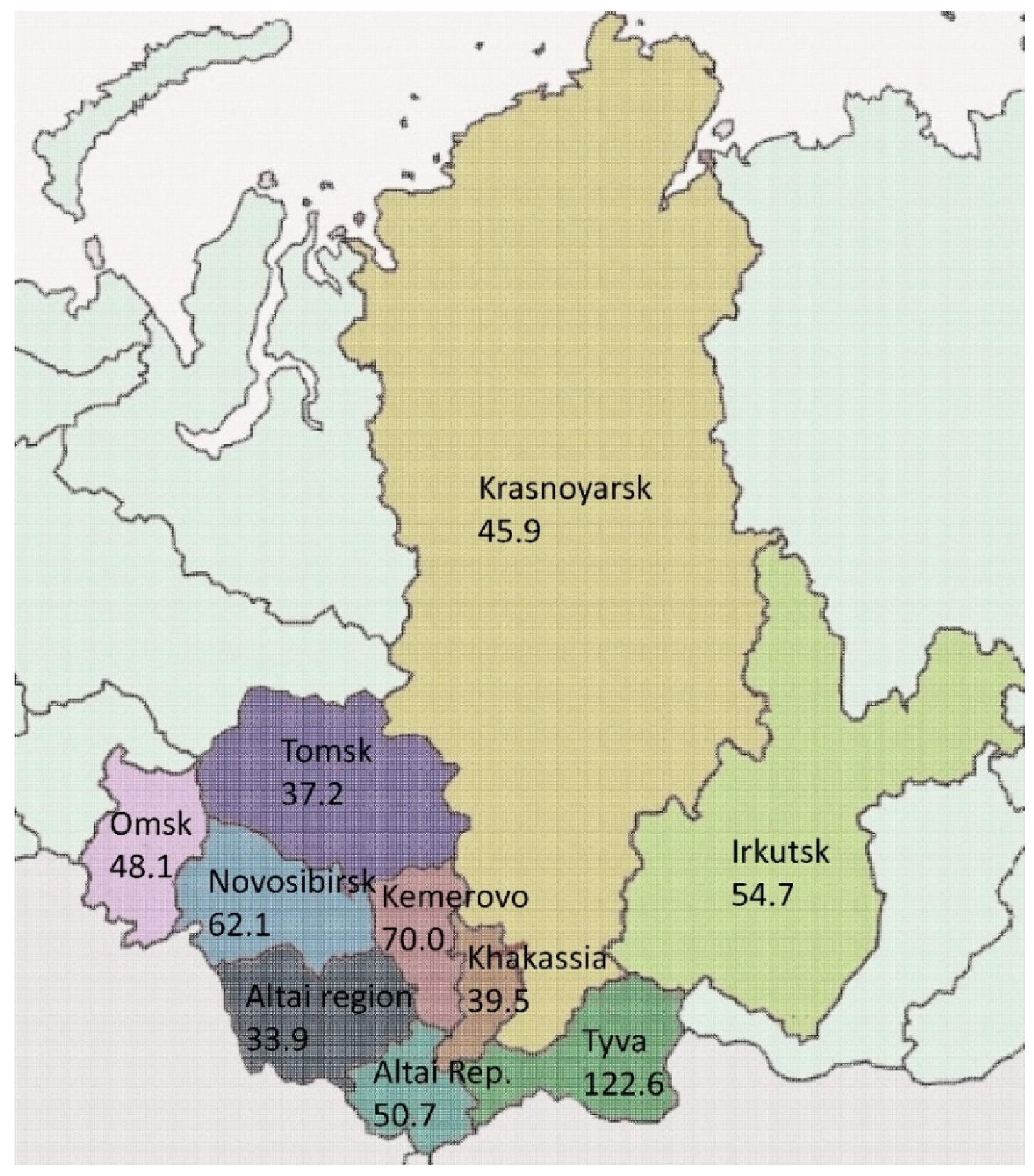
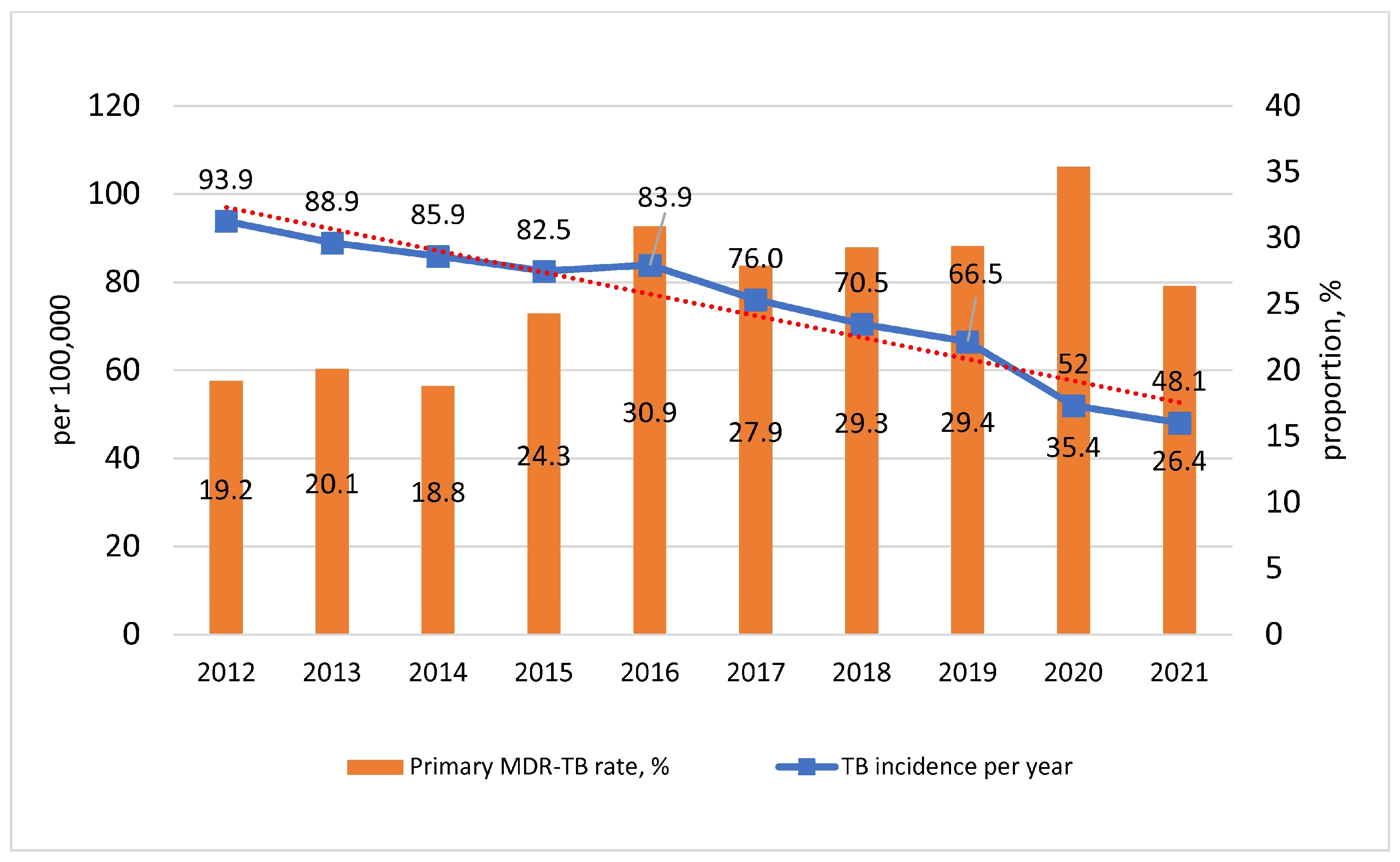
3.1. Characteristics of the Epidemiological Situation
3.2. Characterization of M. tuberculosis Primary Drug Resistance
3.3. Drug Resistance-Associated Mutations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raviglione, M.C.; Korobitsyn, A.A. End TB—The new who strategy in the sdg era, and the contributions from the Russian Federation. Tuberk. Bolezn. Legk. 2016, 11, 7–15. (In Russian) [Google Scholar] [CrossRef]
- Chopra, K.K.; Arora, V.K. End TB—Strategy—A dream to achieve. Indian J. Tuberc. 2019, 1, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.; Sabgayda, T.P.; Radina, T.S. Assessment of Correlation between HIV Infection and Tuberculosis with Multiple Drug Resistance. Tuberc. Lung Dis. 2018, 96, 25–32. [Google Scholar] [CrossRef]
- Sharma, A.; Hill, A.; Kurbatova, E.; van der Walt, M.; Kvasnovsky, C.; Tupasi, T.E.; Caoili, J.C.; Gler, M.T.; Volchenkov, G.V.; Kazennyy, B.Y.; et al. Estimating the future burden of multidrug-resistant and extensively drug-resistant tuberculosis in India, the Philippines, Russia, and South Africa: A mathematical modelling study. Lancet Infect. Dis. 2017, 17, 707–715. [Google Scholar] [CrossRef]
- McQuaid, C.F.; Vassall, A.; Cohen, T.; Fiekert, K.; COVID/TB Modelling Working Group; White, R.G. The impact of COVID-19 on TB: A review of the data. Int. J. Tuberc. Lung Dis. 2021, 25, 436–446. [Google Scholar] [CrossRef]
- WHO. Global Tuberculosis Report 2022. Available online: https://www.who.int/publications/i/item/9789240061729 (accessed on 12 October 2022).
- WHO Consolidated Guidelines on Tuberculosis: Module 4: Treatment: Drug-Susceptible Tuberculosis Treatment. Available online: https://www.who.int/publications/i/item/9789240048126 (accessed on 20 October 2022).
- Tuberculosis in Adults: Clinical Guidelines. 2022. Available online: https://edu.nmrc.ru/wp-content/uploads/2022/09/%D0%9A%D0%A016.pdf. (accessed on 20 October 2022).
- Dean, A.S.; Auguet, O.T.; Glaziou, P.; Zignol, M.; Ismail, N.; Kasaeva, T.; Floyd, K. 25 years of surveillance of drug-resistant tuberculosis: Achievements, challenges, and way forward. Lancet Infect. Dis. 2022, 22, e191–e196. [Google Scholar] [CrossRef]
- WHO. Global Task Force on TB Impact Measurement: Report of a Subgroup Meeting on Methods Used by WHO to Estimate TB Disease Burden, 11–12 May 2022, Geneva, Switzerland. Available online: https://apps.who.int/iris/bitstream/handle/10665/363428/9789240057647-eng.pdf (accessed on 15 October 2022).
- Methods for Estimating the Incidence of Drug-Resistant TB. Available online: https://cdn.who.int/media/docs/default-source/hq-tuberculosis/global-task-force-on-tb-impact-measurement/meetings/2022-05/tf-2022-05-2-background--document-2--dr-tb.pdf?sfvrsn=a8757cfa_3). (accessed on 26 October 2022).
- World Health Organisation. Global Tuberculosis Report 2020. Available online: https://www.who.int/publications/i/item/9789240013131. (accessed on 26 October 2022).
- World Health Organisation. Global Tuberculosis Report 2021. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021 (accessed on 20 October 2022).
- Multidrug and Extensively Drug-Resistant TB (M/XDR-TB): 2010 Global Report on Surveillance and Response. World Health Organisation. 2010. Available online: https://apps.who.int/iris/bitstream/handle/10665/44286/9789241599191_eng.pdf?sequence=1&isAllowed=y (accessed on 20 October 2022).
- Ergeshov, A.E.; Punga, V.V.; Rusakova, L.I.; Sterlikov, S.A.; Yakimova, M.A.; Izmaylova, T.V. Tuberculosis with multidrug-resistant and extensively drug-resistant of Micobacterium tuberculosis in the Russian Federation. Vestn. Avitsenny Avicenna Bull. 2018, 2–3, 314–319. (In Russian) [Google Scholar]
- Pasechnik, O.A.; Vyazovaya, A.A.; Bloch, A.I.; Yarusova, I.V.; Tatarintseva, M.P.; Mokrousov, I.V. Assessment of the Prevalence and Epidemic Spread of Strains of Ancient, and Modern Sublineages of the Mycobacterium tuberculosis Beijing Genotype in Omsk Region. Epidemiol. Vaccinal Prev. 2020, 19, 20–29. [Google Scholar] [CrossRef]
- Shivekar, S.S.; Kaliaperumal, V.; Brammacharry, U.; Sakkaravarthy, A.; Raj, C.K.V.; Alagappan, C.; Muthaiah, M. Prevalence and factors associated with multidrug-resistant tuberculosis in South India. Sci. Rep. 2020, 10, 17552. [Google Scholar] [CrossRef]
- Yang, C.; Luo, T.; Shen, X.; Wu, J.; Gan, M.; Xu, P.; Wu, Z.; Lin, S.; Tian, J.; Liu, Q.; et al. Transmission of multidrug-resistant Mycobacterium tuberculosis in Shanghai, China: A retrospective observational study using whole-genome sequencing and epidemiological investigation. Lancet Infect. Dis. 2016, 17, 275–284. [Google Scholar] [CrossRef]
- Martinez, L.; Shen, Y.; Mupere, E.; Kizza, A.; Hill, P.C.; Whalen, C.C. Transmission of Mycobacterium Tuberculosis in Households and the Community: A Systematic Review and Meta-Analysis. Am. J. Epidemiol. 2017, 185, 1327–1339. [Google Scholar] [CrossRef]
- Migliori, G.B.; Ortmann, J.; Girardi, E.; Besozzi, G.; Lange, C.; Cirillo, D.M.; Ferrarese, M.; De Iaco, G.; Gori, A.; Raviglione, M.; et al. Extensively Drug-resistant Tuberculosis, Italy and Germany. Emerg. Infect. Dis. 2007, 13, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Velayati, A.A.; Masjedi, M.R.; Farnia, P.; Tabarsi, P.; Ghanavi, J.; Ziazarifi, A.H.; Hoffner, S.E. Emergence of new forms of to-tally drug-resistant tuberculosis bacilli: Super extensively drug-resistant tuberculosis or totally drug-resistant strains in Iran. Chest 2009, 136, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Klopper, M.; Warren, R.M.; Hayes, C.; Gey Van Pittius, N.C.; Streicher, E.M.; Müller, B.; Sirgel, F.A.; Chabula-Nxiweni, M.; Hoosain, E.; Coetzee, G.; et al. Emergence and spread of extensively and totally drug-resistant tuberculosis, South Africa. Emerg. Infect. Dis. 2013, 19, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Frolova, O.P.; Butylchenko, O.V.; Gadzhieva, P.G.; Timofeeva, M.Y.; Basangova, V.A.; Petrova, V.O.; Fadeeva, I.A.; Kashutina, M.I.; Zabroda, N.N.; Basov, A.A.; et al. Medical Care for Tuberculosis-HIV-Coinfected Patients in Russia with Respect to a Changeable Patients’ Structure. Trop. Med. Infect Dis. 2022, 6, 86. [Google Scholar] [CrossRef]
- Nazarova, O.I. (Ed.) Epidemiological Manifestations of HIV Infection in the Omsk Region for 2021: Information Bulletin; Omsk, Russia, 2022; 25p, (In Russian). Available online: https://aidsomsk.ru/sites/default/uploads/informacionnyi_byulleten_2022.pdf (accessed on 27 January 2023).
- Shugaeva, S.N.; Savilov, E.D. Criteria for the integration of epidemic processes of HIV infection and tuberculosis. Tuberk Bolezn Legk. 2019, 5, 43–49. (In Russian) [Google Scholar] [CrossRef]
- Nechaeva, O.B. Tuberculosis in Russia. Available online: https://last.mednet.ru/images/materials/CMT/tuberkulez-2019.pdf (accessed on 9 November 2022).
- Pasechnik, O.; Zimoglyad, A.; Yarusova, I.; Vitriv, S.; Blokh, A. Multidrug-resistant and extensively drug-resistant tuberculosis in the Omsk region: Trends and characteristics. Tihookeanskiy. Med. Zh. 2018, 4, 95–100. (In Russian) [Google Scholar] [CrossRef]
- The Main Indicators of Anti-Tuberculosis Activity in the Siberian and Far Eastern Federal Districts (Statistical Materials). Novosibirsk. 2022. Available online: http://nsk-niit.ru/ftpgetfile.php?id=351 (accessed on 2 November 2022).
- Sevastyanova, E.V.; Larionova, E.E.; Andrievskaya, I.Y.; Smirnova, T.G. Detection of Mycobacteria by culture inoculation. Decontamination of diagnostic samples. CTRI Bull. 2020, 2, 89–99. (In Russian) [Google Scholar]
- Larionova, E.E.; Andrievskaya, I.Y.; Andreevskaya, S.N.; Smirnova, T.G.; Sevastyanova, E.V. The culture method for Mycobacteria studies. Solid growth media. CTRI Bull. 2020, 3, 75–86. (In Russian) [Google Scholar]
- Larionova, E.E.; Andreevskaya, S.N.; Smirnova, T.G.; Sevastyanova, E.V.; Chernousova, L.N. Methods for the identification of Mycobacterium Species. CTRI Bull. 2021, 1, 87–89. (In Russian) [Google Scholar]
- On the improvement of antitb measures in the Russian Federation: Order №109. 21 March 2003. Available online: https://roszdravnadzor.gov.ru/documents/40804 (accessed on 27 January 2023).
- World Health Organization. Meeting Report of the WHO Expert Consultation on the Definition of Extensively Drug-Resistant Tuberculosis, 27–29 October 2020; WHO: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240018662 (accessed on 27 January 2023).
- Singh, V.; Chibale, K. Strategies to Combat Multi-Drug Resistance in Tuberculosis. Accounts Chem. Res. 2021, 54, 2361–2376. [Google Scholar] [CrossRef]
- Brynildsrud, O.B.; Pepperell, C.S.; Suffys, P.; Grandjean, L.; Monteserin, J.; Debech, N.; Bohlin, J.; Alfsnes, K.; Pettersson, J.O.-H.; Kirkeleite, I.; et al. Global expansion of mycobacterium tuberculosis lineage 4 shaped by colonial migration and local adaptation. Sci. Adv. 2018, 4, eaat5869. [Google Scholar] [CrossRef]
- Ektefaie, Y.; Dixit, A.; Freschi, L.; Farhat, M.R. Globally diverse mycobacterium tuberculosis resistance acquisition: A retrospective geographical and temporal analysis of whole genome sequences. Lancet Microbe 2021, 2, e96–e104. [Google Scholar] [CrossRef]
- WHO/HTM/TB/2016.13; Global Tuberculosis Report; WHO: Geneva, Switzerland, 2016.
- Caulfield, A.J.; Wengenack, N.L. Diagnosis of active tuberculosis disease: From microscopy to molecular techniques. J. Clin. Tuberc. Other Mycobact. Dis. 2016, 4, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Gholoobi, A.; Masoudi-Kazemabad, A.; Meshkat, M.; Meshkat, Z. Comparison of Culture and PCR Methods for Diagnosis of Mycobacterium tuberculosis in Different Clinical Specimens. Jundishapur. J. Microbiol. 2014, 7, e8939. [Google Scholar] [CrossRef] [PubMed]
- Pasechnik, O.; Vyazovaya, A.; Vitriv, S.; Tatarintseva, M.; Blokh, A.; Stasenko, V.; Mokrousov, I. Major genotype families and epidemic clones of Mycobacterium tuberculosis in Omsk region, Western Siberia, Russia, marked by a high burden of tuberculosis-HIV coinfection. Tuberculosis 2018, 108, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organisation. Tuberculosis in the Americas 2018; Pan American Health Organisation: Washington, DC, USA, 2018. [Google Scholar]
- Chiang, C.-Y.; Centis, R.; Migliori, G.B. Drug-resistant tuberculosis: Past, present, future. Respirology 2010, 15, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Crofton, J.; Mitchison, D.A. Streptomycin Resistance in Pulmonary Tuberculosis. BMJ 1948, 2, 1009–1015. [Google Scholar] [CrossRef]
- Mitchison, D.A. Problems of drug resistance. Br. Med. Bull. 1954, 10, 115–124. [Google Scholar] [CrossRef]
- Long, R.; Chong, H.; Hoeppner, V.; Shanmuganathan, H.; Kowalewska-Grochowska, K.; Shandro, C.; Manfreda, J.; Senthilselvan, A.; Elzainy, A.; Marrie, T. Empirical Treatment of Community-Acquired Pneumonia and the Development of Fluoroquinolone-Resistant Tuberculosis. Clin. Infect. Dis. 2009, 48, 1354–1360. [Google Scholar] [CrossRef]
- Gill, A.; Ugalde, I.; Febres-Aldana, C.A.; Tuda, C. Fluoroquinolone resistant tuberculosis: A case report and literature review. Respir. Med. Case Rep. 2019, 27, 100829. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Lin, S.-M.; Shie, S.-S.; Chou, P.-C.; Huang, C.-D.; Chung, F.-T.; Kuo, C.-H.; Chang, P.-J.; Kuo, H.-P. Clinical Characteristics and Treatment Out-comes of Patients with Low- and High-Concentration Isoniazid-Monoresistant Tuberculosis. PLoS ONE 2014, 9, e86316. [Google Scholar]
- Zhang, Y.; Yew, W.W. Mechanisms of drug resistance in Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 2009, 13, 1320–1330. [Google Scholar]
- Chen, J.; Chen, L.; Zhou, M.; Wu, G.; Yi, F.; Jiang, C.; Duan, Q.; Zhou, M. Transmission of multidrug-resistant tuberculosis within family households by DTM-PCR and MIRU-VNTR genotyping. BMC Infect. Dis. 2022, 22, 192. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.A.; Abeel, T.; Manson McGuire, A.; Desjardins, C.A.; Munsamy, V.; Shea, T.P.; Walker, B.J.; Bantubani, N.; Almeida, D.V.; Alvarado, L.; et al. Evolution of Extensively Drug-Resistant Tuberculosis over Four Decades: Whole Genome Sequencing and Dating Analysis of Mycobacterium tuberculosis Isolates from KwaZulu-Natal. PLoS Med. 2015, 12, e1001880. [Google Scholar] [CrossRef]
- Pietersen, E.; Ignatius, E.; Streicher, E.M.; Mastrapa, B.; Padanilam, X.; Pooran, A.; Badri, M.; Lesosky, M.; van Helden, P.; Sirgel, F.A.; et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: A cohort study. Lancet 2014, 383, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Tuberculosis Report 2013. Available online: https://apps.who.int/iris/bitstream/handle/10665/91355/9789241564656_eng.pdf?sequence=1&isAllowed=y (accessed on 27 January 2023).
- Falzon, D.; Mirzayev, F.; Wares, F.; Baena, I.G.; Zignol, M.; Linh, N.; Weyer, K.; Jaramillo, E.; Floyd, K.; Raviglione, M. Multi-drug-resistant tuberculosis around the world: What progress has been made? Eur. Respir. J. 2015, 45, 150–160. [Google Scholar] [CrossRef]
- Dye, C.; Bassili, A.; Bierrenbach, A.L.; Broekmans, J.F.; Chadha, V.K.; Glaziou, P.; Gopi, P.G.; Hosseini, M.; Kim, S.J.; Manissero, D.; et al. Measuring tuberculosis burden, trends, and the impact of control programmes. Lancet Infect Dis. 2008, 8, 233–243. [Google Scholar] [CrossRef] [PubMed]
- TB Country, Regional and Global Profiles. Available online: https://worldhealthorg.shinyapps.io/tb_profiles/?_inputs_&entity_type=%22country%22&lan=%22EN%22&iso2=%22AF%22 (accessed on 27 January 2023).
- Drug-Resistant TB: Surveillance and Response: Supplement to Global Tuberculosis Report 2014. Available online: https://www.who.int/publications/i/item/WHO-HQ-TB-2014.12 (accessed on 27 January 2023).
- Centers for Disease Control and Prevention (CDC). Plan to combat extensively drug-resistant tuberculosis: Recommendations of the Federal Tuberculosis Task Force. MMWR Recomm. Rep. 2009, 58, 1–43. [Google Scholar]
- Yao, C.; Guo, H.; Li, Q.; Zhang, X.; Shang, Y.; Li, T.; Wang, Y.; Xue, Z.; Wang, L.; Li, L.; et al. Prevalence of extensively drug-resistant tuberculosis in a Chinese multidrug-resistant TB cohort after redefinition. Antimicrob. Resist. Infect. Control. 2021, 10, 126. [Google Scholar] [CrossRef]
| Drug Resistance | Number of Isolates | % of All Resistant |
|---|---|---|
| Monoresistance | 33 | 13.9 |
| Polyresistance | 43 | 18.2 |
| MDR, including | 161 | 67.9 |
| Pre-XDR | 65 | 27.4 |
| XDR | 31 | 13.0 |
| Total | 237 | 100 |
| MDR Pattern (Resistance to Particular Drugs) a | Number | % (of All MDR) |
|---|---|---|
| MDR (H + R) | 65 | 40.4 |
| H + R | 5 | 3.1 |
| H + R + S | 16 | 9.9 |
| H + R + S + E | 20 | 12.4 |
| H + R + S + Z | 11 | 6.8 |
| H + R + S + Eto | 1 | 0.6 |
| H + R + S + E + Eto | 2 | 1.2 |
| H + R + S + Z + PAS | 1 | 0.6 |
| H + R + S + E + Z | 8 | 4.9 |
| H + R + S + E + PAS | 1 | 0.6 |
| Pre-XDR (MDR + Ofx or Km.Am.Cm) | 65 | 40.4 |
| H + R + Am | 2 | 0.8 |
| H + R + S + Ofx | 6 | 3.7 |
| H + R + S + Km | 2 | 1.2 |
| H + R + S + Am | 3 | 1.8 |
| H + R + S + Cm | 1 | 0.6 |
| H + R + S + E + Ofx | 17 | 10.5 |
| H + R + S + E + Km | 6 | 3.7 |
| H + R + S + Km + Cm | 1 | 0.6 |
| H + R + S + E + Am | 3 | 1.8 |
| H + R + S + Ofx + Z | 1 | 0.6 |
| H + R + S + Ofx + PAS | 1 | 0.6 |
| H + R + E + Km + Eto | 1 | 0.6 |
| H + R + S + Eto + Cm | 1 | 0.6 |
| H + R + S + Z + Am | 2 | 1.2 |
| H + R + S + E + Z + Km | 4 | 2.4 |
| H + R + S + Ofx + Z + PAS | 1 | 0.6 |
| H + R + S + E + Km + Cm | 1 | 0.6 |
| H + R + S + E + Km + Am | 1 | 0.6 |
| H + R + S + E + Ofx + Z | 2 | 1.2 |
| H + R + S + E + Z + Km + Cm | 1 | 0.6 |
| H + R + S + E + Ofx + Z + PAS | 1 | 0.6 |
| H + R + S + E + Z + Km + Eto | 1 | 0.6 |
| H + R + S + E + Km + Eto + PAS | 1 | 0.6 |
| H + R + S + E + Ofx + Z + Lfx | 1 | 0.6 |
| H + R + S + E + Ofx + Eto + Cs + PAS | 1 | 0.6 |
| H + R + S + E + Z + Km + Eto +Cm | 1 | 0.6 |
| H + R + S + E + Z + Km + Am + Cm | 2 | 1.2 |
| XDR (MDR + Ofx + Km and/or Am, Cm) | 31 | 19.2 |
| H + R + S + Ofx + Km | 2 | 1.2 |
| H + R + S + Ofx + Cm | 1 | 0.6 |
| H + R + S + E + Ofx + Km | 5 | 3.1 |
| H + R + S + Ofx + Cm + PAS | 1 | 0.6 |
| H + R + S + E + Ofx + Km + Cm | 2 | 1.2 |
| H + R + S + E + Ofx + Km + Pas | 1 | 0.6 |
| H + R + S + E + Ofx + Z + Km | 2 | 1.2 |
| H + R + S + Ofx + Eto + Cm + Pas | 1 | 0.6 |
| H + R + S + Ofx + Z + Am + Cm | 1 | 0.6 |
| H + R + S + E + Ofx + Cm + Lfx | 1 | 0.6 |
| H + R + S + Z + Am + Lfx + Mfx + Lzd | 1 | 0.6 |
| H + R + S + E + Z + Am + Lfx + Mfx | 2 | 1.2 |
| H + R + S + E + Ofx + Z + Am + Cm | 1 | 0.6 |
| H + R + S + E + Ofx + Z + Km + Cm | 1 | 0.6 |
| H + R + S + E + Ofx + Z + Eto + Cm | 1 | 0.6 |
| H + R + S + E + Ofx + Z + Km + Eto | 1 | 0.6 |
| H + R + S + Ofx + Z + Km + Eto + Cm | 1 | 0.6 |
| H + R + S + E + Ofx + Km + Eto + Cm + PAS | 1 | 0.6 |
| H + R + S + E + Ofx + Z + Km + Am + Cm | 1 | 0.6 |
| H + R + S + E + Ofx + Km + Eto + Cm + Cs + PAS | 1 | 0.6 |
| H + R + S + E + Ofx + Z + Km + Am + Cm + Cs + Lzd | 1 | 0.6 |
| H + R + S + E + Ofx + Z + Km + Am + Cm + Lfx + Mfx | 2 | 1.2 |
| Total MDR | 161 | 100.0 |
| Gene | Codon | Anti-TB Drug | Number | % of All Tested |
|---|---|---|---|---|
| katG | 315 | isoniazid | 103 | 81.1 |
| inhA | 209 | isoniazid | 2 | 1.6 |
| rpoB | 531 | rifampicin | 22 | 17.3 |
| gyrA | 90 | fluoroquinolones | 12 | 9.4 |
| gyrA | 94 | fluoroquinolones | 9 | 7.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostyukova, I.; Pasechnik, O.; Mokrousov, I. Epidemiology and Drug Resistance Patterns of Mycobacterium tuberculosis in High-Burden Area in Western Siberia, Russia. Microorganisms 2023, 11, 425. https://doi.org/10.3390/microorganisms11020425
Kostyukova I, Pasechnik O, Mokrousov I. Epidemiology and Drug Resistance Patterns of Mycobacterium tuberculosis in High-Burden Area in Western Siberia, Russia. Microorganisms. 2023; 11(2):425. https://doi.org/10.3390/microorganisms11020425
Chicago/Turabian StyleKostyukova, Irina, Oksana Pasechnik, and Igor Mokrousov. 2023. "Epidemiology and Drug Resistance Patterns of Mycobacterium tuberculosis in High-Burden Area in Western Siberia, Russia" Microorganisms 11, no. 2: 425. https://doi.org/10.3390/microorganisms11020425
APA StyleKostyukova, I., Pasechnik, O., & Mokrousov, I. (2023). Epidemiology and Drug Resistance Patterns of Mycobacterium tuberculosis in High-Burden Area in Western Siberia, Russia. Microorganisms, 11(2), 425. https://doi.org/10.3390/microorganisms11020425






