Long-Term Manure Amendment Sustains Black Soil Biodiversity by Mitigating Acidification Induced by Chemical N Fertilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Long-Term Field Experiment and Soil Sampling
2.2. Molecular Methods
2.3. Processing of the Sequencing Data
2.4. Statistical Analysis
3. Results
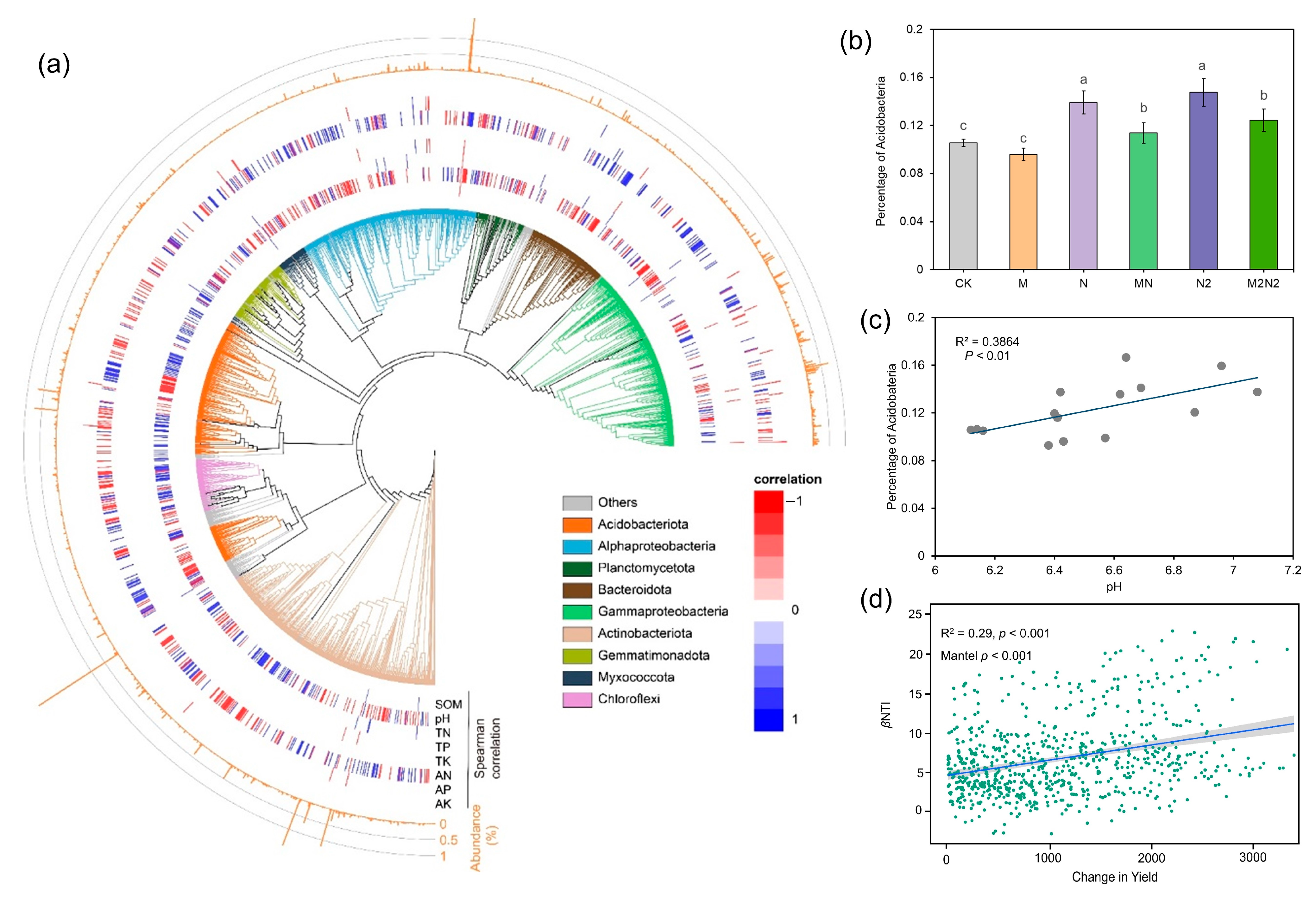
3.1. Soil Microbial Diversity under Different Fertilizations
3.2. Microbial Phylogenetic Diversity and the Inferred Ecology Process under Different Fertilizations
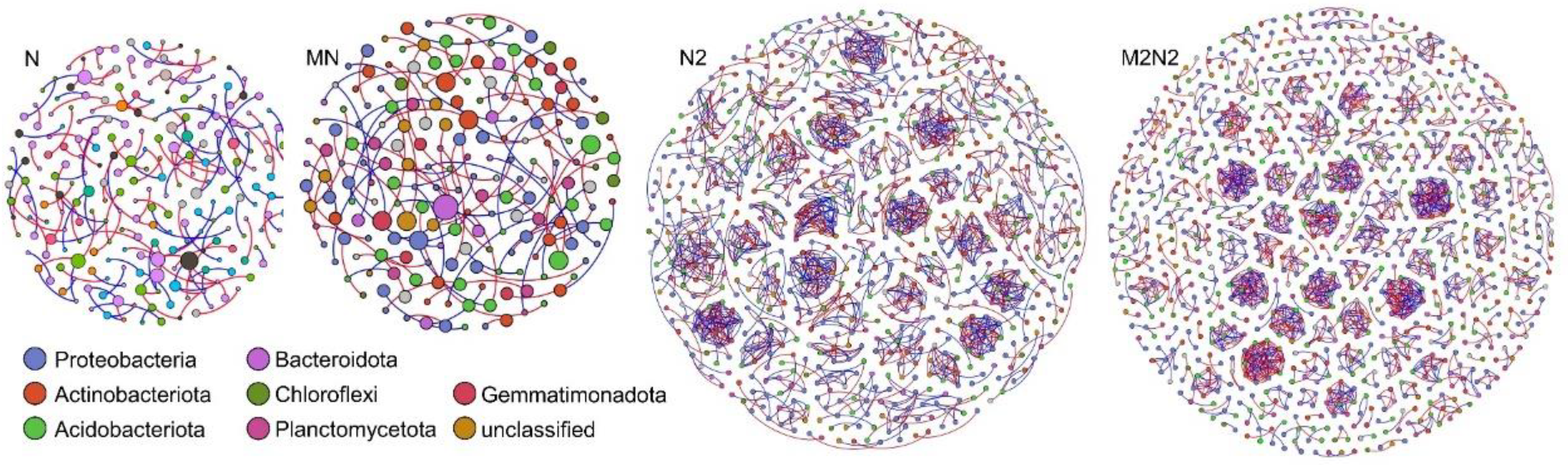
3.3. Response of the Bacterial Phylotype Co-Occurrence to Different Fertilization Regimes
3.4. The Responses of Microbial Phylotypes to Soil Properties under Different Fertilizations
4. Discussion
4.1. Organic Fertilization Mitigated the Decline of the Soil Bacterial α-Diversity Caused by Chemical N Fertilization in Black Soils
4.2. Organic Fertilization Mitigated the Role of Environmental Filtering on Microbial Community Assembly in the Black Soils
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du, E.Z.; Terrer, C.; Pellegrini, A.F.A.; Ahlstrom, A.; Van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221. [Google Scholar] [CrossRef]
- Mbuthia, L.W.; Acosta-Martinez, V.; DeBruyn, J.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walker, F.; Eash, N. Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: Implications for soil quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Morugan-Coronado, A.; Linares, C.; Gomez-Lopez, M.D.; Faz, A.; Zornoza, R. The impact of intercropping, tillage and fertilizer type on soil and crop yield in fruit orchards under Mediterranean conditions: A meta-analysis of field studies. Agric. Syst. 2020, 178, 102736. [Google Scholar] [CrossRef]
- Thilakarathna, S.K.; Hernandez-Ramirez, G.; Puurveen, D.; Kryzanowski, L.; Lohstraeter, G.; Powers, L.-A.; Quan, N.; Tenuta, M. Nitrous oxide emissions and nitrogen use efficiency in wheat: Nitrogen fertilization timing and formulation, soil nitrogen, and weather effects. Soil Sci. Soc. Am. J. 2020, 84, 1910–1927. [Google Scholar] [CrossRef]
- Qaswar, M.; Jing, H.; Ahmed, W.; Li, D.C.; Liu, S.J.; Lu, Z.; Cai, A.D.; Liu, L.S.; Xu, Y.M.; Gao, J.S.; et al. Yield sustainability, soil organic carbon sequestration and nutrients balance under long-term combined application of manure and inorganic fertilizers in acidic paddy soil. Soil Tillage Res. 2020, 198, 104569. [Google Scholar] [CrossRef]
- Qiu, C.; Bao, Y.; Petropoulos, E.; Wang, Y.; Zhong, Z.; Jiang, Y.; Ye, X.; Lin, X.; Feng, Y. Organic and inorganic amendments shape bacterial indicator communities that can, in Yurn, promote rice yield. Microorganisms 2022, 10, 482. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.H.; El-Sawah, A.M.; Ali, D.F.I.; Hamoud, Y.A.; Shaghaleh, H.; Sheteiwy, M.S. The Integration of Bio and Organic Fertilizers Improve Plant Growth, Grain Yield, Quality and Metabolism of Hybrid Maize (Zea mays L.). Agronomy 2020, 10, 319. [Google Scholar] [CrossRef]
- Luo, G.W.; Li, L.; Friman, V.P.; Guo, J.J.; Guo, S.W.; Shen, Q.R.; Ling, N. Organic amendments increase crop yields by improving microbe-mediated soil functioning of agroecosystems: A meta-analysis. Soil Biol. Biochem. 2018, 124, 105–115. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.N.; Lu, M.; Qin, C.; Chen, Y.H.; Yang, L.; Huang, Q.W.; Wang, J.C.; Shen, Z.G.; Shen, Q.R. Microbial communities of an arable soil treated for 8 years with organic and inorganic fertilizers. Biol. Fertil. Soils 2016, 52, 455–467. [Google Scholar] [CrossRef]
- Sun, R.B.; Zhang, X.-X.; Guo, X.S.; Wang, D.Z.; Chu, H.H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Yu, Y.J.; Wu, M.; Petropoulos, E.; Zhang, J.W.; Nie, J.; Liao, Y.L.; Li, Z.P.; Lin, X.G.; Feng, Y.Z. Responses of paddy soil bacterial community assembly to different long-term fertilizations in southeast China. Sci. Total Environ. 2019, 656, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Z.; Heinonsalo, J.; Zhang, Y.; Liu, G. Soil organic carbon stocks and dynamics in a mollisol region: A 1980s–2010s study. Sci. Total Environ. 2022, 807, 150910. [Google Scholar] [CrossRef] [PubMed]
- Xun, W.B.; Li, W.; Xiong, W.; Ren, Y.; Zhang, R.F. Diversity-triggered deterministic bacterial assembly constrains community functions. Nat. Commun. 2019, 10, 3833. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.Y.; Zou, H.T.; Zhang, C.F.; Zhu, B.G.; Wang, N.N.; Yang, X.H.; Gai, Z.J.; Han, Y.Y. Soil mixing with organic matter amendment improves Albic soil physicochemical properties and crop yield in Heilongjiang province, China. PLoS ONE 2020, 15, e0239788. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Jansson, J.K.; Knight, R. The Earth Microbiome project: Successes and aspirations. BMC Biol. 2014, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. The Earth Microbiome Project Consortium. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Kembel, S.W. Disentangling niche and neutral influences on community assembly: Assessing the performance of community phylogenetic structure tests. Ecol. Lett. 2009, 12, 949–960. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and Community Ecology. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef]
- Xiao, N.; Zhou, A.; Kempher, M.L.; Zhou, B.Y.; Shi, Z.J.; Yuan, M.; Guo, X.; Wu, L.; Ning, D.; Nostrand, J.V.; et al. Disentangling direct from indirect relationships in association networks. Proc. Natl. Acad. Sci. USA 2022, 119, e2109995119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Z.; Deng, Y.; Luo, F.; He, Z.L.; Tu, Q.C.; Zhi, X.Y. Functional molecular ecological networks. mBio 2011, 1, e00169-10. [Google Scholar] [CrossRef] [PubMed]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An open source software for exploring and manipulating networks. In Proceedings of the International AAAI Conference on Weblogs and Social Media, San Jose, CA, USA, 17–20 May 2009; pp. 361–362. [Google Scholar]
- Barak, P.; Jobe, B.O.; Krueger, A.R.; Peterson, L.A.; Laird, D.A. Effects of long-term soil acidification due to nitrogen fertilizer inputs in Wisconsin. Plant Soil 1997, 197, 61–69. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.N.; Zhang, Y.; Hess, F.; Huang, B.; Chen, Z. Nutrient balance and soil changes in plastic greenhouse vegetable production. Nutr. Cycl. Agroecosystems 2020, 117, 77–92. [Google Scholar] [CrossRef]
- Han, J.; Shi, J.; Zeng, L.; Xu, J.; Wu, L. Effects of nitrogen fertilization on the acidity and salinity of greenhouse soils. Environ. Sci. Pollut. Res. 2015, 22, 2976–2986. [Google Scholar] [CrossRef]
- Xie, X.; Pu, L.; Zhu, M.; Xu, Y.; Wang, X. Linkage between soil salinization indicators and physicochemical properties in a long-term intensive agricultural coastal reclamation area, Eastern China. J. Soils Sediments 2019, 19, 3699–3707. [Google Scholar] [CrossRef]
- Liu, J.L.; Wang, Q.Q.; Ku, Y.L.; Zhang, W.W.; Zhu, H.L.; Zhao, Z. Precipitation and soil pH drive the soil microbial spatial patterns in the Robinia pseudoacacia forests at the regional scale. Catena 2022, 212, 106120. [Google Scholar] [CrossRef]
- Shen, C.C.; Xiong, J.B.; Zhang, H.Y.; Feng, Y.Z.; Lin, X.G.; Li, X.Y.; Liang, W.J.; Chu, H.Y. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem. 2013, 57, 204–211. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.-J.; Banerjee, S.; Zhou, N.; Zhao, Z.-Y.; Zhang, K.; Tian, C.-Y. Soil pH is equally important as salinity in shaping bacterial communities in saline soils under halophytic vegetation. Sci. Rep. 2018, 8, 4550. [Google Scholar] [CrossRef]
- Fujii, K.; Toma, T. Comparison of soil acidification rates under different land uses in Indonesia. Plant Soil 2021, 465, 1–17. [Google Scholar] [CrossRef]
- Pang, G.; Cai, F.; Li, R.X.; Zhao, Z.; Li, R.; Gu, X.L.; Shen, Q.R.; Chen, W. Trichoderma-enriched organic fertilizer can mitigate microbiome degeneration of monocropped soil to maintain better plant growth. Plant Soil 2017, 416, 181–192. [Google Scholar] [CrossRef]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Monnig, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef]
- Kirkby, E.A.; Knight, A.H. Influence of the level of nitrate nutrition on ion uptake and assimilation, organic Acid accumulation, and cation-anion balance in whole tomato plants. Plant Physiol. 1977, 60, 349–353. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.M.; Su, W.Q.; Chen, H.H.; Barberan, A.; Zhao, H.C.; Yu, M.J.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X.; et al. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Change Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Yu, Y.; Petropoulos, E.; Cui, X.; Wang, S. Long-Term Manure Amendment Sustains Black Soil Biodiversity by Mitigating Acidification Induced by Chemical N Fertilization. Microorganisms 2023, 11, 64. https://doi.org/10.3390/microorganisms11010064
Sun L, Yu Y, Petropoulos E, Cui X, Wang S. Long-Term Manure Amendment Sustains Black Soil Biodiversity by Mitigating Acidification Induced by Chemical N Fertilization. Microorganisms. 2023; 11(1):64. https://doi.org/10.3390/microorganisms11010064
Chicago/Turabian StyleSun, Lei, Yongjie Yu, Evangelos Petropoulos, Xiaoyang Cui, and Shuang Wang. 2023. "Long-Term Manure Amendment Sustains Black Soil Biodiversity by Mitigating Acidification Induced by Chemical N Fertilization" Microorganisms 11, no. 1: 64. https://doi.org/10.3390/microorganisms11010064
APA StyleSun, L., Yu, Y., Petropoulos, E., Cui, X., & Wang, S. (2023). Long-Term Manure Amendment Sustains Black Soil Biodiversity by Mitigating Acidification Induced by Chemical N Fertilization. Microorganisms, 11(1), 64. https://doi.org/10.3390/microorganisms11010064






