Long COVID-19 Pathophysiology: What Do We Know So Far?
Abstract
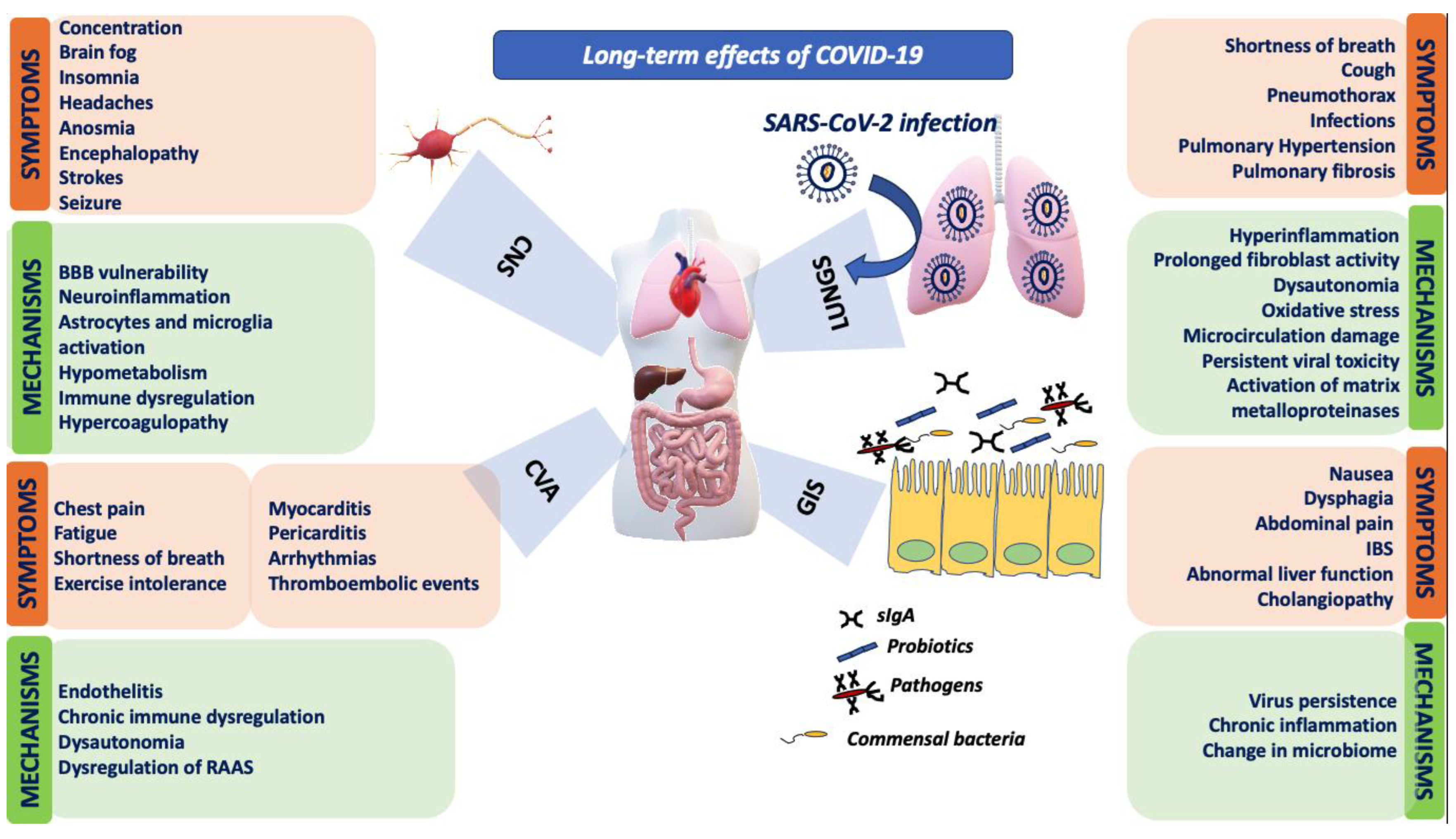
:1. Introduction
2. Cardiovascular System
3. Respiratory System
4. Central Nervous System
5. Immune System
6. Other Systems
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Robertson, M.M.; Qasmieh, S.A.; Kulkarni, S.G.; Teasdale, C.A.; Jones, H.E.; McNairy, M.; Borrell, L.N.; Nash, D. The Epidemiology of Long Coronavirus Disease in US Adults. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2023, 76, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Human Services, Office of the Assistant Secretary for Health. National Research Action Plan on Long COVID; 200 Independence Ave SW: Washington, DC, USA, 2022. [Google Scholar]
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of Post-Acute COVID-19 in Primary Care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, A.V. Long COVID-19: Challenges in the Diagnosis and Proposed Diagnostic Criteria. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major Findings, Mechanisms and Recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Cutler, D.M.; Summers, L.H. The COVID-19 Pandemic and the $16 Trillion Virus. JAMA 2020, 324, 1495. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An Overview. Diabetes Metab. Syndr. 2021, 15, 869–875. [Google Scholar] [CrossRef]
- Pretorius, E.; Venter, C.; Laubscher, G.J.; Kotze, M.J.; Oladejo, S.O.; Watson, L.R.; Rajaratnam, K.; Watson, B.W.; Kell, D.B. Prevalence of Symptoms, Comorbidities, Fibrin Amyloid Microclots and Platelet Pathology in Individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC). Cardiovasc. Diabetol. 2022, 21, 148. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Twomey, R.; DeMars, J.; Franklin, K.; Culos-Reed, S.N.; Weatherald, J.; Wrightson, J.G. Chronic Fatigue and Postexertional Malaise in People Living With Long COVID: An Observational Study. Phys. Ther. 2022, 102, pzac005. [Google Scholar] [CrossRef]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and Risk Factors for Long COVID in Non-Hospitalized Adults. Nat. Med. 2022, 28, 1706–1714. [Google Scholar] [CrossRef]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated With Post−COVID-19 Condition: A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2023, 183, 566. [Google Scholar] [CrossRef] [PubMed]
- Haunhorst, S.; Bloch, W.; Wagner, H.; Ellert, C.; Krüger, K.; Vilser, D.C.; Finke, K.; Reuken, P.; Pletz, M.W.; Stallmach, A.; et al. Long COVID: A Narrative Review of the Clinical Aftermaths of COVID-19 with a Focus on the Putative Pathophysiology and Aspects of Physical Activity. Oxf. Open Immunol. 2022, 3, iqac006. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Pavli, A.; Tsakris, A. Post-COVID Syndrome: An Insight on Its Pathogenesis. Vaccines 2021, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Lammi, V.; Nakanishi, T.; Jones, S.E.; Andrews, S.J.; Karjalainen, J.; Cortés, B.; O’Brien, H.E.; Fulton-Howard, B.E.; Haapaniemi, H.H.; Schmidt, A.; et al. Genome-Wide Association Study of Long COVID. medRxiv 2023, 2023-06. [Google Scholar] [CrossRef]
- Roca-Fernandez, A.; Wamil, M.; Telford, A.; Carapella, V.; Borlotti, A.; Monteiro, D.; Thomaides-Brears, H.; Kelly, M.; Dennis, A.; Banerjee, R.; et al. Cardiac Abnormalities in Long COVID 1-Year Post-SARS-CoV-2 Infection. Open Heart 2023, 10, e002241. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-Term Cardiovascular Outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.-Y.; Wang, S.-I.; Wei, J.C.-C. Long-Term Cardiovascular Outcomes in COVID-19 Survivors among Non-Vaccinated Population: A Retrospective Cohort Study from the TriNetX US Collaborative Networks. eClinicalMedicine 2022, 53, 101619. [Google Scholar] [CrossRef]
- Evans, P.C.; Rainger, G.E.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial Dysfunction in COVID-19: A Position Paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020, 116, 2177–2184. [Google Scholar] [CrossRef]
- Castro, P.; Palomo, M.; Moreno-Castaño, A.B.; Fernández, S.; Torramadé-Moix, S.; Pascual, G.; Martinez-Sanchez, J.; Richardson, E.; Téllez, A.; Nicolas, J.M.; et al. Is the Endothelium the Missing Link in the Pathophysiology and Treatment of COVID-19 Complications? Cardiovasc. Drugs Ther. 2022, 36, 547–560. [Google Scholar] [CrossRef]
- Lambadiari, V.; Mitrakou, A.; Kountouri, A.; Thymis, J.; Katogiannis, K.; Korakas, E.; Varlamos, C.; Andreadou, I.; Tsoumani, M.; Triantafyllidi, H.; et al. Association of COVID-19 with Impaired Endothelial Glycocalyx, Vascular Function and Myocardial Deformation 4 Months after Infection. Eur. J. Heart Fail. 2021, 23, 1916–1926. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Alvarez, K.N.; Weissfeld, L.A.; Angus, D.C.; Chirinos, J.A.; Chang, C.-C.H.; Newman, A.; Loehr, L.; Folsom, A.R.; Elkind, M.S.; et al. Association between Hospitalization for Pneumonia and Subsequent Risk of Cardiovascular Disease. JAMA 2015, 313, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.-S.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Welson, N.N. Pathophysiology of Post-COVID Syndromes: A New Perspective. Virol. J. 2022, 19, 158. [Google Scholar] [CrossRef] [PubMed]
- Szotowski, B.; Antoniak, S.; Poller, W.; Schultheiss, H.-P.; Rauch, U. Procoagulant Soluble Tissue Factor Is Released from Endothelial Cells in Response to Inflammatory Cytokines. Circ. Res. 2005, 96, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T. The Interactions between Inflammation and Coagulation. Br. J. Haematol. 2005, 131, 417–430. [Google Scholar] [CrossRef]
- Kruger, A.; Vlok, M.; Turner, S.; Venter, C.; Laubscher, G.J.; Kell, D.B.; Pretorius, E. Proteomics of Fibrin Amyloid Microclots in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) Shows Many Entrapped pro-Inflammatory Molecules That May Also Contribute to a Failed Fibrinolytic System. Cardiovasc. Diabetol. 2022, 21, 190. [Google Scholar] [CrossRef]
- Østergaard, L. SARS CoV-2 Related Microvascular Damage and Symptoms during and after COVID-19: Consequences of Capillary Transit-time Changes, Tissue Hypoxia and Inflammation. Physiol. Rep. 2021, 9, e14726. [Google Scholar] [CrossRef]
- Fogarty, H.; Townsend, L.; Morrin, H.; Ahmad, A.; Comerford, C.; Karampini, E.; Englert, H.; Byrne, M.; Bergin, C.; O’Sullivan, J.M.; et al. Persistent Endotheliopathy in the Pathogenesis of Long COVID Syndrome. J. Thromb. Haemost. JTH 2021, 19, 2546–2553. [Google Scholar] [CrossRef]
- Chioh, F.W.; Fong, S.-W.; Young, B.E.; Wu, K.-X.; Siau, A.; Krishnan, S.; Chan, Y.-H.; Carissimo, G.; Teo, L.L.; Gao, F.; et al. Convalescent COVID-19 Patients Are Susceptible to Endothelial Dysfunction Due to Persistent Immune Activation. eLife 2021, 10, e64909. [Google Scholar] [CrossRef]
- Vassiliou, A.G.; Vrettou, C.S.; Keskinidou, C.; Dimopoulou, I.; Kotanidou, A.; Orfanos, S.E. Endotheliopathy in Acute COVID-19 and Long COVID. Int. J. Mol. Sci. 2023, 24, 8237. [Google Scholar] [CrossRef]
- Ahamed, J.; Laurence, J. Long COVID Endotheliopathy: Hypothesized Mechanisms and Potential Therapeutic Approaches. J. Clin. Investig. 2022, 132, e161167. [Google Scholar] [CrossRef]
- Cooper, S.L.; Boyle, E.; Jefferson, S.R.; Heslop, C.R.A.; Mohan, P.; Mohanraj, G.G.J.; Sidow, H.A.; Tan, R.C.P.; Hill, S.J.; Woolard, J. Role of the Renin-Angiotensin-Aldosterone and Kinin-Kallikrein Systems in the Cardiovascular Complications of COVID-19 and Long COVID. Int. J. Mol. Sci. 2021, 22, 8255. [Google Scholar] [CrossRef] [PubMed]
- Gyöngyösi, M.; Alcaide, P.; Asselbergs, F.W.; Brundel, B.J.J.M.; Camici, G.G.; Martins, P.D.C.; Ferdinandy, P.; Fontana, M.; Girao, H.; Gnecchi, M.; et al. Long COVID and the Cardiovascular System—Elucidating Causes and Cellular Mechanisms in Order to Develop Targeted Diagnostic and Therapeutic Strategies: A Joint Scientific Statement of the ESC Working Groups on Cellular Biology of the Heart and Myocardial and Pericardial Diseases. Cardiovasc. Res. 2023, 119, 336–356. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Deitchman, A.N.; Torres, L.; Iyer, N.S.; Munter, S.E.; Nixon, C.C.; Donatelli, J.; Thanh, C.; Takahashi, S.; Hakim, J.; et al. Long-Term SARS-CoV-2-Specific Immune and Inflammatory Responses in Individuals Recovering from COVID-19 with and without Post-Acute Symptoms. Cell Rep. 2021, 36, 109518. [Google Scholar] [CrossRef] [PubMed]
- Schultheiß, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Henkes, S.-S.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; et al. The IL-1β, IL-6, and TNF Cytokine Triad Is Associated with Post-Acute Sequelae of COVID-19. Cell Rep. Med. 2022, 3, 100663. [Google Scholar] [CrossRef] [PubMed]
- Low, R.N.; Low, R.J.; Akrami, A. A Cytokine-Based Model for the Pathophysiology of Long COVID Symptoms; Open Science Framework: Charlottesville, VA, USA, 2020. [Google Scholar]
- Wallukat, G.; Hohberger, B.; Wenzel, K.; Fürst, J.; Schulze-Rothe, S.; Wallukat, A.; Hönicke, A.-S.; Müller, J. Functional Autoantibodies against G-Protein Coupled Receptors in Patients with Persistent Long-COVID-19 Symptoms. J. Transl. Autoimmun. 2021, 4, 100100. [Google Scholar] [CrossRef] [PubMed]
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Lim, P.B. Autonomic Dysfunction in “Long COVID”: Rationale, Physiology and Management Strategies. Clin. Med. Lond. Engl. 2021, 21, e63–e67. [Google Scholar] [CrossRef]
- Abrams, R.M.C.; Simpson, D.M.; Navis, A.; Jette, N.; Zhou, L.; Shin, S.C. Small Fiber Neuropathy Associated with SARS-CoV-2 Infection. Muscle Nerve 2022, 65, 440–443. [Google Scholar] [CrossRef]
- Vernino, S.; Bourne, K.M.; Stiles, L.E.; Grubb, B.P.; Fedorowski, A.; Stewart, J.M.; Arnold, A.C.; Pace, L.A.; Axelsson, J.; Boris, J.R.; et al. Postural Orthostatic Tachycardia Syndrome (POTS): State of the Science and Clinical Care from a 2019 National Institutes of Health Expert Consensus Meeting—Part 1. Auton. Neurosci. 2021, 235, 102828. [Google Scholar] [CrossRef]
- Perlis, R.H.; Santillana, M.; Ognyanova, K.; Safarpour, A.; Lunz Trujillo, K.; Simonson, M.D.; Green, J.; Quintana, A.; Druckman, J.; Baum, M.A.; et al. Prevalence and Correlates of Long COVID Symptoms among US Adults. JAMA Netw. Open 2022, 5, e2238804. [Google Scholar] [CrossRef]
- Jacobs, L.G.; Gourna Paleoudis, E.; Lesky-Di Bari, D.; Nyirenda, T.; Friedman, T.; Gupta, A.; Rasouli, L.; Zetkulic, M.; Balani, B.; Ogedegbe, C.; et al. Persistence of Symptoms and Quality of Life at 35 Days after Hospitalization for COVID-19 Infection. PLoS ONE 2020, 15, e0243882. [Google Scholar] [CrossRef]
- Xiong, Q.; Xu, M.; Li, J.; Liu, Y.; Zhang, J.; Xu, Y.; Dong, W. Clinical Sequelae of COVID-19 Survivors in Wuhan, China: A Single-Centre Longitudinal Study. Clin. Microbiol. Infect. 2021, 27, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Cortinovis, M.; Perico, N.; Remuzzi, G. Long-Term Follow-up of Recovered Patients with COVID-19. Lancet 2021, 397, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shang, Y.; Song, W.; Li, Q.; Xie, H.; Xu, Q.; Jia, J.; Li, L.; Mao, H.; Zhou, X.; et al. Follow-up Study of the Pulmonary Function and Related Physiological Characteristics of COVID-19 Survivors Three Months after Recovery. EClinicalMedicine 2020, 25, 100463. [Google Scholar] [CrossRef]
- Huang, W.; Wu, Q.; Chen, Z.; Xiong, Z.; Wang, K.; Tian, J.; Zhang, S. The Potential Indicators for Pulmonary Fibrosis in Survivors of Severe COVID-19. J. Infect. 2021, 82, e5–e7. [Google Scholar] [CrossRef]
- Ojo, A.S.; Balogun, S.A.; Williams, O.T.; Ojo, O.S. Pulmonary Fibrosis in COVID-19 Survivors: Predictive Factors and Risk Reduction Strategies. Pulm. Med. 2020, 2020, 6175964. [Google Scholar] [CrossRef] [PubMed]
- Tanni, S.E.; Fabro, A.T.; De Albuquerque, A.; Ferreira, E.V.M.; Verrastro, C.G.Y.; Sawamura, M.V.Y.; Ribeiro, S.M.; Baldi, B.G. Pulmonary Fibrosis Secondary to COVID-19: A Narrative Review. Expert Rev. Respir. Med. 2021, 15, 791–803. [Google Scholar] [CrossRef]
- Guizani, I.; Fourti, N.; Zidi, W.; Feki, M.; Allal-Elasmi, M. SARS-CoV-2 and Pathological Matrix Remodeling Mediators. Inflamm. Res. 2021, 70, 847–858. [Google Scholar] [CrossRef]
- Yong, S.J. Persistent Brainstem Dysfunction in Long-COVID: A Hypothesis. ACS Chem. Neurosci. 2021, 12, 573–580. [Google Scholar] [CrossRef]
- Dhawan, R.T.; Gopalan, D.; Howard, L.; Vicente, A.; Park, M.; Manalan, K.; Wallner, I.; Marsden, P.; Dave, S.; Branley, H.; et al. Beyond the Clot: Perfusion Imaging of the Pulmonary Vasculature after COVID-19. Lancet Respir. Med. 2021, 9, 107–116. [Google Scholar] [CrossRef]
- Ooi, M.W.X.; Rajai, A.; Patel, R.; Gerova, N.; Godhamgaonkar, V.; Liong, S.Y. Pulmonary Thromboembolic Disease in COVID-19 Patients on CT Pulmonary Angiography—Prevalence, Pattern of Disease and Relationship to D-Dimer. Eur. J. Radiol. 2020, 132, 109336. [Google Scholar] [CrossRef]
- Van Kruijsdijk, R.C.; De Jong, P.A.; Abrahams, A.C. Pulmonary Vein Thrombosis in COVID-19. BMJ Case Rep. 2020, 13, e239986. [Google Scholar] [CrossRef]
- Silva Andrade, B.; Siqueira, S.; de Assis Soares, W.R.; de Souza Rangel, F.; Santos, N.O.; Dos Santos Freitas, A.; Ribeiro da Silveira, P.; Tiwari, S.; Alzahrani, K.J.; Góes-Neto, A.; et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses 2021, 13, 700. [Google Scholar] [CrossRef] [PubMed]
- Astin, R.; Banerjee, A.; Baker, M.R.; Dani, M.; Ford, E.; Hull, J.H.; Lim, P.B.; McNarry, M.; Morten, K.; O’Sullivan, O.; et al. Long COVID: Mechanisms, Risk Factors and Recovery. Exp. Physiol. 2023, 108, 12–27. [Google Scholar] [CrossRef]
- Desimmie, B.A.; Raru, Y.Y.; Awadh, H.M.; He, P.; Teka, S.; Willenburg, K.S. Insights into SARS-CoV-2 Persistence and Its Relevance. Viruses 2021, 13, 1025. [Google Scholar] [CrossRef] [PubMed]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-Acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Rebman, A.W.; Aucott, J.N. Post-Treatment Lyme Disease as a Model for Persistent Symptoms in Lyme Disease. Front. Med. 2020, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple Early Factors Anticipate Post-Acute COVID-19 Sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, J.; Chen, L.; Fu, C.; Kang, Y.; Zhang, W.; Fakhri, G.E.; Gu, J.; Shao, F.; Wang, M. Inflammatory Response in Lungs and Extrapulmonary Sites Detected by [18F] Fluorodeoxyglucose PET/CT in Convalescing COVID-19 Patients Tested Negative for Coronavirus. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2531–2542. [Google Scholar] [CrossRef]
- Song, W.-J.; Hui, C.K.M.; Hull, J.H.; Birring, S.S.; McGarvey, L.; Mazzone, S.B.; Chung, K.F. Confronting COVID-19-Associated Cough and the Post-COVID Syndrome: Role of Viral Neurotropism, Neuroinflammation, and Neuroimmune Responses. Lancet Respir. Med. 2021, 9, 533–544. [Google Scholar] [CrossRef]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of Post-Acute COVID-19 Syndrome Symptoms at Different Follow-up Periods: A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2022, 28, 657–666. [Google Scholar] [CrossRef]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.-M. Mid and Long-Term Neurological and Neuropsychiatric Manifestations of Post-COVID-19 Syndrome: A Meta-Analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef] [PubMed]
- García-Moncó, J.C.; Cabrera-Muras, A.; Collía-Fernández, A.; Erburu-Iriarte, M.; Rodrigo-Armenteros, P.; Oyarzun-Irazu, I.; Martínez-Condor, D.; Bilbao-González, A.; Carmona-Abellán, M.; Caballero-Romero, I.; et al. Neurological Reasons for Consultation and Hospitalization during the COVID-19 Pandemic. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2020, 41, 3031–3038. [Google Scholar] [CrossRef] [PubMed]
- Stefanou, M.-I.; Palaiodimou, L.; Bakola, E.; Smyrnis, N.; Papadopoulou, M.; Paraskevas, G.P.; Rizos, E.; Boutati, E.; Grigoriadis, N.; Krogias, C.; et al. Neurological Manifestations of Long-COVID Syndrome: A Narrative Review. Ther. Adv. Chronic Dis. 2022, 13, 20406223221076890. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, Co-Occurrence, and Evolution of Long-COVID Features: A 6-Month Retrospective Cohort Study of 273,618 Survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent Neurologic Symptoms and Cognitive Dysfunction in Non-Hospitalized COVID-19 “Long Haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef]
- Staffolani, S.; Iencinella, V.; Cimatti, M.; Tavio, M. Long COVID-19 Syndrome as a Fourth Phase of SARS-CoV-2 Infection. Infez. Med. 2022, 30, 22–29. [Google Scholar] [CrossRef]
- Parotto, M.; Myatra, S.N.; Munblit, D.; Elhazmi, A.; Ranzani, O.T.; Herridge, M.S. Recovery after Prolonged ICU Treatment in Patients with COVID-19. Lancet Respir. Med. 2021, 9, 812–814. [Google Scholar] [CrossRef]
- Whitaker, M.; Elliott, J.; Chadeau-Hyam, M.; Riley, S.; Darzi, A.; Cooke, G.; Ward, H.; Elliott, P. Persistent Symptoms Following SARS-CoV-2 Infection in a Random Community Sample of 508,707 People. medRxiv 2021. [Google Scholar] [CrossRef]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory Transmucosal SARS-CoV-2 Invasion as a Port of Central Nervous System Entry in Individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Boldrini, M.; Canoll, P.D.; Klein, R.S. How COVID-19 Affects the Brain. JAMA Psychiatry 2021, 78, 682–683. [Google Scholar] [CrossRef]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.-Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 Infection and Persistence in the Human Body and Brain at Autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.L.; Bain, W.; Naqvi, A.; Staines, B.; Castanha, P.M.S.; Yang, H.; Boltz, V.F.; Barratt-Boyes, S.; Marques, E.T.A.; Mitchell, S.L.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 Viremia Is Associated With Coronavirus Disease 2019 Severity and Predicts Clinical Outcomes. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 74, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Deffner, F.; Scharr, M.; Klingenstein, S.; Klingenstein, M.; Milazzo, A.; Scherer, S.; Wagner, A.; Hirt, B.; Mack, A.F.; Neckel, P.H. Histological Evidence for the Enteric Nervous System and the Choroid Plexus as Alternative Routes of Neuroinvasion by SARS-CoV-2. Front. Neuroanat. 2020, 14, 596439. [Google Scholar] [CrossRef]
- Yang, A.C.; Kern, F.; Losada, P.M.; Agam, M.R.; Maat, C.A.; Schmartz, G.P.; Fehlmann, T.; Stein, J.A.; Schaum, N.; Lee, D.P.; et al. Dysregulation of Brain and Choroid Plexus Cell Types in Severe COVID-19. Nature 2021, 595, 565–571. [Google Scholar] [CrossRef] [PubMed]
- DeMarino, C.; Lee, M.-H.; Cowen, M.; Steiner, J.P.; Inati, S.; Shah, A.H.; Zaghloul, K.A.; Nath, A. Detection of SARS-CoV-2 Nucleocapsid and Microvascular Disease in the Brain: A Case Report. Neurology 2023, 100, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K.; Chan, D.; Watermeyer, T. The Cognitive Consequences of the COVID-19 Epidemic: Collateral Damage? Brain Commun. 2020, 2, fcaa069. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Geng, D.; Mei, N.; Wu, P.-Y.; Huang, C.-C.; Jia, T.; Zhao, Y.; Wang, D.; Xiao, A.; et al. Cerebral Micro-Structural Changes in COVID-19 Patients—An MRI-Based 3-Month Follow-up Study. EClinicalMedicine 2020, 25, 100484. [Google Scholar] [CrossRef]
- Guedj, E.; Campion, J.Y.; Dudouet, P.; Kaphan, E.; Bregeon, F.; Tissot-Dupont, H.; Guis, S.; Barthelemy, F.; Habert, P.; Ceccaldi, M.; et al. 18F-FDG Brain PET Hypometabolism in Patients with Long COVID. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2823–2833. [Google Scholar] [CrossRef]
- Kas, A.; Soret, M.; Pyatigoskaya, N.; Habert, M.-O.; Hesters, A.; Le Guennec, L.; Paccoud, O.; Bombois, S.; Delorme, C.; on the behalf of CoCo-Neurosciences Study Group and COVID SMIT PSL Study Group; et al. The Cerebral Network of COVID-19-Related Encephalopathy: A Longitudinal Voxel-Based 18F-FDG-PET Study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2543–2557. [Google Scholar] [CrossRef]
- Stefano, G.B.; Büttiker, P.; Weissenberger, S.; Martin, A.; Ptacek, R.; Kream, R.M. Editorial: The Pathogenesis of Long-Term Neuropsychiatric COVID-19 and the Role of Microglia, Mitochondria, and Persistent Neuroinflammation: A Hypothesis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e933015. [Google Scholar] [CrossRef]
- Ortelli, P.; Ferrazzoli, D.; Sebastianelli, L.; Engl, M.; Romanello, R.; Nardone, R.; Bonini, I.; Koch, G.; Saltuari, L.; Quartarone, A.; et al. Neuropsychological and Neurophysiological Correlates of Fatigue in Post-Acute Patients with Neurological Manifestations of COVID-19: Insights into a Challenging Symptom. J. Neurol. Sci. 2021, 420, 117271. [Google Scholar] [CrossRef] [PubMed]
- Trougakos, I.P.; Stamatelopoulos, K.; Terpos, E.; Tsitsilonis, O.E.; Aivalioti, E.; Paraskevis, D.; Kastritis, E.; Pavlakis, G.N.; Dimopoulos, M.A. Insights to SARS-CoV-2 Life Cycle, Pathophysiology, and Rationalized Treatments That Target COVID-19 Clinical Complications. J. Biomed. Sci. 2021, 28, 9. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Oscos, F.; Salgado, H.; Hall, S.; Thomas, F.; Farmer, G.E.; Bermeo, J.; Galindo, L.C.; Ramirez, R.D.; D’Mello, S.; Rose-John, S.; et al. The Stress-Induced Cytokine Interleukin-6 Decreases the Inhibition/Excitation Ratio in the Rat Temporal Cortex via Trans-Signaling. Biol. Psychiatry 2012, 71, 574–582. [Google Scholar] [CrossRef]
- Alpert, O.; Begun, L.; Garren, P.; Solhkhah, R. Cytokine Storm Induced New Onset Depression in Patients with COVID-19. A New Look into the Association between Depression and Cytokines-Two Case Reports. Brain Behav. Immun.—Health 2020, 9, 100173. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Tang, N.; Peluso, M.J.; Iyer, N.S.; Torres, L.; Donatelli, J.L.; Munter, S.E.; Nixon, C.C.; Rutishauser, R.L.; Rodriguez-Barraquer, I.; et al. Characterization and Biomarker Analyses of Post-COVID-19 Complications and Neurological Manifestations. Cells 2021, 10, 386. [Google Scholar] [CrossRef]
- Koumpa, F.S.; Forde, C.T.; Manjaly, J.G. Sudden Irreversible Hearing Loss Post COVID-19. BMJ Case Rep. 2020, 13, e238419. [Google Scholar] [CrossRef]
- Dalakas, M.C. Guillain-Barré Syndrome: The First Documented COVID-19–Triggered Autoimmune Neurologic Disease: More to Come with Myositis in the Offing. Neurol.—Neuroimmunol. Neuroinflamm. 2020, 7, e781. [Google Scholar] [CrossRef]
- Bowles, L.; Platton, S.; Yartey, N.; Dave, M.; Lee, K.; Hart, D.P.; MacDonald, V.; Green, L.; Sivapalaratnam, S.; Pasi, K.J.; et al. Lupus Anticoagulant and Abnormal Coagulation Tests in Patients with COVID-19. N. Engl. J. Med. 2020, 383, 288–290. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, W.; Jiang, W.; Xiao, M.; Li, Y.; Tang, N.; Liu, Z.; Yan, X.; Zhao, Y.; Li, T.; et al. Profile of Natural Anticoagulant, Coagulant Factor and Anti-Phospholipid Antibody in Critically Ill COVID-19 Patients. J. Thromb. Thrombolysis 2020, 50, 580–586. [Google Scholar] [CrossRef]
- Lee, M.-H.; Perl, D.P.; Nair, G.; Li, W.; Maric, D.; Murray, H.; Dodd, S.J.; Koretsky, A.P.; Watts, J.A.; Cheung, V.; et al. Microvascular Injury in the Brains of Patients with COVID-19. N. Engl. J. Med. 2021, 384, 481–483. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.; Sandoval Valencia, H.; Barbella-Aponte, R.A.; Collado-Jiménez, R.; Ayo-Martín, Ó.; Barrena, C.; Molina-Nuevo, J.D.; García-García, J.; Lozano-Setién, E.; Alcahut-Rodriguez, C.; et al. Cerebrovascular Disease in Patients with COVID-19: Neuroimaging, Histological and Clinical Description. Brain J. Neurol. 2020, 143, 3089–3103. [Google Scholar] [CrossRef] [PubMed]
- Gulko, E.; Oleksk, M.L.; Gomes, W.; Ali, S.; Mehta, H.; Overby, P.; Al-Mufti, F.; Rozenshtein, A. MRI Brain Findings in 126 Patients with COVID-19: Initial Observations from a Descriptive Literature Review. AJNR Am. J. Neuroradiol. 2020, 41, 2199–2203. [Google Scholar] [CrossRef] [PubMed]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.-H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-Associated Coagulopathy: Evidence from a Single-Centre, Cross-Sectional Study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef] [PubMed]
- Chopra, V.; Flanders, S.A.; O’Malley, M.; Malani, A.N.; Prescott, H.C. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann. Intern. Med. 2021, 174, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.T.; Hamilton, F.W.; Milne, A.; Morley, A.J.; Viner, J.; Attwood, M.; Noel, A.; Gunning, S.; Hatrick, J.; Hamilton, S.; et al. Patient Outcomes after Hospitalisation with COVID-19 and Implications for Follow-up: Results from a Prospective UK Cohort. Thorax 2021, 76, 399–401. [Google Scholar] [CrossRef]
- Garrigues, E.; Janvier, P.; Kherabi, Y.; Le Bot, A.; Hamon, A.; Gouze, H.; Doucet, L.; Berkani, S.; Oliosi, E.; Mallart, E.; et al. Post-Discharge Persistent Symptoms and Health-Related Quality of Life after Hospitalization for COVID-19. J. Infect. 2020, 81, e4–e6. [Google Scholar] [CrossRef]
- Heneka, M.T.; Golenbock, D.; Latz, E.; Morgan, D.; Brown, R. Immediate and Long-Term Consequences of COVID-19 Infections for the Development of Neurological Disease. Alzheimers Res. Ther. 2020, 12, 69. [Google Scholar] [CrossRef]
- Kaseda, E.T.; Levine, A.J. Post-Traumatic Stress Disorder: A Differential Diagnostic Consideration for COVID-19 Survivors. Clin. Neuropsychol. 2020, 34, 1498–1514. [Google Scholar] [CrossRef]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and Depression in COVID-19 Survivors: Role of Inflammatory and Clinical Predictors. Brain. Behav. Immun. 2020, 89, 594–600. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and Neuropsychiatric Presentations Associated with Severe Coronavirus Infections: A Systematic Review and Meta-Analysis with Comparison to the COVID-19 Pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Romero-Sánchez, C.M.; Díaz-Maroto, I.; Fernández-Díaz, E.; Sánchez-Larsen, Á.; Layos-Romero, A.; García-García, J.; González, E.; Redondo-Peñas, I.; Perona-Moratalla, A.B.; Del Valle-Pérez, J.A.; et al. Neurologic Manifestations in Hospitalized Patients with COVID-19: The ALBACOVID Registry. Neurology 2020, 95, e1060–e1070. [Google Scholar] [CrossRef] [PubMed]
- Reichard, R.R.; Kashani, K.B.; Boire, N.A.; Constantopoulos, E.; Guo, Y.; Lucchinetti, C.F. Neuropathology of COVID-19: A Spectrum of Vascular and Acute Disseminated Encephalomyelitis (ADEM)-like Pathology. Acta Neuropathol. 2020, 140, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Halaris, A. Inflammation and Depression but Where Does the Inflammation Come From? Curr. Opin. Psychiatry 2019, 32, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Enache, D.; Pariante, C.M.; Mondelli, V. Markers of Central Inflammation in Major Depressive Disorder: A Systematic Review and Meta-Analysis of Studies Examining Cerebrospinal Fluid, Positron Emission Tomography and Post-Mortem Brain Tissue. Brain. Behav. Immun. 2019, 81, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Wostyn, P. COVID-19 and Chronic Fatigue Syndrome: Is the Worst yet to Come? Med. Hypotheses 2021, 146, 110469. [Google Scholar] [CrossRef]
- Ameres, M.; Brandstetter, S.; Toncheva, A.A.; Kabesch, M.; Leppert, D.; Kuhle, J.; Wellmann, S. Association of Neuronal Injury Blood Marker Neurofilament Light Chain with Mild-to-Moderate COVID-19. J. Neurol. 2020, 267, 3476–3478. [Google Scholar] [CrossRef]
- Dotan, A.; Muller, S.; Kanduc, D.; David, P.; Halpert, G.; Shoenfeld, Y. The SARS-CoV-2 as an Instrumental Trigger of Autoimmunity. Autoimmun. Rev. 2021, 20, 102792. [Google Scholar] [CrossRef]
- Arthur, J.M.; Forrest, J.C.; Boehme, K.W.; Kennedy, J.L.; Owens, S.; Herzog, C.; Liu, J.; Harville, T.O. Development of ACE2 Autoantibodies after SARS-CoV-2 Infection. PLoS ONE 2021, 16, e0257016. [Google Scholar] [CrossRef]
- Verano-Braga, T.; Martins, A.L.V.; Motta-Santos, D.; Campagnole-Santos, M.J.; Santos, R.A.S. ACE2 in the Renin–Angiotensin System. Clin. Sci. 2020, 134, 3063–3078. [Google Scholar] [CrossRef]
- Koning, R.; Bastard, P.; Casanova, J.-L.; Brouwer, M.C.; Van De Beek, D.; with the Amsterdam U.M.C. COVID-19 Biobank Investigators; Van Agtmael, M.; Algera, A.G.; Appelman, B.; Van Baarle, F.; et al. Autoantibodies against Type I Interferons Are Associated with Multi-Organ Failure in COVID-19 Patients. Intensive Care Med. 2021, 47, 704–706. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Banerjee, M. Immune Thrombocytopenia Secondary to COVID-19: A Systematic Review. SN Compr. Clin. Med. 2020, 2, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Son, K.; Jamil, R.; Chowdhury, A.; Mukherjee, M.; Venegas, C.; Miyasaki, K.; Zhang, K.; Patel, Z.; Salter, B.; Yuen, A.C.Y.; et al. Circulating Anti-Nuclear Autoantibodies in COVID-19 Survivors Predict Long-COVID Symptoms. Eur. Respir. J. 2022, 61, 2200970. [Google Scholar] [CrossRef] [PubMed]
- Caress, J.B.; Castoro, R.J.; Simmons, Z.; Scelsa, S.N.; Lewis, R.A.; Ahlawat, A.; Narayanaswami, P. COVID-19–associated Guillain-Barré Syndrome: The Early Pandemic Experience. Muscle Nerve 2020, 62, 485–491. [Google Scholar] [CrossRef]
- Cervia, C.; Zurbuchen, Y.; Taeschler, P.; Ballouz, T.; Menges, D.; Hasler, S.; Adamo, S.; Raeber, M.E.; Bächli, E.; Rudiger, A.; et al. Immunoglobulin Signature Predicts Risk of Post-Acute COVID-19 Syndrome. Nat. Commun. 2022, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, L.B.; Brook, J.B.; Walters, A.S.; Goris, A.; Afrin, L.B.; Molderings, G.J. Mast Cell Activation Symptoms Are Prevalent in Long-COVID. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2021, 112, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, J.B.; Butuci, M.; Wong, A.; Kamboj, A.P.; Youngblood, B.A. Mast Cell Activation Is Associated with Post-Acute COVID-19 Syndrome. Allergy 2022, 77, 1288–1291. [Google Scholar] [CrossRef]
- Richter, A.G.; Shields, A.M.; Karim, A.; Birch, D.; Faustini, S.E.; Steadman, L.; Ward, K.; Plant, T.; Reynolds, G.; Veenith, T.; et al. Establishing the Prevalence of Common Tissue-Specific Autoantibodies Following Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Clin. Exp. Immunol. 2021, 205, 99–105. [Google Scholar] [CrossRef]
- Hohberger, B.; Harrer, T.; Mardin, C.; Kruse, F.; Hoffmanns, J.; Rogge, L.; Heltmann, F.; Moritz, M.; Szewczykowski, C.; Schottenhamml, J.; et al. Case Report: Neutralization of Autoantibodies Targeting G-Protein-Coupled Receptors Improves Capillary Impairment and Fatigue Symptoms After COVID-19 Infection. Front. Med. 2021, 8, 754667. [Google Scholar] [CrossRef]
- Sapkota, H.R.; Nune, A. Long COVID from Rheumatology Perspective—A Narrative Review. Clin. Rheumatol. 2022, 41, 337–348. [Google Scholar] [CrossRef]
- Gavrilova, N.; Soprun, L.; Lukashenko, M.; Ryabkova, V.; Fedotkina, T.V.; Churilov, L.P.; Shoenfeld, Y. New Clinical Phenotype of the Post-Covid Syndrome: Fibromyalgia and Joint Hypermobility Condition. Pathophysiology 2022, 29, 24–29. [Google Scholar] [CrossRef]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological Dysfunction Persists for 8 Months Following Initial Mild-to-Moderate SARS-CoV-2 Infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Glynne, P.; Tahmasebi, N.; Gant, V.; Gupta, R. Long COVID Following Mild SARS-CoV-2 Infection: Characteristic T Cell Alterations and Response to Antihistamines. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2022, 70, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Schietinger, A.; Greenberg, P.D. Tolerance and Exhaustion: Defining Mechanisms of T Cell Dysfunction. Trends Immunol. 2014, 35, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J.; Kurachi, M. Molecular and Cellular Insights into T Cell Exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Saheb Sharif-Askari, N.; Saheb Sharif-Askari, F.; Mdkhana, B.; Al Heialy, S.; Alsafar, H.S.; Hamoudi, R.; Hamid, Q.; Halwani, R. Enhanced Expression of Immune Checkpoint Receptors during SARS-CoV-2 Viral Infection. Mol. Ther.—Methods Clin. Dev. 2021, 20, 109–121. [Google Scholar] [CrossRef]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional Exhaustion of Antiviral Lymphocytes in COVID-19 Patients. Cell. Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef]
- Zheng, H.-Y.; Zhang, M.; Yang, C.-X.; Zhang, N.; Wang, X.-C.; Yang, X.-P.; Dong, X.-Q.; Zheng, Y.-T. Elevated Exhaustion Levels and Reduced Functional Diversity of T Cells in Peripheral Blood May Predict Severe Progression in COVID-19 Patients. Cell. Mol. Immunol. 2020, 17, 541–543. [Google Scholar] [CrossRef]
- Raman, B.; Cassar, M.P.; Tunnicliffe, E.M.; Filippini, N.; Griffanti, L.; Alfaro-Almagro, F.; Okell, T.; Sheerin, F.; Xie, C.; Mahmod, M.; et al. Medium-Term Effects of SARS-CoV-2 Infection on Multiple Vital Organs, Exercise Capacity, Cognition, Quality of Life and Mental Health, Post-Hospital Discharge. EClinicalMedicine 2021, 31, 100683. [Google Scholar] [CrossRef]
- Gabarre, P.; Dumas, G.; Dupont, T.; Darmon, M.; Azoulay, E.; Zafrani, L. Acute Kidney Injury in Critically Ill Patients with COVID-19. Intensive Care Med. 2020, 46, 1339–1348. [Google Scholar] [CrossRef]
- Lim, M.A.; Pranata, R.; Huang, I.; Yonas, E.; Soeroto, A.Y.; Supriyadi, R. Multiorgan Failure with Emphasis on Acute Kidney Injury and Severity of COVID-19: Systematic Review and Meta-Analysis. Can. J. Kidney Health Dis. 2020, 7, 205435812093857. [Google Scholar] [CrossRef]
- Wu, H.; Larsen, C.P.; Hernandez-Arroyo, C.F.; Mohamed, M.M.B.; Caza, T.; Sharshir, M.; Chughtai, A.; Xie, L.; Gimenez, J.M.; Sandow, T.A.; et al. AKI and Collapsing Glomerulopathy Associated with COVID-19 and APOL 1 High-Risk Genotype. J. Am. Soc. Nephrol. 2020, 31, 1688–1695. [Google Scholar] [CrossRef]
- Gaillard, F.; Ismael, S.; Sannier, A.; Tarhini, H.; Volpe, T.; Greze, C.; Verpont, M.C.; Zouhry, I.; Rioux, C.; Lescure, F.-X.; et al. Tubuloreticular Inclusions in COVID-19–Related Collapsing Glomerulopathy. Kidney Int. 2020, 98, 241. [Google Scholar] [CrossRef]
- Velez, J.C.Q.; Caza, T.; Larsen, C.P. COVAN Is the New HIVAN: The Re-Emergence of Collapsing Glomerulopathy with COVID-19. Nat. Rev. Nephrol. 2020, 16, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Xu, D.; Zhang, H.; Zhou, W.; Wang, L.; Cui, X. Identification of a Potential Mechanism of Acute Kidney Injury during the COVID-19 Outbreak: A Study Based on Single-Cell Transcriptome Analysis. Intensive Care Med. 2020, 46, 1114–1116. [Google Scholar] [CrossRef] [PubMed]
- Montefusco, L.; Ben Nasr, M.; D’Addio, F.; Loretelli, C.; Rossi, A.; Pastore, I.; Daniele, G.; Abdelsalam, A.; Maestroni, A.; Dell’Acqua, M.; et al. Acute and Long-Term Disruption of Glycometabolic Control after SARS-CoV-2 Infection. Nat. Metab. 2021, 3, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Apicella, M.; Campopiano, M.C.; Mantuano, M.; Mazoni, L.; Coppelli, A.; Del Prato, S. COVID-19 in People with Diabetes: Understanding the Reasons for Worse Outcomes. Lancet Diabetes Endocrinol. 2020, 8, 782–792. [Google Scholar] [CrossRef]
- Morieri, M.L.; Fadini, G.P.; Boscari, F.; Fioretto, P.; Maran, A.; Busetto, L.; Crepaldi, M.C.; Vedovato, M.; Bonora, B.M.; Selmin, E.; et al. Hyperglycemia, Glucocorticoid Therapy, and Outcome of COVID-19. Diabetes Res. Clin. Pract. 2020, 168, 108449. [Google Scholar] [CrossRef]
- Müller, J.A.; Groß, R.; Conzelmann, C.; Krüger, J.; Merle, U.; Steinhart, J.; Weil, T.; Koepke, L.; Bozzo, C.P.; Read, C.; et al. SARS-CoV-2 Infects and Replicates in Cells of the Human Endocrine and Exocrine Pancreas. Nat. Metab. 2021, 3, 149–165. [Google Scholar] [CrossRef]
- Brancatella, A.; Ricci, D.; Viola, N.; Sgrò, D.; Santini, F.; Latrofa, F. Subacute Thyroiditis After SARS-CoV-2 Infection. J. Clin. Endocrinol. Metab. 2020, 105, 2367–2370. [Google Scholar] [CrossRef]
- Vanherwegen, A.-S.; Gysemans, C.; Mathieu, C. Regulation of Immune Function by Vitamin D and Its Use in Diseases of Immunity. Endocrinol. Metab. Clin. North Am. 2017, 46, 1061–1094. [Google Scholar] [CrossRef]
- Pizzini, A.; Aichner, M.; Sahanic, S.; Böhm, A.; Egger, A.; Hoermann, G.; Kurz, K.; Widmann, G.; Bellmann-Weiler, R.; Weiss, G.; et al. Impact of Vitamin D Deficiency on COVID-19—A Prospective Analysis from the CovILD Registry. Nutrients 2020, 12, 2775. [Google Scholar] [CrossRef] [PubMed]
- Sapra, L.; Saini, C.; Garg, B.; Gupta, R.; Verma, B.; Mishra, P.K.; Srivastava, R.K. Long-Term Implications of COVID-19 on Bone Health: Pathophysiology and Therapeutics. Inflamm. Res. 2022, 71, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Lau, H.E.; Xie, H.; Poon, V.K.-M.; Chan, C.C.-S.; Chu, H.; Yuan, S.; Yuen, T.T.-T.; Chik, K.K.-H.; Tsang, J.O.-L.; et al. SARS-CoV-2 Infection Induces Inflammatory Bone Loss in Golden Syrian Hamsters. Nat. Commun. 2022, 13, 2539. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged Presence of SARS-CoV-2 Viral RNA in Faecal Samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef] [PubMed]
- Ridruejo, E.; Soza, A. The Liver in Times of COVID-19: What Hepatologists Should Know. Ann. Hepatol. 2020, 19, 353–358. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.-B.; Lyu, J.-R.; Lei, X.-M.; Li, W.; Wu, G.; Lyu, J.; Dai, Z.-M. Specific ACE2 Expression in Small Intestinal Enterocytes May Cause Gastrointestinal Symptoms and Injury after 2019-NCoV Infection. Int. J. Infect. Dis. 2020, 96, 19–24. [Google Scholar] [CrossRef]
- Carvalho-Schneider, C.; Laurent, E.; Lemaignen, A.; Beaufils, E.; Bourbao-Tournois, C.; Laribi, S.; Flament, T.; Ferreira-Maldent, N.; Bruyère, F.; Stefic, K.; et al. Follow-up of Adults with Noncritical COVID-19 Two Months after Symptom Onset. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 258–263. [Google Scholar] [CrossRef]
- Park, S.; Lee, C.-W.; Park, D.-I.; Woo, H.-Y.; Cheong, H.S.; Shin, H.C.; Ahn, K.; Kwon, M.-J.; Joo, E.-J. Detection of SARS-CoV-2 in Fecal Samples from Patients with Asymptomatic and Mild COVID-19 in Korea. Clin. Gastroenterol. Hepatol. 2021, 19, 1387–1394.e2. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut Microbiota Composition Reflects Disease Severity and Dysfunctional Immune Responses in Patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Wanner, N.; Andrieux, G.; Badia-i-Mompel, P.; Edler, C.; Pfefferle, S.; Lindenmeyer, M.T.; Schmidt-Lauber, C.; Czogalla, J.; Wong, M.N.; Okabayashi, Y.; et al. Molecular Consequences of SARS-CoV-2 Liver Tropism. Nat. Metab. 2022, 4, 310–319. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, X. The Role of Gut–Liver Axis in Gut Microbiome Dysbiosis Associated NAFLD and NAFLD-HCC. Biomedicines 2022, 10, 524. [Google Scholar] [CrossRef]
- Ferreira-Junior, A.S.; Borgonovi, T.F.; De Salis, L.V.V.; Leite, A.Z.; Dantas, A.S.; De Salis, G.V.V.; Cruz, G.N.F.; De Oliveira, L.F.V.; Gomes, E.; Penna, A.L.B.; et al. Detection of Intestinal Dysbiosis in Post-COVID-19 Patients One to Eight Months after Acute Disease Resolution. Int. J. Environ. Res. Public. Health 2022, 19, 10189. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, L.; Wang, Y.; Dai, T.; Qin, Z.; Zhou, F.; Zhang, L. Alterations in Microbiota of Patients with COVID-19: Potential Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2022, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Yanny, B.; Alkhero, M.; Alani, M.; Stenberg, D.; Saharan, A.; Saab, S. Post-COVID-19 Cholangiopathy: A Systematic Review. J. Clin. Exp. Hepatol. 2023, 13, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Nordvig, A.S.; Fong, K.T.; Willey, J.Z.; Thakur, K.T.; Boehme, A.K.; Vargas, W.S.; Smith, C.J.; Elkind, M.S.V. Potential Neurologic Manifestations of COVID-19. Neurol. Clin. Pract. 2021, 11, e135–e146. [Google Scholar] [CrossRef]
- Guo, Q.; Zheng, Y.; Shi, J.; Wang, J.; Li, G.; Li, C.; Fromson, J.A.; Xu, Y.; Liu, X.; Xu, H.; et al. Immediate Psychological Distress in Quarantined Patients with COVID-19 and Its Association with Peripheral Inflammation: A Mixed-Method Study. Brain. Behav. Immun. 2020, 88, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Roy, D.; Sinha, K.; Parveen, S.; Sharma, G.; Joshi, G. Impact of COVID-19 and Lockdown on Mental Health of Children and Adolescents: A Narrative Review with Recommendations. Psychiatry Res. 2020, 293, 113429. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tziolos, N.-R.; Ioannou, P.; Baliou, S.; Kofteridis, D.P. Long COVID-19 Pathophysiology: What Do We Know So Far? Microorganisms 2023, 11, 2458. https://doi.org/10.3390/microorganisms11102458
Tziolos N-R, Ioannou P, Baliou S, Kofteridis DP. Long COVID-19 Pathophysiology: What Do We Know So Far? Microorganisms. 2023; 11(10):2458. https://doi.org/10.3390/microorganisms11102458
Chicago/Turabian StyleTziolos, Nikolaos-Renatos, Petros Ioannou, Stella Baliou, and Diamantis P. Kofteridis. 2023. "Long COVID-19 Pathophysiology: What Do We Know So Far?" Microorganisms 11, no. 10: 2458. https://doi.org/10.3390/microorganisms11102458
APA StyleTziolos, N.-R., Ioannou, P., Baliou, S., & Kofteridis, D. P. (2023). Long COVID-19 Pathophysiology: What Do We Know So Far? Microorganisms, 11(10), 2458. https://doi.org/10.3390/microorganisms11102458





