LRRC25 Inhibits IFN-γ Secretion by Microglia to Negatively Regulate Anti-Tuberculosis Immunity in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Revival and Culture of H37Rv and GFP-H37Rv
2.3. Transfection and Silencing
2.4. Mtb Infection in BV2 Cells
2.5. Quantitative Real-Time PCR (Q-PCR)
2.6. Western Blot Analysis
2.7. Laser Confocal Microscopy Inspection
2.8. Cellular Immunofluorescence
2.9. Flow Cytometry
2.10. Pathological Section
2.11. ELISA
2.12. Establishment of Mouse Model with Tuberculous Meningitis
2.13. Cell Viability Detection
2.14. Data Analysis
2.15. Ethical Approval
3. Results
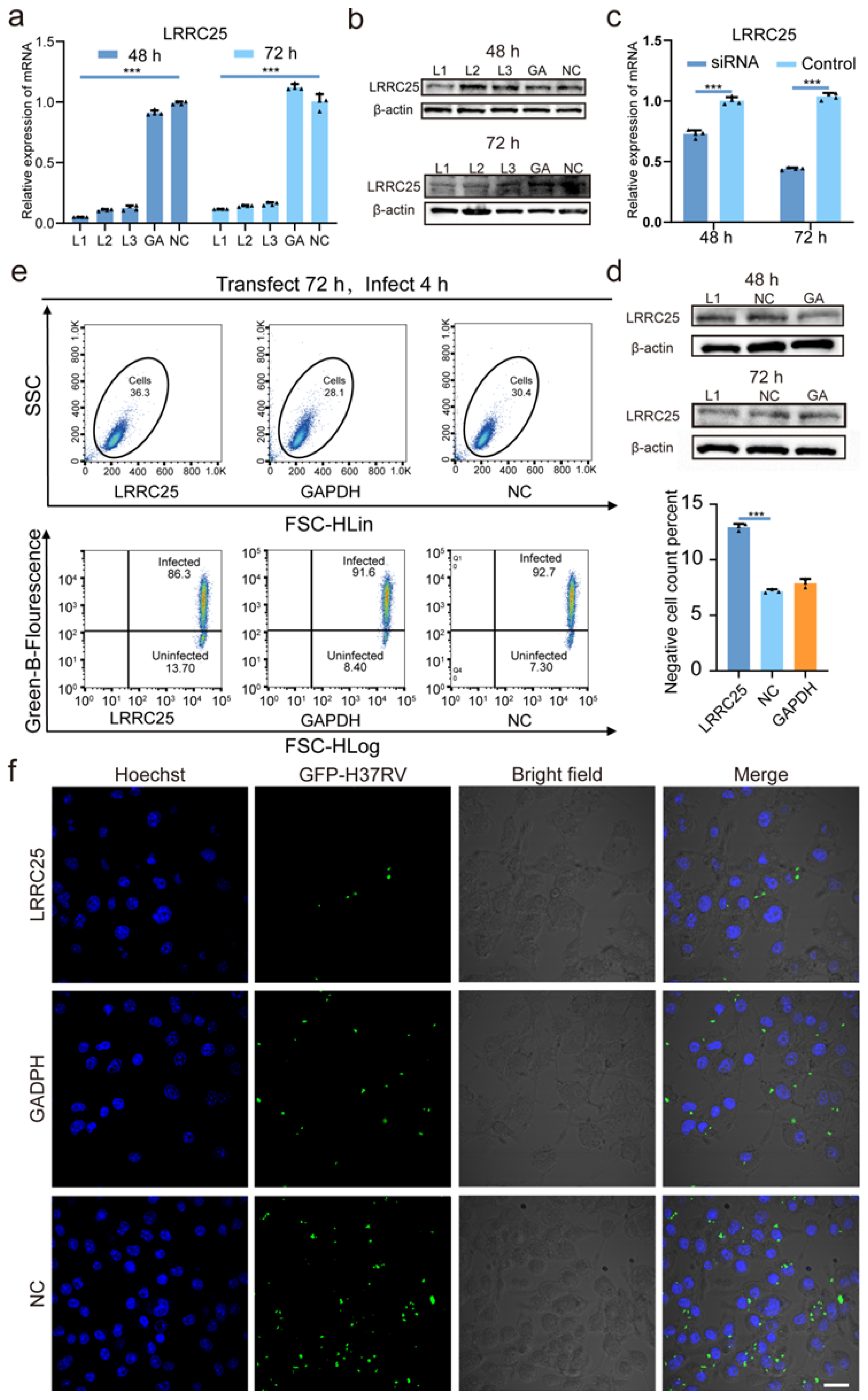
3.1. LRRC25 Is Involved in the Anti-Mtb Natural Immunity of Microglia
3.2. LRRC25 Deficiency Enhances the Anti-Mtb Immunity of Microglia
3.3. LRRC25 Is an Important Negative Regulatory Signal for Microglia to Release IFN-γ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, Y.; Wang, J.; Cai, J.; Kelley, N.; He, Y. The leucine-rich repeat (LRR) domain of NLRP3 is required for NLRP3 inflammasome activation in macrophages. J. Biol. Chem. 2022, 298, 102717. [Google Scholar] [CrossRef]
- Ng, A.; Xavier, R.J. Leucine-rich repeat (LRR) proteins: Integrators of pattern recognition and signaling in immunity. Autophagy 2011, 7, 1082–1084. [Google Scholar] [CrossRef] [PubMed]
- Kufer, T.A.; Fritz, J.H.; Philpott, D.J. NACHT-LRR proteins (NLRs) in bacterial infection and immunity. Trends Microbiol. 2005, 13, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.C.; Eisenberg, J.M.; Heath, R.J.; Huett, A.; Robinson, C.M.; Nau, G.J.; Xavier, R.J. Human leucine-rich repeat proteins: A genome-wide bioinformatic categorization and functional analysis in innate immunity. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4631–4638. [Google Scholar] [CrossRef]
- Feng, Y.; Duan, T.; Du, Y.; Jin, S.; Wang, M.; Cui, J.; Wang, R.F. LRRC25 Functions as an Inhibitor of NF-κB Signaling Pathway by Promoting p65/RelA for Autophagic Degradation. Sci. Rep. 2017, 7, 13448. [Google Scholar] [CrossRef]
- Du, Y.; Duan, T.; Feng, Y.; Liu, Q.; Lin, M.; Cui, J.; Wang, R.F. LRRC25 inhibits type I IFN signaling by targeting ISG15-associated RIG-I for autophagic degradation. EMBO J. 2018, 37, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.; Figaji, A.; Imran, D.; Phu, N.H.; Rohlwink, U.; Thwaites, G.E. The neurocritical care of tuberculous meningitis. Lancet Neurol. 2019, 18, 771–783. [Google Scholar] [CrossRef]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and non-immune functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef]
- Qin, Y.; Sun, X.; Shao, X.; Cheng, C.; Feng, J.; Sun, W.; Gu, D.; Liu, W.; Xu, F.; Duan, Y. Macrophage-Microglia Networks Drive M1 Microglia Polarization After Mycobacterium Infection. Inflammation 2015, 38, 1609–1616. [Google Scholar] [CrossRef]
- Lmolda, B.; Gonzalez, B.; Castellano, B. Antigen presentation in EAE: Role of microglia, macrophages and dendritic cells. Front. Biosci. Landmark Ed. 2011, 16, 1157–1171. [Google Scholar] [CrossRef]
- Conti, P.; Lauritano, D.; Caraffa, A.; Gallenga, C.E.; Kritas, S.K.; Ronconi, G.; Martinotti, S. Microglia and mast cells generate proinflammatory cytokines in the brain and worsen inflammatory state: Suppressor effect of IL-37. Eur. J. Pharmacol. 2020, 15, 173035. [Google Scholar] [CrossRef] [PubMed]
- Tucker, E.W.; Pokkali, S.; Zhang, Z.; DeMarco, V.P.; Klunk, M.; Smith, E.S.; Ordonez, A.A.; Penet, M.F.; Bhujwalla, Z.; Jain, S.K.; et al. Microglia activation in a pediatric rabbit model of tuberculous meningitis. Dis. Model. Mech. 2016, 9, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.G.; Rohlwink, U.K.; Proust, A.; Figaji, A.A.; Wilkinson, R.J. The pathogenesis of tuberculous meningitis. J. Leukocyte Biol. 2019, 105, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Koutsouras, G.W.; Ramos, R.L.; Martinez, L.R. Role of microglia in fungal infections of the central nervous system. Virulence 2017, 8, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Kosoy, R.; Fullard, J.F.; Zeng, B.; Bendl, J.; Dong, P.; Rahman, S.; Kleopoulos, S.P.; Shao, Z.; Girdhar, K.; Humphrey, J.; et al. Genetics of the human microglia regulome refines Alzheimer’s disease risk loci. Nat. Genet. 2022, 54, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Qiao, N.; Zhang, J.; Zhang, Y.; Liu, X. Synergistic regulation of microglia differentiation by CD93 and integrin β1 in the rat pneumococcal meningitis model. Immunol. Lett. 2022, 251–252, 63–74. [Google Scholar] [CrossRef]
- Lu, H.J.; Guo, D.; Wei, Q.Q. Potential of Neuroinflammation-Modulating Strategies in Tuberculous Meningitis: Targeting Microglia. Aging Dis. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Nau, R.; Brück, W. Neuronal injury in bacterial meningitis: Mechanisms and implications for therapy. Trends Neurosci. 2002, 25, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, S.; Qiao, Y.; Hoshino, Y.; Weiden, M.; Canova, A.; Giacomini, E.; Coccia, E.; Pine, R. Inhibition of response to alpha interferon by Mycobacterium tuberculosis. Infect. Immun. 2003, 71, 2487–2497. [Google Scholar] [CrossRef]
- Mezouar, S.; Mege, J.L. Changing the paradigm of IFN-γ at the interface between innate and adaptive immunity: Macrophage-derived IFN-γ. J. Leukocyte Biol. 2020, 108, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Stanley, S.A.; Johndrow, J.E.; Manzanillo, P.; Cox, J.S. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J. Immunol. 2007, 178, 3143–3152. [Google Scholar] [CrossRef]
- Speer, S.D.; Li, Z.; Buta, S.; Payelle-Brogard, B.; Qian, L.; Vigant, F.; Rubino, E.; Gardner, T.J.; Wedeking, T.; Hermann, M.; et al. ISG15 deficiency and increased viral resistance in humans but not mice. Nat. Commun. 2016, 7, 11496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bogunovic, D.; Payelle-Brogard, B.; Francois-Newton, V.; Speer, S.D.; Yuan, C.; Volpi, S.; Li, Z.; Sanal, O.; Mansouri, D.; et al. Human intracellular ISG15 prevents interferon-α/β over-amplification and auto-inflammation. Nature 2015, 517, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, T.; Gong, Z.; Xie, J. Role of ISG15 post-translational modification in immunity against Mycobacterium tuberculosis infection. Cell Signal. 2022, 94, 110329. [Google Scholar] [CrossRef] [PubMed]
- Bogunovic, D.; Boisson-Dupuis, S.; Casanova, J.L. ISG15: Leading a double life as a secreted molecule. Exp. Mol. Med. 2013, 45, e18. [Google Scholar] [CrossRef] [PubMed]
- Swaim, C.D.; Scott, A.F.; Canadeo, L.A.; Huibregtse, J.M. Extracellular ISG15 Signals Cytokine Secretion through the LFA-1 Integrin Receptor. Mol. Cell. 2017, 68, 581–590.e5. [Google Scholar] [CrossRef]
- Yángüez, E.; García-Culebras, A.; Frau, A.; Llompart, C.; Knobeloch, K.P.; Gutierrez-Erlandsson, S.; García-Sastre, A.; Esteban, M.; Nieto, A.; Guerra, S. ISG15 regulates peritoneal macrophages functionality against viral infection. PLoS Pathog. 2013, 9, e1003632. [Google Scholar] [CrossRef]
- Christensen, L.B.; Woods, T.A.; Carmody, A.B.; Caughey, B.; Peterson, K.E. Age-related differences in neuroinflammatory responses associated with a distinct profile of regulatory markers on neonatal microglia. J. Neuroinflamm. 2014, 11, 70. [Google Scholar] [CrossRef]
- Neumann, J.; Gunzer, M.; Gutzeit, H.O.; Ullrich, O.; Reymann, K.G.; Dinkel, K. Microglia provide neuroprotection after ischemia. FASEB J. 2006, 20, 714–716. [Google Scholar] [CrossRef]
- Guo, H.; Tong, N.; Turner, T.; Epstein, L.G.; McDermott, M.P.; Kilgannon, P.; Gelbard, H.A. Release of the neuronal glycoprotein ICAM-5 in serum after hypoxic-ischemic injury. Ann. Neurol. 2000, 48, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Zilka, N.; Stozicka, Z.; Kovac, A.; Pilipcinec, E.; Bugos, O.; Novak, M. Human misfolded truncated tau protein promotes activation of microglia and leukocyte infiltration in the transgenic rat model of tauopathy. J. Neuroimmunol. 2009, 209, 16–25. [Google Scholar] [CrossRef]
- Bogunovic, D.; Byun, M.; Durfee, L.A.; Abhyankar, A.; Sanal, O.; Mansouri, D.; Salem, S.; Radovanovic, I.; Grant, A.V.; Adimi, P.; et al. Mycobacterial disease and impaired IFN-γ immunity in humans with inherited ISG15 deficiency. Science 2012, 337, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Casanova, J.L.; Abel, L. Genetic dissection of immunity to mycobacteria: The human model. Annu. Rev. Immunol. 2002, 20, 581–620. [Google Scholar] [CrossRef] [PubMed]
- Mirzalieva, O.; Juncker, M.; Schwartzenburg, J.; Desai, S. ISG15 and ISGylation in Human Diseases. Cells 2022, 11, 538. [Google Scholar] [CrossRef] [PubMed]
- Susta, L.; Cornax, I.; Diel, D.G.; Garcia, S.C.; Miller, P.J.; Liu, X.; Hu, S.; Brown, C.C.; Afonso, C.L. Expression of interferon gamma by a highly virulent strain of Newcastle disease virus decreases its pathogenicity in chickens. Microb. Pathog. 2013, 61–62, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Hardison, S.E.; Ravi, S.; Wozniak, K.L.; Young, M.L.; Olszewski, M.A.; Wormlmley, F.L., Jr. Pulmonary infection with an interferon-gamma-producing Cryptococcus neoformans strain results in classical macrophage activation and protection. Am. J. Pathol. 2010, 176, 774–785. [Google Scholar] [CrossRef]
- Ramon-Luing, L.A.; Olvera, Y.; Flores-Gonzalez, J.; Palacios, Y.; Carranza, C.; Aguilar-Duran, Y.; Vargas, M.A.; Gutierrez, N.; Medina-Quero, K.; Chavez-Galan, L. Cell Death Mechanisms Are Simultaneously Activated in Macrophages Infected by Virulent Mycobacterium tuberculosis. Pathogens 2022, 11, 492. [Google Scholar] [CrossRef]
- Jung, B.G.; Vankayalapati, R.; Samten, B. Mycobacterium tuberculosis stimulates IL-1β production by macrophages in an ESAT-6 dependent manner with the involvement of serum amyloid A3. Mol. Immunol. 2021, 135, 285–293. [Google Scholar] [CrossRef]
- Cooper, A.M.; Solache, A.; Khader, S.A. Interleukin-12 and tuberculosis: An old story revisited. Curr. Opin. Immunol. 2007, 19, 441–447. [Google Scholar] [CrossRef]
- Zhang, J.A.; Lu, Y.B.; Wang, W.D.; Liu, G.B.; Chen, C.; Shen, L.; Luo, H.L.; Xu, H.; Peng, Y.; Luo, H.; et al. BTLA-Expressing Dendritic Cells in Patients With Tuberculosis Exhibit Reduced Production of IL-12/IFN-α and Increased Production of IL-4 and TGF-β, Favoring Th2 and Foxp3+ Treg Polarization. Front. Immunol. 2020, 11, 518. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, Y.; Lao, S.; Yang, B.; Yu, S.; Zhang, Y.; Wu, C. Mycobacterium tuberculosis-Specific IL-21+IFN-γ+CD4+ T Cells Are Regulated by IL-12. PLoS ONE 2016, 11, e0147356. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.T.; Torres, M.; Nevels, D.; Perez-Redondo, C.N.; Ellner, J.J.; Sada, E.; Schwander, S.K. Compartmentalized bronchoalveolar IFN-gamma and IL-12 response in human pulmonary tuberculosis. Tuberculosis 2009, 89, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Barricarte, R.; Markle, J.G.; Ma, C.S.; Deenick, E.K.; Ramírez-Alejo, N.; Mele, F.; Latorre, D.; Mahdaviani, S.A.; Aytekin, C.; Mansouri, D.; et al. Human IFN-γ immunity to mycobacteria is governed by both IL-12 and IL-23. Sci. Immunol. 2018, 3, eaau6759. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, G.; Chu, H.; Duan, H.; Wang, W.; Tian, N.; Liu, D.; Sun, H.; Sun, Z. LRRC25 Inhibits IFN-γ Secretion by Microglia to Negatively Regulate Anti-Tuberculosis Immunity in Mice. Microorganisms 2023, 11, 2500. https://doi.org/10.3390/microorganisms11102500
Sheng G, Chu H, Duan H, Wang W, Tian N, Liu D, Sun H, Sun Z. LRRC25 Inhibits IFN-γ Secretion by Microglia to Negatively Regulate Anti-Tuberculosis Immunity in Mice. Microorganisms. 2023; 11(10):2500. https://doi.org/10.3390/microorganisms11102500
Chicago/Turabian StyleSheng, Gang, Hongqian Chu, Huijuan Duan, Wenjing Wang, Na Tian, Dingyi Liu, Hong Sun, and Zhaogang Sun. 2023. "LRRC25 Inhibits IFN-γ Secretion by Microglia to Negatively Regulate Anti-Tuberculosis Immunity in Mice" Microorganisms 11, no. 10: 2500. https://doi.org/10.3390/microorganisms11102500




