Controlling Multi-Drug-Resistant Traits of Salmonella Obtained from Retail Poultry Shops Using Metal–Organic Framework (MOF) as a Novel Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Study Area and Period
2.3. Sample Collection
2.4. Isolation, Identification, and Serotyping of Salmonella Isolates
2.5. Antimicrobial Susceptibility Testing
2.6. Molecular Detection of Salmonella-Related Biofilm Gene
2.7. Synthesis of MOF
2.8. Characterization of MOF
2.9. Antimicrobial Assessment of MOF
2.9.1. Inculum Preparation
2.9.2. Well Agar Preparation
2.10. Statistical Analysis
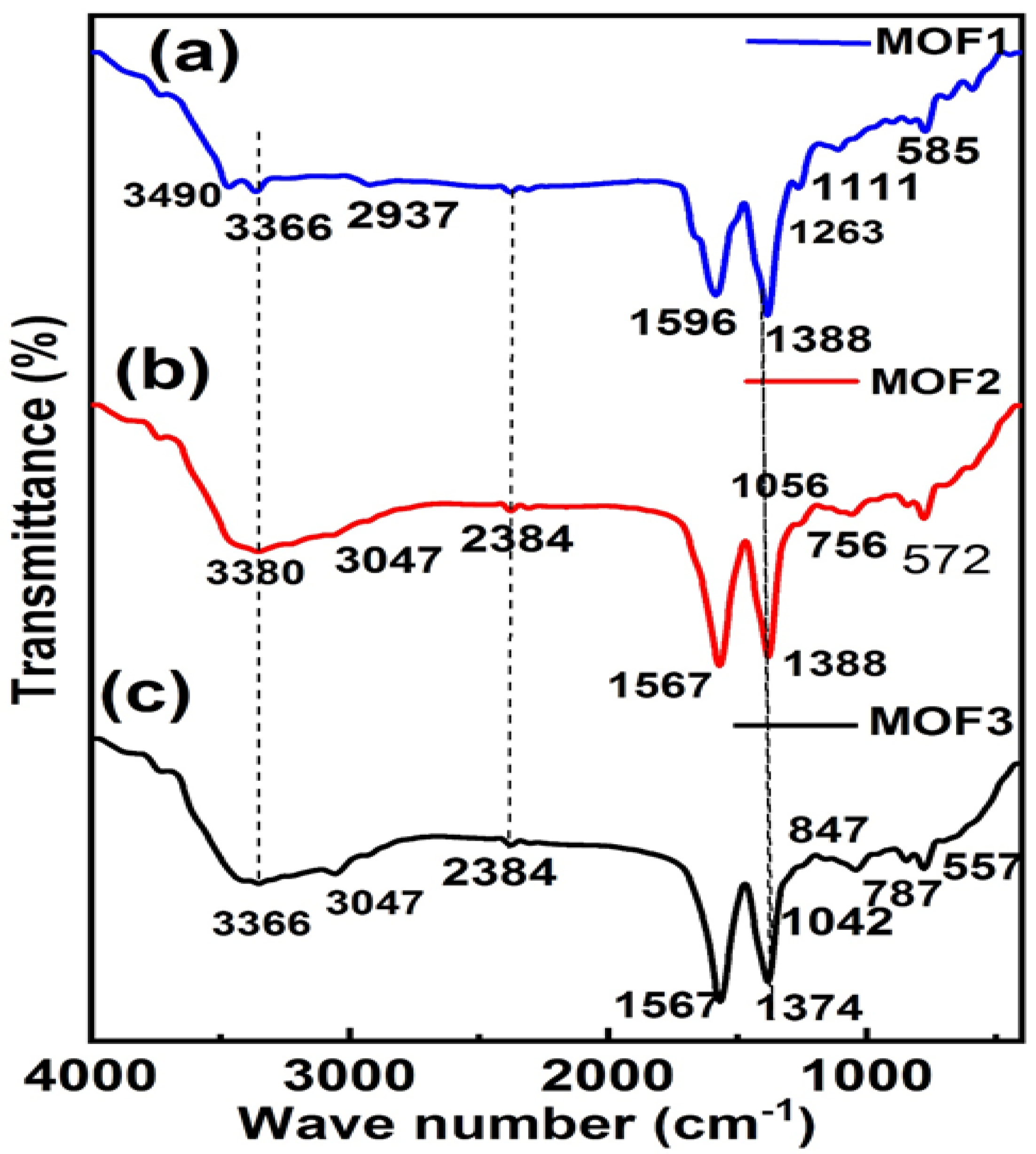
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MOFs | Metal Organic Frameworks |
| MOF1 | Cu-MOF |
| MOF2 | Cu/Ni-MOF |
| MOF3 | Cu/Ni/Co-MOF |
| SEM | Scan Electron Microscope |
| XRD | X-Ray Diffraction |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| R | Resistance |
| I | Intermediate |
| S | Susceptible |
References
- Attia, M.M.; Salem, H.M. Morphological and molecular characterization of Pseudolynchia canariensis (Diptera: Hippoboscidae) infesting domestic pigeons. Int. J. Trop. Insect Sci. 2022, 42, 733–740. [Google Scholar] [CrossRef]
- Barrow, P.A.; Huggins, M.B.; Lovell, M.A.; Simpson, J.M. Observations on the pathogenesis of experimental Salmonella typhimurium infection in chickens. Res. Vet. Sci. 1987, 42, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Vose, J.M.; Sun, G.; Ford, C.R.; Bredemeier, M.; Otsuki, K.; Wei, X.; Zhang, Z.; Zhang, L. Forest ecohydrological research in the 21st century: What are the critical needs ? Ecohydrology 2011, 158, 146–158. [Google Scholar] [CrossRef]
- Donado-Godoy, P.; Clavijo, V.; León, M.; Tafur, M.A.; Gonzales, S.; Hume, M.; Alali, W.; Walls, I.; Wong, D.M.L.F.; Doyle, M.P. Prevalence of Salm onella on Retail Broiler Chicken Meat Carcasses in Colombia. J. Food Prot. 2012, 75, 1134–1138. [Google Scholar] [CrossRef]
- Goh, K.; Heng, C.; Lin, Z. Social Media Brand Community and Consumer Behavior: Quantifying the Relative Impact of User- and Marketer- Generated Content. Inf. Syst. Res. 2013, 24, 88–107. [Google Scholar] [CrossRef]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef]
- Zwe, Y.H.; Tang, V.C.Y.; Aung, K.T.; Gutiérrez, R.A.; Ng, L.C.; Yuk, H.G. Prevalence, sequence types, antibiotic resistance and, gyrA mutations of Salmonella isolated from retail fresh chicken meat in Singapore. Food Control 2018, 90, 233–240. [Google Scholar] [CrossRef]
- Marquardt, R.R.; Li, S. Antimicrobial resistance in livestock: Advances and alternatives to antibiotics. Anim. Front. 2018, 8, 30–37. [Google Scholar] [CrossRef]
- Tessema, F.B.; Gonfa, Y.H.; Asfaw, T.B.; Tadesse, T.G.; Tadesse, M.G.; Bachheti, A.; Pandey, D.P.; Wabaidur, S.M.; Dahlous, K.A.; Širić, I.; et al. Flavonoids and Phenolic Acids from Aerial Part of Ajugaintegrifolia (Buch.-Ham. Ex D. Don): Anti-Shigellosis Activity and In Silico Molecular Docking Studies. Molecules 2023, 28, 1111. [Google Scholar] [CrossRef]
- Tessema, F.B.; Gonfa, Y.H.; Asfaw, T.B.; Tadesse, M.G.; Tadesse, T.G.; Bachheti, A.; Alshaharni, M.O.; Kumar, P.; Kumar, V.; Širić, I.; et al. Targeted HPTLC Profile, Quantification of Flavonoids and Phenolic Acids, and Antimicrobial Activity of Dodonaea angustifolia (L.f.) Leaves and Flowers. Molecules 2023, 28, 2870. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, F.; Wang, D. MOFs and MOF-Derived Materials for Antibacterial Application. J. Funct. Biomater. 2022, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Zhao, X.; Yan, X. Vancomycin-Functionalized Porphyrinic Metal-Organic Framework PCN-224 with Enhanced Antibacterial Activity against Staphylococcus Aureus. Chem. Asian J. 2021, 16, 2022–2026. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, E.; Zafar, F. Introductory Chapter: Metal Organic Frameworks (MOFs). In Metal-Organic Frameworks; InTechOpen: London, UK, 2016; pp. 3–16. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef]
- Meek, S.T.; Greathouse, J.A.; Allendorf, M.D. Metal-organic frameworks: A rapidly growing class of versatile nanoporous materials. Adv. Mater. 2011, 23, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.D.; Jiang, H.L. Metal-Organic Frameworks for Photocatalysis and Photothermal Catalysis. Acc. Chem. Res. 2019, 52, 356–366. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Jiang, H.L.; Xu, Q. Metal–Organic Frameworks as Platforms for Catalytic Applications. Adv. Mater. 2018, 30, e1703663. [Google Scholar] [CrossRef]
- Cui, Y.; Li, B.; He, H.; Zhou, W.; Chen, B.; Qian, G. Metal-Organic Frameworks as Platforms for Functional Materials. Acc. Chem. Res. 2016, 49, 483–493. [Google Scholar] [CrossRef]
- McKinlay, A.C.; Morris, R.E.; Horcajada, P.; Férey, G.; Gref, R.; Couvreur, P.; Serre, C. BioMOFs: Metal-organic frameworks for biological and medical applications. Angew. Chemie Int. Ed. 2010, 49, 6260–6266. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.W. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef]
- Claes, B.; Boudewijns, T.; Muchez, L.; Hooyberghs, G.; Van der Eycken, E.V.; Vanderleyden, J.; Steenackers, H.P.; De Vos, D.E. Smart Metal-Organic Framework Coatings: Triggered Antibiofilm Compound Release. ACS Appl. Mater. Interfaces 2017, 9, 4440–4449. [Google Scholar] [CrossRef]
- Miller, S.R.; Heurtaux, D.; Baati, T.; Horcajada, P.; Grenèche, J.M.; Serre, C. Biodegradable therapeutic MOFs for the delivery of bioactive molecules. Chem. Commun. 2010, 46, 4526–4528. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.N.; Gwon, K.; Kim, Y.; Cho, H.; Lee, S. Immobilization of antibacterial copper metal-organic framework containing glutarate and 1,2-bis(4-pyridyl)ethylene ligands on polydimethylsiloxane and its low cytotoxicity. J. Ind. Eng. Chem. 2021, 102, 135–145. [Google Scholar] [CrossRef]
- Yilgor, E.; Nugay, I.I.; Bakan, M.; Yilgor, I. Antibacterial silicone-urea/organoclay nanocomposites. Silicon 2009, 1, 183–190. [Google Scholar] [CrossRef]
- Duan, F.; Feng, X.; Jin, Y.; Liu, D.; Yang, X.; Zhou, G.; Liu, D.; Li, Z.; Liang, X.-J.; Zhang, J. Metal–carbenicillin framework-based nanoantibiotics with enhanced penetration and highly efficient inhibition of MRSA. Biomaterials 2017, 144, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Junaid, M.; Chen, G.; Wang, J. Interactions and associated resistance development mechanisms between microplastics, antibiotics and heavy metals in the aquaculture environment. Rev. Aquac. 2022, 14, 1028–1045. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, Y.; Zhou, B.; Mei, L.; Wang, Q.; Zhang, B. Porous metal-organic framework (MOF) Carrier for incorporation of volatile antimicrobial essential oil. Food Control 2019, 98, 174–178. [Google Scholar] [CrossRef]
- Gowriboy, N.; Kalaivizhi, R.; Kaleekkal, N.J.; Ganesh, M.R.; Aswathy, K.A. Fabrication and characterization of polymer nanocomposites membrane (Cu-MOF@CA/PES) for water treatment. J. Environ. Chem. Eng. 2022, 10, 108668. [Google Scholar] [CrossRef]
- Li, W.; Zhou, S.; Gao, S.; Chen, S.; Huang, M.; Cao, R. Spatioselective Fabrication of Highly Effective Antibacterial Layer by Surface-Anchored Discrete Metal-Organic Frameworks. Adv. Mater. Interfaces 2015, 2, 1400405. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano 2010, 4, 5471–5479. [Google Scholar] [CrossRef]
- Miao, H.; Teng, Z.; Wang, C.; Chong, H.; Wang, G. Recent Progress in Two-Dimensional Antimicrobial Nanomaterials. Chem. A Eur. J. 2019, 25, 929–944. [Google Scholar] [CrossRef]
- Pang, L.; Dai, C.; Bi, L.; Guo, Z.; Fan, J. Biosafety and Antibacterial Ability of Graphene and Graphene Oxide In Vitro and In Vivo. Nanoscale Res. Lett. 2017, 12, 564. [Google Scholar] [CrossRef]
- Liu, J.; Wu, D.; Zhu, N.; Wu, Y.; Li, G. Antibacterial mechanisms and applications of metal-organic frameworks and their derived nanomaterials. Trends Food Sci. Technol. 2021, 109, 413–434. [Google Scholar] [CrossRef]
- Overend, C.; Yuan, L.; Peccoud, J. The synthetic futures of vesicular stomatitis virus. Trends Biotechnol. 2012, 30, 497–498. [Google Scholar] [CrossRef]
- Zhuang, W.; Yuan, D.; Li, J.-R.; Luo, Z.; Zhou, H.-C.; Bashir, S.; Liu, J. Highly potent bactericidal activity of porous metal-organic frameworks. Adv. Healthc. Mater. 2012, 1, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Argueta-figueroa, L.; Morales-luckie, R.A.; Scougall-vilchis, R.J. Synthesis, characterization and antibacterial activity of copper, nickel and bimetallic Cu–Ni nanoparticles for potential use in dental materials. Prog. Nat. Sci. Mater. Int. 2014, 24, 321–328. [Google Scholar] [CrossRef]
- Senthil Raja, D.; Huang, C.L.; Chen, Y.A.; Choi, Y.M.; Lu, S.Y. Composition-balanced trimetallic MOFs as ultra-efficient electrocatalysts for oxygen evolution reaction at high current densities. Appl. Catal. B Environ. 2020, 279, 119375. [Google Scholar] [CrossRef]
- Quinn, P.J.; Markey, B.K.; Leonard, F.C.; Hartigan, P.; Fanning, S.; Fitzpatrick, E. Veterinary Microbiology and Microbial Disease; Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar]
- Popoff, M.Y.; Bockemühl, J.; Gheesling, L.L. Supplement 2002 (no. 46) to the Kauffmann–White scheme. Res. Microbiol. 2004, 155, 568–570. [Google Scholar] [CrossRef] [PubMed]
- CLSI M100-S27; Performance Standards for Antimicrobial Susceptibility Testing: 28th Edition. CLSI: Wayne, PA, USA, 2018.
- Bhowmick, P.P.; Devegowda, D.; Ruwandeepika, H.D.; Fuchs, T.M.; Srikumar, S.; Karunasagar, I.; Karunasagar, I. GcpA (stm1987) is critical for cellulose production and biofilm formation on polystyrene surface by Salmonella enterica serovar Weltevreden in both high and low nutrient medium. Microb. Pathog. 2011, 50, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, N.; Vishwavidyalaya, U.B.K.; Banik, B.C. Influence of pre-harvest foliar application of growth regulators and micronutrients on mango cv. Himsagar. Indian J. Hortic. 2011, 86, 103–107. [Google Scholar]
- Ahmed, H.; Gharieb, R.M.; Mohamed, M.E.; Amin, M.A.; Mohamed, R.E. Bacteriological and Molecular Characterization of Salmonella Species Isolated from Humans and Chickens in Sharkia Governorate, Egypt Heba. Zagazig Vet. J. 2017, 45, 48–61. [Google Scholar] [CrossRef]
- Orabi, A.; Armanious, W.; Radwan, I.A.; Girh, Z.M.S.A.; Hammad, E. Genetic Correlation of Virulent Salmonella Serovars (Extended Spectrum β -Lactamases) Isolated from Broiler Chickens and Human: A Public Health Concern. Pathogens 2022, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.A.; Albraikan, A.A.; Alzahrani, J.S.; Dhahbi, S.; Al-Turaiki, I.; Al Duhayyim, M.; Yaseen, I.; Eldesouki, M.I. Optimal Deep Transfer Learning-Based Human-Centric Biomedical Diagnosis for Acute Lymphoblastic Leukemia Detection. Comput. Intell. Neurosci. 2022, 2022, 7954111. [Google Scholar] [CrossRef] [PubMed]
- Thiruppathi, S.; Abdulla, M.H.; Lakshmanaperumalsamy, P. Salmonella Cross-contamination in Retail Chicken Outlets and the Efficacy of Spice Extracts on Salmonella enteritidis Growth Inhibition on Various Surfaces Salmonella Cross-contamination in Retail Chicken Outlets and the Efficacy of Spice Extracts on Salm. Microbes Environ. 2004, 19, 286–291. [Google Scholar] [CrossRef]
- Sharada, R.; Krishnappa, G.; Raghavan, R.; Gowda, R.S. Isolation and serotyping of Escherichia coli from different pathological conditions in poultry. Indian J. Poult. Sci. 1999, 34, 366–369. [Google Scholar]
- Akl, N.O.; Cliver, D.O.; Kasparl, C.W. Decontamination of Plastic and Wooden Cutting Boards for Kitchen Use. J. Food Prot. 1994, 57, 23–30. [Google Scholar]
- Srey, S.; Jahid, I.K.; Ha, S. Biofilm formation in food industries: A food safety concern. Food Control 2013, 31, 572–585. [Google Scholar] [CrossRef]
- Dawson, P.; Han, I.; Cox, M.; Black, C.; Simmons, L. Residence time and food contact time effects on transfer of Salmonella Typhimurium from tile, wood and carpet: Testing the five-second rule. J. Appl. Microbiol. 2007, 102, 945–953. [Google Scholar] [CrossRef]
- Steenackers, H.; Hermans, K.; Vanderleyden, J.; De Keersmaecker, S.C. Salmonella biofilms: An overview on occurrence, structure, regulation and eradication. Food Res. Int. 2012, 45, 502–531. [Google Scholar] [CrossRef]
- Shi, X. Biofilm formation and food safety in food industries. Trends Food Sci. Technol. 2009, 20, 407–413. [Google Scholar] [CrossRef]
- Nilsson, R.E.; Ross, T.; Bowman, J.P. International Journal of Food Microbiology Variability in bio fi lm production by Listeria monocytogenes correlated to strain origin and growth conditions. Int. J. Food Microbiol. 2011, 150, 14–24. [Google Scholar] [CrossRef]
- Wang, R.; Schmidt, J.W.; Harhay, D.M.; Bosilevac, J.M.; King, D.A.; Arthur, T.M. Biofilm Formation, Antimicrobial Resistance, and Sanitizer Tolerance of Salmonella enterica. Foodborne Pathog. Dis. 2017, 14, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Soomro, A.H.; Bhutto, B.; Shah, G.; Azizullah, M. Prevalence and antimicrobial resistance of Salmonella serovars isolated from poultry meat in Hyderabad, Pakistan Prevalence and antimicrobial resistance of Salmonella serovars isolated from poultry meat in Hyderabad, Pakistan. J. Vet. Anim. Sci. 2010, 34, 455–460. [Google Scholar] [CrossRef]
- Zishiri, O.T.; Mkhize, N.; Mukaratirwa, S. Prevalence of virulence and antimicrobial resistance genes in Salmonella spp. isolated from commercial chickens and human clinical isolates from South Africa and Brazil. Onderstepoort J. Vet. Res. 2016, 83, a1067. [Google Scholar] [CrossRef] [PubMed]
- Nabil, N.M.; Yonis, A.E. Isolation of Salmonella Characterized by Biofilm Formation and Disinfectant Resistance from Broiler Chickens. Alex. J. Vet. Sci. 2019, 62, 26–36. [Google Scholar] [CrossRef]
- Gharieb, R.M.; Tartor, Y.H.; Khedr, M.H.E. Non-Typhoidal Salmonella in poultry meat and diarrhoeic patients: Prevalence, antibiogram, virulotyping, molecular detection and sequencing of class I integrons in multidrug resistant strains. Gut Pathog. 2015, 7, 34. [Google Scholar] [CrossRef]
- Radwan, I.A.; Abed, A.H.; Abd Al-Wanis, S.A.; Abd El-Aziz, G.G.; El-Shemy, A. Antibacterial effect of cinnamon and oreganium oils on multidrug resistant Escherichia coli and Salmonellae isolated from broiler chickens. J. Egy. Vet. Med. Assoc. 2016, 76, 169–186. [Google Scholar]
- Wyszogrodzka, G.; Marszałek, B.; Gil, B.; Dorożyński, P. Metal-organic frameworks: Mechanisms of antibacterial action and potential applications. Drug Discov. Today 2016, 21, 1009–1018. [Google Scholar] [CrossRef]
- Beg, S.; Rahman, M.; Jain, A.; Saini, S.; Midoux, P.; Pichon, C.; Ahmad, F.J.; Akhter, S. Nanoporous metal organic frameworks as hybrid polymer—Metal composites for drug delivery and biomedical applications. Drug Discov. Today 2016, 22, 625–637. [Google Scholar] [CrossRef]
- Shafeeulla, R.M.; Krishnamurthy, G.; Bhojynaik, H.S.; Manjuraj, T.; Poojari, S. Synthesis of (4-Bromo-3-Fluorophenyl)(Pyrimidin-5-yl)Methanol and their Transition Metal Complexes, Spectral, X-ray Powder Diffraction, Cytotoxicity, Molecular Docking, and Biological Evaluation. Sci. Indian J. Adv. Chem. 2017, 5, 268–279. [Google Scholar] [CrossRef]
- Nagalakshmi, G.; Nandeesh, I.M.; Yallur, B.C.; Adimule, V.; Batakurki, S. Synthesis and Optical Properties of Copper Terephthalate Metal Organic Frame Works. Eng. Chem. 2023, 2, 3–11. [Google Scholar] [CrossRef]
- Otte, H.M. Lattice parameter determinations with an X-ray spectrogoniometer by the debye-scherrer method and the effect of specimen condition. J. Appl. Phys. 1961, 32, 1536–1546. [Google Scholar] [CrossRef]
- Karthik, C.; Radha, K.V. Silver nanoparticle loaded activated carbon: An escalated nanocomposite with antimicrobial property. Orient. J. Chem. 2016, 32, 735–741. [Google Scholar] [CrossRef]
- Etacheri, V.; Wang, C.; O’Connell, M.J.; Chan, C.K.; Pol, V.G. Porous carbon sphere anodes for enhanced lithium-ion storage. J. Mater. Chem. A 2015, 3, 9861–9868. [Google Scholar] [CrossRef]
- Nivetha, R.; Sajeev, A.; Paul, A.M.; Gothandapani, K.; Gnanasekar, S.; Bhardwaj, P.; Jacob, G.; Sellappan, R.; Raghavan, V.; Pitchaimuthu, S.; et al. Cu based Metal Organic Framework (Cu-MOF) for electrocatalytic hydrogen evolution reaction. Mater. Res. Express 2020, 7, 114001. [Google Scholar] [CrossRef]
- Pamei, M.; Achumi, A.G.; Kahmei, R.; Sarkar, A.; PUZARI, A. Functionalized Cu-Bdc Mof with Peroxidase-Mimetic Activity as an Adsorbent for Efficient Removal of Noxious Organic Dye from Aqueous Solution. Microporous Mesoporous Mater. 2022, 340, 112031. [Google Scholar] [CrossRef]
- Gopi, S.; Al-Mohaimeed, A.M.; Al-onazi, W.A.; Soliman Elshikh, M.; Yun, K. Metal organic framework-derived Ni-Cu bimetallic electrocatalyst for efficient oxygen evolution reaction. J. King Saud Univ. Sci. 2021, 33, 101379. [Google Scholar] [CrossRef]
- Li, H.; Xu, S.; Du, J.; Tang, J.; Zhou, Q. Cu@Co-MOFs as a novel catalyst of peroxymonosulfate for the efficient removal of methylene blue. RSC Adv. 2019, 9, 9410–9420. [Google Scholar] [CrossRef]
- Zhao, S.; Zeng, L.; Cheng, G.; Yu, L.; Zeng, H. Ni/Co-based metal-organic frameworks as electrode material for high performance supercapacitors. Chin. Chem. Lett. 2019, 30, 605–609. [Google Scholar] [CrossRef]
- El-Trass, A.; Elshamy, H.; El-Mehasseb, I.; El-Kemary, M. CuO nanoparticles: Synthesis, characterization, optical properties and interaction with amino acids. Appl. Surf. Sci. 2012, 258, 2997–3001. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Liu, B.; Zhao, Q.; Chen, G. Hexagonal microspindle of NH2-MIL-101(Fe) metal-organic frameworks with visible-light-induced photocatalytic activity for the degradation of toluene. RSC Adv. 2016, 6, 4289–4295. [Google Scholar] [CrossRef]
- Wang, F.; Hu, J.; Peng, Y.; Wu, X.; Xue, H.; Pang, H. Application and modification of nickel-based metal-organic frameworks in electrochemical sensing. Adv. Sens. Energy Mater. 2023, 2, 100053. [Google Scholar] [CrossRef]
- Ye, C.; Qin, Q.; Liu, J.; Mao, W.; Yan, J.; Wang, Y.; Cui, J.; Zhang, Q.; Yang, L.; Wu, Y. Coordination derived stable Ni-Co MOFs for foldable all-solid-state supercapacitors with high specific energy. J. Mater. Chem. A 2019, 7, 4998–5008. [Google Scholar] [CrossRef]
- Gao, H.; Wang, G.; Yang, M.; Tan, L.; Yu, J. Novel tunable hierarchical Ni-Co hydroxide and oxide assembled from two-wheeled units. Nanotechnology 2012, 23, 15607. [Google Scholar] [CrossRef]
- Salama, R.S.; El-Hakama, S.A.; Samraa, S.E.; El-Dafrawya, S.M.; Ahmeda, A.I. Adsorption, Equilibrium and kinetic studies on the removal of methyl orange dye from aqueous solution by the use of copper metal organic framework (Cu-BDC). Int. J. Mod. Chem. 2018, 10, 195–207. [Google Scholar]
- Zhang, W.; Yin, C.; Jin, Y.; Feng, X.; Li, X.; Xu, A. Co-MOF as a highly efficient catalyst for contaminants degradation via sulfite activation. Inorg. Chem. Commun. 2021, 126, 108498. [Google Scholar] [CrossRef]



| Target Gene | Primers Sequences | Amplified Segment (bp) | References |
|---|---|---|---|
| csgd | TTACCGCCTGAGATTATCGT | 651 | [42] |
| ATGTTTAATGAAGTCCATAG |
| Samples/Swabs | No. Examined | No. Positive | Percentage (%) |
|---|---|---|---|
| Intestinal | 140 | 30 | 21.4 |
| Hand | 50 | 11 | 22.0 |
| Surface (Cutting broad) | 40 | 11 | 27.5 |
| Cutting knives | 40 | 6 | 15.0 |
| Rinsing water | 40 | 9 | 22.5 |
| Defeathering machine | 40 | 8 | 20.0 |
| Total | 350 | 75 | 25.0 |
| Antibiotic Sensitivity | Positive Samples (No.) | Antibiotics Used | |||||||||||||||||||||
| Samples/Swabs | |||||||||||||||||||||||
| - | Cefotaxime (CTX, 30 μg) | Ceftriaxone (CRO, 30 μg) | Ampicillin-Sulbactam (SAM, 10 μg) | Amoxicillin-Clavulanic (AMC, 30 μg) | Sulfamethoxazole-Trimethoprim (SXT, 25 μg) | Gentamicin (CN, 10 μg) | Ciprofloxacin (CIP, 5 μg) | ||||||||||||||||
| - | S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | ||
| Intestinal swab | 30 | 9 30.0 | 4 13.3 | 17 56.7 | 10 33.3 | - 0.0 | 20 66.7 | 8 26.7 | 1 3.3 | 21 70.0 | - 0.0 | - 0.0 | 30 100.0 | 4 13.3 | 2 6.7 | 24 80.0 | - 0.0 | 2 6.7 | 28 93.3 | 2 6.7 | 4 13.3 | 24 80.0 | |
| Hand swab | 11 | 1 9.1 | 3 27.3 | 7 63.6 | 4 36.4 | 2 18.2 | 5 72.7 | 2 18.2 | 1 9.1 | 8 72.7 | 1 9.1 | - 0.0 | 10 90.1 | - 0.0 | 2 18.2 | 9 81.8 | - 0.0 | 1 9.1 | 10 90.1 | - 0.0 | 1 9.1 | 10 90.1 | |
| Cutting broad Swab | 9 | 1 11.1 | 1 11.1 | 7 63.6 | 2 22.2 | 1 11.1 | 6 66.7 | - 0.0 | 1 11.1 | 8 88.9 | - 0.0 | 1 11.1 | 8 88.9 | 2 22.2 | - 0.0 | 7 63.6 | 1 11.1 | - 0.0 | 8 88.9 | 2 22.2 | 1 11.1 | 6 66.7 | |
| Cutting knives swab | 6 | 2 33.3 | - 0.0 | 4 66.7 | 1 16.7 | 1 16.7 | 4 66.7 | 2 33.3 | 1 16.7 | 3 50.0 | - 0.0 | - 0.0 | 6 100.0 | 2 33.3 | - 0.0 | 4 66.7 | - 0.0 | - 0.0 | 6 100.0 | 2 33.3 | - 0.0 | 4 66.7 | |
| Rinsing water | 11 | 2 18.2 | - 0.0 | 9 81.8 | 1 9.1 | 3 27.3 | 7 63.6 | 2 18.2 | - 0.0 | 9 81.8 | 1 9.1 | 1 9.1 | 9 81.8 | 3 27.3 | - 0.0 | 8 72.7 | 2 18.2 | - 0.0 | 9 81.8 | 3 27.3 | - 0.0 | 8 72.7 | |
| Defeathering machine | 8 | 2 25.0 | 1 12.5 | 5 62.5 | - 0.0 | 1 12.5 | 7 87.5 | 2 25.0 | 1 12.5 | 5 62.5 | 2 25.0 | - 0.0 | 6 75.5 | 1 12.0 | - 0.0 | 7 87.5 | 2 25.0 | 1 12.5 | 5 62.5 | - 0.0 | 1 12.5 | 7 87.5 | |
| Samples | Positive Samples (No.) | Cu/MOF | Cu/Ni/MOF | Co/Cu/Ni/MOF | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 | 0.05 | 0.01 | 0.05 | 0.01 | 0.05 | |||||||||
| R | S | R | S | R | S | R | S | R | S | R | S | |||
| Intestinal swab | 30 | 12 40.0 | 18 60.0 | 10 33.3 | 20 66.7 | 14 46.7 | 16 53.3 | 13 43.3 | 17 56.6 | 8 26.7 | 22 73.3 | 6 20.0 | 24 80.0 | 0.000 |
| Hand swab | 11 | 4 76.7 | 7 23.3 | 3 27.3 | 8 72.7 | 5 45.4 | 6 54.5 | 4 36.4 | 7 63.6 | 3 27.3 | 8 72.7 | 2 18.2 | 9 81.2 | 0.000 |
| Cutting broad Swab | 9 | 7 77.7 | 2 22.2 | 5 55.5 | 4 44.4 | 4 44.4 | 5 55.6 | 3 33.3 | 6 66.7 | 1 11.1 | 8 72.7 | 2 18.2 | 7 77.8 | 0.004 |
| Cutting knives swab | 6 | 6 66.7 | 3 33.3 | 4 44.4 | 5 55.6 | 3 50.0 | 3 50.0 | 3 50.0 | 3 50.0 | 2 33.3 | 4 66.7 | 2 33.3 | 4 66.7 | 0.002 |
| Rinsing water | 11 | 5 45.4 | 6 64.5 | 4 36.3 | 7 63.6 | 5 45.4 | 6 54.5 | 4 36.4 | 7 63.6 | 3 27.3 | 8 72.7 | 3 27.3 | 8 72.7 | 0.014 |
| Defeathering machine | 8 | 5 62.5 | 3 37.5 | 4 50.0 | 4 50.0 | 4 50.0 | 4 50.0 | 2 25.0 | 6 75.0 | 1 12.5 | 7 87.5 | 1 12.5 | 7 87.5 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, W.; Mahmoud, R.; Allah, A.E.; Farghali, A.A.; Abdelwahab, A.; Alkhalifah, D.H.M.; Hozzein, W.N.; Mohamed, M.B.E.D.; Abdel Aziz, S.A.A. Controlling Multi-Drug-Resistant Traits of Salmonella Obtained from Retail Poultry Shops Using Metal–Organic Framework (MOF) as a Novel Technique. Microorganisms 2023, 11, 2506. https://doi.org/10.3390/microorganisms11102506
Kamal W, Mahmoud R, Allah AE, Farghali AA, Abdelwahab A, Alkhalifah DHM, Hozzein WN, Mohamed MBED, Abdel Aziz SAA. Controlling Multi-Drug-Resistant Traits of Salmonella Obtained from Retail Poultry Shops Using Metal–Organic Framework (MOF) as a Novel Technique. Microorganisms. 2023; 11(10):2506. https://doi.org/10.3390/microorganisms11102506
Chicago/Turabian StyleKamal, W., Rehab Mahmoud, Abeer Enaiet Allah, Ahmed A. Farghali, Abdalla Abdelwahab, Dalal Hussien M. Alkhalifah, Wael N. Hozzein, Manar Bahaa El Din Mohamed, and Sahar Abdel Aleem Abdel Aziz. 2023. "Controlling Multi-Drug-Resistant Traits of Salmonella Obtained from Retail Poultry Shops Using Metal–Organic Framework (MOF) as a Novel Technique" Microorganisms 11, no. 10: 2506. https://doi.org/10.3390/microorganisms11102506





