Mycoviruses: Antagonistic Potential, Fungal Pathogenesis, and Their Interaction with Rhizoctonia solani
Abstract
1. Introduction
2. Pathogenesis Biology of Rhizoctonia solani
3. Mycoviruses
Symptoms of Mycoviruses and Their Interactions with Hosts
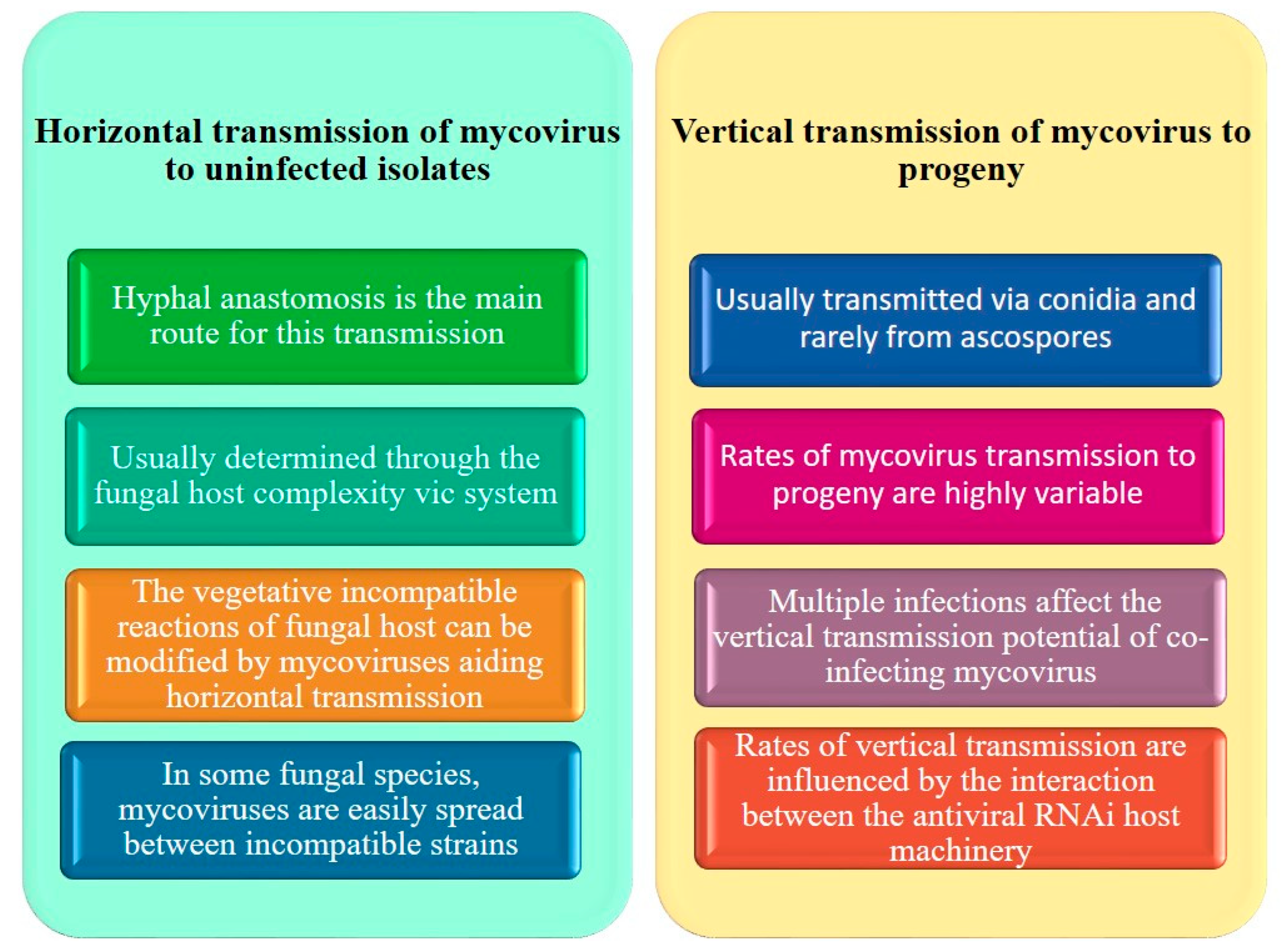
4. Transmission Properties of Fungi
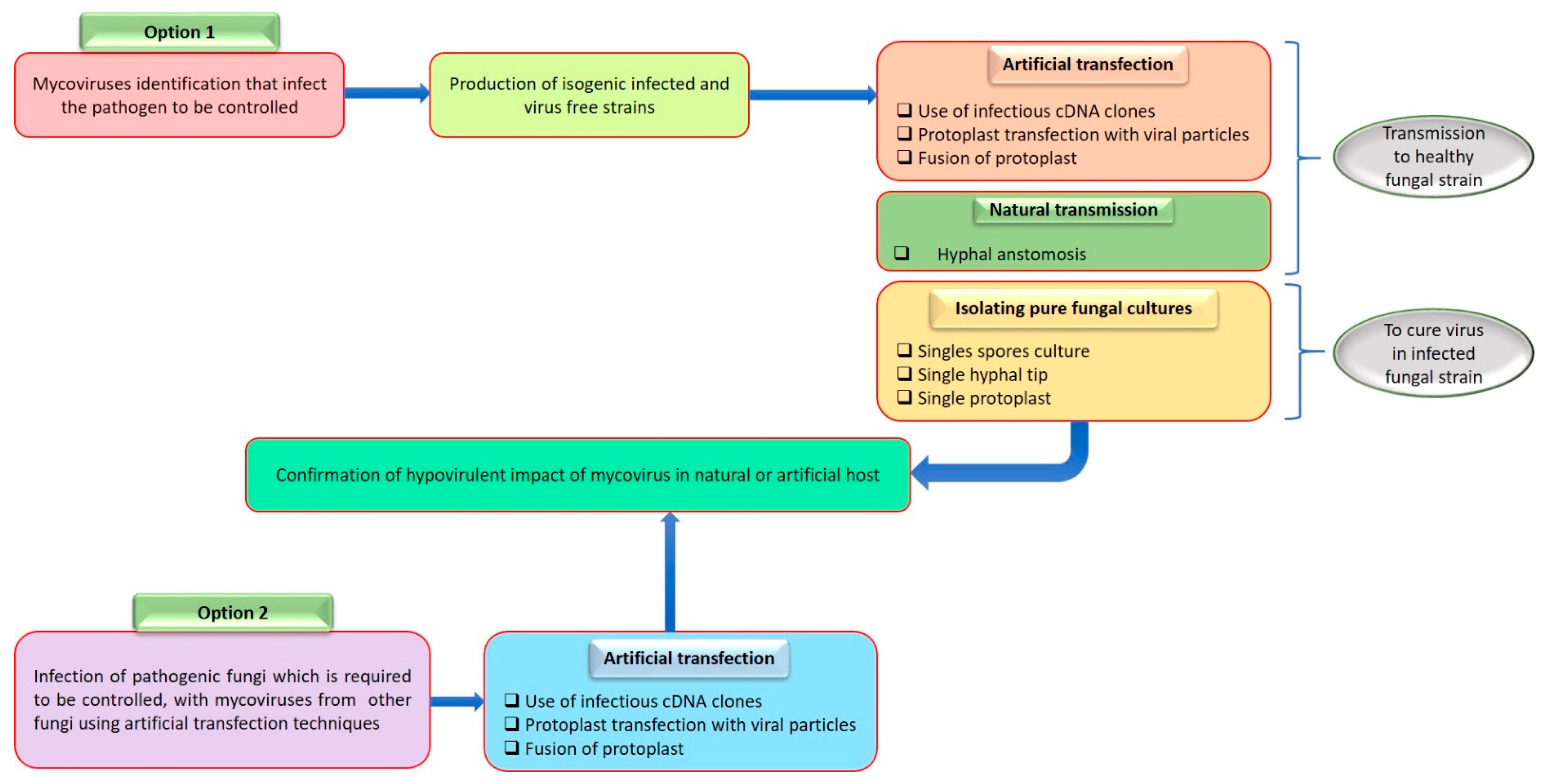
5. Transmission of Viruses in Rhizoctonia solani
6. Mycoviruses of Rhizoctonia solani
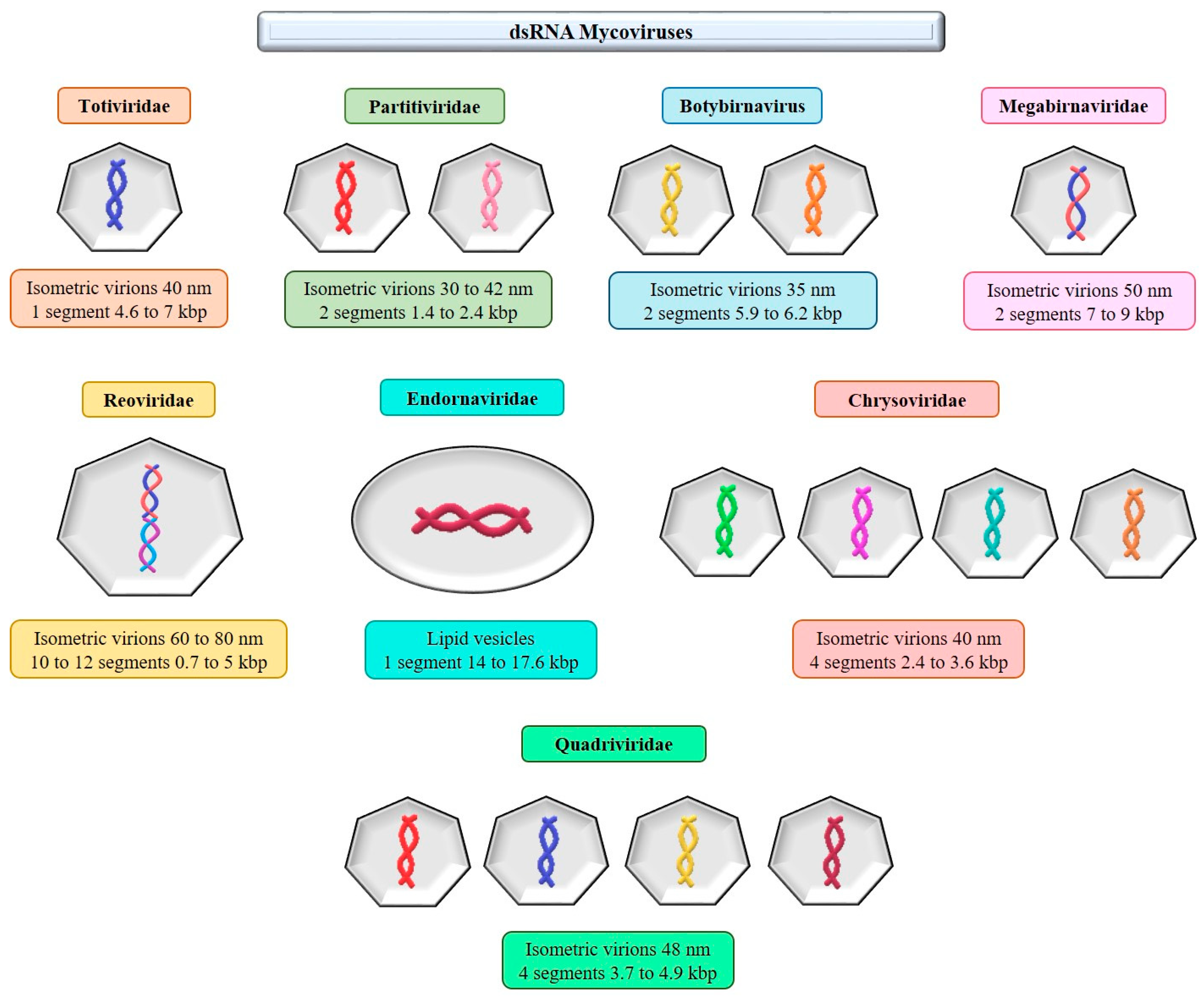
7. dsRNA Mycoviruses
7.1. Family Megabirnaviridae
7.2. Family Partitiviridae
8. Unclassified dsRNA Mycoviruses
9. ssRNA Mycoviruses
9.1. Family Barnaviridae
9.2. Family Benyviridae
9.3. Family Botourmiaviridae
9.4. Family Bromoviridae
9.5. Tymoviridae, Deltaflexiviridae, and Unidentified Viruses of the Tymovirales Order Are Mycoviruses
9.6. Family Endornaviridae
9.7. Family Hypoviridae
9.8. Mitoviridae and Narnaviridae Families
9.9. Family Togaviridae
9.10. Order Serpentovirales
9.11. Order Bunyavirales
10. Effects of Viral Infection in Rhizoctonia solani
11. Conclusions
12. Future Aspects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Genome | Family/Sub-Family | Genus | Species |
|---|---|---|---|
| ssDNA | Genomoviridae | Gemycircularvirus | Sclerotinia gemycircularvirus 1 |
| dsRNA | Amalgaviridae | Zybavirus | Zygosaccharomyces bailii virus Z |
| Alphachrysovirus | Penicillium chrysogenum virus | ||
| Chrysoviridae | Betachrysovirus | Botryosphaeria dothidea chrysovirus | |
| Megabirnaviridae | Megabirnavirus | Rosellinia necatrix megabirnavirus 1 | |
| Alphapartitivirus | Rosellinia necatrix partitivirus 2 | ||
| Betapartitivirus | Fusarium poae virus 1 | ||
| Partitiviridae | Cryspovirus | Cryptosporidium parvum virus 1 | |
| Gammapartitivirus | Penicillium stoloniferum virus S | ||
| Quadriviridae | Quadrivirus | Rosellinia necatrix quadrivirus 1 | |
| Reoviridae/Spinareovirinae | Mycoreovirus | Mycoreovirus 1 | |
| Totivirus | Saccharomyces cerevisiae virus L-A | ||
| Totiviridae | Victorivirus | Magnaporthe oryzae virus 1 | |
| Unclassified | Botybirnavirus | Botrytis porri botybirnavirus 1 | |
| (+) ssRNA | Alphaflexiviridae | Botrexvirus | Botrytis virus X |
| Sclerodarnavirus | Sclerotinia sclerotiorum debilitation-associated RNA virus | ||
| Barnaviridae | Barnavirus | Mushroom bacilliform virus | |
| Botourmiaviridae | Botoulivirus | Botrytis botoulivirus | |
| Magoulivirus | Magnaporthe magoulivirus | ||
| Scleroulivirus | Sclerotinia scleroulivirus 1 | ||
| Deltaflexiviridae | Deltaflexivirus | Sclerotinia deltaflexivirus 1 | |
| Endornaviridae | Alphaendornavirus | Oryza sativa alphaendornavirus | |
| Betaendornavirus | Sclerotinia sclerotiorum betaendornavirus 1 | ||
| Gammaflexiviridae | Mycoflexivirus | Botrytis virus F | |
| Hypoviridae | Hypovirus | Cryphonectria hypovirus 1 | |
| Narnaviridae | Mitovirus | Cryphonectria mitovirus 1 | |
| Narnavirus | Saccharomyces 20S RNA narnavirus | ||
| (−) ssRNA | Mymonaviridae | Sclerotimonavirus | Sclerotinia sclerotimonavirus |
| ssRNA-RT | Metaviridae | Metavirus | Saccharomyces cerevisiae Ty3 virus |
| Pseudoviridae | Hemivirus | Saccharomyces cerevisiae Ty5 virus | |
| Pseudovirus | Saccharomyces cerevisiae Ty1 virus |

References
- Srivastava, S.; Bist, V.; Srivastava, S.; Singh, P.C.; Trivedi, P.K.; Asif, M.H.; Chauhan, P.S.; Nautiyal, C.S. Unraveling Aspects of Bacillus amyloliquefaciens Mediated Enhanced Production of Rice under Biotic Stress of Rhizoctonia solani. Front. Plant Sci. 2016, 7, 587. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Sang, T.; Lu, B.R.; Hong, D.Y. Phylogeny of Rice Genomes with Emphasis on Origins of Allotetraploid Species. Proc. Natl. Acad. Sci. USA 1999, 96, 14400–14405. [Google Scholar] [CrossRef]
- Sanchez, P.L.; Wing, R.A.; Brar, D.S. The Wild Relative of Rice: Genomes and Genomics. In Genetics and Genomics of Rice; Zhang, Q., Wing, R.A., Eds.; Springer: New York, NY, USA, 2013; pp. 9–25. [Google Scholar] [CrossRef]
- Vaughan, D.A. The Wild Relatives of Rice: A Genetic Resources Handbook; IRRI, International Rice Research Institute: Manila, Philippines, 1994. [Google Scholar]
- Sreenivasaprasad, S.; Johnson, R.; Banniza, S.; Holderness, M. Rice Sheath Blight—Pathogen Biology and Diversity. In Major Fungal Diseases of Rice; Springer: Dordrecht, The Netherlands, 2001; pp. 201–2011. [Google Scholar]
- Padasht-Dehkaei, F.; Ceresini, P.C.; Zala, M.; Okhovvat, S.M.; Nikkhah, M.J.; McDonald, B.A. Population genetic evidence that basidiospores play an important role in the disease cycle of rice-infecting populations of Rhizoctonia solani AG-1 IA in Iran. Plant Pathol. 2012, 62, 49–58. [Google Scholar] [CrossRef]
- Lee, F.N. Rice Sheath Blight: A Major Rice Disease. Plant Dis. 1983, 67, 829. [Google Scholar] [CrossRef]
- Xia, Y.; Fei, B.; He, J.; Zhou, M.; Zhang, D.; Pan, L.; Li, S.; Liang, Y.; Wang, L.; Zhu, J.; et al. Transcriptome Analysis Reveals the Host Selection Fitness Mechanisms of the Rhizoctonia solani Ag1ia Pathogen. Sci. Rep. 2017, 7, 10120. [Google Scholar] [CrossRef]
- Gunnell, P.S.; Webster, R.K. Aggregate sheath spot of rice in California. Plant Dis. 1984, 68, 529–531. [Google Scholar] [CrossRef]
- Johanson, A. A PCR-Based Method to Distinguish Fungi of the Rice Sheath-Blight Complex, Rhizoctonia solani, R. oryzae and R. oryzae-sativae. FEMS Microbiol. Lett. 1998, 162, 289–294. [Google Scholar] [CrossRef]
- Andika, I.B.; Wei, S.; Cao, C.; Salaipeth, L.; Kondo, H.; Sun, L. Phytopathogenic Fungus Hosts a Plant Virus: A Naturally Occurring Cross-Kingdom Viral Infection. Proc. Natl. Acad. Sci. USA 2017, 114, 12267–12272. [Google Scholar] [CrossRef]
- Abdoulaye, A.H.; Foda, M.F.; Kotta-Loizou, I. Viruses Infecting the Plant Pathogenic Fungus Rhizoctonia solani. Viruses 2019, 11, 1113. [Google Scholar] [CrossRef]
- Slaton, N.A.; Cartwright, R.D.; Meng, J.; Gbur, E.E.; Norman, R.J. Sheath Blight Severity and Rice Yield as Affected by Nitrogen Fertilizer Rate, Application Method, and Fungicide. Agron. J. 2003, 95, 1489–1496. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, D. New Insights into Mycoviruses and Exploration for the Biological Control of Crop Fungal Diseases. Annu. Rev. Phytopathol. 2014, 52, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Gobbin, D.; Hoegger, P.J.; Heiniger, U.; Rigling, D. Sequence Variation and Evolution of Cryphonectria hypovirus 1 (CHV-1) in Europe. Virus Res. 2003, 97, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Umer, M.; Qadeer, A.; Razaq, Z.; Anwar, N.; Kiptoo, J.J. Mycovirus: Biocontrol Agent against S. sclerotiorum of Rapeseed. Phytopathogenom. Dis. Control 2022, 1, 97–108. [Google Scholar]
- Ram, R.M.; Singh, H. Rhizoctonia bataticola: A Serious Threat to Chickpea Production. Int. J. Chem. Stud. 2018, 6, 715–723. [Google Scholar]
- Anderson, J.P.; Kidd, B.N.; Garg, G.; Singh, K.B. Transcriptome Analysis Reveals Class IX Ethylene Response Factors Show Specific Up-Regulation in Resistant but Not Susceptible Medicago truncatula Lines Following Infection with Rhizoctonia solani. Eur. J. Plant Pathol. 2018, 152, 549–554. [Google Scholar] [CrossRef]
- Chi, Y.; Xu, M.; Yang, J.; Wang, F.; Wu, J. First Report of Rhizoctonia solani Causing Peanut Pod Rot in China. Plant Dis. 2016, 100, 1008. [Google Scholar] [CrossRef]
- Kiptoo, J.J.; Abbas, A.; Bhatti, A.M.; Usman, H.M.; Shad, M.A.; Umer, M.; Atiq, M.N.; Alam, S.M.; Ateeq, M.; Khan, M.; et al. Rhizoctonia solani of Potato and its Management: A Review. Plant Protect. 2021, 5, 157–169. [Google Scholar] [CrossRef]
- Agrios, G. Plant Pathology, 5th ed.; Elsevier Academic Press: Burlington, MA, USA, 2005; pp. 79–103. [Google Scholar]
- Uppala, S.; Zhou, X. Rice Sheath Blight. Plant Health Instr. 2018. [Google Scholar] [CrossRef]
- Ghosh, P.; Sen, S.; Chakraborty, J.; Das, S. Monitoring the Efficacy of Mutated Allium sativum Leaf Lectin in Transgenic Rice against Rhizoctonia solani. BMC Biotechnol. 2016, 16, 24. [Google Scholar] [CrossRef]
- Costanzo, S.; Jackson, A.K.; Brooks, S.A. High-resolution Mapping of Rsn1, A Locus Controlling Sensitivity of Rice to a Necrosis-Inducing Phytotoxin from Rhizoctonia solani AG1-IA. Theor. Appl. Genet. 2011, 123, 33–41. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, J.; Yang, X.; Zhang, A.-F.; Gao, T.-C. Sensitivity of Rhizoctonia solani Causing Rice Sheath Blight to Fuxapyroxad in China. Eur. J. Plant Pathol. 2014, 140, 419–428. [Google Scholar] [CrossRef]
- de França, S.K.S.; Cardoso, A.F.; Lustosa, D.C.; Ramos, E.M.L.S.; de Filippi, M.C.C.; da Silva, G.B. Biocontrol of Sheath Blight by Trichoderma asperellum in Tropical Lowland Rice. Agron. Sustain. Dev. 2014, 35, 317–324. [Google Scholar] [CrossRef]
- Son, M.; Yu, J.; Kim, K.-H. Five Questions about Mycoviruses. PLoS Pathog. 2015, 11, e1005172. [Google Scholar] [CrossRef] [PubMed]
- Shafik, K.; Umer, M.; You, H.; Aboushedida, H.; Wang, Z.; Ni, D.; Xu, W. Characterization of a Novel Mitovirus Infecting Melanconiella theae Isolated from Tea Plants. Front. Microbiol. 2021, 12, 757556. [Google Scholar] [CrossRef]
- Li, Z.; Chen, L.; Meiling, Z.; Mei, Y.; Erxun, Z. Diversity of DsRNA Viruses Infecting Rice Sheath Blight Fungus Rhizoctonia solani AG-1 IA. Rice Sci. 2018, 25, 57–60. [Google Scholar] [CrossRef]
- Bartholomäus, A.; Wibberg, D.; Winkler, A.; Pühler, A.; Schlüter, A.; Varrelmann, M. Deep Sequencing Analysis Reveals the Mycoviral Diversity of the Virome of an Avirulent Isolate of Rhizoctonia solani AG-2-2 IV. PLoS ONE 2016, 11, e0165965. [Google Scholar] [CrossRef]
- Abbas, A. A Review Paper on Mycoviruses. J. Plant Pathol. Microbiol. 2016, 7, 12. [Google Scholar] [CrossRef]
- Kotta-Loizou, I.; Coutts, R.H.A. Mycoviruses in Aspergilli: A Comprehensive Review. Front. Microbiol. 2017, 8, 1699. [Google Scholar] [CrossRef]
- Hillman, B.I.; Annisa, A.; Suzuki, N. Viruses of Plant-Interacting Fungi. Adv. Virus Res. 2018, 100, 99–116. [Google Scholar] [CrossRef]
- Eusebio-Cope, A.; Sun, L.; Tanaka, T.; Chiba, S.; Kasahara, S.; Suzuki, N. The Chestnut Blight Fungus for Studies on Virus/Host and Virus/Virus Interactions: From a Natural to a Model Host. Virology 2015, 477, 164–175. [Google Scholar] [CrossRef]
- Feau, N.; Dutech, C.; Brusini, J.; Rigling, D.; Robin, C. Multiple Introductions and Recombination in Cryphonectria hypovirus 1: Perspective for a Sustainable Biological Control of Chestnut Blight. Evol. Appl. 2014, 7, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Brusini, J.; Robin, C. Mycovirus Transmission Revisited by in Situ Pairings of Vegetatively Incompatible Isolates of Cryphonectria parasitica. J. Virol. Methods 2013, 187, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Zilio, G.; Thiévent, K.; Koella, J.C. Host Genotype and Environment Affect the Trade-off between Horizontal and Vertical Transmission of the Parasite Edhazardia aedis. BMC Evol. Biol. 2018, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.; Saldierna Guzmán, J.; Shay, J. Transmission of Bacterial Endophytes. Microorganisms 2017, 5, 70. [Google Scholar] [CrossRef]
- Ghabrial, S.A.; Castón, J.R.; Jiang, D.; Nibert, M.L.; Suzuki, N. 50-plus Years of Fungal Viruses. Virology 2015, 479–480, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Chan, D.; Xiang, Y.; Williams, H.; Li, X.-R.; Sniezko, R.A.; Sturrock, R.N. Characterization of Five Novel Mitoviruses in the White Pine Blister Rust Fungus Cronartium Ribicola. PLoS ONE 2016, 11, e0154267. [Google Scholar] [CrossRef][Green Version]
- Zheng, L.; Zhang, M.; Chen, Q.; Zhu, M.; Zhou, E. A Novel Mycovirus Closely Related to Viruses in the Genus Alphapartitivirus Confers Hypovirulence in the Phytopathogenic Fungus Rhizoctonia solani. Virology 2014, 456–457, 220–226. [Google Scholar] [CrossRef]
- Jian, J.; Lakshman, D.K.; Tavantzis, S.M. Association of Distinct Double-Stranded RNAs with Enhanced or Diminished Virulence in Rhizoctonia solani Infecting Potato. Mol. Plant Microbe Interact. 1997, 10, 1002–1009. [Google Scholar] [CrossRef]
- Chen, Y.; Tong Gai, X.; Xing Chen, R.; Li, C.X.; Zhao, G.K.; Yuan Xia, Z.; Zou, C.M.; Zhong, J. Characterization of Three Novel Betapartitiviruses Co-Infecting the Phytopathogenic Fungus Rhizoctonia solani. Virus Res. 2019, 270, 197649. [Google Scholar] [CrossRef]
- Lakshman, D.K.; Jian, J.; Tavantzis, S.M. A Double-Stranded RNA Element from a Hypovirulent Strain of Rhizoctonia solani Occurs in DNA Form and Is Genetically Related to the Pentafunctional AROM Protein of the Shikimate Pathway. Proc. Natl. Acad. Sci. USA 1998, 95, 6425–6429. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Fujita, M.; Chiba, S.; Hyodo, K.; Andika, I.B.; Suzuki, N.; Kondo, H. Two Novel Fungal Negative-Strand RNA Viruses Related to Mymonaviruses and Phenuiviruses in the Shiitake Mushroom (Lentinula edodes). Virology 2019, 533, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Liu, H.; Zhang, M.; Cao, X.; Zhou, E. The Complete Genomic Sequence of a Novel Mycovirus from Rhizoctonia solani AG-1 IA Strain B275. Arch. Virol. 2013, 158, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Chen, C.-Y.; Gao, B.-D. Genome Sequence of a Novel Mycovirus of Rhizoctonia solani, a Plant Pathogenic Fungus. Virus Genes 2015, 51, 167–170. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, L.; Liu, C.; Shu, C.; Zhou, E. Characterization of a Novel DsRNA Mycovirus Isolated from Strain A105 of Rhizoctonia solani AG-1 IA. Arch. Virol. 2018, 163, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Shu, C.; Zhang, M.; Yang, M.; Zhou, E. Molecular characterization of a novel endornavirus conferring hypovirulence in rice sheath blight fungus Rhizoctonia solani AG- 1 IA Strain GD-2. Viruses 2019, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Falloon, R.E.; Stewart, A.; Pitman, A.R. Molecular Characterization of an Endornavirus from Rhizoctonia solani AG-3PT Infecting Potato. Fungal Biol. 2014, 118, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Falloon, R.E.; Stewart, A.; Pitman, A.R. Novel Mitoviruses in Rhizoctonia solani AG- 3PT Infecting Potato. Fungal Biol. 2016, 120, 338–350. [Google Scholar] [CrossRef]
- Jia, H.; Dong, K.; Zhou, L.; Wang, G.; Hong, N.; Jiang, D.; Xu, W. A DsRNA Virus with Filamentous Viral Particles. Nat. Commun. 2017, 8, 168. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, J.F.; Yang, J.; Jiang, L.; Li, D. Characterization of a Novel Bipartite Double- Stranded RNA Mycovirus Conferring Hypovirulence in the Phytopathogenic Fungus Botrytis porri. J. Virol. 2012, 86, 6605–6619. [Google Scholar] [CrossRef]
- Zhai, L.; Zhang, M.; Hong, N.; Xiao, F.; Fu, M.; Xiang, J.; Wang, G. Identification and Characterization of a Novel Hepta-Segmented DsRNA Virus from the Phytopathogenic Fungus Colletotrichum fructicola. Front. Microbiol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Lo, G.C.S.; Chow, F.W.N.; Fan, R.Y.Y.; Cai, J.J.; Yuen, K.-Y.; Woo, P.C.Y. Novel Partitivirus Enhances Virulence of and Causes Aberrant Gene Expression in Talaromyces marneffei. MBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Miyazaki, N.; Kanematsu, S.; Xie, J.; Ghabrial, S.A.; Hillman, B.I.; Suzuki, N.; Ictv Report Consortium. ICTV Virus Taxonomy Profile: Megabirnaviridae. J. Gen. Virol. 2019, 100, 1269–1270. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Salaipeth, L.; Lin, Y.-H.; Sasaki, A.; Kanematsu, S.; Suzuki, N. A Novel Bipartite Double- Stranded RNA Mycovirus from the White Root Rot Fungus Rosellinia necatrix: Molecular and Biological Characterization, Taxonomic Considerations, and Potential for Biological Control. J. Virol. 2009, 83, 12801–12812. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Sun, X.; Cheng, J.; Fu, Y.; Liu, H.; Jiang, D.; Ghabrial, S.A.; Xie, J. Characterization of a Novel Megabirnavirus from Sclerotinia Sclerotiorum Reveals Horizontal Gene Transfer from Single-Stranded RNA Virus to Double-Stranded RNA Virus. J. Virol. 2015, 89, 8567–8579. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Nakamura, H.; Suzuki, N.; Kanematsu, S. Characterization of a New Megabirnavirus That Confers Hypovirulence with the Aid of a Co-Infecting Partitivirus to the Host Fungus, Rosellinia necatrix. Virus Res. 2016, 219, 73–82. [Google Scholar] [CrossRef]
- Nerva, L.; Ciuffo, M.; Vallino, M.; Margaria, P.; Varese, G.C.; Gnavi, G.; Turina, M. Multiple Approaches for the Detection and Characterization of Viral and Plasmid Symbionts from a Collection of Marine Fungi. Virus Res. 2016, 219, 22–38. [Google Scholar] [CrossRef]
- Velasco, L.; Arjona-Girona, I.; Cretazzo, E.; López-Herrera, C. Viromes in Xylariaceae Fungi Infecting Avocado in Spain. Virology 2019, 532, 11–21. [Google Scholar] [CrossRef]
- Vainio, E.J.; Chiba, S.; Ghabrial, S.A.; Maiss, E.; Roossinck, M.; Sabanadzovic, S.; Suzuki, N.; Xie, J.; Nibert, M.; Ictv Report Consortium. ICTV Virus Taxonomy Profile: Partitiviridae. J. Gen. Virol. 2018, 99, 17–18. [Google Scholar] [CrossRef]
- Xiao, X.; Cheng, J.; Tang, J.; Fu, Y.; Jiang, D.; Baker, T.S.; Ghabrial, S.A.; Xie, J. A Novel Partitivirus That Confers Hypovirulence on Plant Pathogenic Fungi. J. Virol. 2014, 88, 10120–10133. [Google Scholar] [CrossRef] [PubMed]
- Picarelli, M.A.S.C.; Forgia, M.; Rivas, E.B.; Nerva, L.; Chiapello, M.; Turina, M.; Colariccio, A. Extreme Diversity of Mycoviruses Present in Isolates of Rhizoctonia solani AG2-2 LP from Zoysia japonica from Brazil. Front. Cell. Infect. Microbiol. 2019, 9, 244. [Google Scholar] [CrossRef]
- Jian, J.; Lakshman, D.K.; Tavantzis, S.M. A Virulence-Associated, 6.4-Kb, Double-Stranded RNA from Rhizoctonia solani Is Phylogenetically Related to Plant Bromoviruses and Electron Transport Enzymes. Mol. Plant Microbe Interact. 1998, 11, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Zoll, J.; Verweij, P.E.; Melchers, W.J.G. Discovery and Characterization of Novel Aspergillus Fumigatus Mycoviruses. PLoS ONE 2018, 13, e0200511. [Google Scholar] [CrossRef] [PubMed]
- Usui, K.; Ichihashi, N.; Yomo, T. A Design Principle for a Single-Stranded RNA Genome That Replicates with Less Double-Strand Formation. Nucleic Acids Res. 2015, 43, 8033–8043. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Dolja, V.V. Evolution and Taxonomy of Positive-Strand RNA Viruses: Implications of Comparative Analysis of Amino Acid Sequences. Crit. Rev. Biochem. Mol. Biol. 1993, 28, 375–430. [Google Scholar] [CrossRef] [PubMed]
- Reguera, J.; Gerlach, P.; Cusack, S. Towards a Structural Understanding of RNA Synthesis by Negative Strand RNA Viral Polymerases. Curr. Opin. Struct. Biol. 2016, 36, 75–84. [Google Scholar] [CrossRef]
- Chen, X.; He, H.; Yang, X.; Zeng, H.; Qiu, D.; Guo, L. The Complete Genome Sequence of a Novel Fusarium graminearum RNA Virus in a New Proposed Family within the Order Tymovirales. Arch. Virol. 2016, 161, 2899–2903. [Google Scholar] [CrossRef]
- Liu, L.; Xie, J.; Cheng, J.; Fu, Y.; Li, G.; Yi, X.; Jiang, D. Fungal Negative-Stranded RNA Virus That Is Related to Bornaviruses and Nyaviruses. Proc. Natl. Acad. Sci. USA 2014, 111, 12205–12210. [Google Scholar] [CrossRef]
- Revill, P.A.; Davidson, A.D.; Wright, P.J. The Nucleotide Sequence and Genome Organization of Mushroom Bacilliform Virus: A Single-Stranded RNA Virus of Agaricus bisporus (Lange) Imbach. Virology 1994, 202, 904–911. [Google Scholar] [CrossRef]
- Marzano, S.-Y.L.; Domier, L.L. Novel Mycoviruses Discovered from Metatranscriptomics Survey of Soybean Phyllosphere Phytobiomes. Virus Res. 2016, 213, 332–342. [Google Scholar] [CrossRef]
- Gilmer, D.; Ratti, C.; Ictv Report Consortium. ICTV Virus Taxonomy Profile: Benyviridae. J. Gen. Virol. 2017, 98, 1571–1572. [Google Scholar] [CrossRef]
- Kondo, H.; Hirano, S.; Chiba, S.; Andika, I.B.; Hirai, M.; Maeda, T.; Tamada, T. Characterization of Burdock Mottle Virus, a Novel Member of the Genus Benyvirus, and the Identification of Benyvirus-Related Sequences in the Plant and Insect Genomes. Virus Res. 2013, 177, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Marzano, S.-Y.L.; Nelson, B.D.; Ajayi-Oyetunde, O.; Bradley, C.A.; Hughes, T.J.; Hartman, G.L.; Eastburn, D.M.; Domier, L.L. Identification of Diverse Mycoviruses through Metatranscriptomics Characterization of the Viromes of Five Major Fungal Plant Pathogens. J. Virol. 2016, 90, 6846–6863. [Google Scholar] [CrossRef] [PubMed]
- Sztuba-Solinska, J.; Bujarski, J.J. Insights into the Single-Cell Reproduction Cycle of Members of the Family Bromoviridae: Lessons from the Use of Protoplast Systems. J. Virol. 2008, 82, 10330–10340. [Google Scholar] [CrossRef]
- King, A.M.; Lefkowitz, E.; Adams, M.J.; Carstens, E.B. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier: Amsterdam, The Netherlands, 2011; Volume 9. [Google Scholar]
- Ghanem-Sabanadzovic, N.A.; Tzanetakis, I.E.; Sabanadzovic, S. Rubus Canadensis Virus 1, a Novel Betaflexivirus Identified in Blackberry. Arch. Virol. 2013, 158, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Howitt, R.L.; Beever, R.E.; Pearson, M.N.; Forster, R.L. Genome Characterization of Botrytis Virus F, a Flexuous Rod-Shaped Mycovirus Resembling Plant ‘Potex-like’ Viruses. J. Gen. Virol. 2001, 82, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Charles, J.; Tangudu, C.S.; Hurt, S.L.; Tumescheit, C.; Firth, A.E.; Garcia-Rejon, J.E.; Machain-Williams, C.; Blitvich, B.J. Discovery of a Novel Tymoviridae-like Virus in Mosquitoes from Mexico. Arch. Virol. 2019, 164, 649–652. [Google Scholar] [CrossRef]
- Davison, A.J. Journal of General Virology-Introduction to ‘ICTV Virus Taxonomy Profiles. J. Gen. Virol. 2017, 98, 1. [Google Scholar] [CrossRef]
- Roossinck, M.J.; Sabanadzovic, S.; Okada, R.; Valverde, R.A. The Remarkable Evolutionary History of Endornaviruses. J. Gen. Virol. 2011, 92, 2674–2678. [Google Scholar] [CrossRef]
- Okada, R.; Kiyota, E.; Moriyama, H.; Fukuhara, T.; Valverde, R.A. Molecular and Biological Properties of an Endornavirus Infecting Winged Bean (Psophocarpus tetragonolobus). Virus Genes 2017, 53, 141–145. [Google Scholar] [CrossRef]
- Okada, R.; Kiyota, E.; Moriyama, H.; Toshiyuki, F.; Valverde, R.A. A New Endornavirus Species Infecting Malabar Spinach (Basella alba L.). Arch. Virol. 2014, 159, 807–809. [Google Scholar] [CrossRef]
- Okada, R.; Yong, C.K.; Valverde, R.A.; Sabanadzovic, S.; Aoki, N.; Hotate, S.; Kiyota, E.; Moriyama, H.; Fukuhara, T. Molecular Characterization of Two Evolutionarily Distinct Endornaviruses Co-Infecting Common Bean (Phaseolus vulgaris). J. Gen. Virol. 2013, 94, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Hillman, B.I.; Halpern, B.T.; Brown, M.P. A Viral dsRNA Element of the Chestnut Blight Fungus with a Distinct Genetic Organization. Virology 1994, 201, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Linder-Basso, D.; Dynek, J.N.; Hillman, B.I. Genome Analysis of Cryphonectria hypovirus 4, the Most Common Hypovirus Species in North America. Virology 2005, 337, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Aulia, A.; Andika, I.B.; Kondo, H.; Hillman, B.I.; Suzuki, N. A Symptomless Hypovirus, CHV4, Facilitates Stable Infection of the Chestnut Blight Fungus by a Coinfecting Reovirus Likely through Suppression of Antiviral RNA Silencing. Virology 2019, 533, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xiao, X.; Fu, Y.; Liu, H.; Cheng, J.; Ghabrial, S.A.; Li, G.; Jiang, D. A Novel Mycovirus Closely Related to Hypoviruses That Infects the Plant Pathogenic Fungus Sclerotinia sclerotiorum. Virology 2011, 418, 49–56. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, S.; Chiba, S.; Kondo, H.; Kanematsu, S.; Suzuki, N. A Novel Single-Stranded RNA Virus Isolated from a Phytopathogenic Filamentous Fungus, Rosellinia necatrix, with Similarity to Hypo-like Viruses. Front. Microbiol. 2014, 5, 360. [Google Scholar] [CrossRef]
- Niu, Y.; Yuan, Y.; Mao, J.; Yang, Z.; Cao, Q.; Zhang, T.; Wang, S.; Liu, D. Characterization of Two Novel Mycoviruses from Penicillium digitatum and the Related Fungicide Resistance Analysis. Sci. Rep. 2018, 8, 5513. [Google Scholar] [CrossRef]
- Nerva, L.; Forgia, M.; Ciuffo, M.; Chitarra, W.; Chiapello, M.; Vallino, M.; Varese, G.C.; Turina, M. The Mycovirome of a Fungal Collection from the Sea Cucumber Holothuria polii. Virus Res. 2019, 273, 197737. [Google Scholar] [CrossRef]
- Marais, A.; Nivault, A.; Faure, C.; Theil, S.; Comont, G.; Candresse, T.; Corio-Costet, M.-F. Determination of the Complete Genomic Sequence of Neofusicoccum luteum Mitovirus 1 (NLMV1), a Novel Mitovirus Associated with a Phytopathogenic Botryosphaeriaceae. Arch. Virol. 2017, 162, 2477–2480. [Google Scholar] [CrossRef][Green Version]
- Das, S. Rhizoctonia solani on Potato in New Zealand: Pathogen Characterization and Identification of Double-Stranded RNA Viruses That May Affect Their Virulence. Ph.D. Thesis, Lincoln University, Jefferson City, MO, USA, 2013. [Google Scholar]
- Khan, A.H.; Morita, K.; Parquet, M.D.C.; Hasebe, F.; Mathenge, E.G.M.; Igarashi, A. Complete Nucleotide Sequence of Chikungunya Virus and Evidence for an Internal Polyadenylation Site. J. Gen. Virol. 2002, 83, 3075–3084. [Google Scholar] [CrossRef]
- Chen, R.; Mukhopadhyay, S.; Merits, A.; Bolling, B.; Nasar, F.; Coffey, L.L.; Powers, A.; Weaver, S.C.; Ictv Report Consortium. ICTV Virus Taxonomy Profile: Togaviridae. J. Gen. Virol. 2018, 99, 761–762. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jose, J.; Xiang, Y.; Kuhn, R.J.; Rossmann, M.G. Structural Changes of Envelope Proteins during Alphavirus Fusion. Nature 2010, 468, 705–708. [Google Scholar] [CrossRef] [PubMed]
- García, M.L.; Bó, D.; Da Graça, E.; Gago-Zachert, J.V.; Hammond, S.; Moreno, J.; Natsuaki, P.; Pallás, T.; Navarro, V.; Reyes, J.A. ICTV Virus Taxonomy Profile: Ophioviridae. J. Gen. Virol. 2017, 98, 1161–1162. [Google Scholar] [CrossRef] [PubMed]
- Dawe, A.L.; Nuss, D.L. Hypovirus Molecular Biology: From Koch’s Postulates to Host Self- Recognition Genes That Restrict Virus Transmission. Adv. Virus Res. 2013, 86, 109–147. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umer, M.; Mubeen, M.; Shakeel, Q.; Ali, S.; Iftikhar, Y.; Bajwa, R.T.; Anwar, N.; Rao, M.J.; He, Y. Mycoviruses: Antagonistic Potential, Fungal Pathogenesis, and Their Interaction with Rhizoctonia solani. Microorganisms 2023, 11, 2515. https://doi.org/10.3390/microorganisms11102515
Umer M, Mubeen M, Shakeel Q, Ali S, Iftikhar Y, Bajwa RT, Anwar N, Rao MJ, He Y. Mycoviruses: Antagonistic Potential, Fungal Pathogenesis, and Their Interaction with Rhizoctonia solani. Microorganisms. 2023; 11(10):2515. https://doi.org/10.3390/microorganisms11102515
Chicago/Turabian StyleUmer, Muhammad, Mustansar Mubeen, Qaiser Shakeel, Sajjad Ali, Yasir Iftikhar, Rabia Tahir Bajwa, Naureen Anwar, Muhammad Junaid Rao, and Yuejun He. 2023. "Mycoviruses: Antagonistic Potential, Fungal Pathogenesis, and Their Interaction with Rhizoctonia solani" Microorganisms 11, no. 10: 2515. https://doi.org/10.3390/microorganisms11102515
APA StyleUmer, M., Mubeen, M., Shakeel, Q., Ali, S., Iftikhar, Y., Bajwa, R. T., Anwar, N., Rao, M. J., & He, Y. (2023). Mycoviruses: Antagonistic Potential, Fungal Pathogenesis, and Their Interaction with Rhizoctonia solani. Microorganisms, 11(10), 2515. https://doi.org/10.3390/microorganisms11102515











