Lipid Production from Native Oleaginous Yeasts Isolated from Southern Chilean Soil Cultivated in Industrial Vinasse Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast and Culture Conditions
2.2. Industrial Residual Substrate Vinasse (IRV) P5
2.3. Evaluation of Lipid Accumulation of the Six Yeast Strains in Synthetic Mineral Medium (SMM) and Selection of Two Oleaginous Strains
2.4. Effect of Physicochemical Variables on Lipid Accumulation in Selected Strains
2.5. Kinetics of Growth and Lipid Accumulation in Optimized Industrial Residual
2.6. Analytical Methods
2.6.1. Quantification of Cell Biomass (Yeast) and Lipids
2.6.2. PCR Amplification and Yeast DNA Sequencing
2.6.3. Molecular identification of yeast strains
2.7. Statistical Analysis
3. Results
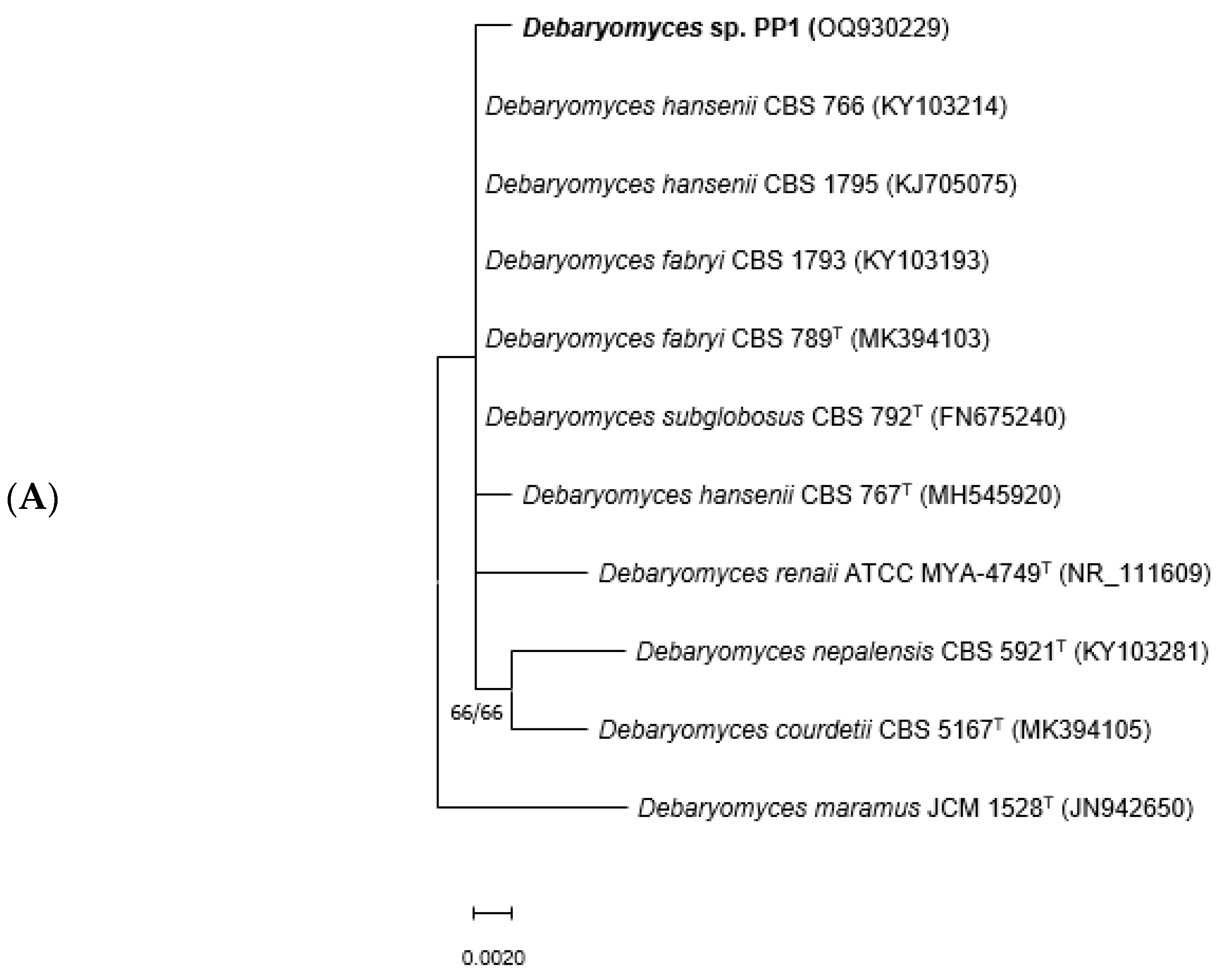
3.1. Molecular Identification of Strains
3.2. Yeast Culture in Synthetic Mineral Medium (SMM)
3.3. Effect of Physicochemical Variables on Lipid Accumulation in Selected Strains Cultured in IRV
3.4. Determination of Growth Kinetics of Yeasts Cultured in Vinasse
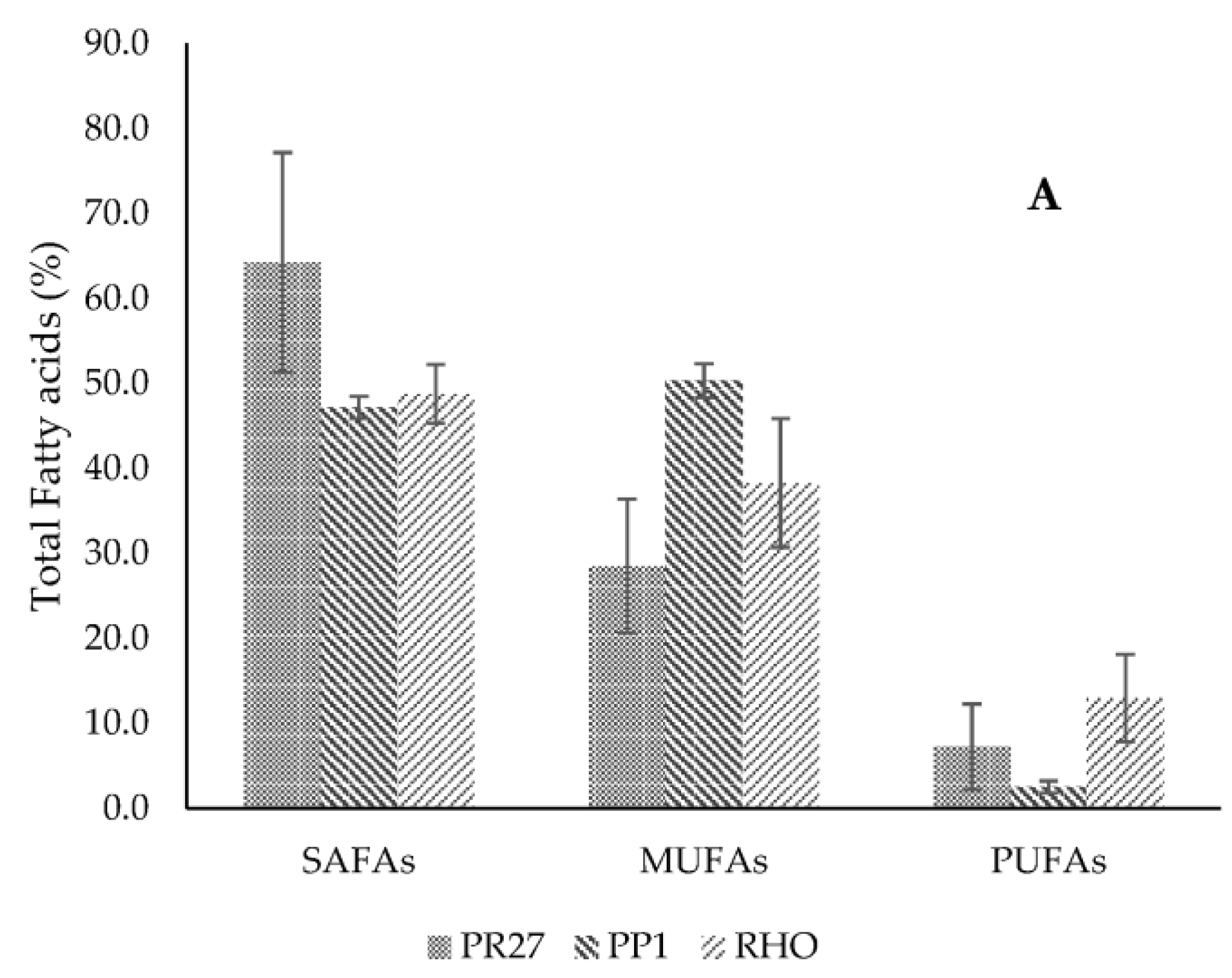
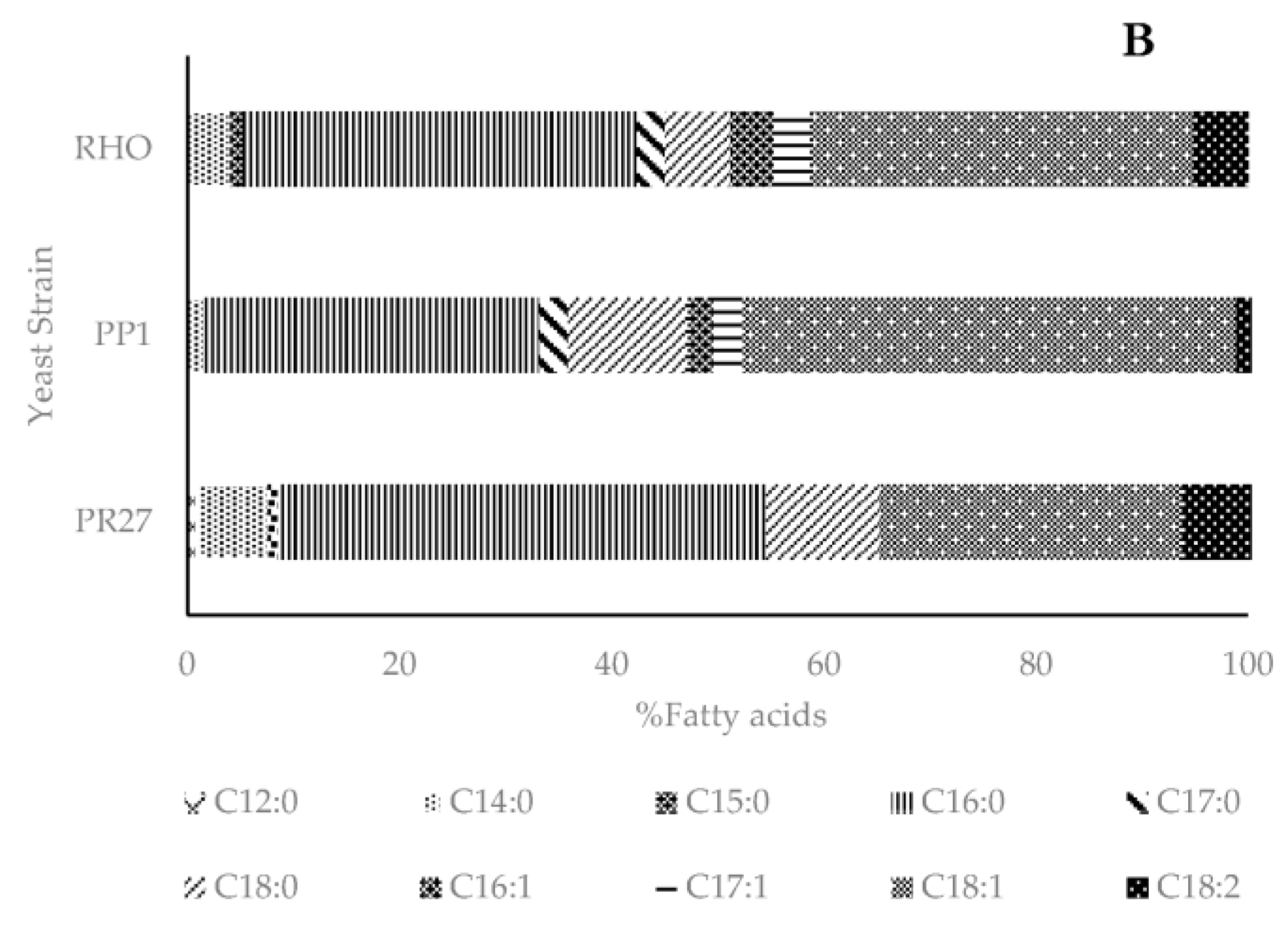
Lipid Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karayannis, D.; Papanikolaou, S.; Vatistas, C.; Paris, C.; Chevalot, I. Yeast Lipid Produced through Glycerol Conversions and Its Use for Enzymatic Synthesis of Amino Acid-Based Biosurfactants. Int. J. Mol. Sci. 2023, 24, 714. [Google Scholar] [CrossRef]
- Fabiszewska, A.; Paplińska-Goryca, M.; Misiukiewicz-Stępień, P.; Wołoszynowska, M.; Nowak, D.; Zieniuk, B. Expression Profile of Selected Genes Involved in Storage Lipid Synthesis in a Model Oleaginous Yeast Species Yarrowia lipolytica. Int. J. Mol. Sci. 2022, 23, 1041. [Google Scholar] [CrossRef] [PubMed]
- Gemperlein, K.; Dietrich, D.; Kohlstedt, M.; Zipf, G.; Bernauer, H.S.; Wittmann, C.; Wenzel, S.C.; Müller, R. Polyunsaturated fatty acid production by Yarrowia lipolytica employing designed myxobacterial PUFA synthases. Nat. Commun. 2019, 10, 4055. [Google Scholar] [CrossRef]
- Grubišić, M.; Mihajlovski, K.; Gruičić, A.M.; Beluhan, S.; Šantek, B.; Ivančić Šantek, M. Strategies for Improvement of Lipid Production by Yeast Trichosporon oleaginosus from Lignocellulosic Biomass. J. Fungi 2021, 7, 934. [Google Scholar] [CrossRef] [PubMed]
- Jach, M.E.; Malm, A. Yarrowia lipolytica as an alternative and valuable source of nutritional and bioactive compounds for humans. Molecules 2022, 27, 2300. [Google Scholar]
- Sirakov, I.; Velichkova, K.; Stoyanova, S.; Staykov, Y. The importance of microalgae for aquaculture industry. Review. Int. J. Fish. Aquat. Stud. 2015, 2, 81–84. [Google Scholar]
- Tacon, P.; Auclair, E. Yeast in aquaculture from nutrition to well-being. Aqua Cult. Asia Pac. 2010, 6, 22–24. [Google Scholar]
- Sahlmann, C.; Djordjevic, B.; Lagos, L.; Mydland, L.T.; Morales-Lange, B.; Hansen, J.Ø.; Øverland, M. Yeast as a protein source during smoltification of Atlantic salmon (Salmo salar L.), enhances performance and modulates health. Aquaculture 2019, 513, 734396. [Google Scholar]
- Mekonnen, M.M.; Hoekstra, A.Y. Water footprint benchmarks for crop production: A first global assessment. Ecol. Indic. 2014, 46, 214–223. [Google Scholar]
- Agboola, J.O.; Mensah, D.D.; Hansen, J.Ø.; Lapeña, D.; Mydland, L.T.; Arntzen, M.Ø.; Horn, S.J.; Øyås, O.; Press, C.M.; Øverland, M. Effects of Yeast Species and Processing on Intestinal Health and Transcriptomic Profiles of Atlantic Salmon (Salmo salar) Fed Soybean Meal-Based Diets in Seawater. Int. J. Mol. Sci. 2022, 23, 1675. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Matsakas, L.; Sartaj, K.; Chandra, R. Extraction of lipids from algae using supercritical carbon dioxide. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–39. [Google Scholar]
- Sprague, M.; Betancor, M.B.; Tocher, D.R. Microbial and genetically engineered oils as replacements for fish oil in aquaculture feeds. Biotechnol. Lett. 2017, 39, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Rajak, R.C.; Rajlakshmi; Saravanabhupathy, S.; Banerjee, R. Chapter 9 Microbial lipids production by oleaginous yeasts. In Biomass Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 161–189. [Google Scholar] [CrossRef]
- Karamerou, E. Colin Webb, Cultivation modes for microbial oil production using oleaginous yeasts—A review. Biochem. Eng. J. 2019, 151, 107322. [Google Scholar] [CrossRef]
- Morales-Palomo, S.; Tomás-Pejó, E.; González-Fernández, C. Phosphate limitation as crucial factor to enhance yeast lipid production from short-chain fatty acids. Microb. Biotechnol. 2023, 16, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Poorinmohammad, N.; Fu, J.; Wabeke, B.; Kerkhoven, E.J. Validated Growth Rate-Dependent Regulation of Lipid Metabolism in Yarrowia lipolytica. Int. J. Mol. Sci. 2022, 23, 8517. [Google Scholar] [CrossRef] [PubMed]
- Arous, F.; Triantaphyllidou, I.; Mechichi, T.; Azabou, S.; Nasri, M.; Aggelis, G. Lipid accumulation in the new oleaginous yeast Debaryomyces etchellsii correlates with ascosporogenesis. Biomass Bioenergy 2015, 80, 307–315. [Google Scholar] [CrossRef]
- Amaretti, A.; Raimondi, S.; Sala, M.; Roncaglia, L.; De Lucia, M.; Leonardi, A.; Rossi, M. Single cell oils of the cold-adapted oleaginous yeast Rhodotorula glacialis DBVPG 4785. Microb. Cell Fact. 2010, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Aggelis, G. Lipid productionby Yarrowia lipolytica growing on industrial glycerol in a single-stage continuous culture. Bioresour. Technol. 2002, 82, 43–49. [Google Scholar] [CrossRef]
- Rakicka, M.; Lazar, Z.; Dulermo, T.; Fickers, P.; Nicaud, J.M. Lipid production by the oleaginous yeast Yarrowia lipolytica using industrial by-products under different culture conditions. Biotechnol Biofuels. 2015, 8, 104. [Google Scholar] [CrossRef]
- Tanimura, A.; Takashima, M.; Sugita, T.; Endoh, R.; Kikukawa, M.; Yamaguchi, S.; Sakuradani, E.; Ogawa, J.; Shima, J. Selection of oleaginous yeasts with high lipid productivity for practical biodiesel production. Bioresour. Technol. 2014, 153, 30–235. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, S.; Odoh, C.K.; Jin, M.; Zhao, Z.K. Rhodosporidium toruloides—A potential red yeast chassis for lipids and beyond. FEMS Yeast Res. 2020, 20, foaa038. [Google Scholar] [CrossRef]
- Beopoulos, A.; Haddouche, R.; Kabran, P.; Dulermo, T.; Chardot, T.; Nicaud, J.M. Identification and characterization of DGA2, an acyltransferase of the DGAT1 acyl-CoA: Diacylglycerol acyltransferase family in the oleaginous yeast Yarrowia lipolytica. New insights into the storage lipid metabolism of oleaginous yeasts. Appl. Microbiol. Biotechnol. 2012, 93, 1523–1537. [Google Scholar] [CrossRef]
- Evans, C.T.; Ratledge, C. Influence of nitrogen metabolism on lipid accumulation by Rhodosporidium toruloides CBS 14. Microbiology 1984, 130, 1705–1710. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Bryś, J.; Reczek, L.; Pobiega, K. Effect of exogenous stress factors on the biosynthesis of carotenoids and lipids by Rhodotorula yeast strains in media containing agro-industrial waste. World J. Microbiol. Biotechnol. 2019, 35, 157. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Vázquez, D.; Orozco-Nunnelly, D.A.; Yebra-Montes, C.; Senés-Guerrero, C.; Gradilla-Hernández, M.S. Using yeast cultures to valorize tequila vinasse waste: An example of a circular bioeconomy approach in the agro-industrial sector. Biomass Bioenergy 2022, 161, 106471. [Google Scholar] [CrossRef]
- Gientka, I.; Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Janowicz, M.; Reczek, L.; Synowiec, A.; Błażejak, S. Enhancing Red Yeast Biomass Yield and Lipid Biosynthesis by Using Waste Nitrogen Source by Glucose Fed-Batch at Low Temperature. Microorganisms 2022, 10, 1253. [Google Scholar] [CrossRef]
- Younes, S.; Bracharz, F.; Awad, D.; Qoura, F.; Mehlmer, N.; Brueck, T. Microbial lipid production by oleaginous yeasts grown on Scenedesmus obtusiusculus microalgae biomass hydrolysate. Bioprocess Biosyst. Eng. 2020, 43, 1629–1638. [Google Scholar] [CrossRef]
- Muñoz-Tamayo, R.; Aceves-Lara, C.; Bideaux, C. Optimization of lipid production by oleaginous yeast in continuous culture. IFAC Proc. Vol. 2014, 47, 6210–6215. [Google Scholar] [CrossRef]
- Soto-Sánchez, O.; Hidalgo, P.; González, A.; Oliveira, P.E.; Hernández Arias, A.J.; Dantagnan, P. Microalgae as Raw Materials for Aquafeeds: Growth Kinetics and Improvement Strategies of Polyunsaturated Fatty Acids Production. Aquac. Nutr. 2023, 2023, 5110281. [Google Scholar] [CrossRef]
- Ledesma-Amaro, R.; Nicaud, J.M. Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids. Prog. Lipid Res. 2016, 61, 40–50. [Google Scholar]
- Dyaa, A.; Soliman, H.; Abdelrazak, A.; Samra, B.N.; Khojah, E.; Ahmed, A.F.; El-Esawi, M.A.; Elsayed, A. Optimization of Carotenoids Production from Rhodotorula sp. Strain ATL72 for Enhancing Its Biotechnological Applications. J. Fungi 2022, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.N.; Múgica, P.; Sá-Correia, I. Exploring Yeast Diversity to Produce Lipid-Based Biofuels from Agro-Forestry and Industrial Organic Residues. J. Fungi 2022, 8, 687. [Google Scholar] [CrossRef]
- Donzella, S.; Serra, I.; Fumagalli, A.; Pellegrino, L.; Mosconi, G.; Scalzo, R.L.; Compagno, C. Recycling industrial food wastes for lipid production by oleaginous yeasts Rhodosporidiobolus azoricus and Cutaneotrichosporon oleaginosum. Biotechnol. Biofuels Bioprod. 2022, 15, 51. [Google Scholar] [CrossRef]
- Chang, Y.; Chang, K.; Hsu, C.; Chuang, L.; Chen, C.; Huang, F.; Jang, H. A comparative study on batch and fed-batch cultures of oleaginous yeast Cryptococcus sp. in glucose-based media and corncob hydrolysate for microbial oil production. Fuel 2013, 105, 711–717. [Google Scholar] [CrossRef]
- Acevedo, F.; Gentina, J.C.; Illanes, A. Fundamentos de Ingeniería Bioquímica, 347; Ediciones Universitarias de Valparaíso: Valparaíso, Chile, 2002. [Google Scholar]
- Zhang, L.; Lim, E.Y.; Loh, K.-C.; Dai, Y.; Tong, Y.W. Two-Stage Fermentation of Lipomyces starkeyi for Production of Microbial Lipids and Biodiesel. Microorganisms 2021, 9, 1724. [Google Scholar] [CrossRef]
- Poontawee, R.; Yongmanitchai, W.; Limtong, S. Lipid production from a mixture of sugarcane top hydrolysate and biodiesel-derived crude glycerol by the oleaginous red yeast, Rhodosporidiobolus fluvialis. Process Biochem. 2018, 66, 150–161. [Google Scholar] [CrossRef]
- Lapeña, D.; Olsen, P.M.; Arntzen, M.Ø.; Kosa, G.; Passoth, V.; Eijsink, V.G.; Horn, S.J. Spruce sugars and poultry hydrolysate as growth medium in repeated fed-batch fermentation processes for production of yeast biomass. Bioprocess Biosyst. Eng. 2020, 43, 723–736. [Google Scholar] [PubMed]
- Brandenburg, J.; Blomqvist, J.; Shapaval, V.; Kohler, A.; Sampels, S.; Sandgren, M.; Passoth, V. Oleaginous yeasts respond differently to carbon sources present in lignocellulose hydrolysate. Biotechnol. Biofuels 2021, 14, 124. [Google Scholar] [CrossRef]
- Gorte, O.; Kugel, M.; Ochsenreither, K. Optimization of carbon source efficiency for lipid production with the oleaginous yeast Saitozyma podzolica DSM 27192 applying automated continuous feeding. Biotechnol. Biofuels 2020, 13, 181. [Google Scholar] [CrossRef]
- Lin, J.; Shen, H.; Tan, H.; Zhao, X.; Wu, S.; Hu, C.; Zhao, Z.K. Lipid production by Lipomyces starkeyi cells in glucose solution without auxiliary nutrients. J. Biotechnol. 2011, 152, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhang, S.; Wang, Q.; Tan, H.; Zhao, Z.K. The isocitrate dehydrogenase gene of oleaginous yeast Lipomyces starkeyi is linked to lipid accumulation. Can. J. Microbiol. 2012, 55, 1062–1069. [Google Scholar] [CrossRef]
- Xue, Z.; Sharpe, P.L.; Hong, S.P.; Yadav, N.S.; Xie, D.; Short, D.R.; Damude, H.G.; Rupert, R.A.; Seip, J.E.; Wang, J.; et al. Production of omega-3 eicosapentaenoic acid by metabolic engineering of Yarrowia lipolytica. Nat. Biotechnol. 2013, 31, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wei, Y.; Ji, B.; Nielsen, J. Advances in Metabolic Engineering of Saccharomyces cerevisiae for Cocoa Butter Equivalent Production. Front. Bioeng. Biotechnol. 2020, 8, 594081. [Google Scholar] [CrossRef]
- Hu, C.; Wu, S.; Wang, Q.; Jin, G.; Shen, H.; Zhao, Z.K. Simultaneous utilization of glucose and xylose for lipid production by trichosporon cutaneum. Biotechnol. Biofuels 2011, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Konzock, O.; Matsushita, Y.; Zaghen, S.; Sako, A.; Norbeck, J. Altering the fatty acid profile of Yarrowia lipolytica to mimic cocoa butter by genetic engineering of desaturases. Microb. Cell Fact. 2022, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Siewers, V.; Nielsen, J. Cocoa butter-like lipid production ability of non-oleaginous and oleaginous yeasts under nitrogen-limited culture conditions. Appl. Microbiol. Biotechnol. 2017, 101, 3577–3585. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.E.; Aranda, C.; Martínez, O.; Godoy, R.; Gonzales, A.; Valenzuela, E. Characterization of yeast in hapludands soil with biotechnological potential. J. Soil Sci. Plant Nutr. 2017, 17, 948–965. [Google Scholar] [CrossRef]
- Adel, A.; El-Baz, A.; Shetaia, Y.; Sorour, N.M. Biosynthesis of polyunsaturated fatty acids by two newly cold-adapted Egyptian marine yeast. 3 Biotech 2021, 11, 461. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byprod-UCTS, and Degradation Products in Liquid Fraction Process Samples; National Renewable Energy Laboratory: Golden, CO, USA, 2006; Volume 11, pp. 65–71. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Maza, D.D.; Viñarta, S.C.; Su, Y.; Guillamón, J.M.; Aybar, M.J. Growth and lipid production of R C/N R4, in comparison to other oleaginous yeasts. J. Biotechnol. 2020, 310, 21–31. [Google Scholar] [CrossRef]
- Poontawee, R.; Limtong, S. Feeding Strategies of Two-Stage Fed-Batch Cultivation Processes for Micro-bial Lipid Production from Sugarcane Top Hydrolysate and Crude Glycerol by the Oleaginous Red Yeast Rhodosporidiobolus fluvialis. Microorganisms 2020, 8, 151. [Google Scholar] [CrossRef]
- Guerreiro, F.; Constantino, A.; Lima-Costa, E.; Raposo, S. A new combined approach to improved lipid production using a strictly aerobic and oleaginous yeast. Eng. Life Sci. 2018, 19, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Vu, D.; Groenewald, M.; Szöke, S.; Cardinali, G.; Eberhardt, U.; Stielow, B.; de Vries, M.; Verkleij, G.J.M.; Crous, P.W.; Boekhout, T.; et al. DNA barcoding analysis of more than 9000 yeast isolates contributes to quantitative thresholds for yeast species and genera delimitation. Stud. Mycol. 2016, 85, 91–105. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Groenewald, M.; Daniel, H.-M.; Robert, V.; Poot, G.A.; Smith, M.T. Polyphasic re-examination of Debaryomyces hansenii strains and reinstatement of D. hansenii, D. fabryi and D. subglobosus. Persoonia 2008, 21, 17–27. [Google Scholar] [CrossRef]
- Silva, C.F.; Arcuri, S.L.; Campos, C.R.; Vilela, D.M.; Alves, J.G.; Schwan, R.F. Using the residue of spirit production and bio-ethanol for protein production by yeasts. Waste Manag. 2011, 31, 108–114. [Google Scholar] [PubMed]
- García, A.; Rojas, C. Posibilidades de Uso de la Vinaza en la Agricultura de Acuerdo con su Modo de Acción en los Suelos. 2006. Available online: www.tecnicana.org/pdf/2006/tec_v10_no17_2006_p3-13.pdf (accessed on 10 May 2023).
- Fernandes, B.S.; Vieira, J.P.F.; Contesini, F.J.; Mantelatto, P.E.; Zaiat, M.; Pradella, J.G.D.C. High value added lipids produced by microorganisms: A potential use of sugarcane vinasse. Crit. Rev. Biotechnol. 2017, 37, 1048–1061. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, I.; Belghith, H.; Gargouri, A.; Guerfali, M. Utilization of Wheat Bran Acid Hydrolysate by Rhodotorula mucilaginosa Y-MG1 for Microbial Lipid Production as Feedstock for Biodiesel Synthesis. BioMed Res. Int. 2019, 2019, 3213521. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, Y.; Dorgan, K.M.; Chen, S. Oil production by oleaginous yeasts using the hydrolysate from pretreatment of wheat straw with dilute sulfuric acid. Bioresour. Technol. 2011, 102, 6134–6140. [Google Scholar]
- Patel, A.; Sartaj, K.; Arora, N.; Pruthi, V.; Pruthi, P.A. Biodegradation of phenol via meta cleavage pathway triggers de novo TAG biosynthesis pathway in oleaginous yeast. J. Hazard. Mater. 2017, 340, 47–56. [Google Scholar] [CrossRef]
- Takashima, M.; Kurakado, S.; Cho, O.; Kikuchi, K.; Sugiyama, J.; Sugita, T. Description of four Apiotrichum and two Cutaneotrichosporon species isolated from guano samples from bat-infested caves in Japan. Int. J. Syst. Evol. Microbiol. 2020, 70, 4458–4469. [Google Scholar] [CrossRef]
- Li, A.-H.; Yuan, F.-X.; Groenewald, M.; Bensch, K.; Yurkov, A.M.; Li, K.; Han, P.-J.; Guo, L.-D.; Aime, M.C.; Sampaio, J.; et al. Diversity and phylogeny of basidiomycetous yeasts from plant leaves and soil: Proposal of two new orders, three new families, eight new genera and one hundred and seven new species. Stud. Mycol. 2020, 96, 17–140. [Google Scholar] [CrossRef]
- Li, Q.; Xiao, W.; Wu, P.; Zhang, T.; Xiang, P.; Wu, Q.; Zou, L.; Gui, M. The first two mitochondrial genomes from Apiotrichum reveal mitochondrial evolution and different taxonomic assignment of Trichosporonales. IMA Fungus 2023, 14, 1–16. [Google Scholar] [CrossRef]
- Liu, X.Z.; Wang, G.M.; Göker, M.; Groenewald, M.; Kachalkin, A.V.; Lumbsch, H.T.; Millanes, A.M.; Wedin, M.; Yurkov, A.M.; Bai, F.Y.; et al. Towards an integrated phylogenetic classification of the Tremellomycetes. Stud. Mycol. 2015, 81, 85–147. [Google Scholar] [PubMed]
- Aliyu, H.; Gorte, O.; de Maayer, P.; Neumann, A.; Ochsenreither, K. Genomic insights into the lifestyles, functional capacities and oleagenicity of members of the fungal family Trichosporonaceae. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Qian, X.; Zhou, X.; Chen, L.; Zhang, X.; Xin, F.; Dong, W.; Zhang, W.; Ochsenreither, K.; Jiang, M. Bioconversion of volatile fatty acids into lipids by the oleaginous yeast Apiotrichum porosum DSM27194. Fuel 2020, 290, 119811. [Google Scholar] [CrossRef]
- Park, W.-S.; Murphy, P.A.; Glatz, B.A. Lipid metabolism and cell composition of the oleaginous yeast Apiotrichum curvatum grown at different carbon to nitrogen ratios. Can. J. Microbiol. 1990, 36, 318–326. [Google Scholar] [CrossRef]
- Gorte, O.; Aliyu, H.; Neumann, A.; Ochsenreither, K. Draft Genome Sequence of the Oleaginous Yeast Apiotrichum porosum (syn. Trichosporon porosum) DSM 27194. J. Genom. 2019, 7, 11–13. [Google Scholar] [CrossRef]
- Šantek, M.I.; Lisičar, J.; Mušak, L.; Špoljarić, I.V.; Beluhan, S.; Šantek, B. Lipid Production by Yeast Trichosporon oleaginosus on the Enzymatic Hydrolysate of Alkaline Pre-treated Corn Cobs for Biodiesel Production. Energy Fuels 2018, 32, 12501–12513. [Google Scholar]
- Shen, Q.; Lin, H.; Wang, Q.; Fan, X.; Yang, Y.; Zhao, Y. Sweetpotato vines hydrolysate promotes single cell oils production of Trichosporon fermentans in high-density molasses fermentation. Bioresour. Technol. 2015, 176, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.; Huang, Q.; Li, Q.; Chu, Y.; Yu, X.; Limtong, S.; Xue, S.; Zhao, Z.K. Conversion of Arthrospira platensis Biomass into Microbial Lipids by the Oleaginous Yeast Cryptococcus curvatus. ACS Sustain. Chem. Eng. 2021, 9, 11011–11021. [Google Scholar] [CrossRef]
- Shaigani, P.; Awad, D.; Redai, V.; Fuchs, M.; Haack, M.; Mehlmer, N.; Brueck, T. Oleaginous yeasts- substrate preference and lipid productivity: A view on the performance of microbial lipid producers. Microb. Cell Fact. 2021, 20, 220. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Quantity * |
|---|---|
| Water | 94.1 ± 3 g/L |
| Total soluble solids | 10° ± 0.5° BRIX |
| Density | 1.0047 ± 0.2 g/mL |
| pH | 6.6 ± 0.2 |
| Total protein | 2.6 ± 0.3% |
| Fat matter | 0.004 ± 0% |
| Soluble carbohydrates | 8 ± 0.9% |
| Glucose | 1.8 ± 0.2% |
| Fructose | 1.8 ± 0.2% |
| Factor | Level | Biomass g/L | Lipid Productivity (g L−1 Day) | Lipid Content (g/L Lipids) | |||
|---|---|---|---|---|---|---|---|
| PR27 | PP1 | PR27 | PP1 | PR27 | PP1 | ||
| T°C | 25 | 5.21 ± 0.37 * | 7.52 ± 0.86 * | 0.47 ± 0.11 | 0.31 ± 0.09 | 2.83 ± 0.65 | 1.87 ± 0.49 |
| 30 | 4.28 ± 0.73 | 6.35 ± 0.12 | 0.41 ± 0.17 | 0.32 ± 0.02 | 2.417 ± 1.07 | 1.975 ± 0.97 | |
| 37 | 4.80 ± 0.85 | 6.03 ± 0.40 | 0.116 ± 0.10 | 0.1695 ± 0.02 | 0.699 ± 0.06 | 1.017 ± 0.13 | |
| pH | 4 | 5.783 ± 0.85 | 13.86 ± 1.12 * | 0.113 ± 0.01 | 0.286 ± 0.14 | 0.68 ± 0.06 | 1.72 ± 0.86 |
| 5.5 | 7.64 ± 1.29 * | 6.49 ± 0.22 | 0.176 ± 0.02 | 0.243 ± 0.09 | 1.056 ± 0.12 | 1.459 ± 0.59 | |
| 7 | 4.32 ± 0.67 | 6.53 ± 0.52 | 0.43 ± 0.12 | 0.328 ± 0.09 | 2.63 ± 0.69 | 1.97 ± 0.58 | |
| C/N ratio | 20 | 7.3 ± 0.65 | 6.03 ± 0.56 | 0.33 ± 0.13 | 0.295 ± 0.08 | 2.03 ± 0.78 | 1.77 ± 0.50 |
| 50 | 11.46 ± 0.71 | 15.83 ± 0.57 * | 0.34 ± 0.14 | 0.38 ± 0.13 | 2.04 ± 0.69 | 2.29 ± 0.78 | |
| 80 | 14.8 ± 1.51 * | 14.8 ± 1.52 | 0.65 ± 0.36 | 0.76 ± 0.02 | 3.981 ± 0.22 | 4.614 ± 0.14 | |
| Parameter | PP1 Strain | PR27 Strain | ||||||
|---|---|---|---|---|---|---|---|---|
| Biomass | Lipids | Biomass | Lipids | |||||
| Temperature | −0.301 | * | −0.379 | −0.413 | * | −0.406 | * | |
| C:N ratio | 0.876 | * | 0.833 | * | 0.895 | * | 0.655 | * |
| pH | 0.033 | 0.271 | 0.105 | 0.586 | * | |||
| Yeast | Dry Weight g/L | YX * | ** Lipid Productivity (g L−1 d−1) | µ (h−1) |
|---|---|---|---|---|
| PP1 | 14.9 ± 1.52 | 0.11 | 0.69 | 0.14 |
| PR27 | 11.3 ± 1.71 | 0.07 | 0.57 | 0.12 |
| RHO | 16.7 ± 1.11 | 0.12 | 0.70 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Navarrete, P.; Marileo, L.; Madrid, H.; Belezaca-Pinargote, C.; Dantagnan, P. Lipid Production from Native Oleaginous Yeasts Isolated from Southern Chilean Soil Cultivated in Industrial Vinasse Residues. Microorganisms 2023, 11, 2516. https://doi.org/10.3390/microorganisms11102516
Díaz-Navarrete P, Marileo L, Madrid H, Belezaca-Pinargote C, Dantagnan P. Lipid Production from Native Oleaginous Yeasts Isolated from Southern Chilean Soil Cultivated in Industrial Vinasse Residues. Microorganisms. 2023; 11(10):2516. https://doi.org/10.3390/microorganisms11102516
Chicago/Turabian StyleDíaz-Navarrete, Paola, Luis Marileo, Hugo Madrid, Carlos Belezaca-Pinargote, and Patricio Dantagnan. 2023. "Lipid Production from Native Oleaginous Yeasts Isolated from Southern Chilean Soil Cultivated in Industrial Vinasse Residues" Microorganisms 11, no. 10: 2516. https://doi.org/10.3390/microorganisms11102516
APA StyleDíaz-Navarrete, P., Marileo, L., Madrid, H., Belezaca-Pinargote, C., & Dantagnan, P. (2023). Lipid Production from Native Oleaginous Yeasts Isolated from Southern Chilean Soil Cultivated in Industrial Vinasse Residues. Microorganisms, 11(10), 2516. https://doi.org/10.3390/microorganisms11102516






