A Uniform Design Method Can Optimize the Combinatorial Parameters of Antimicrobial Photodynamic Therapy, Including the Concentrations of Methylene Blue and Potassium Iodide, Light Dose, and Methylene Blue’s Incubation Time, to Improve Fungicidal Effects on Candida Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cells and Culture Conditions
2.3. Light Source
2.4. Study Design
2.5. aPDT Studies
2.5.1. U7(74) Uniform Design Method
2.5.2. U6(64) Uniform Design Method
2.5.3. aPDT Applications
2.6. Statistical Analysis
3. Results
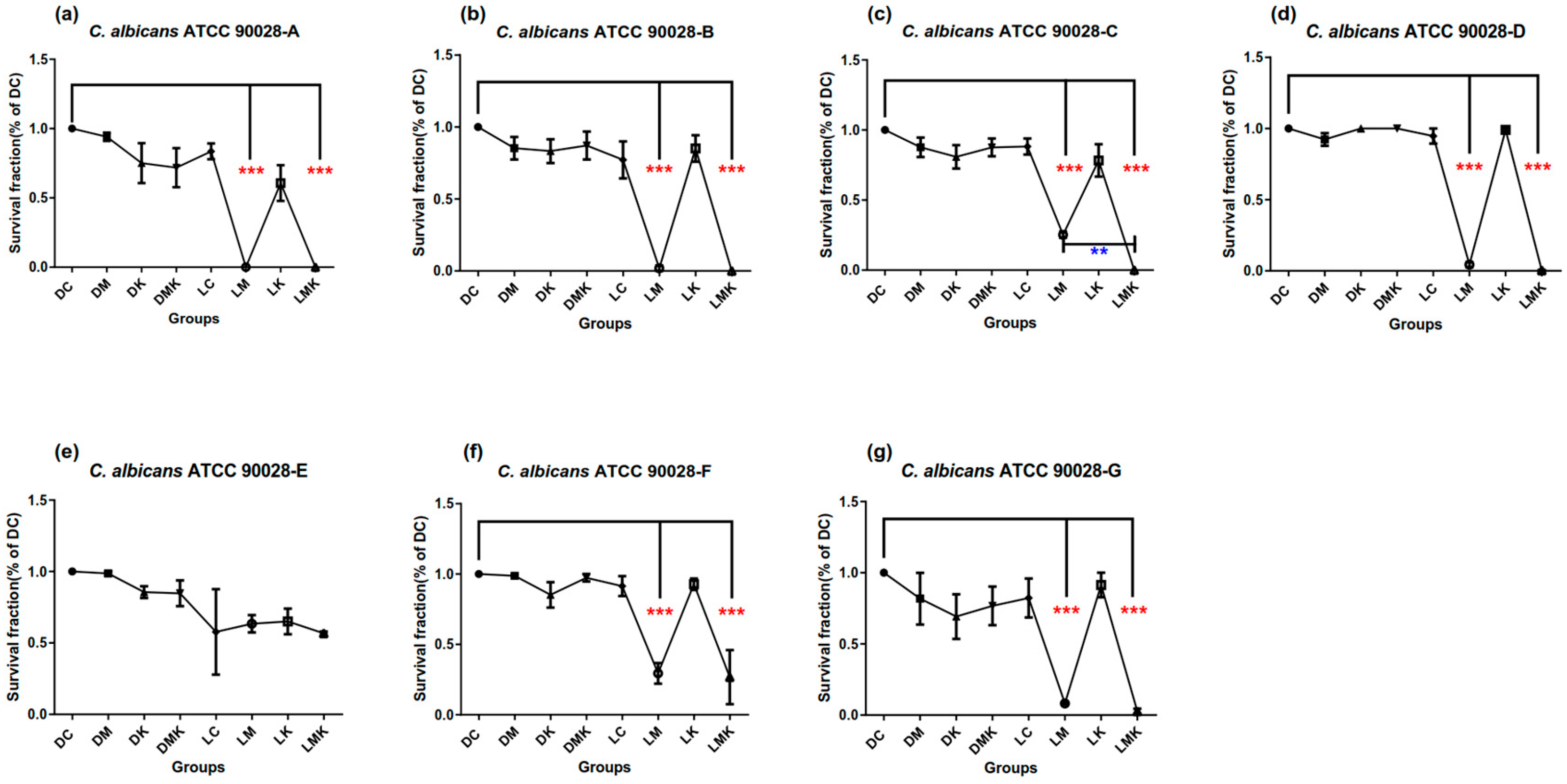
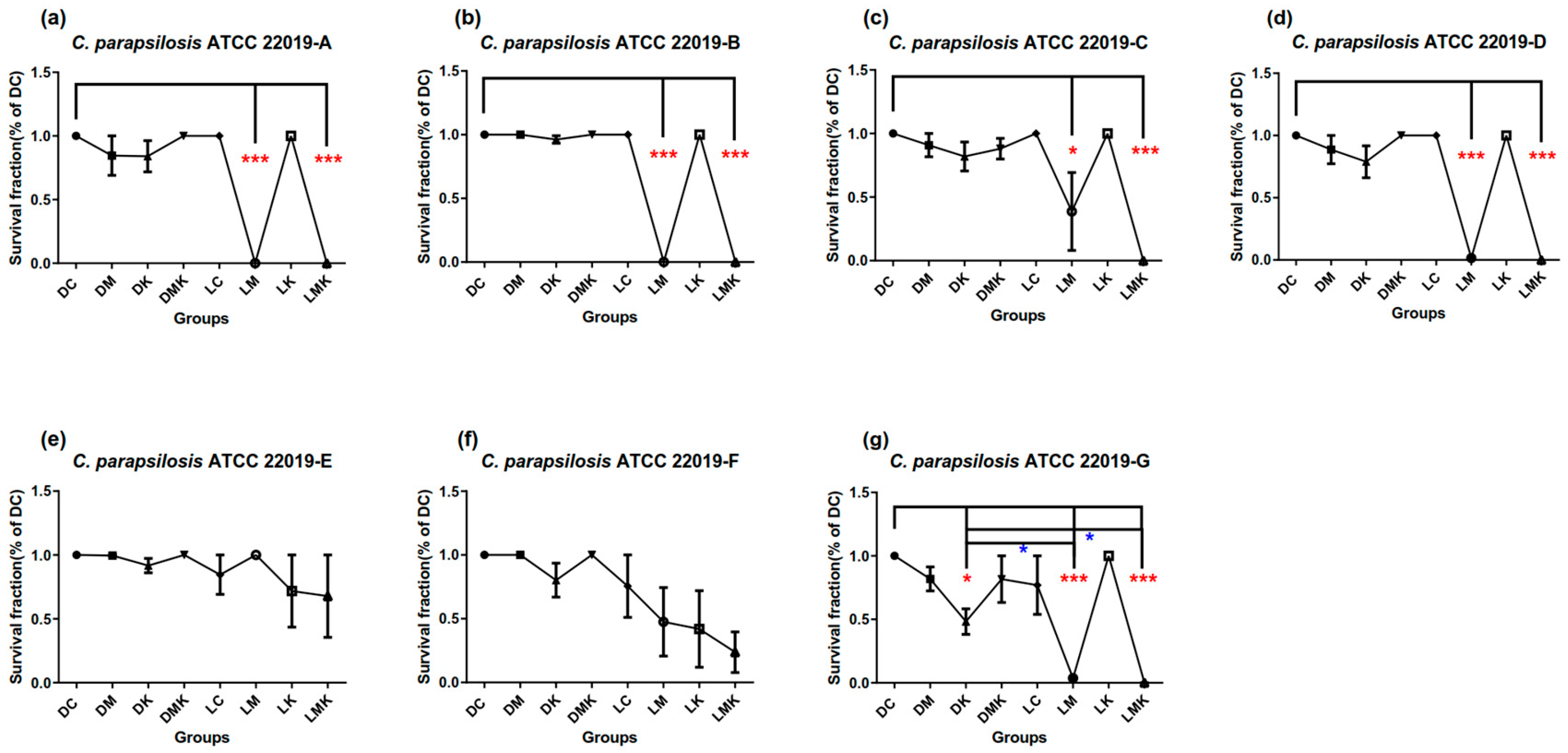
3.1. Survival Fractions of aPDT Schemes A to G on Three Candida Strains
3.1.1. Survival Fractions of aPDT Schemes A–G on C. albicans ATCC 90028
3.1.2. Survival Fractions of aPDT Schemes A–G on C. parapsilosis ATCC 22019
3.1.3. Survival Fractions of aPDT Schemes A–G on C. glabrata ATCC 2950
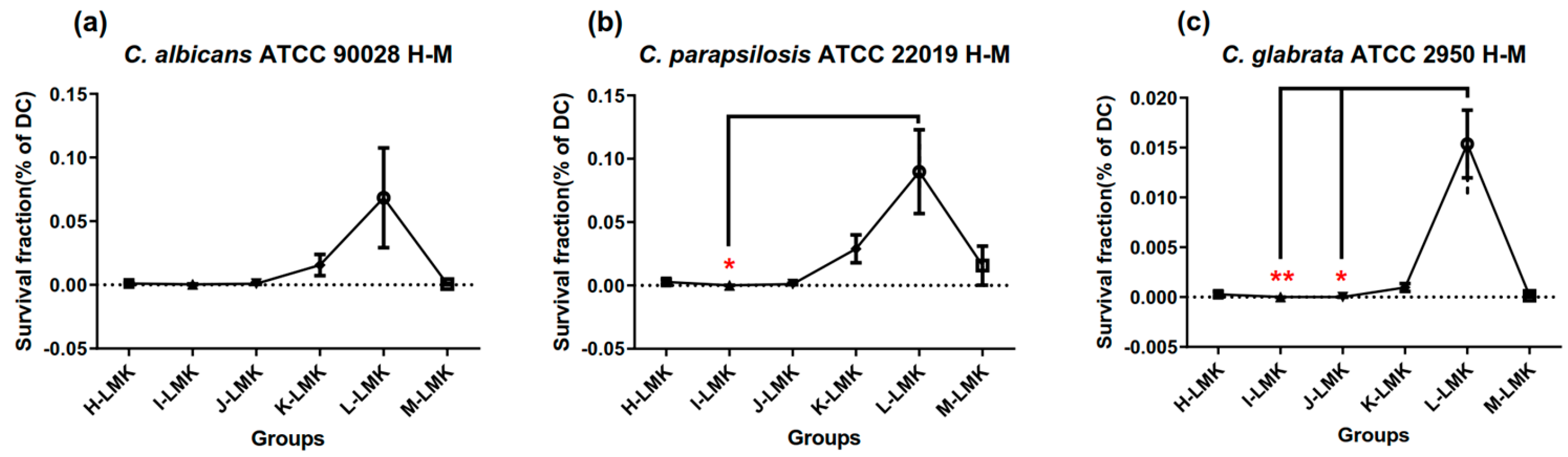
3.2. Survival Fractions of aPDT Schemes H to M on Three Candida Strains
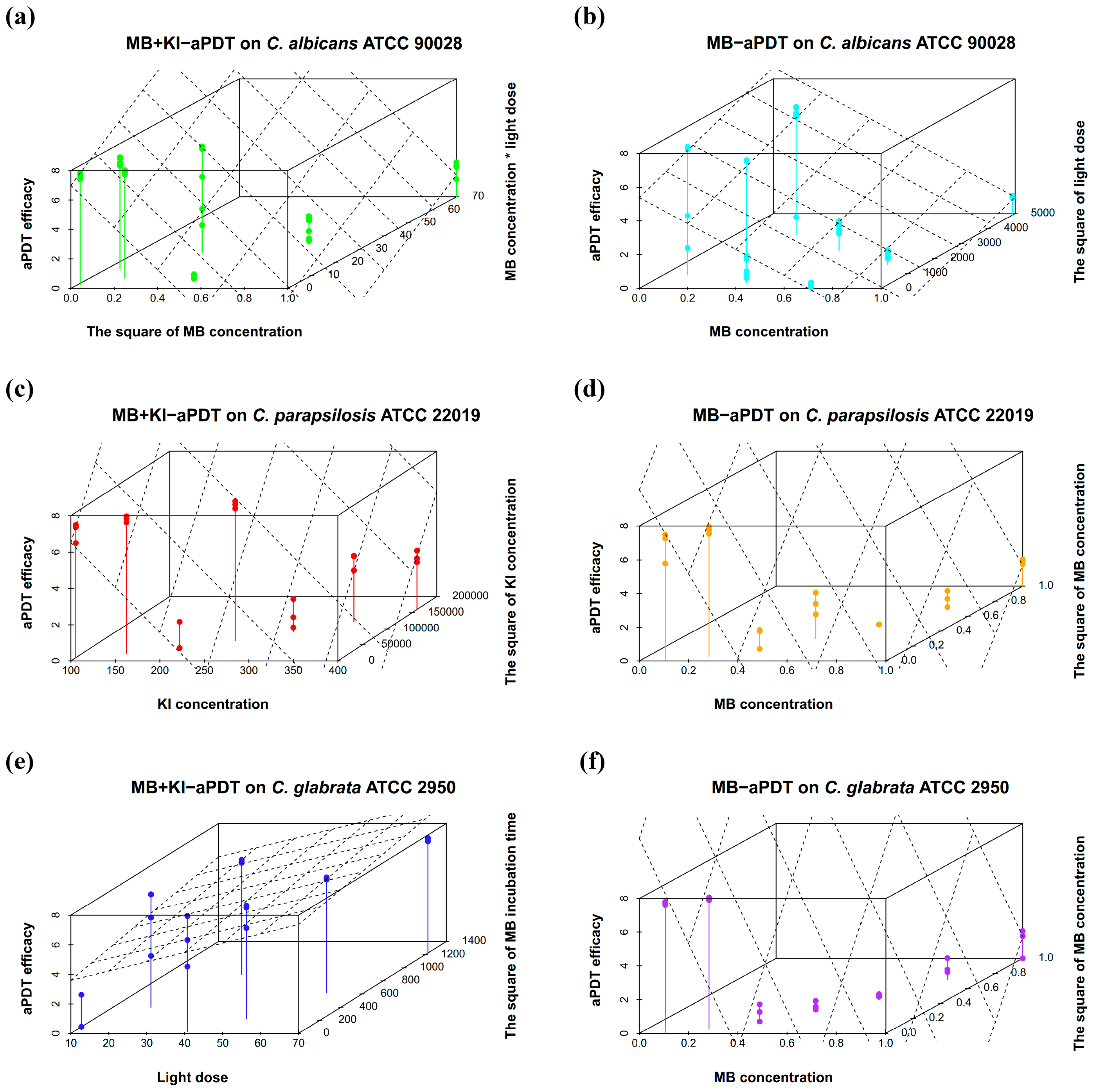
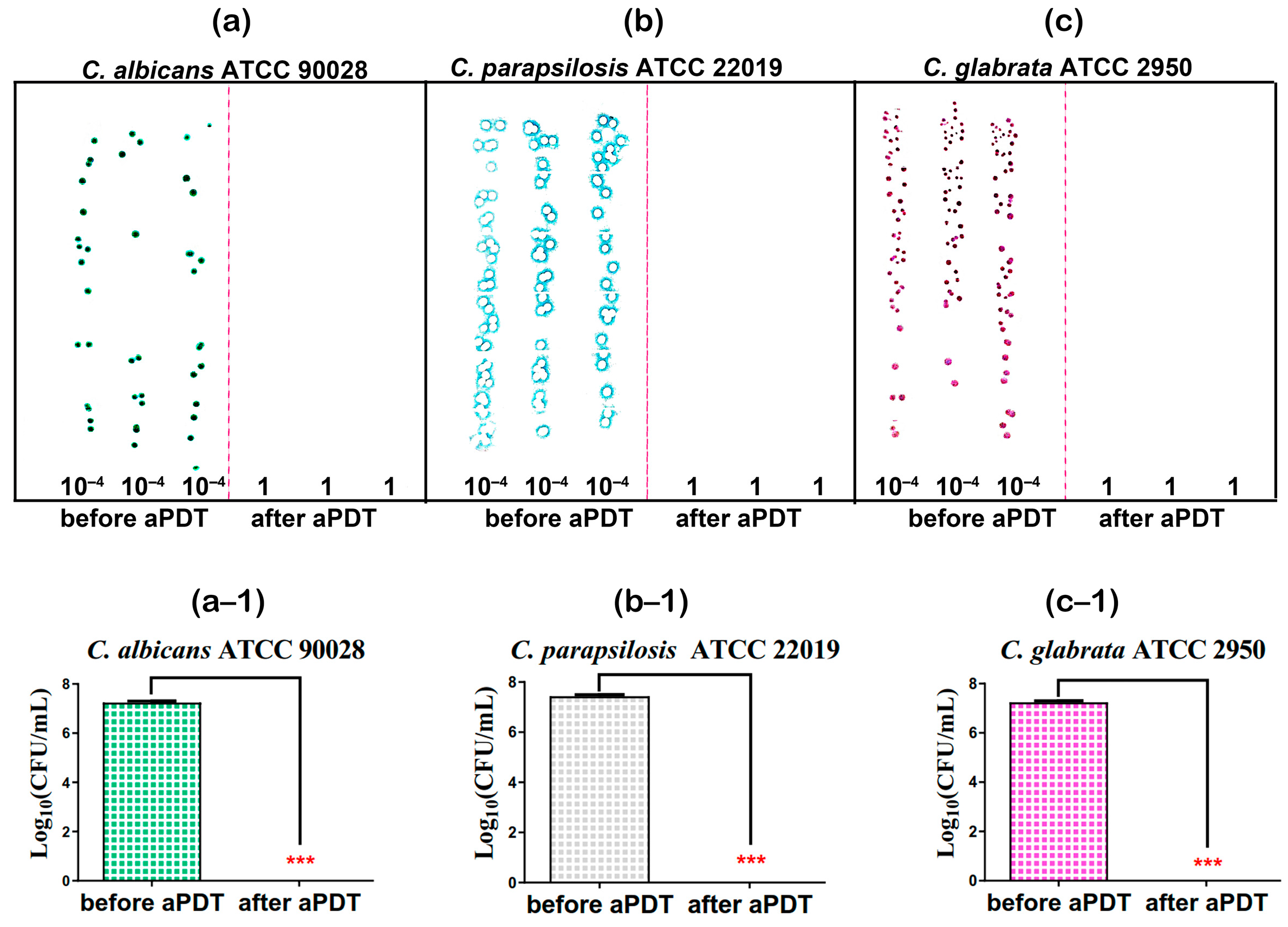
3.3. Results for Optimized MB + KI-aPDT Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| aPDT | antimicrobial photodynamic therapy |
| MB | methylene blue |
| KI | potassium iodide |
| C. albicans | Candida albicans |
| C. glabrata | Candida glabrata |
| C. parapsilosis | Candida parapsilosis |
| MRSA | methicillin-resistant Staphylococcus aureus |
| ROS | reactive oxygen species |
| LED | light-emitting diode |
| ATCC | American Type Culture Collection |
| PBS | phosphate-buffered saline |
| PDA | potato dextrose agar |
| SDA | Sabouraud glucose agar with chloramphenicol |
| CFU | counting forming unit |
| ABC | ATP-binding cassette |
| MFS | major facilitator superfamily |
References
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Antinori, S.; Milazzo, L.; Sollima, S.; Galli, M.; Corbellino, M. Candidemia and invasive candidiasis in adults: A narrative review. Eur. J. Intern. Med. 2016, 34, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Badiee, P.; Boekhout, T.; Haddadi, P.; Mohammadi, R.; Ghadimi-Moghadam, A.; Soltani, J.; Zarei Mahmoudabadi, A.; Ayatollahi Mousavi, S.A.; Najafzadeh, M.J.; Diba, K.; et al. Epidemiology and Antifungal Susceptibility of Candida Species Isolated from 10 Tertiary Care Hospitals in Iran. Microbiol. Spectr. 2022, 10, e0245322. [Google Scholar] [CrossRef]
- Kaur, J.; Nobile, C.J. Antifungal drug-resistance mechanisms in Candida biofilms. Curr. Opin. Microbiol. 2023, 71, 102237. [Google Scholar] [CrossRef]
- Martins, N.; Ferreira, I.C.; Barros, L.; Silva, S.; Henriques, M. Candidiasis: Predisposing factors, prevention, diagnosis and alternative treatment. Mycopathologia 2014, 177, 223–240. [Google Scholar] [CrossRef]
- Lopes, J.P.; Lionakis, M.S. Pathogenesis and virulence of Candida albicans. Virulence 2022, 13, 89–121. [Google Scholar] [CrossRef]
- Branco, J.; Miranda, I.M.; Rodrigues, A.G. Candida parapsilosis Virulence and Antifungal Resistance Mechanisms: A Comprehensive Review of Key Determinants. J. Fungi 2023, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Kan, S.; Song, N.; Pang, Q.; Mei, H.; Zheng, H.; Li, D.; Cui, F.; Lv, G.; An, R.; Li, P.; et al. In Vitro Antifungal Activity of Azoles and Other Antifungal Agents Against Pathogenic Yeasts from Vulvovaginal Candidiasis in China. Mycopathologia 2023, 188, 99–109. [Google Scholar] [CrossRef]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal Drug Resistance: Molecular Mechanisms in Candida albicans and Beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef]
- Piksa, M.; Lian, C.; Samuel, I.C.; Pawlik, K.J.; Samuel, I.D.W.; Matczyszyn, K. The role of the light source in antimicrobial photodynamic therapy. Chem. Soc. Rev. 2023, 52, 1697–1722. [Google Scholar] [CrossRef]
- Liu, X.; Fang, R.; Feng, R.; Li, Q.; Su, M.; Hou, C.; Zhuang, K.; Dai, Y.; Lei, N.; Jiang, Y.; et al. Cage-modified hypocrellin against multidrug-resistant Candida spp. with unprecedented activity in light-triggered combinational photodynamic therapy. Drug Resist. Updat. 2022, 65, 100887. [Google Scholar] [CrossRef] [PubMed]
- Sellera, F.P.; Sabino, C.P.; Nunez, S.C.; Ribeiro, M.S. Clinical acceptance of antimicrobial photodynamic therapy in the age of WHO global priority pathogens: So what we need to move forward? Photodiagnosis Photodyn. Ther. 2022, 40, 103158. [Google Scholar] [CrossRef] [PubMed]
- Sabino, C.P.; Wainwright, M.; Ribeiro, M.S.; Sellera, F.P.; Dos Anjos, C.; Baptista, M.D.S.; Lincopan, N. Global priority multidrug-resistant pathogens do not resist photodynamic therapy. J. Photochem. Photobiol. B 2020, 208, 111893. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Gomes, A.; Mesquita, M.Q.; Moura, N.M.M.; Neves, M.; Faustino, M.A.F.; Almeida, A. An Insight Into the Potentiation Effect of Potassium Iodide on aPDT Efficacy. Front. Microbiol. 2018, 9, 2665. [Google Scholar] [CrossRef]
- Hamblin, M.R. Potentiation of antimicrobial photodynamic inactivation by inorganic salts. Expert Rev. Anti Infect. Ther. 2017, 15, 1059–1069. [Google Scholar] [CrossRef]
- Xuan, W.; He, Y.; Huang, L.; Huang, Y.Y.; Bhayana, B.; Xi, L.; Gelfand, J.A.; Hamblin, M.R. Antimicrobial Photodynamic Inactivation Mediated by Tetracyclines in Vitro and in Vivo: Photochemical Mechanisms and Potentiation by Potassium Iodide. Sci. Rep. 2018, 8, 17130. [Google Scholar] [CrossRef]
- Boltes Cecatto, R.; Siqueira de Magalhaes, L.; Fernanda Setubal Destro Rodrigues, M.; Pavani, C.; Lino-Dos-Santos-Franco, A.; Teixeira Gomes, M.; Fatima Teixeira Silva, D. Methylene blue mediated antimicrobial photodynamic therapy in clinical human studies: The state of the art. Photodiagn. Photodyn. Ther. 2020, 31, 101828. [Google Scholar] [CrossRef]
- Lopes, M.M.; Bartolomeu, M.; Gomes, A.; Figueira, E.; Pinto, R.; Reis, L.; Balcao, V.M.; Faustino, M.A.F.; Neves, M.; Almeida, A. Antimicrobial Photodynamic Therapy in the Control of Pseudomonas syringae pv. actinidiae Transmission by Kiwifruit Pollen. Microorganisms 2020, 8, 1022. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef]
- Perez-Laguna, V.; Barrena-Lopez, Y.; Gilaberte, Y.; Rezusta, A. In Vitro Effect of Photodynamic Therapy with Different Lights and Combined or Uncombined with Chlorhexidine on Candida spp. Pharmaceutics 2021, 13, 1176. [Google Scholar] [CrossRef]
- Melendez-Celis, U.; Spezzia-Mazzocco, T.; Persheyev, S.; Lian, C.; Samuel, I.; Ramirez-San-Juan, J.C.; Ramos-Garcia, R. Organic light emitting diode for in vitro antimicrobial photodynamic therapy of Candida strains. Photodiagn. Photodyn. Ther. 2021, 36, 102567. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lyu, P.; Huang, Y.Y.; Du, N.; Qi, W.; Hamblin, M.R.; Wang, Y. Potassium iodide enhances the photobactericidal effect of methylene blue on Enterococcus faecalis as planktonic cells and as biofilm infection in teeth. J. Photochem. Photobiol. B 2020, 203, 111730. [Google Scholar] [CrossRef] [PubMed]
- Brenes-Alvarado, A.; Soto-Montero, J.; Farias-da-Silva, F.F.; Benine-Warlet, J.; Ribeiro, A.F.; Groppo, F.C.; Steiner-Oliveira, C. Does potassium iodide help in the microbial reduction of oral microcosm biofilms after photodynamic therapy with methylene blue and red laser? Photodiagnosis Photodyn. Ther. 2022, 40, 103123. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, D.; Gupta, A.; Huang, L.; Landi, G.; Avci, P.; Rodas, A.; Hamblin, M.R. Bacterial photodynamic inactivation mediated by methylene blue and red light is enhanced by synergistic effect of potassium iodide. Antimicrob. Agents Chemother 2015, 59, 5203–5212. [Google Scholar] [CrossRef] [PubMed]
- Freire, F.; Ferraresi, C.; Jorge, A.O.; Hamblin, M.R. Photodynamic therapy of oral Candida infection in a mouse model. J. Photochem. Photobiol. B 2016, 159, 161–168. [Google Scholar] [CrossRef]
- Du, M.; Xuan, W.; Zhen, X.; He, L.; Lan, L.; Yang, S.; Wu, N.; Qin, J.; Zhao, R.; Qin, J.; et al. Antimicrobial photodynamic therapy for oral Candida infection in adult AIDS patients: A pilot clinical trial. Photodiagnosis Photodyn. Ther. 2021, 34, 102310. [Google Scholar] [CrossRef]
- Ziental, D.; Mlynarczyk, D.T.; Czarczynska-Goslinska, B.; Lewandowski, K.; Sobotta, L. Photosensitizers Mediated Photodynamic Inactivation against Fungi. Nanomaterials 2021, 11, 2883. [Google Scholar] [CrossRef]
- de Carvalho Leonel, L.; Carvalho, M.L.; da Silva, B.M.; Zamuner, S.; Alberto-Silva, C.; Silva Costa, M. Photodynamic Antimicrobial Chemotherapy (PACT) using methylene blue inhibits the viability of the biofilm produced by Candida albicans. Photodiagn. Photodyn. Ther. 2019, 26, 316–323. [Google Scholar] [CrossRef]
- Cernakova, L.; Dizova, S.; Bujdakova, H. Employment of methylene blue irradiated with laser light source in photodynamic inactivation of biofilm formed by Candida albicans strain resistant to fluconazole. Med. Mycol. 2017, 55, 748–753. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Abrahamse, H. Inorganic Salts and Antimicrobial Photodynamic Therapy: Mechanistic Conundrums? Molecules 2018, 23, 3190. [Google Scholar] [CrossRef]
- Rossoni, R.D.; Barbosa, J.O.; de Oliveira, F.E.; de Oliveira, L.D.; Jorge, A.O.; Junqueira, J.C. Biofilms of Candida albicans serotypes A and B differ in their sensitivity to photodynamic therapy. Lasers Med. Sci. 2014, 29, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.C.; Junqueira, J.C.; Rossoni, R.D.; Pereira, C.A.; Munin, E.; Jorge, A.O. Comparison of the photodynamic fungicidal efficacy of methylene blue, toluidine blue, malachite green and low-power laser irradiation alone against Candida albicans. Lasers Med. Sci. 2010, 25, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Machado-de-Sena, R.M.; Correa, L.; Kato, I.T.; Prates, R.A.; Senna, A.M.; Santos, C.C.; Picanco, D.A.; Ribeiro, M.S. Photodynamic therapy has antifungal effect and reduces inflammatory signals in Candida albicans-induced murine vaginitis. Photodiagn. Photodyn. Ther. 2014, 11, 275–282. [Google Scholar] [CrossRef]
- Jett, B.D.; Hatter, K.L.; Huycke, M.M.; Gilmore, M.S. Simplified agar plate method for quantifying viable bacteria. Biotechniques 1997, 23, 648–650. [Google Scholar] [CrossRef]
- Matveev, A.T.; Varlamova, L.A.; Konopatsky, A.S.; Leybo, D.V.; Volkov, I.N.; Sorokin, P.B.; Fang, X.; Shtansky, D.V. A New Insight into the Mechanisms Underlying the Discoloration, Sorption, and Photodegradation of Methylene Blue Solutions with and without BNO(x) Nanocatalysts. Materials 2022, 15, 8169. [Google Scholar] [CrossRef]
- Junqueira, M.V.; Borghi-Pangoni, F.B.; Ferreira, S.B.; Rabello, B.R.; Hioka, N.; Bruschi, M.L. Functional Polymeric Systems as Delivery Vehicles for Methylene Blue in Photodynamic Therapy. Langmuir 2016, 32, 19–27. [Google Scholar] [CrossRef]
- Mehraban, N.; Freeman, H.S. Developments in PDT Sensitizers for Increased Selectivity and Singlet Oxygen Production. Materials 2015, 8, 4421–4456. [Google Scholar] [CrossRef] [PubMed]
- Baptista, A.; Sabino, C.P.; Nunez, S.C.; Miyakawa, W.; Martin, A.A.; Ribeiro, M.S. Photodynamic damage predominates on different targets depending on cell growth phase of Candida albicans. J. Photochem. Photobiol. B 2017, 177, 76–84. [Google Scholar] [CrossRef]
- Polat, E.; Kang, K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Abrahamse, H. Oxygen-Independent Antimicrobial Photoinactivation: Type III Photochemical Mechanism? Antibiotics 2020, 9, 53. [Google Scholar] [CrossRef]
- Toth, R.; Nosek, J.; Mora-Montes, H.M.; Gabaldon, T.; Bliss, J.M.; Nosanchuk, J.D.; Turner, S.A.; Butler, G.; Vagvolgyi, C.; Gacser, A. Candida parapsilosis: From Genes to the Bedside. Clin. Microbiol. Rev. 2019, 32, e00111-18. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.W.; Kraneveld, E.A.; Yin, Q.Y.; Dekker, H.L.; Gross, U.; Crielaard, W.; de Koster, C.G.; Bader, O.; Klis, F.M.; Weig, M. The cell wall of the human pathogen Candida glabrata: Differential incorporation of novel adhesin-like wall proteins. Eukaryot Cell 2008, 7, 1951–1964. [Google Scholar] [CrossRef] [PubMed]
- Prates, R.A.; Kato, I.T.; Ribeiro, M.S.; Tegos, G.P.; Hamblin, M.R. Influence of multidrug efflux systems on methylene blue-mediated photodynamic inactivation of Candida albicans. J. Antimicrob. Chemother. 2011, 66, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Edlind, T.; Katiyar, S. Intrinsically High Resistance of Candida glabrata to Hydrogen Peroxide and Its Reversal in a Fluconazole-Resistant Mutant. Antimicrob. Agents Chemother. 2022, 66, e0072122. [Google Scholar] [CrossRef]
- Ramirez-Quijas, M.D.; Lopez-Romero, E.; Cuellar-Cruz, M. Proteomic analysis of cell wall in four pathogenic species of Candida exposed to oxidative stress. Microb. Pathog. 2015, 87, 1–12. [Google Scholar] [CrossRef]
- Medrano-Diaz, C.L.; Vega-Gonzalez, A.; Ruiz-Baca, E.; Moreno, A.; Cuellar-Cruz, M. Moonlighting proteins induce protection in a mouse model against Candida species. Microb. Pathog. 2018, 124, 21–29. [Google Scholar] [CrossRef]
- Redhu, A.K.; Shah, A.H.; Prasad, R. MFS transporters of Candida species and their role in clinical drug resistance. FEMS Yeast Res. 2016, 16, fow043. [Google Scholar] [CrossRef]
- Prasad, R.; Nair, R.; Banerjee, A. Multidrug transporters of Candida species in clinical azole resistance. Fungal Genet. Biol. 2019, 132, 103252. [Google Scholar] [CrossRef]



| Parameters | Levels | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| X1 = MB (mM) | 0.10 | 0.25 | 0.40 | 0.55 | 0.70 | 0.85 | 1.00 |
| X2 = KI (mM) | 100 | 150 | 200 | 250 | 300 | 350 | 400 |
| X3 = Light dose (J/cm2) | 10 | 20 | 30 | 40 | 50 | 60 | 70 |
| X4 = incubation time (min) | 5 | 10 | 15 | 20 | 25 | 30 | 35 |
| Detection Schemes | Parameters and Levels | |||
|---|---|---|---|---|
| X1 = MB (mM) | X2 = KI (mM) | X3 = Light Dose (J/cm2) | X4 = Incubation Time (min) | |
| A | 0.10 | 150 | 30 | 30 |
| B | 0.25 | 250 | 60 | 25 |
| C | 0.40 | 350 | 20 | 20 |
| D | 0.55 | 100 | 50 | 15 |
| E | 0.70 | 200 | 10 | 10 |
| F | 0.85 | 300 | 40 | 5 |
| G | 1.00 | 400 | 70 | 35 |
| Parameters | Levels | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| X1 = MB (mM) | 0.05 | 0.06 | 0.07 | 0.08 | 0.09 | 0.10 |
| X2 = KI (mM) | 50 | 60 | 70 | 80 | 90 | 100 |
| X3 = Light dose(J/cm2) | 5 | 6 | 7 | 8 | 9 | 10 |
| X4 = incubation time (s) | 50 | 100 | 150 | 200 | 250 | 300 |
| Detection Schemes | Parameters | |||
|---|---|---|---|---|
| X1 = MB (mM) | X2 = KI (mM) | X3 = Light Dose (J/cm2) | X4 = Incubation Time (s) | |
| H | 0.05 | 60 | 7 | 300 |
| I | 0.06 | 80 | 10 | 250 |
| J | 0.07 | 100 | 6 | 200 |
| K | 0.08 | 50 | 9 | 150 |
| L | 0.09 | 70 | 5 | 100 |
| M | 0.10 | 90 | 8 | 50 |
| Equations | Strains | Regression Model | Model p Value | Model Fit |
|---|---|---|---|---|
| (1) | C. albicans ATCC 90028 | Y = 7.126 + 1.199X1X3 − 1.742X12 + 0.206X22 −0.361X32 (MB + KI-aPDT) | <0.001 | R = 0.959, adjusted R2 = 0.913 |
| (2) | C. albicans ATCC 90028 | Y = 5.392− 0.743 X1 + 0.361 X32 (MB-aPDT) | <0.001 | R = 0.771, adjusted R2 = 0.575 |
| (3) | C. parapsilosis ATCC 22019 | Y = 10.724 − 0.867X1 − 1.497X2 + 0.560X3 + 1.298X22 (MB + KI-aPDT) | <0.001 | R = 0.987, adjusted R2 = 0.968 |
| (4) | C. parapsilosis ATCC 22019 | Y = 10.577 − 3.237X1 + 1.075 X32 + 2.836X12 − 0.876X1X3 − 0.401X42 (MB-aPDT) | <0.001 | R = 0.987, adjusted R2 = 0.966 |
| (5) | C. glabrata ATCC 2950 | Y = 0.892 − 0.956X1 + 2.296X3 + 1.299X42 −3.316 X3X4 (MB + KI-aPDT) | <0.001 | R = 0.914, adjusted R2 = 0.769 |
| (6) | C. glabrata ATCC 2950 | Y = 13.04 − 3.809X1 + 3.674X12 + 1.038X32 −1.172X1X3 − 0.402X4 2(MB-aPDT) | <0.001 | R = 0.994, adjusted R2 = 0.984 |
| Equations | Strains | Regression Model | Model p Value | Model Fit |
|---|---|---|---|---|
| (7) | C. albicans ATCC 90028 | Y = −0.997 + 0.635X2X3 | 0.001 | R = 0.635, adjusted R2 = 0.376 |
| (8) | C. parapsilosis ATCC 22019 | Y = −1.727 + 0.707X2X3 | <0.001 | R = 0.707, adjusted R2 = 0.478 |
| (9) | C. glabrata ATCC 2950 | Y = −1.511 + 0.559 X2X3 + 0.457X2X4 | <0.001 | R = 0.812, adjusted R2 = 0.627 |
| Strains | X1 = MB Concentration (mM) | X2 = KI Concentration (mM) | X3 = Light Dose (J/cm2) | X4 = Incubation Time (min) | Y = MB + KI-aPDT Killing Efficiency (Log10(CFU/mL)) |
|---|---|---|---|---|---|
| C. albicans ATCC 90028 | 0.1 | 400 | 20 | 5 | 16.322 |
| C. parapsilosis ATCC 22019 | 0.1 | 400 | 70 | 5 | 67.08 |
| C. glabrata ATCC 2950 | 0.1 | 100 | 10 | 35 | 42.671 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, M.; Li, F.; Hu, Y. A Uniform Design Method Can Optimize the Combinatorial Parameters of Antimicrobial Photodynamic Therapy, Including the Concentrations of Methylene Blue and Potassium Iodide, Light Dose, and Methylene Blue’s Incubation Time, to Improve Fungicidal Effects on Candida Species. Microorganisms 2023, 11, 2557. https://doi.org/10.3390/microorganisms11102557
Du M, Li F, Hu Y. A Uniform Design Method Can Optimize the Combinatorial Parameters of Antimicrobial Photodynamic Therapy, Including the Concentrations of Methylene Blue and Potassium Iodide, Light Dose, and Methylene Blue’s Incubation Time, to Improve Fungicidal Effects on Candida Species. Microorganisms. 2023; 11(10):2557. https://doi.org/10.3390/microorganisms11102557
Chicago/Turabian StyleDu, Meixia, Feng Li, and Yanwei Hu. 2023. "A Uniform Design Method Can Optimize the Combinatorial Parameters of Antimicrobial Photodynamic Therapy, Including the Concentrations of Methylene Blue and Potassium Iodide, Light Dose, and Methylene Blue’s Incubation Time, to Improve Fungicidal Effects on Candida Species" Microorganisms 11, no. 10: 2557. https://doi.org/10.3390/microorganisms11102557
APA StyleDu, M., Li, F., & Hu, Y. (2023). A Uniform Design Method Can Optimize the Combinatorial Parameters of Antimicrobial Photodynamic Therapy, Including the Concentrations of Methylene Blue and Potassium Iodide, Light Dose, and Methylene Blue’s Incubation Time, to Improve Fungicidal Effects on Candida Species. Microorganisms, 11(10), 2557. https://doi.org/10.3390/microorganisms11102557






