Streptococcal Arginine Deiminase Inhibits T Lymphocyte Differentiation In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Supernatants of Destroyed Streptococcal Cells
2.2. Isolation and Culture of Human Peripheral Blood Mononuclear Cells (PBMCs)
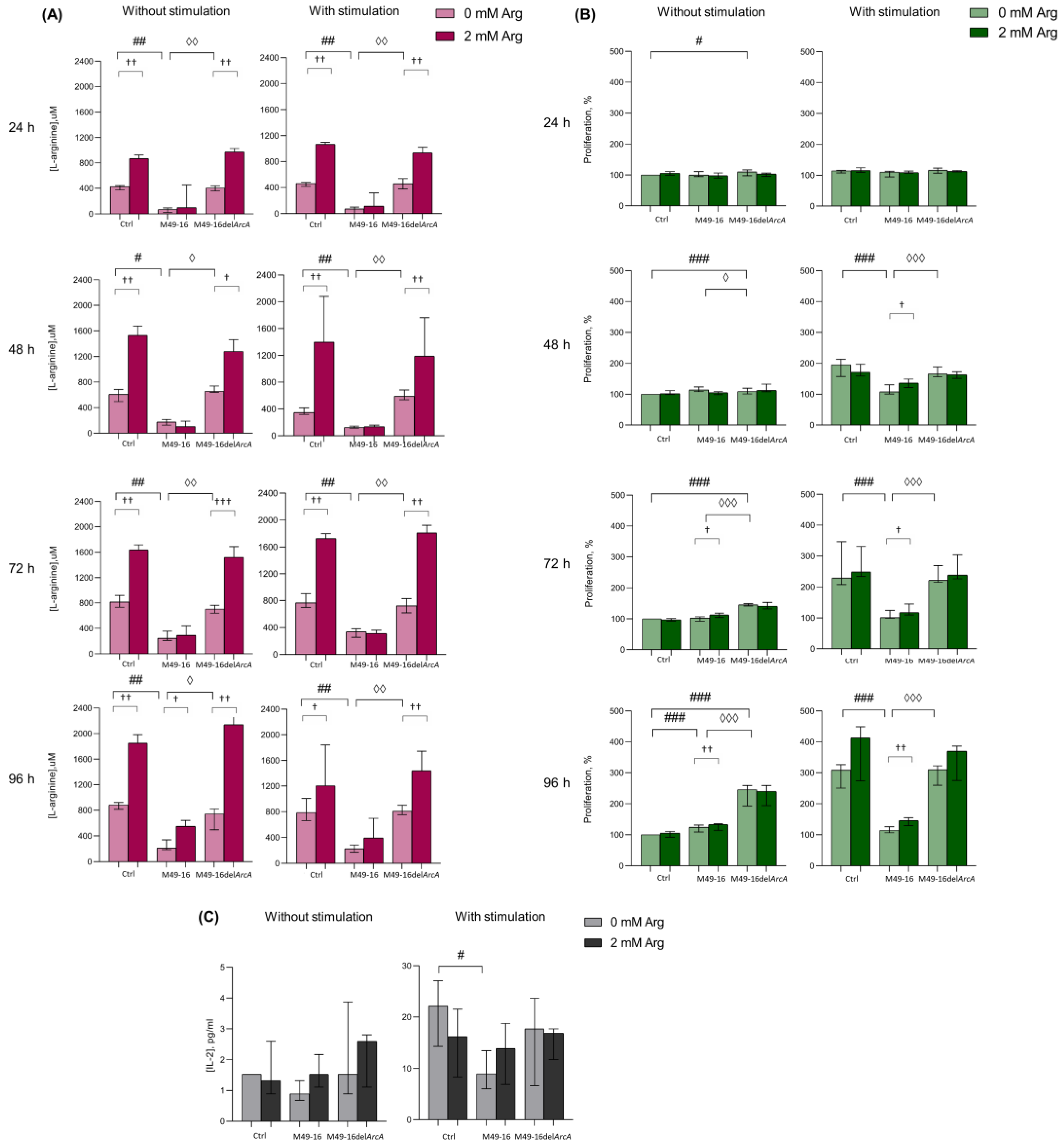
2.3. PBMC Activation Assessed in MTT Assay
2.4. Assessing SDSC-Mediated Effects on PBMCs IL-2 Production
2.5. PBMC Supernatant Arginine Concentration Measurement
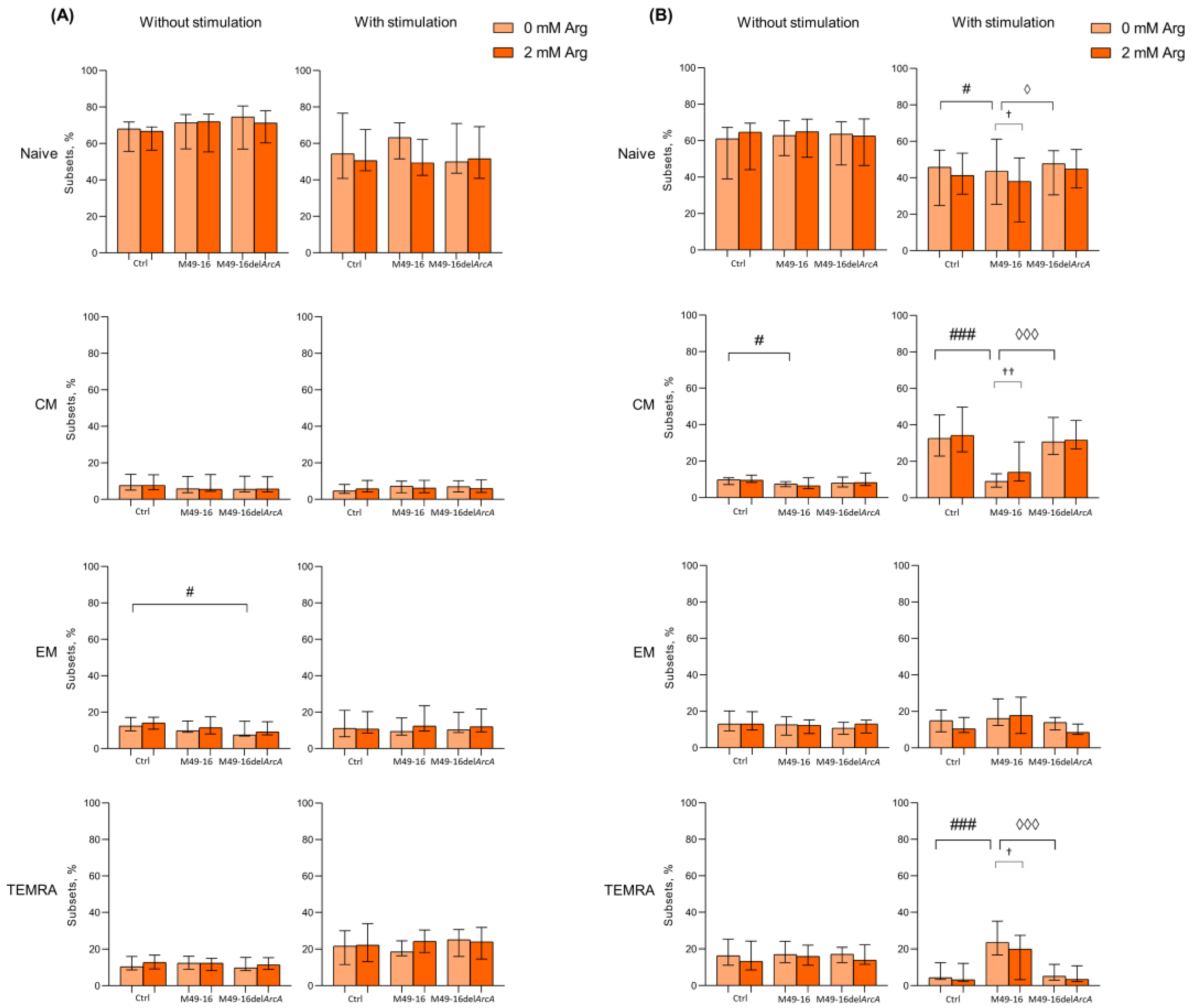
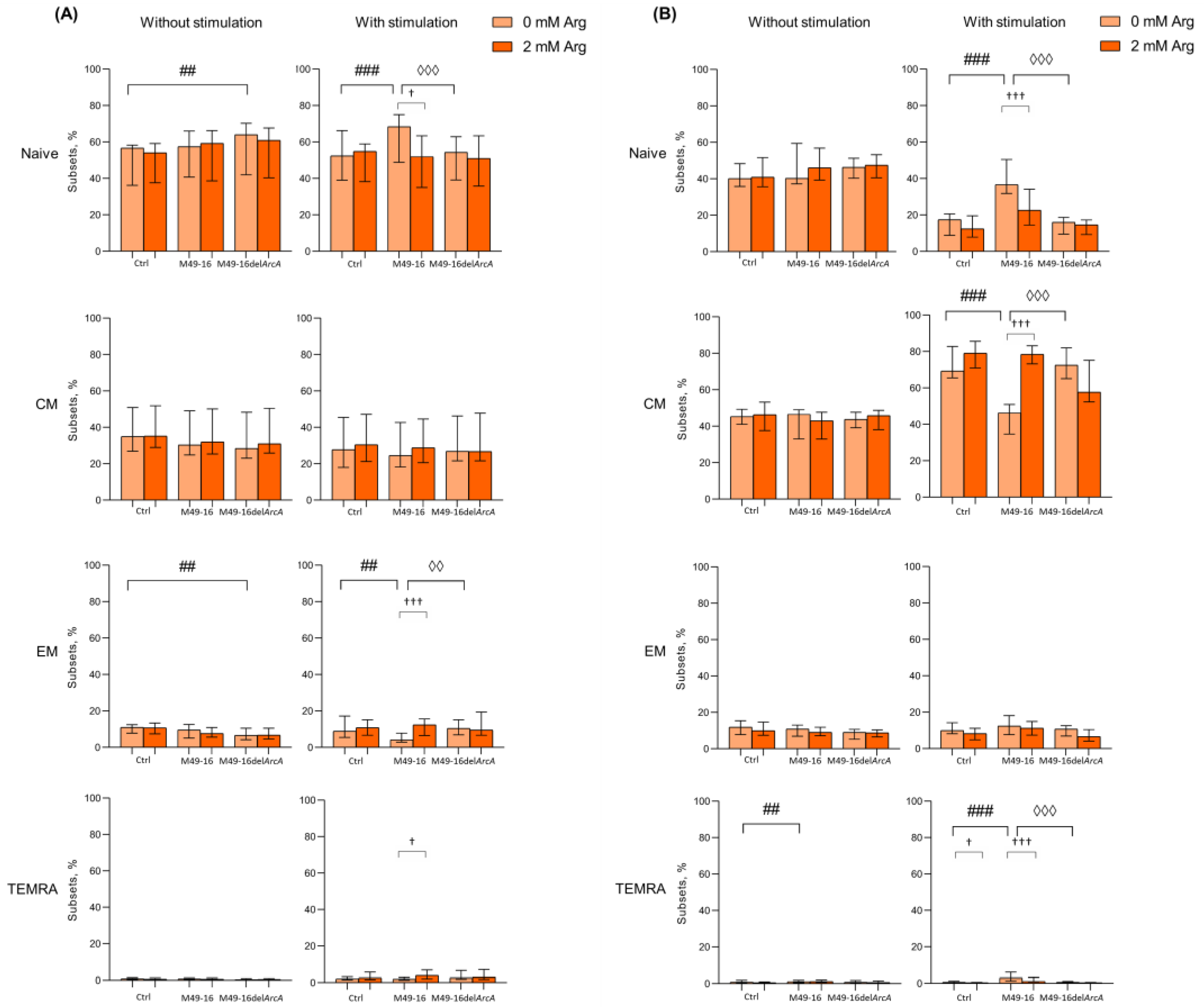
2.6. Assessing SDSC-Mediated Effect on T Cell Differentiation
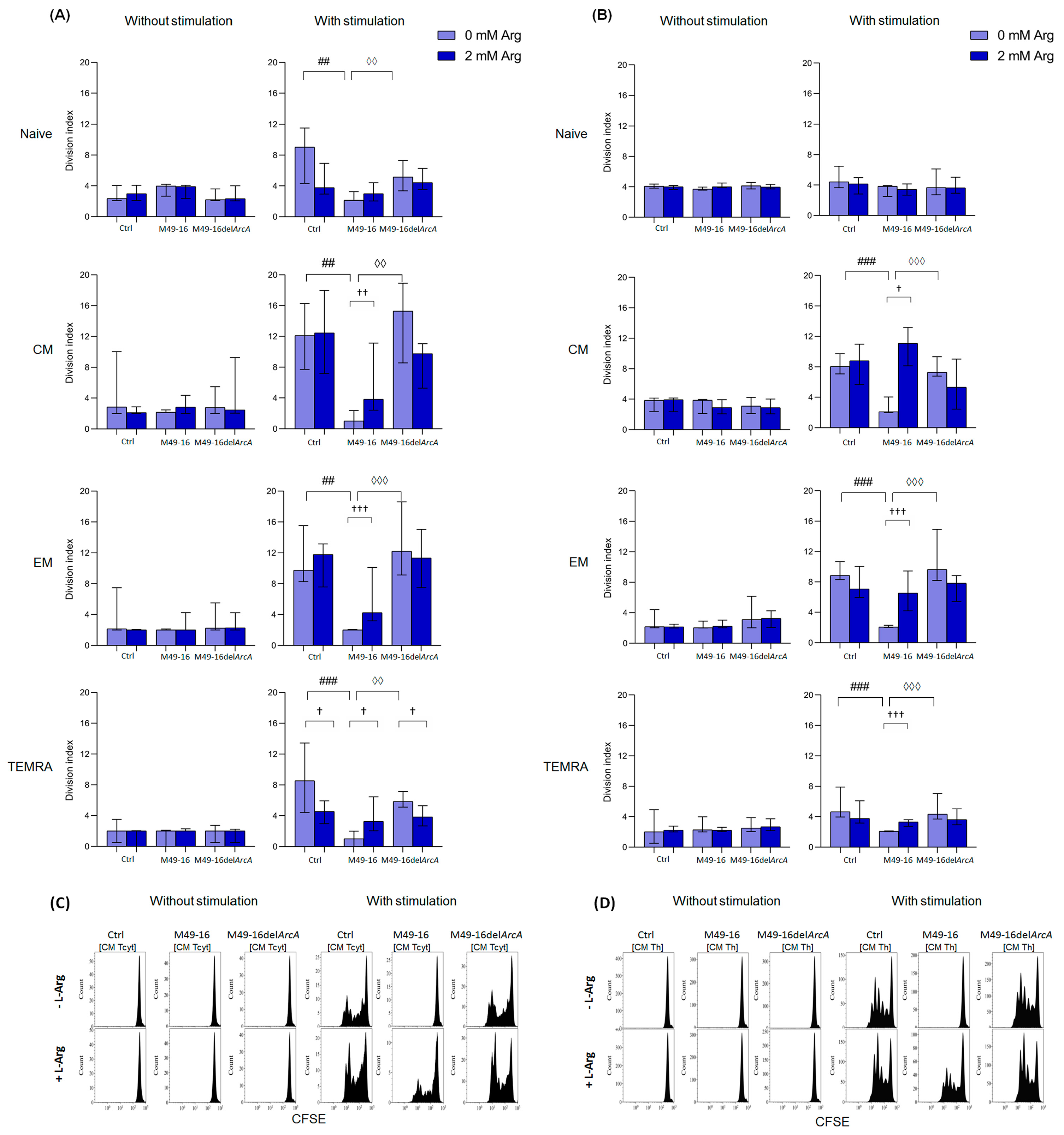
2.7. Assessing T Cell Subset Proliferative Activity
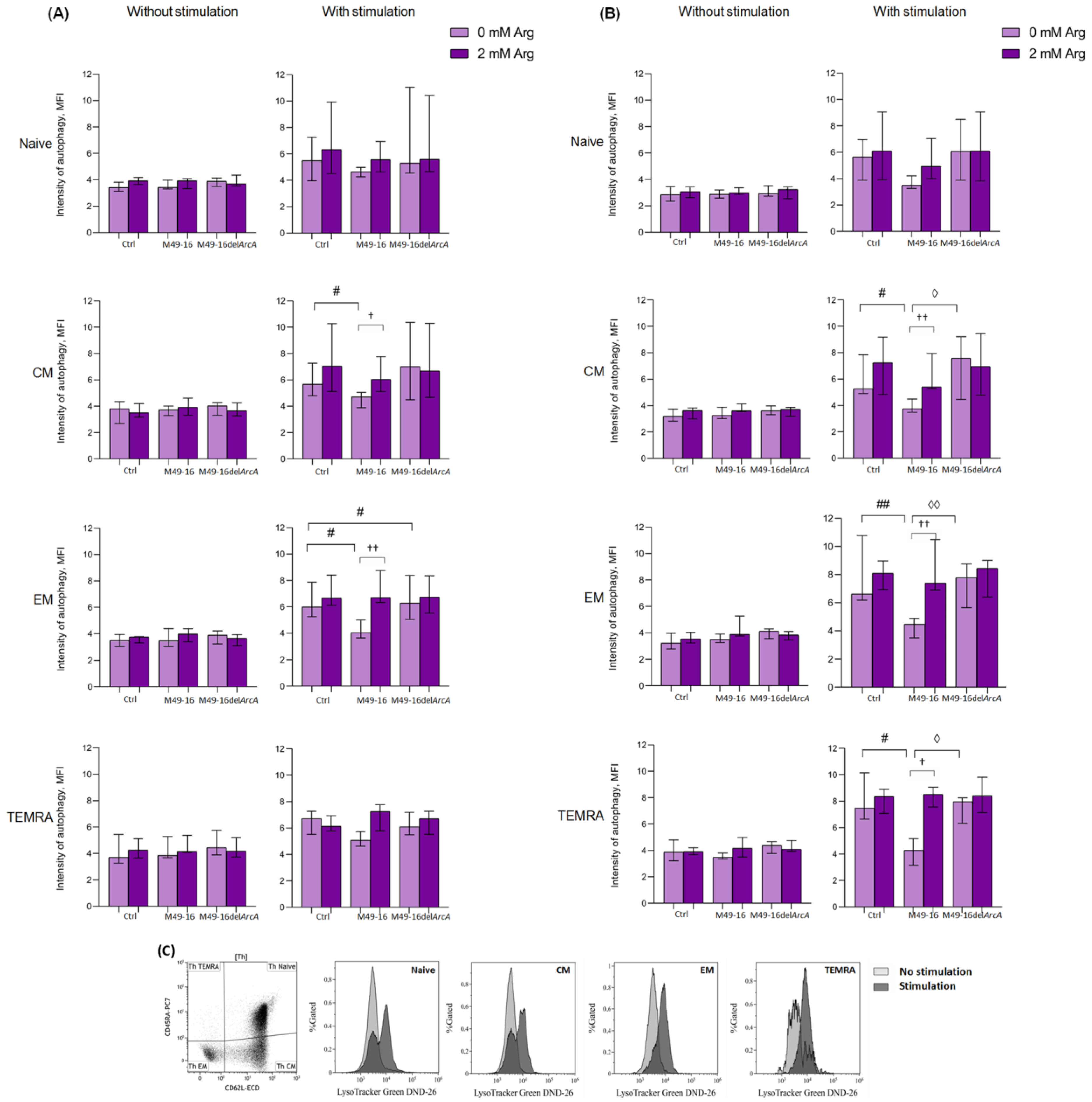
2.8. Assessing ADI Impact on T Cell Autophagy
2.9. Statistical Analysis
3. Results
3.1. ADI Decreased Culture Medium Arginine and Suppressed PBMC Activation and IL-2 Production
3.2. ADI Inhibits Activation-Induced T Cell Differentiation
3.3. ADI Inhibited Anti-CD2/CD3/CD28-Bead Driven Proliferation of CD8+ and CD4+ T Cell Subset
3.4. ADI Affects CD8+ and CD4+ Memory T Cell Autophagy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grohmann, U.; Mondanelli, G.; Belladonna, M.L.; Orabona, C.; Pallotta, M.T.; Iacono, A.; Puccetti, P.; Volpi, C. Amino-Acid Sensing and Degrading Pathways in Immune Regulation. Cytokine Growth Factor Rev. 2017, 35, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Szefel, J.; Danielak, A.; Kruszewski, W.J. Metabolic Pathways of L-Arginine and Therapeutic Consequences in Tumors. Adv. Med. Sci. 2019, 64, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Martí I Líndez, A.-A.; Reith, W. Arginine-Dependent Immune Responses. Cell. Mol. Life Sci. 2021, 78, 5303–5324. [Google Scholar] [CrossRef]
- Munder, M. Arginase: An Emerging Key Player in the Mammalian Immune System. Br. J. Pharmacol. 2009, 158, 638–651. [Google Scholar] [CrossRef]
- Sosnowska, A.; Chlebowska-Tuz, J.; Matryba, P.; Pilch, Z.; Greig, A.; Wolny, A.; Grzywa, T.M.; Rydzynska, Z.; Sokolowska, O.; Rygiel, T.P.; et al. Inhibition of Arginase Modulates T-Cell Response in the Tumor Microenvironment of Lung Carcinoma. Oncoimmunology 2021, 10, 1956143. [Google Scholar] [CrossRef]
- Modolell, M.; Choi, B.-S.; Ryan, R.O.; Hancock, M.; Titus, R.G.; Abebe, T.; Hailu, A.; Müller, I.; Rogers, M.E.; Bangham, C.R.M.; et al. Local Suppression of T Cell Responses by Arginase-Induced L-Arginine Depletion in Nonhealing Leishmaniasis. PLoS Negl. Trop. Dis. 2009, 3, e480. [Google Scholar] [CrossRef]
- Werner, A.; Koschke, M.; Leuchtner, N.; Luckner-Minden, C.; Habermeier, A.; Rupp, J.; Heinrich, C.; Conradi, R.; Closs, E.I.; Munder, M. Reconstitution of T Cell Proliferation under Arginine Limitation: Activated Human T Cells Take Up Citrulline via L-Type Amino Acid Transporter 1 and Use It to Regenerate Arginine after Induction of Argininosuccinate Synthase Expression. Front. Immunol. 2017, 8, 864. [Google Scholar] [CrossRef]
- Bretscher, P.; Cohn, M. A Theory of Self-Nonself Discrimination. Science 1970, 169, 1042–1049. [Google Scholar] [CrossRef]
- Powell, J.D.; Ragheb, J.A.; Kitagawa-Sakakida, S.; Schwartz, R.H. Molecular Regulation of Interleukin-2 Expression by CD28 Co-Stimulation and Anergy. Immunol. Rev. 1998, 165, 287–300. [Google Scholar] [CrossRef]
- Glinos, D.A.; Soskic, B.; Williams, C.; Kennedy, A.; Jostins, L.; Sansom, D.M.; Trynka, G. Genomic Profiling of T-Cell Activation Suggests Increased Sensitivity of Memory T Cells to CD28 Costimulation. Genes Immun. 2020, 21, 390–408. [Google Scholar] [CrossRef]
- Cunin, R.; Glansdorff, N.; Piérard, A.; Stalon, V. Biosynthesis and Metabolism of Arginine in Bacteria. Microbiol. Rev. 1986, 50, 314–352. [Google Scholar] [CrossRef] [PubMed]
- Henningham, A.; Chiarot, E.; Gillen, C.M.; Cole, J.N.; Rohde, M.; Fulde, M.; Ramachandran, V.; Cork, A.J.; Hartas, J.; Magor, G.; et al. Conserved Anchorless Surface Proteins as Group A Streptococcal Vaccine Candidates. J. Mol. Med. 2012, 90, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Winterhoff, N.; Goethe, R.; Gruening, P.; Rohde, M.; Kalisz, H.; Smith, H.E.; Valentin-Weigand, P. Identification and Characterization of Two Temperature-Induced Surface-Associated Proteins of Streptococcus suis with High Homologies to Members of the Arginine Deiminase System of Streptococcus pyogenes. J. Bacteriol. 2002, 184, 6768–6776. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, Z.T.; Watson, M.E.; Caparon, M.G. Streptococcus pyogenes Arginine and Citrulline Catabolism Promotes Infection and Modulates Innate Immunity. Infect. Immun. 2014, 82, 233–242. [Google Scholar] [CrossRef]
- Fulde, M.; Willenborg, J.; Huber, C.; Hitzmann, A.; Willms, D.; Seitz, M.; Eisenreich, W.; Valentin-Weigand, P.; Goethe, R. The Arginine-Ornithine Antiporter ArcD Contributes to Biological Fitness of Streptococcus suis. Front. Cell. Infect. Microbiol. 2014, 4, 107. [Google Scholar] [CrossRef]
- Wimmer, F.; Oberwinkler, T.; Bisle, B.; Tittor, J.; Oesterhelt, D. Identification of the Arginine/Ornithine Antiporter ArcD from Halobacterium salinarum. FEBS Lett. 2008, 582, 3771–3775. [Google Scholar] [CrossRef] [PubMed]
- Starikova, E.A.; Golovin, A.S.; Vasilyev, K.A.; Karaseva, A.B.; Serebriakova, M.K.; Sokolov, A.V.; Kudryavtsev, I.V.; Burova, L.A.; Voynova, I.V.; Suvorov, A.N.; et al. Role of Arginine Deiminase in Thymic Atrophy during Experimental Streptococcus pyogenes Infection. Scand. J. Immunol. 2019, 89, e12734. [Google Scholar] [CrossRef]
- Walker, M.J.; Barnett, T.C.; McArthur, J.D.; Cole, J.N.; Gillen, C.M.; Henningham, A.; Sriprakash, K.S.; Sanderson-Smith, M.L.; Nizet, V. Disease Manifestations and Pathogenic Mechanisms of Group A Streptococcus. Clin. Microbiol. Rev. 2014, 27, 264–301. [Google Scholar] [CrossRef]
- Anderson, J.; Imran, S.; Frost, H.R.; Azzopardi, K.I.; Jalali, S.; Novakovic, B.; Osowicki, J.; Steer, A.C.; Licciardi, P.V.; Pellicci, D.G. Immune Signature of Acute Pharyngitis in a Streptococcus pyogenes Human Challenge Trial. Nat. Commun. 2022, 13, 769. [Google Scholar] [CrossRef]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-Tumor Activity. Cell 2016, 167, 829–842. [Google Scholar] [CrossRef]
- Starikova, E.A.; Rubinstein, A.A.; Mammedova, J.T.; Isakov, D.V.; Kudryavtsev, I.V. Regulated Arginine Metabolism in Immunopathogenesis of a Wide Range of Diseases: Is There a Way to Pass between Scylla and Charybdis? Curr. Issues Mol. Biol. 2023, 45, 3525–3551. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Rahbar, M.R.; Morowvat, M.H.; Nezafat, N.; Negahdaripour, M.; Berenjian, A.; Ghasemi, Y. Arginine Deiminase: Current Understanding and Applications. Recent Pat. Biotechnol. 2019, 13, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Ash, D.E. Structure and Function of Arginases. J. Nutr. 2004, 134, 2760S–2764S. [Google Scholar] [CrossRef] [PubMed]
- Starikova, E.A.; Sokolov, A.V.; Vlasenko, A.Y.; Burova, L.A.; Freidlin, I.S.; Vasilyev, V.B. Biochemical and Biological Activity of Arginine Deiminase from Streptococcus pyogenes M22. Biochem. Cell Biol. 2016, 94, 129–137. [Google Scholar] [CrossRef]
- Mammedova, J.T.; Sokolov, A.V.; Freidlin, I.S.; Starikova, E.A. The Mechanisms of L-Arginine Metabolism Disorder in Endothelial Cells. Biochemistry 2021, 86, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Somani, R.R.; Chaskar, P.K. Arginine Deiminase Enzyme Evolving as a Potential Antitumor Agent. Mini Rev. Med. Chem. 2018, 18, 363–368. [Google Scholar] [CrossRef]
- Savaraj, N.; You, M.; Wu, C.; Wangpaichitr, M.; Kuo, M.T.; Feun, L.G. Arginine Deprivation, Autophagy, Apoptosis (AAA) for the Treatment of Melanoma. Curr. Mol. Med. 2010, 10, 405–412. [Google Scholar] [CrossRef]
- Kremer, J.C.; Prudner, B.C.; Lange, S.E.S.; Bean, G.R.; Schultze, M.B.; Brashears, C.B.; Radyk, M.D.; Redlich, N.; Tzeng, S.-C.; Kami, K.; et al. Arginine Deprivation Inhibits the Warburg Effect and Upregulates Glutamine Anaplerosis and Serine Biosynthesis in ASS1-Deficient Cancers. Cell Rep. 2017, 18, 991–1004. [Google Scholar] [CrossRef]
- Shen, L.-J.; Shen, W.-C. Drug Evaluation: ADI-PEG-20--a PEGylated Arginine Deiminase for Arginine-Auxotrophic Cancers. Curr. Opin. Mol. Ther. 2006, 8, 240–248. [Google Scholar]
- Kim, R.H.; Coates, J.M.; Bowles, T.L.; McNerney, G.P.; Sutcliffe, J.; Jung, J.U.; Gandour-Edwards, R.; Chuang, F.Y.S.; Bold, R.J.; Kung, H.-J. Arginine Deiminase as a Novel Therapy for Prostate Cancer Induces Autophagy and Caspase-Independent Apoptosis. Cancer Res. 2009, 69, 700–708. [Google Scholar] [CrossRef]
- Sahu, S.K.; Kumar, M.; Chakraborty, S.; Banerjee, S.K.; Kumar, R.; Gupta, P.; Jana, K.; Gupta, U.D.; Ghosh, Z.; Kundu, M.; et al. MicroRNA 26a (miR-26a)/KLF4 and CREB-C/EBPβ Regulate Innate Immune Signaling, the Polarization of Macrophages and the Trafficking of Mycobacterium Tuberculosis to Lysosomes during Infection. PLoS Pathog. 2017, 13, e1006410. [Google Scholar] [CrossRef] [PubMed]
- Frauwirth, K.A.; Thompson, C.B. Activation and Inhibition of Lymphocytes by Costimulation. J. Clin. Investig. 2002, 109, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Samji, T.; Khanna, K.M. Understanding Memory CD8+ T Cells. Immunol. Lett. 2017, 185, 32–39. [Google Scholar] [CrossRef]
- Soon, M.S.; Engel, J.A.; Lee, H.J.; Haque, A. Development of Circulating CD4+ T-Cell Memory. Immunol. Cell Biol. 2019, 97, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lakkis, F.G. Memory T Cell Migration. Front. Immunol. 2015, 6, 504. [Google Scholar] [CrossRef]
- Shyer, J.A.; Flavell, R.A.; Bailis, W. Metabolic Signaling in T Cells. Cell Res. 2020, 30, 649–659. [Google Scholar] [CrossRef]
- Macian, F. Autophagy in T Cell Function and Aging. Front. Cell Dev. Biol. 2019, 7, 213. [Google Scholar] [CrossRef]
- Degnan, B.A.; Palmer, J.M.; Robson, T.; Jones, C.E.; Fischer, M.; Glanville, M.; Mellor, G.D.; Diamond, A.G.; Kehoe, M.A.; Goodacre, J.A. Inhibition of Human Peripheral Blood Mononuclear Cell Proliferation by Streptococcus pyogenes Cell Extract Is Associated with Arginine Deiminase Activity. Infect. Immun. 1998, 66, 3050–3058. [Google Scholar] [CrossRef]
- Kanamoto, T.; Sato, S.; Nakashima, H.; Inoue, M. Proliferation of Mitogen-Stimulated Human Peripheral Blood Mononuclear Cells Is Inhibited by Extracellular Arginine Deiminase of Granulicatella Elegans Isolated from the Human Mouth. J. Infect. Chemother. 2007, 13, 353–355. [Google Scholar] [CrossRef]
- Starickova, E.A.; Leveshko, T.A.; Churakina, D.V.; Kudryavtsev, I.V.; Burova, L.A.; Freidlin, I.S.S. Pyogenes M49-16 Arginine Deiminase Inhibits Proliferative Activity of Human Peripheral Blood Lymphocytes. Russ. J. Infect. Immun. 2021, 11, 349–356. [Google Scholar] [CrossRef]
- Brin, E.; Wu, K.; Lu, H.-T.; He, Y.; Dai, Z.; He, W. PEGylated Arginine Deiminase Can Modulate Tumor Immune Microenvironment by Affecting Immune Checkpoint Expression, Decreasing Regulatory T Cell Accumulation and Inducing Tumor T Cell Infiltration. Oncotarget 2017, 8, 58948–58963. [Google Scholar] [CrossRef]
- Mølgaard, K.; Rahbech, A.; Met, Ö.; Svane, I.M.; Thor Straten, P.; Desler, C.; Peeters, M.J.W. Real-Time Monitoring of Mitochondrial Respiration in Cytokine-Differentiated Human Primary T Cells. J. Vis. Exp. 2021, 176, e62984. [Google Scholar] [CrossRef]
- Deacy, A.M.; Gan, S.K.-E.; Derrick, J.P. Superantigen Recognition and Interactions: Functions, Mechanisms and Applications. Front. Immunol. 2021, 12, 731845. [Google Scholar] [CrossRef]
- Marchingo, J.M.; Cantrell, D.A. Protein Synthesis, Degradation, and Energy Metabolism in T Cell Immunity. Cell. Mol. Immunol. 2022, 19, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Horwitz, M.E.; Kwatemaa, A.; Nomicos, E.; Castro, K.; Sokolic, R.; Foster, S.F.; Garofalo, M.; Choi, U.; Ryherd, M.; et al. Unique Abnormalities of CD4(+) and CD8(+) Central Memory Cells Associated with Chronic Graft-versus-Host Disease Improve after Extracorporeal Photopheresis. Biol. Blood Marrow Transplant. 2006, 12 (Suppl. S2), 22–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-Z.; Fang, L.-B.; Hjelmström, P.; Gao, X.-G. Increased CD8+ Central Memory T Cells in Patients with Multiple Sclerosis. Mult. Scler. 2007, 13, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Pender, M.P.; Csurhes, P.A.; Pfluger, C.M.; Burrows, S.R. Deficiency of CD8+ Effector Memory T Cells Is an Early and Persistent Feature of Multiple Sclerosis. Mult. Scler. 2014, 20, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Arbogast, F.; Gros, F. Lymphocyte Autophagy in Homeostasis, Activation, and Inflammatory Diseases. Front. Immunol. 2018, 9, 1801. [Google Scholar] [CrossRef]
- Botbol, Y.; Guerrero-Ros, I.; Macian, F. Key Roles of Autophagy in Regulating T-Cell Function. Eur. J. Immunol. 2016, 46, 1326–1334. [Google Scholar] [CrossRef]
- Xiong, L.; Teng, J.L.L.; Botelho, M.G.; Lo, R.C.; Lau, S.K.P.; Woo, P.C.Y. Arginine Metabolism in Bacterial Pathogenesis and Cancer Therapy. Int. J. Mol. Sci. 2016, 17, 363. [Google Scholar] [CrossRef]
- Carroll, B.; Maetzel, D.; Maddocks, O.D.; Otten, G.; Ratcliff, M.; Smith, G.R.; Dunlop, E.A.; Passos, J.F.; Davies, O.R.; Jaenisch, R.; et al. Control of TSC2-Rheb Signaling Axis by Arginine Regulates mTORC1 Activity. eLife 2016, 5, e11058. [Google Scholar] [CrossRef]
- Goberdhan, D.C.I.; Wilson, C.; Harris, A.L. Amino Acid Sensing by mTORC1: Intracellular Transporters Mark the Spot. Cell Metab. 2016, 23, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Mondino, A.; Mueller, D.L. mTOR at the Crossroads of T Cell Proliferation and Tolerance. Semin. Immunol. 2007, 19, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. Regulation and Function of mTOR Signalling in T Cell Fate Decisions. Nat. Rev. Immunol. 2012, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Green, D.R. Metabolic Checkpoints in Activated T Cells. Nat. Immunol. 2012, 13, 907–915. [Google Scholar] [CrossRef] [PubMed]
- MacIver, N.J.; Michalek, R.D.; Rathmell, J.C. Metabolic Regulation of T Lymphocytes. Annu. Rev. Immunol. 2013, 31, 259–283. [Google Scholar] [CrossRef]
- Pearce, E.L.; Pearce, E.J. Metabolic Pathways in Immune Cell Activation and Quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Zhang, L.; Romero, P. Metabolic Control of CD8+ T Cell Fate Decisions and Antitumor Immunity. Trends Mol. Med. 2018, 24, 30–48. [Google Scholar] [CrossRef]
- Araki, K.; Turner, A.P.; Shaffer, V.O.; Gangappa, S.; Keller, S.A.; Bachmann, M.F.; Larsen, C.P.; Ahmed, R. mTOR Regulates Memory CD8 T-Cell Differentiation. Nature 2009, 460, 108–112. [Google Scholar] [CrossRef]
- García-Navas, R.; Munder, M.; Mollinedo, F. Depletion of L-Arginine Induces Autophagy as a Cytoprotective Response to Endoplasmic Reticulum Stress in Human T Lymphocytes. Autophagy 2012, 8, 1557–1576. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Guan, K.-L. mTOR as a Central Hub of Nutrient Signalling and Cell Growth. Nat. Cell Biol. 2019, 21, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, V.M.; Valdor, R.; Patel, B.; Singh, R.; Cuervo, A.M.; Macian, F. Macroautophagy Regulates Energy Metabolism during Effector T Cell Activation. J. Immunol. 2010, 185, 7349–7357. [Google Scholar] [CrossRef] [PubMed]
- Whang, M.I.; Tavares, R.M.; Benjamin, D.I.; Kattah, M.G.; Advincula, R.; Nomura, D.K.; Debnath, J.; Malynn, B.A.; Ma, A. The Ubiquitin Binding Protein TAX1BP1 Mediates Autophagasome Induction and the Metabolic Transition of Activated T Cells. Immunity 2017, 46, 405. [Google Scholar] [CrossRef]
- Xu, X.; Araki, K.; Li, S.; Han, J.-H.; Ye, L.; Tan, W.G.; Konieczny, B.T.; Bruinsma, M.W.; Martinez, J.; Pearce, E.L.; et al. Autophagy Is Essential for Effector CD8(+) T Cell Survival and Memory Formation. Nat. Immunol. 2014, 15, 1152–1161. [Google Scholar] [CrossRef]
- Murera, D.; Arbogast, F.; Arnold, J.; Bouis, D.; Muller, S.; Gros, F. CD4 T Cell Autophagy Is Integral to Memory Maintenance. Sci. Rep. 2018, 8, 5951. [Google Scholar] [CrossRef]
- Mocholi, E.; Dowling, S.D.; Botbol, Y.; Gruber, R.C.; Ray, A.K.; Vastert, S.; Shafit-Zagardo, B.; Coffer, P.J.; Macian, F. Autophagy Is a Tolerance-Avoidance Mechanism That Modulates TCR-Mediated Signaling and Cell Metabolism to Prevent Induction of T Cell Anergy. Cell Rep. 2018, 24, 1136–1150. [Google Scholar] [CrossRef]
- Jia, W.; He, M.-X.; McLeod, I.X.; Guo, J.; Ji, D.; He, Y.-W. Autophagy Regulates T Lymphocyte Proliferation through Selective Degradation of the Cell-Cycle Inhibitor CDKN1B/p27Kip1. Autophagy 2015, 11, 2335–2345. [Google Scholar] [CrossRef]
- Paul, S.; Kashyap, A.K.; Jia, W.; He, Y.-W.; Schaefer, B.C. Selective Autophagy of the Adaptor Protein Bcl10 Modulates T Cell Receptor Activation of NF-κB. Immunity 2012, 36, 947–958. [Google Scholar] [CrossRef]
- Dowling, S.D.; Macian, F. Autophagy and T Cell Metabolism. Cancer Lett. 2018, 419, 20–26. [Google Scholar] [CrossRef]
- Eibert, S.M.; Lee, K.-H.; Pipkorn, R.; Sester, U.; Wabnitz, G.H.; Giese, T.; Meuer, S.C.; Samstag, Y. Cofilin Peptide Homologs Interfere with Immunological Synapse Formation and T Cell Activation. Proc. Natl. Acad. Sci. USA 2004, 101, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Klemke, M.; Wabnitz, G.H.; Funke, F.; Funk, B.; Kirchgessner, H.; Samstag, Y. Oxidation of Cofilin Mediates T Cell Hyporesponsiveness under Oxidative Stress Conditions. Immunity 2008, 29, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Wabnitz, G.H.; Goursot, C.; Jahraus, B.; Kirchgessner, H.; Hellwig, A.; Klemke, M.; Konstandin, M.H.; Samstag, Y. Mitochondrial Translocation of Oxidized Cofilin Induces Caspase-Independent Necrotic-like Programmed Cell Death of T Cells. Cell Death Dis. 2010, 1, e58. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Lei, L.; Qin, W.; Wang, L.; Zhang, G.; Hu, J. GCN2 Controls the Cellular Checkpoint: Potential Target for Regulating Inflammation. Cell Death Discov. 2018, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Field, G.C.; Pavlyk, I.; Szlosarek, P.W. Bench-to-Bedside Studies of Arginine Deprivation in Cancer. Molecules 2023, 28, 2150. [Google Scholar] [CrossRef]
- Kawatra, A.; Dhankhar, R.; Gulati, P. Microbial Arginine Deiminase: A Multifaceted Green Catalyst in Biomedical Sciences. Int. J. Biol. Macromol. 2022, 196, 151–162. [Google Scholar] [CrossRef]
- Zhang, Y.; Higgins, C.B.; Van Tine, B.A.; Bomalaski, J.S.; DeBosch, B.J. Pegylated Arginine Deiminase Drives Arginine Turnover and Systemic Autophagy to Dictate Energy Metabolism. Cell Rep. Med. 2022, 3, 100498. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starikova, E.A.; Mammedova, J.T.; Ozhiganova, A.; Leveshko, T.A.; Lebedeva, A.M.; Sokolov, A.V.; Isakov, D.V.; Karaseva, A.B.; Burova, L.A.; Kudryavtsev, I.V. Streptococcal Arginine Deiminase Inhibits T Lymphocyte Differentiation In Vitro. Microorganisms 2023, 11, 2585. https://doi.org/10.3390/microorganisms11102585
Starikova EA, Mammedova JT, Ozhiganova A, Leveshko TA, Lebedeva AM, Sokolov AV, Isakov DV, Karaseva AB, Burova LA, Kudryavtsev IV. Streptococcal Arginine Deiminase Inhibits T Lymphocyte Differentiation In Vitro. Microorganisms. 2023; 11(10):2585. https://doi.org/10.3390/microorganisms11102585
Chicago/Turabian StyleStarikova, Eleonora A., Jennet T. Mammedova, Arina Ozhiganova, Tatiana A. Leveshko, Aleksandra M. Lebedeva, Alexey V. Sokolov, Dmitry V. Isakov, Alena B. Karaseva, Larissa A. Burova, and Igor V. Kudryavtsev. 2023. "Streptococcal Arginine Deiminase Inhibits T Lymphocyte Differentiation In Vitro" Microorganisms 11, no. 10: 2585. https://doi.org/10.3390/microorganisms11102585
APA StyleStarikova, E. A., Mammedova, J. T., Ozhiganova, A., Leveshko, T. A., Lebedeva, A. M., Sokolov, A. V., Isakov, D. V., Karaseva, A. B., Burova, L. A., & Kudryavtsev, I. V. (2023). Streptococcal Arginine Deiminase Inhibits T Lymphocyte Differentiation In Vitro. Microorganisms, 11(10), 2585. https://doi.org/10.3390/microorganisms11102585








