Epstein-Barr Virus Encephalitis: A Review of Case Reports from the Last 25 Years
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Epidemiological Analysis
3.2. Clinical Observations
3.3. Biological Data
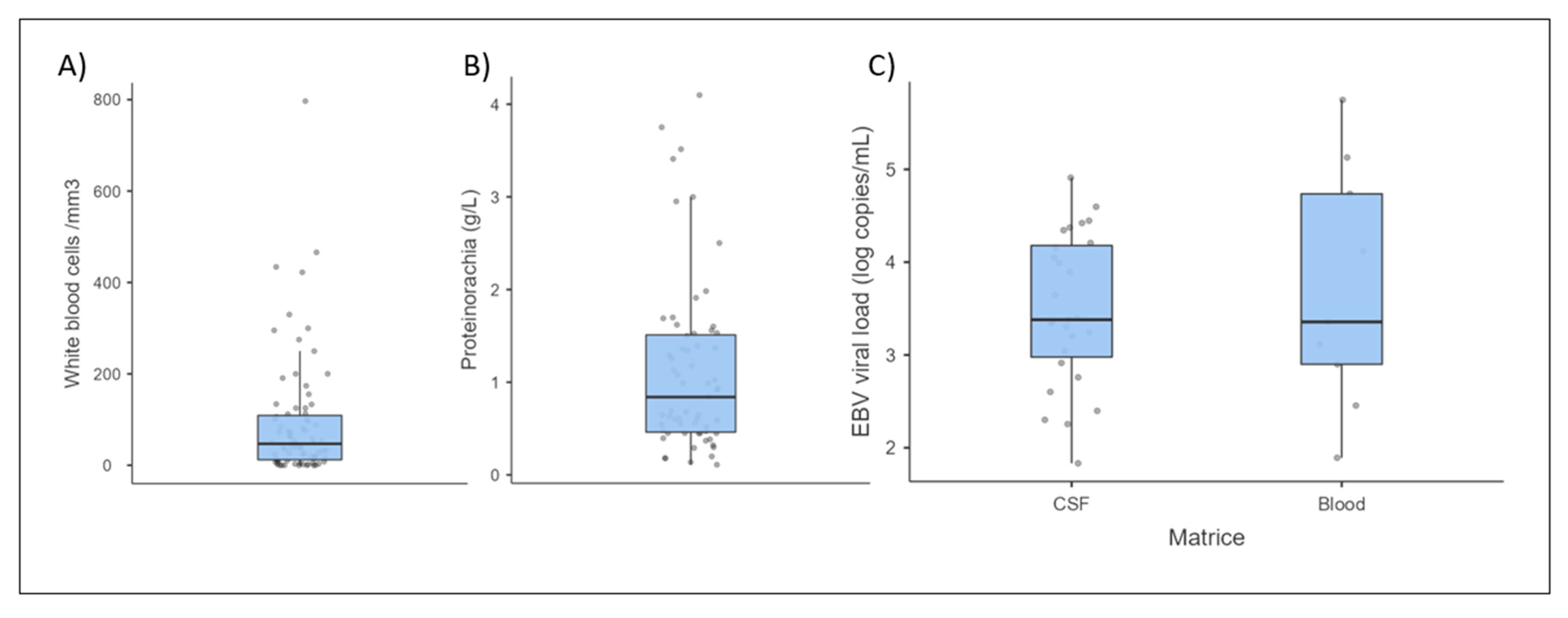
3.3.1. Cerebrospinal Fluid and Brain Analysis
3.3.2. Blood Analysis
3.4. Imaging
3.5. Treatments
4. Discussion
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Dunmire, S.K.; Hogquist, K.A.; Balfour, H.H. Infectious Mononucleosis. In Epstein Barr Virus Volume 1: One Herpes Virus: Many Diseases; Münz, C., Ed.; Current Topics in Microbiology and Immunology; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 211–240. [Google Scholar] [CrossRef]
- Shannon-Lowe, C.; Rickinson, A.B.; Bell, A.I. Epstein-Barr virus-associated lymphomas. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160271. [Google Scholar] [CrossRef]
- Soldan, S.S.; Lieberman, P.M. Epstein-Barr virus infection in the development of neurological disorders. Drug Discov. Today Dis. Models 2020, 32, 35–52. [Google Scholar] [CrossRef]
- Zhang, N.; Zuo, Y.; Jiang, L.; Peng, Y.; Huang, X.; Zuo, L. Epstein-Barr Virus and Neurological Diseases. Front. Mol. Biosci. 2021, 8, 816098. [Google Scholar] [CrossRef]
- Le Maréchal, M.; Mailles, A.; Seigneurin, A.; Tattevin, P.; Stahl, J.-P.; Abgrall, S.; Argaud, L.; Argemi, X.; Asseray, N.; Baille, G.; et al. A Prospective Cohort Study to Identify Clinical, Biological, and Imaging Features That Predict the Etiology of Acute Encephalitis. Clin. Infect. Dis. 2021, 73, 264–270. [Google Scholar] [CrossRef]
- Johansen, A.H. Serous Meningitis and Infectious Mononucleosis. Acta Med. Scand. 1931, 76, 269–272. [Google Scholar] [CrossRef]
- Epstein, S.H.; Dameshek, W. Involvement of the Central Nervous System in a Case of Glandular Fever. N. Engl. J. Med. 1931, 205, 1238–1241. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, D.; Peng, X.; Wu, P.; Jiang, L.; Hu, Y. Clinical characteristics of Epstein-Barr virus infection in the pediatric nervous system. BMC Infect. Dis. 2020, 20, 886. [Google Scholar] [CrossRef]
- Dyachenko, P.; Smiianova, O.; Kurhanskaya, V.; Oleshko, A.; Dyachenko, A. Epstein-barr virus-associated encephalitis in a case-series of more than 40 patients. Wiad. Lek. 2018, 71, 1224–1230. [Google Scholar]
- Barón Sánchez, J.; Herrero Velázquez, S.; Ruiz Piñero, M.; Pedraza Hueso, M.I.; Rojo Rello, S.; Guerrero Peral, Á.L. Encefalitis por el virus de Epstein-Barr: Descripción de un caso clínico y revisión de la bibliografía. Rev. Neurol. 2013, 57, 451. [Google Scholar] [CrossRef]
- Zarlasht, F.; Salehi, M.; Abu Hishmeh, M.; Khan, M. Encephalitis treatment: A case report with long-term follow-up of EBV PCR in cerebrospinal fluid. Int. J. Gen. Med. 2017, 10, 371–373. [Google Scholar] [CrossRef]
- Lau, J.S.Y.; Low, Z.M.; Abbott, I.; Shochet, L.; Kanellis, J.; A Kitching, R.; Korman, T.M. Epstein-Barr virus encephalitis in solid organ transplantation. New Microbiol. 2017, 40, 212–217. [Google Scholar]
- Han, J.; Kang, Z.; Xie, Y.; Li, H.; Yan, H.; Song, X. Acute diffuse edematous-hemorrhagic Epstein-Barr virus meningoencephalitis: A case report. Medicine 2019, 98, e18070. [Google Scholar] [CrossRef]
- Hashemian, S.; Ashrafzadeh, F.; Akhondian, J.; Beiraghi Toosi, M. Epstein-Barr Virus Encephalitis: A Case Report. Iran. J. Child Neurol. 2015, 9, 107–110. [Google Scholar]
- Stone, J.A.; Knoll, B.M.; Farmakiotis, D. Relapsing EBV encephalitis in a renal transplant recipient. IDCases 2017, 10, 83–87. [Google Scholar] [CrossRef]
- Polilli, E.; Sozio, F.; Mazzotta, E.; Consorte, A.; Di Masi, F.; Agostinone, A.; Tontodonati, M.; Cosentino, L.; Parruti, G. Rapidly progressive and fatal EBV-related encephalitis in a patient with advanced HIV-1 infection at presentation: A case report and review of the literature. New Microbiol. 2010, 33, 275–280. [Google Scholar]
- Francisci, D.; Sensini, A.; Fratini, D.; Moretti, M.V.; Luchetta, M.L.; Di Caro, A.; Stagni, G.; Baldelli, F. Acute fatal necrotizing hemorrhagic encephalitis caused by Epstein-Barr virus in a young adult immunocompetent man. J. Neurovirol. 2004, 10, 414–417. [Google Scholar] [CrossRef]
- Derler, F.; Seidel, S.; Bengel, D. Fulminante EBV-Meningoenzephalitis. Nervenarzt 2017, 88, 1186–1191. [Google Scholar] [CrossRef]
- Akkoc, G.; Kadayifci, E.K.; Karaaslan, A.; Atici, S.; Yakut, N.; Demir, S.O.; Soysal, A.; Bakir, M. Epstein-Barr Virus Encephalitis in an Immunocompetent Child: A Case Report and Management of Epstein-Barr Virus Encephalitis. Case Rep. Infect. Dis. 2016, 2016, 7549252. [Google Scholar] [CrossRef]
- Koning, M.T.; Brik, T.; Hagenbeek, R.; van den Wijngaard, I. A case of fulminant Epstein-Barr virus encephalitis in an immune-competent adult. J. Neurovirol. 2019, 25, 422–425. [Google Scholar] [CrossRef]
- Sabat, S.; Agarwal, A.; Zacharia, T.; Labib, S.; Yousef, J. Epstein-Barr virus encephalitis presenting as cerebellar hemorrhage. Neuroradiol. J. 2015, 28, 555–558. [Google Scholar] [CrossRef]
- Komur, M.; Celik, T.; Celik, U.; Tolunay, O.; Baspinar, H.; Yilmaz, C.; Mert, G.; Yildizdas, D. Epstein-Barr virus encephalitis with substantia nigra involvement. J. Pediatr. Neurosci. 2015, 10, 401. [Google Scholar] [CrossRef]
- de Haes, I.; Versluis, J.; Lam, K.H.; Jongen, J.L.M.; Doorduijn, J.K.; Kuipers, S. Epstein-Barr virus infection or malignant lymphoma—What you see is not what you get. Neth. J. Med. 2019, 77, 370–372. [Google Scholar]
- Gurbuz, F.; Gurbuz, B.; Çayir, A.; Tezer, H. Epstein-Barr Virus Encephalitis in Infancy. West Indian Med. J. 2014, 63, 206–207. [Google Scholar] [CrossRef]
- Nordbø, S.A.; Dalen, A. Encefalitt etter akutt Epstein-Barr-virusinfeksjon. Tidsskr. Laegeforen Nr. 2001, 18, 2202–2203. [Google Scholar]
- Tsuruyama, Y.; Mori, N.; Yoshida, S.; Hayashi, T. Epstein-Barr virus-related encephalitis in a young woman: A case report. J. Infect. Chemother. 2020, 26, 741–744. [Google Scholar] [CrossRef]
- Babik, J.M.; Katrak, S.; Miller, S.; Shah, M.; Chin-Hong, P. Epstein-Barr virus encephalitis in a renal transplant recipient manifesting as hemorrhagic, ring-enhancing mass lesions. Transpl. Infect. Dis. 2015, 17, 744–750. [Google Scholar] [CrossRef]
- Ascenção, B.B.; Gonçalves, A.C.; Luís, N.; Sá, J.; Brito, A.P.; Poças, J.M. Epstein-Barr virus hemorrhagic meningoencephalitis: Case report and review of the literature. J. Neurovirol. 2016, 22, 695–698. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, J.; Shi, Y. Transient widespread cortical and splenial lesions in acute encephalitis/encephalopathy associated with primary Epstein-Barr virus infection. Int. J. Infect. Dis. 2016, 42, 7–10. [Google Scholar] [CrossRef]
- Seynaeve, L.; Caekebeke, J.; Cypers, G. A fatal case of Epstein Barr encephalitis presenting as fever of unknown origin. Acta Neurol. Belg. 2013, 113, 91–94. [Google Scholar] [CrossRef]
- Engelmann, I.; Nasser, H.; Belmiloudi, S.; Le Guern, R.; Dewilde, A.; Vallée, L.; Hober, D. Clinically severe Epstein-Barr virus encephalitis with mild cerebrospinal fluid abnormalities in an immunocompetent adolescent: A case report. Diagn. Microbiol. Infect. Dis. 2013, 76, 232–234. [Google Scholar] [CrossRef]
- Borg, S.A.; Tonkin, A.; Kleinig, T.; Waters, M. Unusual presentation of Epstein-Barr virus encephalitis in an older patient with a dramatic clinical response to intravenous immunoglobulin. Intern. Med. J. 2015, 45, 879–881. [Google Scholar] [CrossRef]
- Garré, J.; Sprengers, M.; Van Melkebeke, D.; Laureys, G. EBV-NMDA double positive encephalitis in an immunocompromised patient. J. Neurol. Sci. 2019, 396, 76–77. [Google Scholar] [CrossRef]
- Aznar Laín, G.; Martinez Roig, A.; Castejón Ponce, E.; López Segura, N.; Muñoz Almagro, C.; Bonet alcaina, M. Meningoencefalitis por virus Epstein-Barr en niño sano. An. Pediatr. 2010, 72, 445–446. [Google Scholar] [CrossRef]
- Follet-Bouhamed, C.; Nassimi, A.; Troller, S.; Loiseau-Corvez, M.N.; Berthier, M.; Oriot, D. Une cause d’encéphalite aiguë: La primo-infection à virus d’Epstein-Barr. Arch. Pediatr. 1999, 6, 286–289. [Google Scholar] [CrossRef]
- Ahn, S.W.; Youn, Y.C.; Kwon, O.S.; Ahn, D.W.; Sung, J.J. Acute viral encephalitis co-existing with fulminant hepatitis caused by Epstein-Barr virus. Intern. Med. J. 2014, 44, 710–712. [Google Scholar] [CrossRef]
- Takeuchi, S.; Takasato, Y.; Masaoka, H.; Hayakawa, T.; Otani, N.; Yoshino, Y.; Yatsushige, H.; Sugawara, T. Hemorrhagic encephalitis associated with Epstein-Barr virus infection. J. Clin. Neurosci. 2010, 17, 153–154. [Google Scholar] [CrossRef]
- Trevillyan, J.M.; Mahony, A.A.; McLean, C.; Hoy, J.F. Successful Treatment of Epstein-Barr Virus Encephalitis in the Setting of HIV-Associated Neurocognitive Disorder: A Diagnostic and Therapeutic Challenge. Antivir. Ther. 2013, 18, 257–261. [Google Scholar] [CrossRef]
- Misra, U.; Maurya, P.; Kalita, J.; Kumar, B. Epstein Barr virus encephalitis: Clinical diversity and radiological similarity. Neurol. India 2011, 59, 605. [Google Scholar] [CrossRef]
- Grillo, E.; da Silva, R.J.M.; Barbato Filho, J.H. Epstein-Barr virus acute encephalomyelitis in a 13-year-old boy. Eur. J. Paediatr. Neurol. 2008, 12, 417–420. [Google Scholar] [CrossRef]
- Sanefuji, M.; Ohga, S.; Kira, R.; Nomura, A.; Torisu, H.; Takada, H.; Kusuhara, K.; Hara, T. Epstein-Barr Virus—Associated Meningoencephalomyelitis: Intrathecal Reactivation of the Virus in an Immunocompetent Child. J. Child Neurol. 2008, 23, 1072–1077. [Google Scholar] [CrossRef]
- Khalil, M.; Enzinger, C.; Wallner-Blazek, M.; Scarpatetti, M.; Barth, A.; Horn, S.; Reiter, G. Epstein-Barr virus encephalitis presenting with a tumor-like lesion in an immunosuppressed transplant recipient. J. Neurovirol. 2008, 14, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Biebl, A.; Webersinke, C.; Traxler, B.; Povysil, B.; Furthner, D.; Schmitt, K.; Weis, S. Fatal Epstein-Barr virus encephalitis in a 12-year-old child: An underappreciated neurological complication? Nat. Rev. Neurol. 2009, 5, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Raman, L.; Nelson, M. Cerebral vasculitis and encephalitis due to Epstein-Barr virus in a patient with newly diagnosed HIV infection. J. Clin. Virol. 2014, 59, 264–267. [Google Scholar] [CrossRef]
- Cubo, P.; Abad, M.; Vergas, J.; Estrada, V. Posible encefalitis por el virus de Epstein-Barr en un paciente infectado por el VIH. Enfermedades Infecc. Microbiol. Clin. 2007, 25, 491–492. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.; Schaefer, M.; Littmann, E.; Klingebiel, R.; Heinz, A. Psychiatric symptoms and cognitive dysfunction caused by Epstein-Barr virus-induced encephalitis. Eur. Psychiatry 2006, 21, 521–522. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, X.; Fang, X. Case Report: Epstein-Barr Virus Encephalitis Complicated With Brain Stem Hemorrhage in an Immune-Competent Adult. Front. Immunol. 2021, 12, 618830. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.; Diamond, Y.; McCloskey, K.; Standish, J. Probable acute Epstein-Barr virus encephalitis in a 6-year-old girl: Epstein-Barr virus encephalitis. J. Paediatr. Child Health 2017, 53, 1233–1235. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, R.M.; Lauar, L.Z.; Miyake, C.H.; Abud, L.G.; de Oliveira, R.G.G. Acute Epstein-Barr virus encephalitis in an immunocompetent adolescent patient. Arq. Neuropsiquiatr. 2017, 75, 489–490. [Google Scholar] [CrossRef]
- Bazzino Rubio, F.; Gonzalez Betlza, M.; Gonzalez Rabelino, G.; Bello Pedrosa, O. Optic neuritis following Epstein-Barr virus encephalitis in immunocompetent children: A case report. Neurol. Barc. Spain 2017, 32, 129–131. [Google Scholar] [CrossRef]
- Mathew, A.G.; Parvez, Y. Fulminant Epstein Barr virus encephalitis. Indian Pediatr. 2013, 50, 418–419. [Google Scholar] [CrossRef]
- Hou, R.; Wu, J.; He, D.; Yan, Y.; Li, L. Anti-N-methyl-d-aspartate receptor encephalitis associated with reactivated Epstein-Barr virus infection in pediatric patients: Three case reports. Medicine 2019, 98, e15726. [Google Scholar] [CrossRef]
- Jang, Y.Y.; Lee, K.H. Transient asymptomatic white matter lesions following Epstein-Barr virus encephalitis. Korean J. Pediatr. 2011, 54, 389. [Google Scholar] [CrossRef] [PubMed]
- Vince, A.; Lepej, S.; Kurelac, I.; Baršić, B.; Kozić, S.; Klinar, I.; Žarković, K. Virological and immunological characteristics of fatal Epstein-Barr virus mononucleosis in a 17-year-old Caucasian male presenting with meningoencephalitis and hemophagocytic syndrome. J. Neurovirol. 2007, 13, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, F.; Dueñas, G.; Lees, A. Encephalitis lethargica due to Epstein-Barr virus infection. Mov. Disord. Off. J. Mov. Disord. Soc. 2011, 26, 2132–2134. [Google Scholar] [CrossRef] [PubMed]
- Peña Castellanos, I.M.; Santana Porras, J.D. Encefalitis severa por el virus de Epstein-Barr en paciente inmunocompetente: Reporte de caso. Acta Neurol. Colomb. 2019, 35, 30–35. [Google Scholar] [CrossRef]
- Muhlau, M.; Bulow, S.; Stimmer, H.; Schatzl, H.; Berthele, A. Seronegative Epstein-Barr virus myeloradiculitis in an immunocompetent 72-year-old woman. Neurology 2005, 65, 1329–1330. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, Q.; Chen, Y.D.; Hu, W.L.; Zhao, L. Intracranial Epstein-Barr virus infection appearing as an unusual case of meningitis in an immunocompetent woman: A case report. J. Int. Med. Res. 2020, 48, 030006052090321. [Google Scholar] [CrossRef]
- Hu, E.; Chan, D.K. Possible Epstein-Barr virus encephalitis in an elderly patient. Aust. N. Z. J. Med. 2000, 30, 282. [Google Scholar] [CrossRef]
- Roselli, F.; Russo, I.; Fraddosio, A.; Aniello, M.S.; De Mari, M.; Lamberti, P.; Livrea, P.; Defazio, G. Reversible Parkinsonian syndrome associated with anti-neuronal antibodies in acute EBV encephalitis: A case report. Parkinsonism Relat. Disord. 2006, 12, 257–260. [Google Scholar] [CrossRef]
- MacGinley, R.; Bartley, P.B.; Sloots, T.; Johnson, D.W. Epstein-Barr virus encephalitis in a renal allograft recipient diagnosed by polymerase chain reaction on cerebrospinal fluid and successfully treated with ganciclovir. Nephrol. Dial. Transplant. 2001, 16, 197–198. [Google Scholar] [CrossRef]
- Geurten, C.; De Bilderling, G.; Nassogne, M.C.; Misson, J.P.; Verghote, M. Pseudotumoral cerebellitis with acute hydrocephalus as a manifestation of EBV infection. Rev. Neurol. 2018, 174, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, P.; Trizzino, M.; Titone, L.; Capra, G.; Colletti, P.; Mazzola, G.; Pistoia, D.; Sarno, C. Unusual MRI findings in an immunocompetent patient with EBV encephalitis: A case report. BMC Med. Imaging 2011, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- De Simone, M.; Rocca, R.; Mirandola, L. Diffusion-Weighted Imaging in the Early Diagnosis of EBV Meningoencephalitis: A Case Report. Neuroradiol. J. 2011, 24, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Choi, Y.B.; Lee, K.S. Detection of acute Epstein Barr virus cerebellitis using sequential brain HMPAO-SPECT imaging. Clin. Neurol. Neurosurg. 2004, 106, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Van Lierde, A.; Righini, A.; Tremolati, E. Acute cerebellitis with tonsillar herniation and hydrocephalus in Epstein-Barr virus infection. Eur. J. Pediatr. 2004, 163, 689–691. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kobayashi, Z.; Kotera, M. Leptomeningeal Enhancement in Acute Cerebellitis Associated with Epstein-Barr Virus. Intern. Med. 2008, 47, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Garamendi, I.; Montejo, M.; Cancelo, L.; Lopez, L.; Aguirrebengoa, K.; Martín, A.; Zarraga, S. Encephalitis Caused by Epstein-Barr Virus in a Renal Transplant Recipient. Clin. Infect. Dis. 2002, 34, 287–288. [Google Scholar] [CrossRef]
- Monforte, M.; Reale, G.; Masullo, C.; Rossini, P.M.; Tasca, G. Rinsing after spinning: Plasmapheresis in EBV-related post-infectious cerebellitis. J. Neurol. 2017, 264, 576–577. [Google Scholar] [CrossRef]
- Johkura, K.; Momoo, T.; Kuroiwa, Y. Thalamic involvement of Epstein-Barr virus encephalitis demonstrated by MRI. J. Neurol. 2003, 250, 357–358. [Google Scholar] [CrossRef]
- Angelini, L.; Bugiani, M.; Zibordi, F.; Cinque, P.; Bizzi, A. Brainstem encephalitis resulting from Epstein-Barr virus mimicking an infiltrating tumor in a child. Pediatr. Neurol. 2000, 22, 130–132. [Google Scholar] [CrossRef]
- Lahmer, T.; Hoffmann, D.; Heemann, U.; Küchle, C.; Frank, H. Epstein-Barr virus encephalitis after kidney transplantation and successful treatment with brivudine. Transpl. Int. 2010, 23, e24–e25. [Google Scholar] [CrossRef] [PubMed]
- Hongbo, C.; Hongzhen, M.; Lingzhi, H.; Maosheng, X.; Mei, C. Secondary neuropsychiatric manifestations caused by Epstein-Barr virus encephalitis in a new onset systemic lupus erythematosus patient. Rheumatol. Int. 2012, 32, 2321–2323. [Google Scholar] [CrossRef] [PubMed]
- Calore, E.E.; Pérez, N.M.; Martins, J.F.; Cárdenas, P.G. Inmunohistoquímica en un caso de encefalitis por virus de Epstein Barr. Rev. Chil. Infectol. 2012, 29, 687–688. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Cheng, S.J.; Chou, C.L. Myoclonus as the main presentation of Epstein-Barr virus encephalitis. Acta Neurol. Belg. 2015, 115, 479–480. [Google Scholar] [CrossRef]
- Carman, K.B.; Yakut, A.; Ekici, A.; Isikay, S. Nominal dysphasia and euphoria caused by EBV encephalitis. Case Rep. 2013, 2013, bcr2012007514. [Google Scholar] [CrossRef]
- Barberi, W.; Perrone, S.; Iori, A.P.; Torelli, G.F.; Testi, A.M.; Moleti, M.L.; Ceglie, T.; Papoff, P.; Caresta, E.; Antonelli, M.; et al. Proven Epstein-Barr encephalitis with negative EBV-DNA load in cerebrospinal fluid after allogeneic hematopoietic stem cell transplantation in a child with acute lymphoblastic leukemia. Pediatr. Transplant. 2015, 19, E19–E24. [Google Scholar] [CrossRef]
- Rahhal, H.; Nunes, J.T.; Lopes, L.d.C.; Prokopowitsch, A.S. Simultaneous genital ulcer and meningitis: A case of EBV infection. Autopsy Case Rep. 2016, 6, 45–49. [Google Scholar] [CrossRef]
- Chadaide, Z.; Voros, E.; Horvath, S. Epstein-Barr virus encephalitis mimicking clinical and electroencephalographic characteristics of herpes simplex encephalitis. J. Med. Virol. 2008, 80, 1930–1932. [Google Scholar] [CrossRef]
- Iyer, R.S.; Ramalingam, R.T.C.; Akhtar, S.; Ponnadan, S. Bilateral independent periodic lateralised epileptiform discharges at presentation followed by rapid recovery: Novel observations from a case of Epstein-Barr virus encephalitis. BMJ Case Rep. 2019, 12, e229879. [Google Scholar] [CrossRef]
- Mashima, K.; Yano, S.; Yokoyama, H.; Saito, T.; Machishima, T.; Shimada, T.; Yahagi, Y.; Takahara, S.; Sugiyama, K.; Ogasawara, Y.; et al. Epstein-Barr Virus-associated Lymphoproliferative Disorder with Encephalitis Following Anti-thymocyte Globulin for Aplastic Anemia Resolved with Rituximab Therapy: A Case Report and Literature Review. Intern. Med. 2017, 56, 701–706. [Google Scholar] [CrossRef]
- Wade, C.A.; Toupin, D.N.; Darpel, K.; Jones, K.; Lightner, D. Downbeat Nystagmus in a 7-Year-Old Girl With Epstein-Barr Virus-Associated Meningitis and Cerebellitis. Child Neurol. Open 2021, 8, 2329048X2110004. [Google Scholar] [CrossRef]
- Miyashita, T.; Kobayashi, Z.; Numasawa, Y.; Akaza, M.; Ishihara, S.; Shintani, S. Epstein-Barr virus-Associated Meningitis Presenting with Hearing Impairment. Intern. Med. 2012, 51, 1755–1757. [Google Scholar] [CrossRef] [PubMed]
- Phowthongkum, P.; Phantumchinda, K.; Jutivorakool, K.; Suankratay, C. Basal ganglia and brainstem encephalitis, optic neuritis, and radiculomyelitis in Epstein-Barr virus infection. J. Infect. 2007, 54, e141–e144. [Google Scholar] [CrossRef] [PubMed]
- Hayton, E.; Wakerley, B.; Bowler, I.C.; Bogdanovic, M.; Adcock, J.E. Successful outcome of Epstein-Barr virus encephalitis managed with bilateral craniectomy, corticosteroids and aciclovir. Pract. Neurol. 2012, 12, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Sovinz, P.; Schwinger, W.; Lackner, H.; Benesch, M.; Moser, A.; Raicht, A.; Zobel, G.; Urban, C. Severe Epstein-Barr Virus Encephalitis With Hemophagocytic Syndrome: Rapid Clearance of Virus Following Allogeneic Hematopoietic Stem Cell Transplantation From a Seropositive Donor. Pediatr. Infect. Dis. J. 2010, 29, 553–556. [Google Scholar] [CrossRef]
- Patil, A.K.; Azad, Z.; Mathew, V.; Alexander, M. Chronic meningitis and central nervous system vasculopathy related to Epstein Barr virus. Ann. Indian Acad. Neurol. 2012, 15, 303. [Google Scholar] [CrossRef]
- Pinto, J.; Carvalho, S.; Pereira, C.; Figueira, C.; Robalo, C. A case of Epstein-Barr encephalitis with some curiosities. Neuroradiol. J. 2015, 28, 559–561. [Google Scholar] [CrossRef]
- Greco, F.; Cocuzza, M.D.; Smilari, P.; Sorge, G.; Pavone, L. Nonconvulsive Status Epilepticus Complicating Epstein-Barr Virus Encephalitis in a Child. Case Rep. Pediatr. 2014, 2014, 547396. [Google Scholar] [CrossRef]
- Rho, Y.I. Overlapping Guillain-Barré syndrome and Bickerstaff’s brainstem encephalitis associated with Epstein Barr virus. Korean J. Pediatr. 2014, 57, 457. [Google Scholar] [CrossRef]
- Maiese, A.; La Russa, R.; Passaro, G.; Santoro, P.; De Matteis, A.; Fineschi, V. Fatal Epstein-Barr virus infection in an immunocompetent host: A postmortem diagnosis. Forensic Sci. Med. Pathol. 2020, 16, 714–717. [Google Scholar] [CrossRef]
- Rodrigo-Armenteros, P.; Kapetanovic-García, S.; Antón-Méndez, L.; Gómez-Muga, J.J.; Río, E.B.-D.; Fernández-Cuesta, M.; García-Moncó, J.C. Akinetic mutism and status epilepticus due to Epstein Barr virus encephalitis. Clin. Neurol. Neurosurg. 2019, 185, 105492. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Watanabe, G.; Watari, T. Epstein-Barr Virus-induced Meningitis-Retention Syndrome. Eur. J. Case Rep. Intern. Med. 2020, 7, 002133. [Google Scholar] [CrossRef] [PubMed]
- Tlili-Graiess, K.; Souei, M.M.; Mlaiki, B.; Arifa, N.; Moulahi, H.; Gharbi, H.J.; Yacoub, M.; Essoussi, M. Imagerie des cérébellites aiguës chez l’enfant. J. Neuroradiol. 2006, 33, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Göhlich-Ratmann, G.; Wallot, M.; Baethmann, M.; Schaper, J.; Roggendorf, M.; Roll, C.; Aksu, F.; Voit, T. Acute cerebellitis with near-fatal cerebellar swelling and benign outcome under conservative treatment with high dose steroids. Eur. J. Paediatr. Neurol. 1998, 2, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Muscat, K.; Galea, R.; Vella, M. An Adult Case of Acute EBV Cerebellitis. Eur. J. Case Rep. Intern. Med. 2017, 4, 6. [Google Scholar] [CrossRef]
- Al-Shokri, S.D.; Karumannil, S.A.; Mohammed, S.S.; Sadek, M.S. Post-Epstein-Barr Virus Acute Cerebellitis in an Adult. Am. J. Case Rep. 2020, 21, e918567. [Google Scholar] [CrossRef]
- Hussain, R.S.; Hussain, N.A. Ataxia and Encephalitis in a Young Adult with EBV Mononucleosis: A Case Report. Case Rep. Neurol. Med. 2013, 2013, 516325. [Google Scholar] [CrossRef]
- Teive, H.A.G.; Zavala, J.A.A.; Iwamoto, F.M.; Bertucci-Filho, D.; Werneck, L.C. Cerebelite aguda causada por vírus Epstein-Barr: Relato de caso. Arq. Neuropsiquiatr. 2001, 59, 616–618. [Google Scholar] [CrossRef]
- Liu, W.C.; Chiu, S.K.; Hsiang, C.W.; Lin, T.Y. Acute unilateral cerebellitis, Epstein-Barr virus, and HIV. Lancet Infect. Dis. 2014, 14, 778. [Google Scholar] [CrossRef]
- Saikawa, H.; Nagashima, H.; Maeda, T.; Maemondo, M. Acute cerebellar ataxia due to Epstein-Barr virus under administration of an immune checkpoint inhibitor. BMJ Case Rep. 2019, 12, e231520. [Google Scholar] [CrossRef]
- D’Ambrosio, E.; Khalighinejad, F.; Ionete, C. Intravenous immunoglobulins in an adult case of post-EBV cerebellitis. BMJ Case Rep. 2020, 13, e231661. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Sanchez, C.; Steenerson, K.K.; Kelemen, K.; Orenstein, R.; Kusne, S.; Grill, M.F. Post-transplant primary central nervous system lymphoma after Epstein-Barr virus cerebellitis. J. Neurovirol. 2019, 25, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Daaboul, Y.; Vern, B.; Blend, M. Brain SPECT imaging and treatment with IVlg in acute post-infectious cerebellar ataxia: Case report. Neurol. Res. 1998, 20, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Gosalakkal, J.A. Epstein-Barr virus cerebellitis presenting as obstructive hydrocephalus. Clin. Pediatr. 2001, 40, 229–231. [Google Scholar] [CrossRef]
- Schmahmann, J.D. Plasmapheresis improves outcome in postinfectious cerebellitis induced by Epstein-Barr virus. Neurology 2004, 62, 1443. [Google Scholar] [CrossRef]
- Patnaik, S.; Samal, P.; Sahoo, A.; Mohanty, B.; Turuk, J. A fulminant case of Epstein-Barr Virus encephalitis with multiorgan dysfunction. J. Neurovirol. 2022, 28, 464–466. [Google Scholar] [CrossRef]
- Granerod, J.; Ambrose, H.E.; Davies, N.W.S.; Clewley, J.P.; Walsh, A.L.; Morgan, D.; Cunningham, R.; Zuckerman, M.; Mutton, K.J.; Solomon, T.; et al. Causes of encephalitis and differences in their clinical presentations in England: A multicentre, population-based prospective study. Lancet Infect. Dis. 2010, 10, 835–844. [Google Scholar] [CrossRef]
- Raschilas, F.; Wolff, M.; Delatour, F.; Chaffaut, C.; De Broucker, T.; Chevret, S.; Lebon, P.; Canton, P.; Rozenberg, F.; French Herpes Simplex Encephalitis Study Group. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: Results of a multicenter study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2002, 35, 254–260. [Google Scholar] [CrossRef]
- Lazzarotto, T.; Chiereghin, A.; Piralla, A.; Piccirilli, G.; Girello, A.; Campanini, G.; Gabrielli, L.; Costa, C.; Prete, A.; Bonifazi, F.; et al. Cytomegalovirus and Epstein-Barr Virus DNA Kinetics in Whole Blood and Plasma of Allogeneic Hematopoietic Stem Cell Transplantation Recipients. Biol. Blood Marrow Transplant. 2018, 24, 1699–1706. [Google Scholar] [CrossRef]
- Lazzarotto, T.; Chiereghin, A.; Piralla, A.; Gibertoni, D.; Piccirilli, G.; Turello, G.; Campanini, G.; Gabrielli, L.; Costa, C.; Comai, G.; et al. Kinetics of cytomegalovirus and Epstein-Barr virus DNA in whole blood and plasma of kidney transplant recipients: Implications on management strategies. PLoS ONE 2020, 15, e0238062. [Google Scholar] [CrossRef]
- Andersen, O.; Ernberg, I.; Hedström, A.K. Treatment Options for Epstein-Barr Virus-Related Disorders of the Central Nervous System. Infect. Drug Resist. 2023, 16, 4599–4620. [Google Scholar] [CrossRef] [PubMed]

| Country | Number of Cases/Country | Continent | Number of Cases/Continent | Ref. |
|---|---|---|---|---|
| Italy | 11 | Europe | 43 | [16,17,60,63,64,66,69,71,77,89,91] |
| Spain | 6 | [10,34,45,50,68,92] | ||
| Germany | 5 | [18,46,57,72,95] | ||
| Belgium | 3 | [30,33,62] | ||
| France | 3 | [31,35,94] | ||
| Austria | 3 | [42,43,86] | ||
| United Kingdom | 3 | [36,44,85] | ||
| The Netherlands | 2 | [20,23] | ||
| Portugal | 2 | [28,88] | ||
| Norway | 1 | [25] | ||
| Croatia | 1 | [54] | ||
| Malta | 1 | [96] | ||
| Hungary | 1 | [79] | ||
| Japan | 9 | Asia | 38 | [26,37,41,67,70,81,83,93,101] |
| China | 8 | [13,29,47,52,58,73] | ||
| India | 8 | [21,39,51,80,87,107] | ||
| Turkey | 4 | [19,22,24,76] | ||
| South Korea | 3 | [53,65,90] | ||
| Taiwan | 2 | [75,100] | ||
| Iran | 1 | [14] | ||
| Thailand | 1 | [84] | ||
| United States | 11 | North America | 11 | [11,15,27,82,98,102,103,104,105,106] |
| Brazil | 5 | South America | 7 | [40,49,74,78,99] |
| Columbia | 1 | [56] | ||
| Ecuador | 1 | [55] | ||
| Australia | 6 | Australia | 6 | [12,32,38,48,59,61] |
| Niger | 1 | Africa | 1 | [97] |
| Number of Documented Cases | Number of Positive Cases or > Reference Value (%) | Median Value | Highest Value | |
|---|---|---|---|---|
| Proteins (g/L) | 86 | 52 | 0.84 | 4.1 |
| White blood cells (cells/mm3) | 79 | 66 (83%) | 47 | 797 |
| Lymphocytosis | 46 | 40 (87%) | ||
| Neutrophil polynucleosis | 46 | 6 (13%) | ||
| EBV PCR | 70 | 61 (87%) | ||
| qPCR (copies/mL) | 27 | 2400 | 81,200 |
| EBV Serological Status | Number of Cases | Number of EBV PCR in CSF | Number of Positive EBV PCR in CSF | Number of EBV PCR in Blood | Number of Positive EBV PCR in Blood |
|---|---|---|---|---|---|
| Primary infection | 36/75 (48%) | 28/36 | 21/28 (75%) | 11/36 | 9/11 (81%) |
| Past infection | 21/75 (28%) | 14/21 | 14/14 (100%) | 6/21 | 2/6 (33%) |
| Inconclusive | 18/75 (24%) |
| N | % | |
|---|---|---|
| Abnormal images consistent with encephalitis | ||
| Abnormal MRI/performed MRI | 61/88 | 69.3 |
| Consistency between CT scan and MRI | 12/27 | 44.4 |
| Normal CT scan and abnormal MRI | 14/27 | 51.9 |
| Localizations | ||
| Cerebellum | 19 | |
| Basal ganglia | 12 | |
| Frontal lobe | 8 | |
| Cerebral cortex | 7 | |
| Thalamus/hypothalamus | 6 | |
| Meninges | 6 | |
| Occipital lobe | 5 | |
| Temporal lobe | 5 | |
| Parietal lobe | 4 | |
| Diffuse | 4 | |
| Brainstem | 4 | |
| Corpus callosum | 4 | |
| Hippocampus | 1 | |
| Electroencephalogram | ||
| Number performed | 40 | |
| Normal | 7 | 17.7 |
| Diffuse slowing | 19 | 47.5 |
| Discharges | 11 | 27.5 |
| Diffuse slowing + discharges | 1 |
| (Val) Acyclovir | (Val) Ganciclovir | IVIg | Corticoids | Total | Number of Deaths (%) |
|---|---|---|---|---|---|
| x | 23 | 1 (4) | |||
| x | 5 | 0 | |||
| x | 2 | 0 | |||
| x | 9 | 1 (11) | |||
| x | x | 2 | 1 (50) | ||
| x | x | 19 | 3 (16) | ||
| x | x | x | 1 | 0 | |
| x | x | 5 | 2 (40) | ||
| x | x | x | 4 | 0 | |
| x | x | 5 | 1 (20) | ||
| x | x | x | 1 | 1 (100) | |
| x | x | x | 2 | 2 (100) | |
| x | x | x | x | 2 | 1 (50) |
| 55 | 24 | 12 | 42 | 80 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peuchmaur, M.; Voisin, J.; Vaillant, M.; Truffot, A.; Lupo, J.; Morand, P.; Le Maréchal, M.; Germi, R. Epstein-Barr Virus Encephalitis: A Review of Case Reports from the Last 25 Years. Microorganisms 2023, 11, 2825. https://doi.org/10.3390/microorganisms11122825
Peuchmaur M, Voisin J, Vaillant M, Truffot A, Lupo J, Morand P, Le Maréchal M, Germi R. Epstein-Barr Virus Encephalitis: A Review of Case Reports from the Last 25 Years. Microorganisms. 2023; 11(12):2825. https://doi.org/10.3390/microorganisms11122825
Chicago/Turabian StylePeuchmaur, Marine, Joris Voisin, Mathieu Vaillant, Aurélie Truffot, Julien Lupo, Patrice Morand, Marion Le Maréchal, and Raphaele Germi. 2023. "Epstein-Barr Virus Encephalitis: A Review of Case Reports from the Last 25 Years" Microorganisms 11, no. 12: 2825. https://doi.org/10.3390/microorganisms11122825





