The Research Progress on Immortalization of Human B Cells
Abstract
:1. Introduction
2. Immortalize Human Peripheral Blood B Lymphocytes by Epstein-Barr Virus
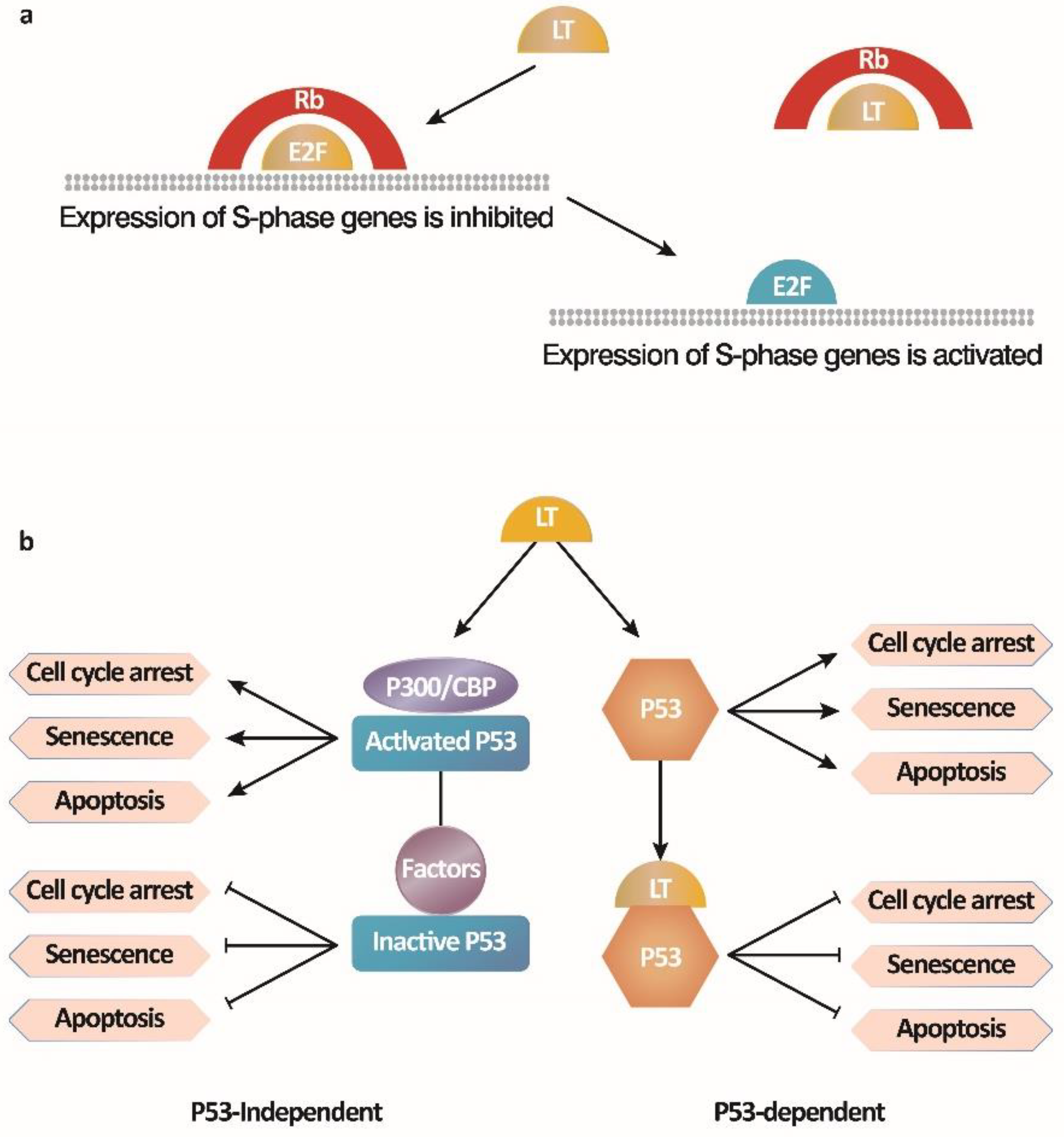
3. Immortalize Human Peripheral B Lymphocytes by Simian Virus 40
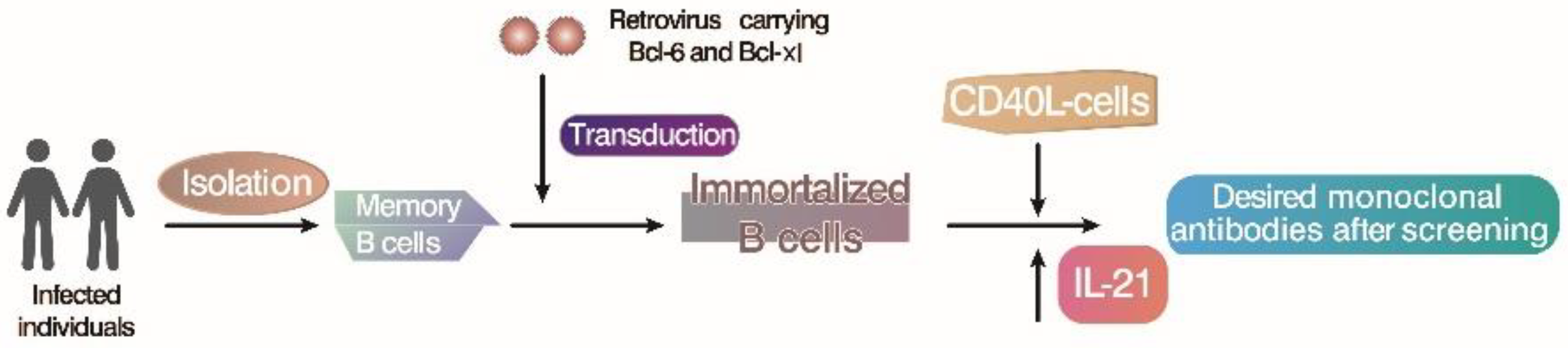
4. Immortalize B Lymphocytes by In Vitro Gene Transduction
5. Immortalize B Lymphocytes by Activation of CD40 Signal
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Obinata, M. The immortalized cell lines with differentiation potentials: Their establishment and possible application. Cancer Sci. 2007, 98, 275–283. [Google Scholar] [CrossRef]
- Chan, S.K.; Rahumatullah, A.; Lai, J.Y.; Lim, T.S. Naive Human Antibody Libraries for Infectious Diseases. Adv. Exp. Med. Biol. 2017, 1053, 35–59. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Y.; Lim, T.S. Construction of Naive and Immune Human Fab Phage Display Library. Methods Mol. Biol. 2023, 2702, 39–58. [Google Scholar] [CrossRef]
- Omar, N.; Lim, T.S. Construction of Naive and Immune Human Fab Phage-Display Library. Methods Mol. Biol. 2018, 1701, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Weitkamp, J.H.; Kallewaard, N.; Kusuhara, K.; Feigelstock, D.; Feng, N.; Greenberg, H.B.; Crowe, J.E., Jr. Generation of recombinant human monoclonal antibodies to rotavirus from single antigen-specific B cells selected with fluorescent virus-like particles. J. Immunol. Methods 2003, 275, 223–237. [Google Scholar] [CrossRef]
- von Boehmer, L.; Liu, C.; Ackerman, S.; Gitlin, A.D.; Wang, Q.; Gazumyan, A.; Nussenzweig, M.C. Sequencing and cloning of antigen-specific antibodies from mouse memory B cells. Nat. Protoc. 2016, 11, 1908–1923. [Google Scholar] [CrossRef]
- Katakura, Y.; Alam, S.; Shirahata, S. Immortalization by gene transfection. Methods Cell Biol. 1998, 57, 69–91. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Yu, S.; Ge, S. Advances in immortalization of human B cells. Sheng Wu Gong Cheng Xue Bao 2021, 37, 30–39. [Google Scholar] [CrossRef]
- Colgin, L.M.; Reddel, R.R. Telomere maintenance mechanisms and cellular immortalization. Curr. Opin. Genet. Dev. 1999, 9, 97–103. [Google Scholar] [CrossRef]
- Lundberg, A.S.; Hahn, W.C.; Gupta, P.; Weinberg, R.A. Genes involved in senescence and immortalization. Curr. Opin. Cell Biol. 2000, 12, 705–709. [Google Scholar] [CrossRef]
- Fridman, A.L.; Tainsky, M.A. Critical pathways in cellular senescence and immortalization revealed by gene expression profiling. Oncogene 2008, 27, 5975–5987. [Google Scholar] [CrossRef]
- Bodnar, A.G.; Ouellette, M.; Frolkis, M.; Holt, S.E.; Chiu, C.P.; Morin, G.B.; Harley, C.B.; Shay, J.W.; Lichtsteiner, S.; Wright, W.E. Extension of life-span by introduction of telomerase into normal human cells. Science 1998, 279, 349–352. [Google Scholar] [CrossRef]
- Ma, C.; Li, X.; Ding, W.; Zhang, X.; Chen, H.; Feng, Y. Effects of hTERT transfection on the telomere and telomerase of Periplaneta americana cells in vitro. AMB Express 2023, 13, 118. [Google Scholar] [CrossRef]
- Sklar, M.D.; White, B.J.; Rowe, W.P. Initiation of oncogenic transformation of mouse lymphocytes in vitro by Abelson leukemia virus. Proc. Natl. Acad. Sci. USA 1974, 71, 4077–4081. [Google Scholar] [CrossRef]
- Muljo, S.A.; Schlissel, M.S. A small molecule Abl kinase inhibitor induces differentiation of Abelson virus-transformed pre-B cell lines. Nat. Immunol. 2003, 4, 31–37. [Google Scholar] [CrossRef]
- Kohler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Parray, H.A.; Shukla, S.; Samal, S.; Shrivastava, T.; Ahmed, S.; Sharma, C.; Kumar, R. Hybridoma technology a versatile method for isolation of monoclonal antibodies, its applicability across species, limitations, advancement and future perspectives. Int. Immunopharmacol. 2020, 85, 106639. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.H. EBV and human cancer. Exp. Mol. Med. 2015, 47, e130. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Robertson, E.S. Mechanisms of B-Cell Oncogenesis Induced by Epstein-Barr Virus. J. Virol. 2019, 93, e00238-19. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.M.; Gewurz, B.E. Epstein-Barr virus oncoprotein-driven B cell metabolism remodeling. PLoS Pathog. 2022, 18, e1010254. [Google Scholar] [CrossRef]
- Yetming, K.D.; Lupey-Green, L.N.; Biryukov, S.; Hughes, D.J.; Marendy, E.M.; Miranda, J.L.; Sample, J.T. The BHLF1 Locus of Epstein-Barr Virus Contributes to Viral Latency and B-Cell Immortalization. J. Virol. 2020, 94, e01215-20. [Google Scholar] [CrossRef]
- Steinitz, M.; Klein, G.; Koskimies, S.; Makel, O. EB virus-induced B lymphocyte cell lines producing specific antibody. Nature 1977, 269, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Neitzel, H. A routine method for the establishment of permanent growing lymphoblastoid cell lines. Hum. Genet. 1986, 73, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Hatton, O.L.; Harris-Arnold, A.; Schaffert, S.; Krams, S.M.; Martinez, O.M. The interplay between Epstein-Barr virus and B lymphocytes: Implications for infection, immunity, and disease. Immunol. Res. 2014, 58, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, A.C.; Devaraju, V.; Velmurugan, P.; Sathiamoorthi, T.; Sivakumar, S.; Subbiah, S.K.; Ravi, A.V. Tumorigenesis and diagnostic practice applied in two oncogenic viruses: Epstein Barr virus and T-cell lymphotropic virus-1-Mini review. Biomed. Pharmacother. 2021, 142, 111974. [Google Scholar] [CrossRef] [PubMed]
- Price, A.M.; Dai, J.; Bazot, Q.; Patel, L.; Nikitin, P.A.; Djavadian, R.; Winter, P.S.; Salinas, C.A.; Barry, A.P.; Wood, K.C.; et al. Epstein-Barr virus ensures B cell survival by uniquely modulating apoptosis at early and late times after infection. Elife 2017, 6, e22509. [Google Scholar] [CrossRef]
- Dolcetti, R.; Dal Col, J.; Martorelli, D.; Carbone, A.; Klein, E. Interplay among viral antigens, cellular pathways and tumor microenvironment in the pathogenesis of EBV-driven lymphomas. Semin. Cancer Biol. 2013, 23, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Ai, P.; Li, Z.; Jiang, Y.; Song, C.; Zhang, L.; Hu, H.; Wang, T. Tumor microenvironment contributes to Epstein-Barr virus anti-nuclear antigen-1 antibody production in nasopharyngeal carcinoma. Oncol. Lett. 2017, 14, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, T.; Bazot, Q.; Leske, D.M.; MacLeod, R.; Mompelat, D.; Tafforeau, L.; Lotteau, V.; Marechal, V.; Baillie, G.S.; Gruffat, H.; et al. Epstein-Barr virus nuclear antigen 1 interacts with regulator of chromosome condensation 1 dynamically throughout the cell cycle. J. Gen. Virol. 2017, 98, 251–265. [Google Scholar] [CrossRef]
- Zimber-Strobl, U.; Strobl, L.J. EBNA2 and Notch signalling in Epstein-Barr virus mediated immortalization of B lymphocytes. Semin. Cancer Biol. 2001, 11, 423–434. [Google Scholar] [CrossRef]
- Dheekollu, J.; Wiedmer, A.; Ayyanathan, K.; Deakyne, J.S.; Messick, T.E.; Lieberman, P.M. Cell-cycle-dependent EBNA1-DNA crosslinking promotes replication termination at oriP and viral episome maintenance. Cell 2021, 184, 643–654.e13. [Google Scholar] [CrossRef] [PubMed]
- Kashuba, E.; Pokrovskaja, K.; Klein, G.; Szekely, L. Epstein-Barr virus-encoded nuclear protein EBNA-3 interacts with the epsilon-subunit of the T-complex protein 1 chaperonin complex. J. Hum. Virol. 1999, 2, 33–37. [Google Scholar] [PubMed]
- Kaye, K.M.; Izumi, K.M.; Kieff, E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 1993, 90, 9150–9154. [Google Scholar] [CrossRef] [PubMed]
- Cahir-McFarland, E.D.; Davidson, D.M.; Schauer, S.L.; Duong, J.; Kieff, E. NF-kappa B inhibition causes spontaneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cells. Proc. Natl. Acad. Sci. USA 2000, 97, 6055–6060. [Google Scholar] [CrossRef]
- Price, A.M.; Tourigny, J.P.; Forte, E.; Salinas, R.E.; Dave, S.S.; Luftig, M.A. Analysis of Epstein-Barr virus-regulated host gene expression changes through primary B-cell outgrowth reveals delayed kinetics of latent membrane protein 1-mediated NF-kappaB activation. J. Virol. 2012, 86, 11096–11106. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, P.A.; Price, A.M.; McFadden, K.; Yan, C.M.; Luftig, M.A. Mitogen-induced B-cell proliferation activates Chk2-dependent G1/S cell cycle arrest. PLoS ONE 2014, 9, e87299. [Google Scholar] [CrossRef] [PubMed]
- Paschos, K.; Smith, P.; Anderton, E.; Middeldorp, J.M.; White, R.E.; Allday, M.J. Epstein-barr virus latency in B cells leads to epigenetic repression and CpG methylation of the tumour suppressor gene Bim. PLoS Pathog. 2009, 5, e1000492. [Google Scholar] [CrossRef]
- Allday, M.J. EBV finds a polycomb-mediated, epigenetic solution to the problem of oncogenic stress responses triggered by infection. Front. Genet. 2013, 4, 212. [Google Scholar] [CrossRef]
- Styles, C.T.; Bazot, Q.; Parker, G.A.; White, R.E.; Paschos, K.; Allday, M.J. EBV epigenetically suppresses the B cell-to-plasma cell differentiation pathway while establishing long-term latency. PLoS Biol. 2017, 15, e2001992. [Google Scholar] [CrossRef]
- Bazot, Q.; Paschos, K.; Skalska, L.; Kalchschmidt, J.S.; Parker, G.A.; Allday, M.J. Epstein-Barr Virus Proteins EBNA3A and EBNA3C Together Induce Expression of the Oncogenic MicroRNA Cluster miR-221/miR-222 and Ablate Expression of Its Target p57KIP2. PLoS Pathog. 2015, 11, e1005031. [Google Scholar] [CrossRef]
- Kamranvar, S.A.; Masucci, M.G. Regulation of Telomere Homeostasis during Epstein-Barr virus Infection and Immortalization. Viruses 2017, 9, 217. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Kavsan, V.M. Immortalization and malignant transformation of eukaryotic cells. Tsitol. Genet. 2012, 46, 36–75. [Google Scholar] [CrossRef] [PubMed]
- Egbuniwe, O.; Idowu, B.D.; Funes, J.M.; Grant, A.D.; Renton, T.; Di Silvio, L. P16/p53 expression and telomerase activity in immortalized human dental pulp cells. Cell Cycle 2011, 10, 3912–3919. [Google Scholar] [CrossRef] [PubMed]
- Kamranvar, S.A.; Chen, X.; Masucci, M.G. Telomere dysfunction and activation of alternative lengthening of telomeres in B-lymphocytes infected by Epstein-Barr virus. Oncogene 2013, 32, 5522–5530. [Google Scholar] [CrossRef]
- Pratt, Z.L.; Zhang, J.; Sugden, B. The latent membrane protein 1 (LMP1) oncogene of Epstein-Barr virus can simultaneously induce and inhibit apoptosis in B cells. J. Virol. 2012, 86, 4380–4393. [Google Scholar] [CrossRef] [PubMed]
- Traggiai, E.; Becker, S.; Subbarao, K.; Kolesnikova, L.; Uematsu, Y.; Gismondo, M.R.; Murphy, B.R.; Rappuoli, R.; Lanzavecchia, A. An efficient method to make human monoclonal antibodies from memory B cells: Potent neutralization of SARS coronavirus. Nat. Med. 2004, 10, 871–875. [Google Scholar] [CrossRef]
- Nogales-Gadea, G.; Saxena, A.; Hoffmann, C.; Hounjet, J.; Coenen, D.; Molenaar, P.; Losen, M.; Martinez-Martinez, P. Generation of Recombinant Human IgG Monoclonal Antibodies from Immortalized Sorted B Cells. J. Vis. Exp. 2015, e52830. [Google Scholar] [CrossRef]
- Tousizadeh, B.; Moghim, S.; Chaleshtori, A.R.S.; Ghanbarian, M.; Mirian, M.; Salehi, M.; Tousizadeh, S.; Zaboli, F. Application of Epstein-Barr Virus for Optimization of Immortalized B-lymphocyte Production as a Positive Control in Genetic Studies. Adv. Biomed. Res. 2017, 6, 80. [Google Scholar] [CrossRef]
- McFadden, K.; Hafez, A.Y.; Kishton, R.; Messinger, J.E.; Nikitin, P.A.; Rathmell, J.C.; Luftig, M.A. Metabolic stress is a barrier to Epstein-Barr virus-mediated B-cell immortalization. Proc. Natl. Acad. Sci. USA 2016, 113, E782–E790. [Google Scholar] [CrossRef]
- Chen, X.; Kamranvar, S.A.; Masucci, M.G. Oxidative stress enables Epstein-Barr virus-induced B-cell transformation by posttranscriptional regulation of viral and cellular growth-promoting factors. Oncogene 2016, 35, 3807–3816. [Google Scholar] [CrossRef]
- Hafez, A.Y.; Messinger, J.E.; McFadden, K.; Fenyofalvi, G.; Shepard, C.N.; Lenzi, G.M.; Kim, B.; Luftig, M.A. Limited nucleotide pools restrict Epstein-Barr virus-mediated B-cell immortalization. Oncogenesis 2017, 6, e349. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Liang, J.; Narita, Y.; Ding, W.; Li, D.; Zhang, L.; Wang, H.; Leong, M.M.L.; Hou, I.; et al. A DNA tumor virus globally reprograms host 3D genome architecture to achieve immortal growth. Nat. Commun. 2023, 14, 1598. [Google Scholar] [CrossRef]
- Zhao, B. Epstein-Barr Virus B Cell Growth Transformation: The Nuclear Events. Viruses 2023, 15, 832. [Google Scholar] [CrossRef]
- Corti, D.; Lanzavecchia, A. Broadly neutralizing antiviral antibodies. Annu. Rev. Immunol. 2013, 31, 705–742. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhao, R.; Lilyestrom, W.; Gai, D.; Zhang, R.; DeCaprio, J.A.; Fanning, E.; Jochimiak, A.; Szakonyi, G.; Chen, X.S. Structure of the replicative helicase of the oncoprotein SV40 large tumour antigen. Nature 2003, 423, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Poulin, D.L.; DeCaprio, J.A. Is there a role for SV40 in human cancer? J. Clin. Oncol. 2006, 24, 4356–4365. [Google Scholar] [CrossRef]
- Pipas, J.M. SV40: Cell transformation and tumorigenesis. Virology 2009, 384, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Fanning, E.; Knippers, R. Structure and function of simian virus 40 large tumor antigen. Annu. Rev. Biochem. 1992, 61, 55–85. [Google Scholar] [CrossRef]
- Sullivan, C.S.; Pipas, J.M. T antigens of simian virus 40: Molecular chaperones for viral replication and tumorigenesis. Microbiol. Mol. Biol. Rev. 2002, 66, 179–202. [Google Scholar] [CrossRef]
- Imperiale, M.J. The human polyomaviruses, BKV and JCV: Molecular pathogenesis of acute disease and potential role in cancer. Virology 2000, 267, 1–7. [Google Scholar] [CrossRef]
- Ahuja, D.; Saenz-Robles, M.T.; Pipas, J.M. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene 2005, 24, 7729–7745. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.T. SV40 large T antigen functions in DNA replication and transformation. Adv. Virus Res. 2000, 55, 75–134. [Google Scholar] [CrossRef] [PubMed]
- Alwin Prem Anand, A.; Gowri Sankar, S.; Kokila Vani, V. Immortalization of neuronal progenitors using SV40 large T antigen and differentiation towards dopaminergic neurons. J. Cell Mol. Med. 2012, 16, 2592–2610. [Google Scholar] [CrossRef]
- DeCaprio, J.A. How the Rb tumor suppressor structure and function was revealed by the study of Adenovirus and SV40. Virology 2009, 384, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Alaribe, F.N.; Mazzoni, E.; Rigolin, G.M.; Rizzotto, L.; Maniero, S.; Pancaldi, C.; Manfrini, M.; Martini, F.; Tognon, M.G. Extended lifespan of normal human B lymphocytes experimentally infected by SV40 or transfected by SV40 large T antigen expression vector. Leuk. Res. 2013, 37, 681–689. [Google Scholar] [CrossRef]
- Ali, S.H.; DeCaprio, J.A. Cellular transformation by SV40 large T antigen: Interaction with host proteins. Semin. Cancer Biol. 2001, 11, 15–23. [Google Scholar] [CrossRef]
- Levine, A.J. The common mechanisms of transformation by the small DNA tumor viruses: The inactivation of tumor suppressor gene products: p53. Virology 2009, 384, 285–293. [Google Scholar] [CrossRef]
- Bright, R.K.; Kimchi, E.T.; Shearer, M.H.; Kennedy, R.C.; Pass, H.I. SV40 Tag-specific cytotoxic T lymphocytes generated from the peripheral blood of malignant pleural mesothelioma patients. Cancer Immunol. Immunother. 2002, 50, 682–690. [Google Scholar] [CrossRef]
- Gutierrez-Guerrero, A.; Cosset, F.L.; Verhoeyen, E. Lentiviral Vector Pseudotypes: Precious Tools to Improve Gene Modification of Hematopoietic Cells for Research and Gene Therapy. Viruses 2020, 12, 1016. [Google Scholar] [CrossRef]
- Chang, C.C.; Ye, B.H.; Chaganti, R.S.; Dalla-Favera, R. BCL-6, a POZ/zinc-finger protein, is a sequence-specific transcriptional repressor. Proc. Natl. Acad. Sci. USA 1996, 93, 6947–6952. [Google Scholar] [CrossRef]
- Klein, U.; Dalla-Favera, R. Germinal centres: Role in B-cell physiology and malignancy. Nat. Rev. Immunol. 2008, 8, 22–33. [Google Scholar] [CrossRef]
- Diehl, S.A.; Schmidlin, H.; Nagasawa, M.; van Haren, S.D.; Kwakkenbos, M.J.; Yasuda, E.; Beaumont, T.; Scheeren, F.A.; Spits, H. STAT3-mediated up-regulation of BLIMP1 Is coordinated with BCL6 down-regulation to control human plasma cell differentiation. J. Immunol. 2008, 180, 4805–4815. [Google Scholar] [CrossRef] [PubMed]
- Shapiro-Shelef, M.; Lin, K.I.; McHeyzer-Williams, L.J.; Liao, J.; McHeyzer-Williams, M.G.; Calame, K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity 2003, 19, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Tunyaplin, C.; Shaffer, A.L.; Angelin-Duclos, C.D.; Yu, X.; Staudt, L.M.; Calame, K.L. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J. Immunol. 2004, 173, 1158–1165. [Google Scholar] [CrossRef]
- Li, M.; Wang, D.; He, J.; Chen, L.; Li, H. Bcl-XL: A multifunctional anti-apoptotic protein. Pharmacol. Res. 2020, 151, 104547. [Google Scholar] [CrossRef]
- Kwakkenbos, M.J.; van Helden, P.M.; Beaumont, T.; Spits, H. Stable long-term cultures of self-renewing B cells and their applications. Immunol. Rev. 2016, 270, 65–77. [Google Scholar] [CrossRef]
- Kwakkenbos, M.J.; Diehl, S.A.; Yasuda, E.; Bakker, A.Q.; van Geelen, C.M.; Lukens, M.V.; van Bleek, G.M.; Widjojoatmodjo, M.N.; Bogers, W.M.; Mei, H.; et al. Generation of stable monoclonal antibody-producing B cell receptor-positive human memory B cells by genetic programming. Nat. Med. 2010, 16, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Westerhuis, B.M.; Benschop, K.S.; Koen, G.; Claassen, Y.B.; Wagner, K.; Bakker, A.Q.; Wolthers, K.C.; Beaumont, T. Human Memory B Cells Producing Potent Cross-Neutralizing Antibodies against Human Parechovirus: Implications for Prevalence, Treatment, and Diagnosis. J. Virol. 2015, 89, 7457–7464. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E. Senescence and immortalization: Role of telomeres and telomerase. Carcinogenesis 2005, 26, 867–874. [Google Scholar] [CrossRef]
- Weng, N.P.; Granger, L.; Hodes, R.J. Telomere lengthening and telomerase activation during human B cell differentiation. Proc. Natl. Acad. Sci. USA 1997, 94, 10827–10832. [Google Scholar] [CrossRef]
- Wennhold, K.; Shimabukuro-Vornhagen, A.; von Bergwelt-Baildon, M. B Cell-Based Cancer Immunotherapy. Transfus. Med. Hemother. 2019, 46, 36–46. [Google Scholar] [CrossRef]
- Hu, B.T.; Lee, S.C.; Marin, E.; Ryan, D.H.; Insel, R.A. Telomerase is up-regulated in human germinal center B cells in vivo and can be re-expressed in memory B cells activated in vitro. J. Immunol. 1997, 159, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, M.; Zentz, C.; Mayr, C.; Wimmer, R.; Hammerschmidt, W.; Zeidler, R.; Moosmann, A. Conditional immortalization of human B cells by CD40 ligation. PLoS ONE 2008, 3, e1464. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; de Paoli, P.; Valle, A.; Garcia, E.; Rousset, F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science 1991, 251, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Kondo, E.; Topp, M.S.; Kiem, H.P.; Obata, Y.; Morishima, Y.; Kuzushima, K.; Tanimoto, M.; Harada, M.; Takahashi, T.; Akatsuka, Y. Efficient generation of antigen-specific cytotoxic T cells using retrovirally transduced CD40-activated B cells. J. Immunol. 2002, 169, 2164–2171. [Google Scholar] [CrossRef]

| Methods | Starting Point | Species | Approaches | Way to Maintain Cell Cultures | Final Products | References |
|---|---|---|---|---|---|---|
| EBV | Isolated mononuclear leukocytes | Human, some primates | Transformation | Latent genes, p53 tumor suppressor, telomerase, etc | LCLs | [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] |
| SV40 | Isolated human B lymphocytes | Mammalian | Infection | LT antigen regulated E2F and p53 down-stream gene, ST antigens | Permanently proliferative cell line | [65,68] |
| Gene transduction | Isolated human B cells | Multiple species | In vitro | Ectopic expression of Bcl-6 and Bcl-xl with proper feeder cells | Variable immortalized B-cell lineage | [72,75] |
| Activation of CD40 signal | PBMC | Human | Exogenous stimulation | Regular and repeated CD40 ligand/IL-4 stimulation plus cyclosporin A | Immortalized B cells with phenotypic characteristics of activated B cells | [82,83,84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Xiang, X.; Ding, W.; Dong, W.; Hu, Y. The Research Progress on Immortalization of Human B Cells. Microorganisms 2023, 11, 2936. https://doi.org/10.3390/microorganisms11122936
Xu H, Xiang X, Ding W, Dong W, Hu Y. The Research Progress on Immortalization of Human B Cells. Microorganisms. 2023; 11(12):2936. https://doi.org/10.3390/microorganisms11122936
Chicago/Turabian StyleXu, Huiting, Xinxin Xiang, Weizhe Ding, Wei Dong, and Yihong Hu. 2023. "The Research Progress on Immortalization of Human B Cells" Microorganisms 11, no. 12: 2936. https://doi.org/10.3390/microorganisms11122936
APA StyleXu, H., Xiang, X., Ding, W., Dong, W., & Hu, Y. (2023). The Research Progress on Immortalization of Human B Cells. Microorganisms, 11(12), 2936. https://doi.org/10.3390/microorganisms11122936






