Abstract
Coffee (Coffea arabica) is among the world’s most economically important crops. Coffee was shown to be highly dependent on arbuscular mycorrhizal fungi (AMF) in traditionally managed coffee plantations in the tropics. The objective of this study was to assess AMF species richness in coffee plantations of four provinces in Perú, to isolate AMF isolates native to these provinces, and to test the effects of selected indigenous AMF strains on coffee growth. AMF species were identified by morphological tools on the genus level, and if possible further to the species level. Two native species, Rhizoglomus variabile and Nanoglomus plukenetiae, recently described from the Peruvian mountain ranges, were successfully cultured in the greenhouse on host plants. In two independent experiments, both species were assessed for their ability to colonize coffee seedlings and improve coffee growth over 135 days. A total of 35 AMF morphospecies were identified from 12 plantations. The two inoculated species effectively colonized coffee roots, which resulted in 3.0–8.6 times higher shoot, root and total biomass, when compared to the non-mycorrhizal controls. R. variabile was superior to N. plukenetiae in all measured parameters, increasing shoot, root, and total biomass dry weight by 4.7, 8.6 and 5.5 times, respectively. The dual inoculation of both species, however, did not further improve plant growth, when compared to single-species inoculations. The colonization of coffee by either R. variabile or N. plukenetiae strongly enhances coffee plant growth. R. variabile, in particular, offers enormous potential for improving coffee establishment and productivity. Assessment of further AMF species, including species from other AMF families should be considered for optimization of coffee growth promotion, both alone and in combination with R. variabile.
1. Introduction
Coffee (Coffea arabica L.) is highly valued for its flavor, fragrance and caffeine content, making it one of the most consumed agricultural commodities worldwide with significant economic and social implications [1]. It is cultivated across the tropics and sub-tropics both on industrial-scale commercial plantations as well as family-owned smallholder plots [2,3]. In South America, Perú is an important producer of coffee and is considered among the ten most important coffee-producing countries globally. Annual exports are currently in the range of 210,000 tons per annum, with marked increases in recent years, representing an annual income of 1.1 billion dollars for the more than 200,000 families of small farmers involved in its production [4,5]. Within Perú, the San Martín and neighboring Amazonia Regions account for approximately 50% of the national coffee production [6].
Worldwide, coffee has traditionally been cultivated under the shade-green system, but with a steady rise in the worldwide demand for coffee, production systems have increasingly shifted to more intensive full-sun systems [7,8]. As a consequence, the majority of coffee plantations are no longer shade-grown, which accounts for approximately 25% of the total production area [8]. However, in Central and South America, shade-grown coffee remains a dominant production system, with the notable exceptions of Brazil and Colombia [8]. In Perú, the majority of coffee farms (‘fincas cafeteleras’) are highly biodiverse with relatively high levels of shade, and cultivation practices characterized by low reliance on agrochemicals [6,8,9]. Most Perúvian coffee growers though, irrespective of the cropping system, rely on some type of organic-based mineral fertilizer to improve crop performance [9]. Perú stands as a principal exporter of ‘organic coffee’ [6], where the cropping systems promote a slower and more equilibrated development of the coffee-cherries or beans [10], which creates a superior organoleptic quality [11,12], increasing its value in the ‘specialty coffee’ market [10,13]. Over recent years, the market for ‘specialty coffee’ has risen, with indications for this to continue increasing [8,12], mainly benefiting smallholder farmers, who represent around 70% of coffee producers worldwide [14].
In organic and biologically-based coffee production systems, the preservation of diverse microbial soil communities towards the maintenance of soil health is a key component for ecosystem stability and multifunctionality [15,16,17]. For example, a reduced soil microbial diversity in coffee monocultures negatively affected several soil functions, suppressing plant growth and reducing coffee production [18]. Additionally, a higher microbiome diversity is also associated with better host plant suppression of diseases and enhanced growth on various crops [19,20]. Within these microbial communities, arbuscular mycorrhizal fungi (herein abbreviated as AM fungi or AMF) (phylum Glomeromycota) are key components [19,21,22], being associated with 70–90% of plant species in terrestrial ecosystems [23] and supporting significantly improved plant growth through their extensive extraradical networks and effective soil nutrient acquisition (P, N, S, K and several microelements [24,25,26,27,28,29,30,31,32,33]. Inoculation of AM fungi can be highly beneficial to crop production [34,35]. Establishing whether single or mixed species applications are more advantageous, however, needs to be assessed for each target crop under prevailing conditions as it is often not initially clear if inoculation with one species is superior to multi-species inoculation [36,37,38,39,40,41]. Berruti et al. [42] concluded that the three globally prevalent AMF species, Rhizoglomus intraradices, Funneliformis mosseae, and R. irregulare, all belonging to the family Glomeraceae, are the most popularly used inocula. These species are widespread around the globe, colonize a large majority of plant species, and are adapted to a large spectrum of edaphoclimatic conditions [43]. Otherwise, it might be more advantageous to use native, and thus more adapted AMF strains, both for ecological-climatological and agronomic reasons [44].
Coffee plants naturally form AMF associations [45,46], showing a high mycotrophy from the early seedling stage to more advanced, mature stages [47,48,49,50]. Various studies have determined a high AMF diversity in the coffee rhizosphere [51,52], and that the application of AMF as biofertilizers improves seedling development and plant growth in the greenhouse [48,50,53,54,55,56]. In total, 70 AMF species have been recorded from the coffee rhizosphere globally [57], but this is most likely a gross underestimation since comprehensive studies to assess AMF diversity in coffee have only recently been undertaken, mostly within the last two decades. To date, just ~330 AMF species have been described [58], with estimates indicating that over 1500–2000 AMF species exist [59,60]. Within Perú, recent studies have led to the description of several new AMF species, such as from the rhizosphere of inka nut (Plukenetia volubilis), cocoa (Theobroma cacao) as well as from coffee [61,62,63,64,65,66]. Studies have also shown that native AMF strains or inocula originating from coffee plantations are more suitable and provide greater benefits to coffee than the commercial, bulk-produced strains/species from abroad [67], an effect similarly observed for other crops [42,44]. In this context, the present study was undertaken to evaluate the AMF diversity in coffee plantations in the San Martín State of Perú and whether single or combined inoculations of prevalent native species provided better biofertilizer effects on seedling development and early coffee growth. We hypothesized that a high AMF diversity can be found in Peruvian coffee plantations and that the combined inoculation of native AMF species, belonging to the Glomeraceae, would be superior for coffee growth than the inoculation with only one AMF species.
2. Materials and Methods
2.1. Coffee Plantations under Study
Between March and April 2016, soil samples (0–20 cm depth) were taken from the coffee rhizosphere of 12 coffee plantations (sites) located in 4 provinces of San Martín State (Table 1). For each site, five plants were selected randomly and sampled separately, with ~1 kg of rhizospheric soil extracted from four equidistant points around the main stem of each plant. Each soil sample was placed in a single plastic bag, constituting five samples in total per site, i.e., five replicates per site. The soil samples were transferred to the laboratory within one day, sieved (5 mm size), air-dried and stored at 4 °C.

Table 1.
Geographic coordinates of coffee plantations in San Martín State (Peru) under AMF diversity study.
2.2. AMF Spore Isolation, Identification and Diversity
The AMF was extracted by wet sieving from 50 g soil according to Gerdemann and Nicolson [68] using 38 and 250 μm sieves, followed by a sucrose density gradient centrifugation as described by Sieverding [69]. Species were morphologically identified and counted as described below, and those species with abundant and ubiquitous occurrence (90–100%) were selected for further multiplication. Species occurrence was calculated from the number of sites each species was detected, divided by the total number of sites investigated [70].
Spores were observed under a compound microscope after mounting in polyvinyl alcohol-lactic acid-glycerol (PVLG) [71], Melzer’s reagent, a mixture of PVLG and Melzer’s reagent [72], a 1:1 mixture of lactic acid and water, and in water [73]. The AMF taxa were identified on the genus level, and, if possible, up to the species level, using described morphological spore characteristics, type of spore formation, and their sub-cellular structures, such as their color, size, number, and structure of walls and wall layers [74,75] using the Glomeromycota system as presented by Wijayawardene et al. [58] and updated by Blaszkowski et al. (2022) [76] and da Silva et al. [77].
The AMF spore abundance and species richness at the 12 sampling sites were determined by counting the number of spores identified by species and counting the number of species detected per site, respectively. From each field site a detailed list of AM fungal species was developed, classifying the spore abundance into four categories (0, 1–2, 3–5 and ≥6 spores per g soil). The most abundant and frequently occurring species were selected for culturing and further assessment.
2.3. Multiplication of Selected AMF Species
The most abundant and ubiquitous species from the field soil sample evaluation were multiplied individually using a polyculture of Sorghum vulgare, Brachiaria brizantha and Medicago sativa. For the multiplication, all 12 field soils were used in 2.5 L pots (~2 kg soil per pot). Each field soil was first mixed with sand (2:1, v/v) before autoclaving at 121 °C for 1 h per day over three days. Five pots per site were used for the cultivation of each AMF species. The pots were maintained in the greenhouse under ambient temperature conditions (Temperature oscillated between 21.4 and 33.2 °C, and relative humidity between 48 and 75%) for three months before harvesting of the AM fungal inocula. The AM fungal species that successfully multiplied were used as inocula in two subsequent experiments, to study their potential to improve coffee plant growth in the greenhouse. These were Rhizoglomus variabile and Nanoglomus plukenetiae (Corazon-Guivin et al. [62,64]), recently described from the study region and firstly isolated from the rhizosphere of Plukenetia volubilis L., an indigenous agroforestry crop of increasing agronomic importance. Both AMF species had also been analyzed phylogenetically to confirm the morphological identification of these AM fungal species (see Corazon-Guivin et al. [62,64]). The two AMF strains used for the present study were isolated from a coffee plantation in Pamashto (R. variabile) and in Pueblo Nuevo (N. plukenetiae; Table 1).
2.4. Effects of AMF Inoculation on Coffee Growth: Experimental Details
The effects of the two species, R. variabile and N. plukenetiae, were assessed on coffee crop growth in the greenhouse of the Laboratorio de Biología y Genética Molecular, Universidad Nacional de San Martín (Distrito Morales, Jr. Amorarca, cdra. 3 s/n), located in the province of San Martín in San Martín State (06°35’28’’ S, 76°18’47’’ W) at 230 m a.s.l. altitude. The experiment was conducted between March and July 2018 and repeated between May and September 2018. The pots were maintained under greenhouse conditions at a mean daily temperature of 29 °C (maximum of 38.2 °C, minimum of 21.4 °C). Mean, maximum and minimum relative humidity were 64.0, 73.8 and 47.9%, respectively during the period March to September 2018.
In both experiments, C. arabica cv. Caturra plants aged aprox. one month was used, which is among the most common cultivars cultivated in Perú. Ripe red coffee cherries were selectively collected from healthy plants, without any obvious pest or disease symptoms, from a field in “Naranjal” ubicate in the Rioja Province of the San Martín Department, Perú in 2018. The berries were manually de-pulped, discarding any small-sized seeds and dried under shade. They were then surface sterilized by dipping in 0.5% sodium hypochlorite for 2 min and 95% ethanol for 2 min, and then rinsed in sterile distilled water three times before placing in germination boxes (1 × 1 × 0.3 m), using autoclaved (121 °C, 15 p.s.i., 30 min) coarse sand as a substrate. The seeds were placed flat-side down onto the sand, spaced 2.5–3.0 cm in regular rows and covered with a thin layer of finely sieved coarse sand (2 mm mesh width). After sowing, the seed beds were mulched with a mesh raschel (80%) to protect the seeds from desiccation and create optimal temperatures of ~25 °C for seed germination. The seed beds were irrigated daily over several weeks until sufficient uniform plants at the “little soldier” growth stage were available (~15 cm high), soon after emergence and before the seed-coat was cast off at ~20 days after emergence (Figure 1a,b).

Figure 1.
Inoculation process of coffee seedlings with arbuscular mycorrhizal fungi (AMF), (a) Growing coffee seeds in nursery beds, (b) Uniform seedlings at the “little soldier” stage, (c) Inoculation and transplantation of coffee seedlings, (d) Vegetative growth of coffee plants.
Field soil mixed with coarse river sand (2:1, v/v) was used after autoclaving for 1 h per day over three consecutive days. The textural classification of this substrate was a sandy-loam, with 4.82 of pH, 0.35 dSm−1 electrical conductivity, 1.66% organic matter, 6.5 mg P kg−1, and 63 mg K kg−1 (0.14 K+meq/100 g). The uniform “little soldier” seedlings were bare-root transplanted singularly into plastic 3 L pots, filled with 3 kg of sterile substrate. The pots were first disinfected with ethanol and rinsed with distilled water. The experiment comprised 4 treatments, each with 36 replications, arranged in a completely randomized design totaling 144 seedlings, which were placed on greenhouse tables. Experimental treatments included: (1) single inoculation of R. variabile (Rv) and of (2) N. plukenetiae (Np), (3) combined inoculation of R. variabile and N. plukenetiae (Rv+Np), (4) non-mycorrhizal control (Ctrl).
In both experiments, R. variabile and N. plukenetiae were inoculated using 20 g freshly chopped pieces of mycorrhizal roots from S. vulgare, B. brizantha and M. sativa, which included hyphae and ~1500 AMF spores; for dual inoculation (Rv+Np), 10 g chopped roots each of the two inocula were mixed and inoculated (Figure 1c). The non-mycorrhizal control treatment received washings of 20 g of the inoculum mixture filtered through Whatman n°. 42 filter paper. Inoculation of the AMF was conducted at transplanting. Holes 10 cm depth and 4 cm diameter were prepared using a trowel, which were first filled with inocula before placing plantlets directly onto the inocula and then completely filling with sterile soil substrate. The soil moisture was increased to maximum water-holding capacity and plants subsequently irrigated every three days to maintain the substrate at field capacity. Fertilizer was applied weekly using 75 mL of the Long Ashton nutrient solution [78], modified to supply 10.25 µg P mL−1 pot.
2.5. Assessment of Plant Characteristics
Plant height (cm), stem diameter (mm) and number of leaves were measured at 30 days after transplanting and then at 15-day intervals until harvest at 135 days. Chlorophyll content (SPAD) was also recorded on the youngest completely expanded leaf of each plant using a chlorophyll analyzer (SPAD-502, Minolta Camera Co. Ltd., Osaka, Japan). The leaf area (cm2) was calculated using ImageJ (https://imagej.nih.gov/ij/). At harvest, the plant’s total fresh weight was determined using a balance (OHAUS, Adventurer™ Parsippany, NJ, USA) and the total dry matter was recorded after oven drying at 80 °C for 48 h.
2.6. Arbuscular Mycorrhizal Root Colonization
The freshly harvested roots were rinsed with tap water, and the percentage of mycorrhizal colonization was estimated using the root clearing and staining method [79], with modifications. Once stained, roots were cut into 1 cm segments, mounted on slides and observed under a compound microscope (NIKON, Eclipse E200, Tokyo, Japan) at 20× magnification [80].
2.7. Analyses of N, P and K Coffee Plant Contents
At harvest, plant N, P and K contents were analyzed using total leaf matter from each plant. The N concentration was obtained using the Kjeldahl method [81], P concentration following digestion in HNO3:HClO4 (4:1), spectr. UV-Vis (λ = 420 nm) and K concentration by digestion in HNO3:HClO4 (4:1) for atomic absorption spectrophotometry analyses (Model Varian, AAS Spectra 55B, Victoria, Australia).
2.8. Statistical Analyses
The results of the two independent experiments showed only minor numerical differences, but no statistical differences (p > 0.05) for each parameter recorded. Thus, the data for the two experiments were combined for analysis. All measured variables were evaluated for normality and homogeneity using Shapiro–Wilk [82] and Levene’s [83] tests, respectively. All the variables evaluated were transformed to a natural logarithm (ln), except for dry biomass and colonization, which were transformed to a square root (√ x) to normalize data. ANOVA analyses were followed by Tukey’s HSD to test for differences among treatments at p < 0.05 significance level [84]. The data were analyzed using INFOSTAT version 2012.1 software [85]. The ANOVA and the mean comparison tests were conducted on transformed data, with data back-transformed to the original units for presentation in results.
3. Results
3.1. AMF Species Richness and Spore Abundance per Species
In total, 35 AMF morphospecies belonging to 13 genera, were identified from the 12 study sites by morphological spore identification (Table 2). Of the 35 species, eleven were not unequivocally identified on the species level. Three of them resembledknown species, while the lasting eight species might be new to science, according to our knowledge, and will be part of future taxonomic analyses. At individual sites, between 6 and 18 species were recorded. Three AMF species were detected across all sites (G. microcarpum, R. variabile and N. plukenetiae), while A. mellea was recovered from 11 sites, and G. brohultii from 10 sites. Five further species were found in at least six sites but with lower abundance than the five most frequently occurring species. The remaining 25 morphospecies were less abundantly and less frequently observed, or only sporadically detected (Table 2).

Table 2.
Arbuscular mycorrhizal fungi species identified from twelve coffee plantations in San Martín State, Peru.
3.2. AMF Species Selected for Further Multiplication and Functional Experiments
The species G. microcarpum, R. variabile, N. plukenetiae and A. mellea were the most abundant and most frequently occurring (92–100% of sites; Table 2). Following three months of culturing in the greenhouse on S. vulgare, B. brizantha and M. sativa in the twelve soils, R. variabile and N. plukenetiae were the only species that multiplied well and with a high spore abundance (Table 3), while A. mellea and G. microcarpum did not multiply well. Therefore, R. variabile and N. plukenetiae only were considered for further functional experiments on coffee crop growth promotion.

Table 3.
Dominant sporulating arbuscular mycorrhizal fungi species isolated after culturing for three months in the screenhouse.
3.3. Effects of AMF on Coffee Plant Growth
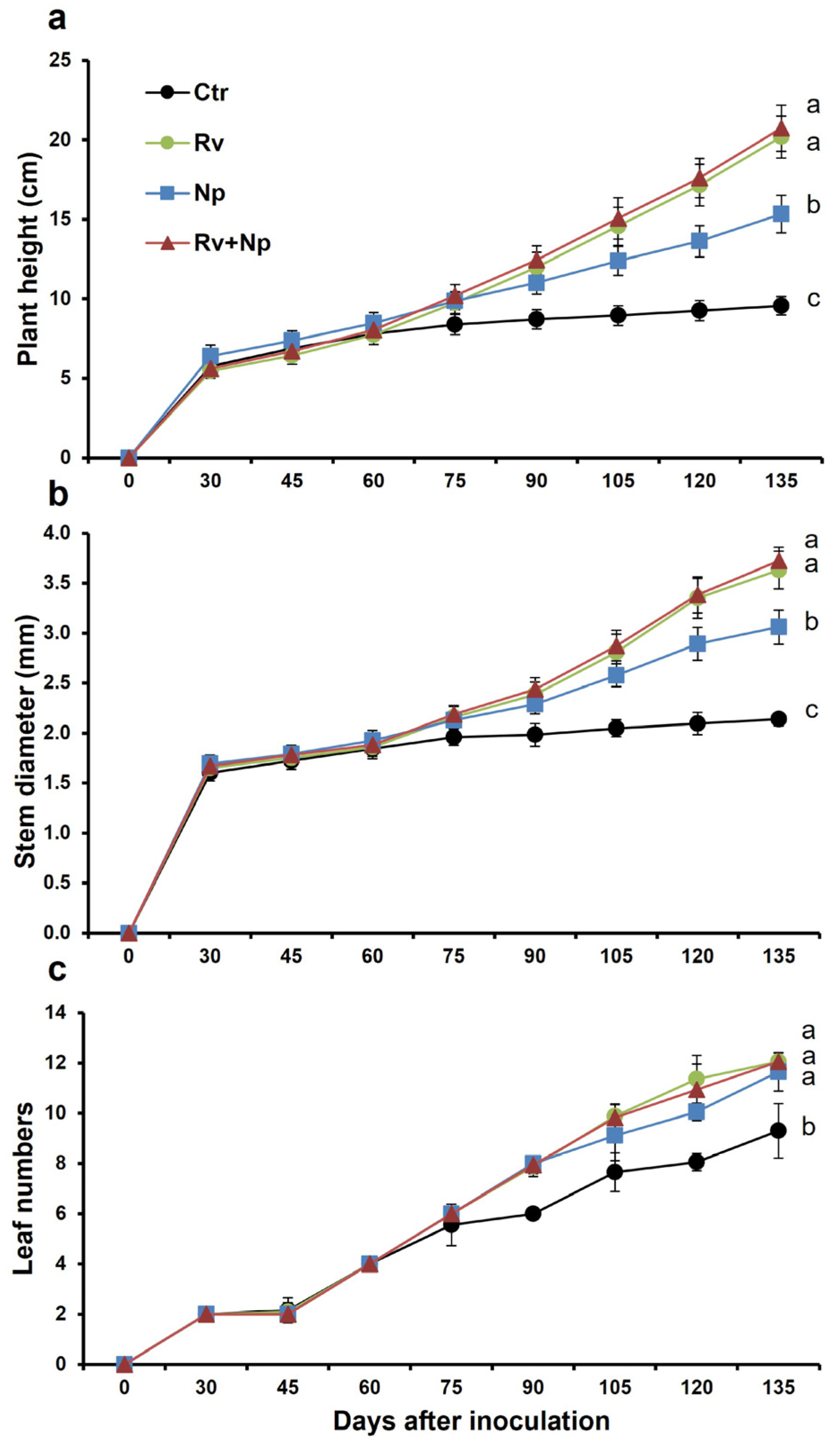
The plant growth parameters began to differentiate between treatments at 60–75 days after inoculation, with mycorrhizal plants having better growth than the controls (Figure 2). At harvest, mycorrhizal plants were taller and had more leaves and thicker stems than non-mycorrhizal plants. Plants dual-inoculated and with R. variabile alone were tallest (mean plant height 20.7 and 20.2 cm, respectively), and 2.2 times higher (p < 0.05) than plants inoculated with N. plukenetiae alone (15.3 cm; 1.5 times higher than control plants), and the non-mycorrhizal control (9.6 cm; Figure 3a, F = 655.38, p < 0.0001). Similarly, stem diameter was higher in the dual-inoculated (3.7 mm) and R. variabile-inoculated (3.6 mm) plants than the N. plukenetiae-inoculated (3.1 mm), or in the non-mycorrhizal control (2.1 mm), with mycorrhizal stem girths 1.4–1.7 times thicker than non-mycorrhizal plants (Figure 3b, F = 1061.76, p < 0.0001). All AMF-treated plants had more leaves than the control (Figure 3c, F = 118.86, p < 0.0001), with approximately 1.3 times more leaves than the non-mycorrhizal control at harvest. In line with leaf number, the leaf area index per plant was greatest in the dual-inoculated (709 cm2) and with R. variabile-inoculated (691 cm2) plants and significantly lower in N. plukenetiae-inoculated plants (434 cm2), and lowest in the non-mycorrhizal control plants (155 cm2), and thus up to 4.6 times higher in the mycorrhizal than non-mycorrhizal treatments (Table 4).
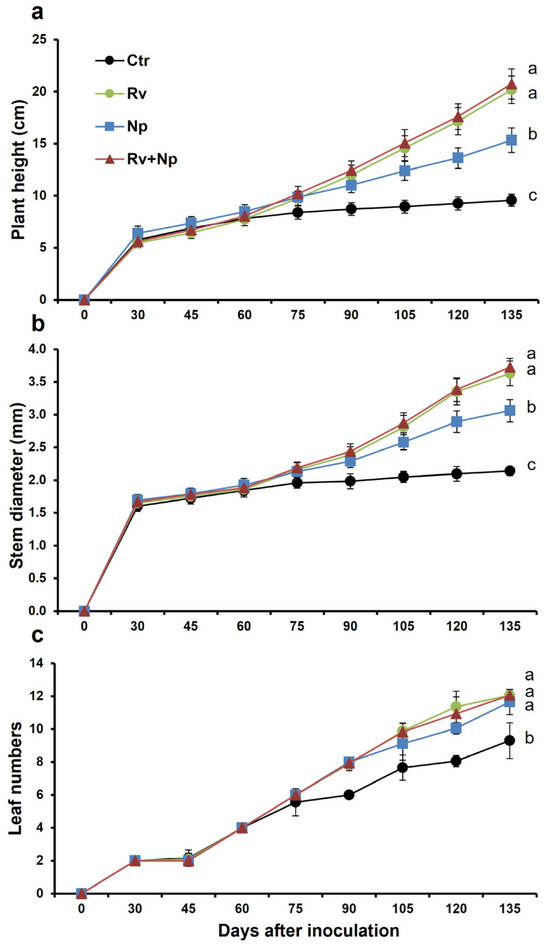
Figure 2.
Effect of single and dual arbuscular mycorrhizal fungal inoculation on (a) plant height (cm), (b) number of coffee leaves per plant and (c) stem diameter (cm) in coffee plants along 135 days of vegetative growth measured (15-day intervals). Mean values per treatment. Error bars indicate standard deviation (±S.D.). Different letters indicate significantly different means (p < 0.05). Ctr = Non-inoculated, Rv = Inoculation with Rhizoglomus variabile, Np = Inoculation with Nanoglomus plukenetiae.

Figure 3.
Illustration of coffee leaf, shoot and root growth 135 days in (a) non-mycorrhizal control plants, after single inoculation with (b) Rhizoglomus variabile, or (c) Nanoglomus plukenetiae, and (d) after dual inoculation with both AMF species.

Table 4.
Impact of AMF single and dual inoculation on Coffea arabica growth and physiology after 135 days.
At harvest, inoculation of coffee seedlings with AMF led to significantly greater shoot and root growth (Table 4; Figure 3). Coffee plant fresh and dry shoot and root weights were consistently heavier after inoculation with dual AMF and single inoculation with R. variabile compared with inoculation of N. plukenetiae alone, and lowest in the non-mycorrhizal control (Table 4; Figure 3). The total coffee fresh plant weight was 6.2 and 6.0 times higher in the dual-inoculated and the R. variabile-inoculated treatments, respectively, compared to the non-mycorrhizal control plants, and total dry weight was 5.5 times heavier for the dual-inoculated plants.
The shoot fresh weight of coffee plants was 3.2–5.3 times higher in the mycorrhizal than non-mycorrhizal treatments (Table 4), and the root fresh weight of the mycorrhizal coffee plants was 5.5–9.8 times higher in the mycorrhizal treatments. The shoot dry weight of the mycorrhizal plants was 2.9–4.7 times higher in the mycorrhizal than non-mycorrhizal treatments, and the root dry weight of mycorrhizal plants was 5.0–8.6 times higher. Also, the leaf area index was highest after dual inoculation and inoculation with R. variabile alone (Leaf area 709 and 691 cm2 per plant, respectively), while it was significantly lower with N. plukenetiae alone (434 cm2), and lowest in the non-mycorrhizal control (155 cm2), meaning that the leaf area of mycorrhizal plants was 2.8–4.6 times higher in the mycorrhizal than non-mycorrhizal treatments (Table 4).
3.4. Arbuscular Mycorrhizal Root Colonization in the Growth-Response Experiments
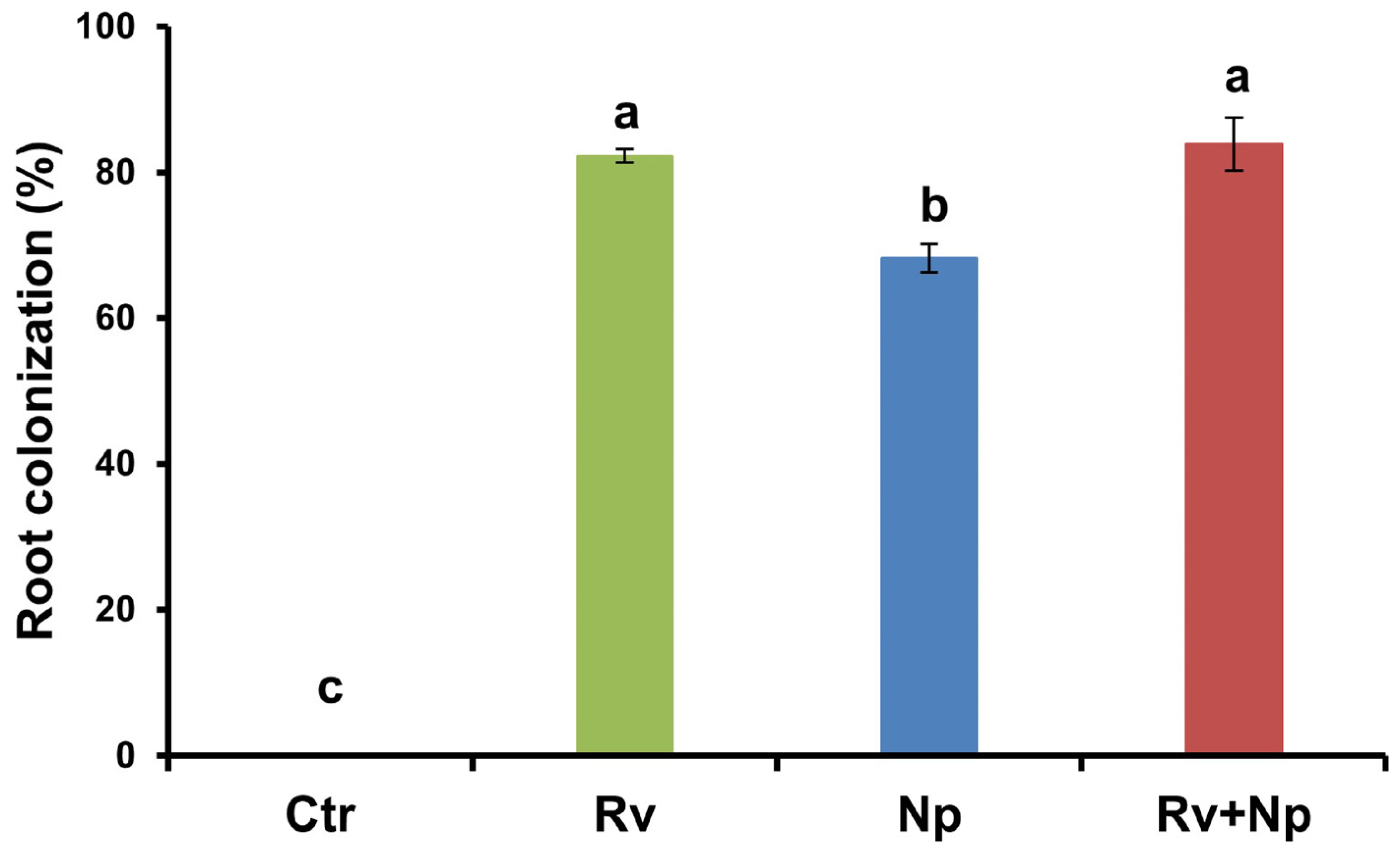
At harvest, all mycorrhizal treatments colonized roots to a high percentage (68–84%) by R. variabile, N. plukenetiae, or both fungi, while the non-mycorrhizal control was absent of any colonization. The highest (p < 0.05) colonization occurred with R. variabile (82%) and R. variabile + N. plukenetiae (84%), compared to N. plukenetiae alone (68%). See Figure 4, F = 4864.4, p < 0.0001.
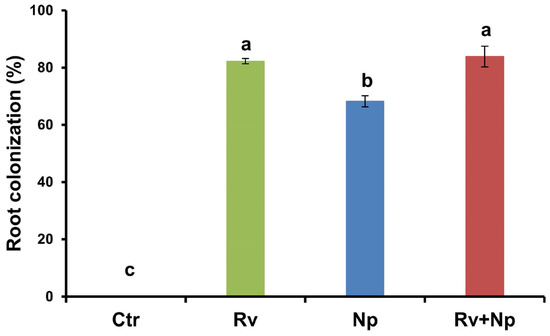
Figure 4.
AM fungal root colonization of coffee plants inoculated with Rhizoglomus variabile, Nanoglomus plukenetiae or a combination in the greenhouse after 135 days. Mean values per treatment (N = 34). Error bars indicate standard deviation (±S.D.). Columns sharing the same letter were not significantly different (p < 0.05). Ctr = Non-inoculated, Rv = Inoculation with Rhizoglomus variabile, Np = Inoculation with Nanoglomus plukenetiae.
3.5. Impact of AMF on Chlorophyll and Mineral Nutrient Contents in Coffee Leaves
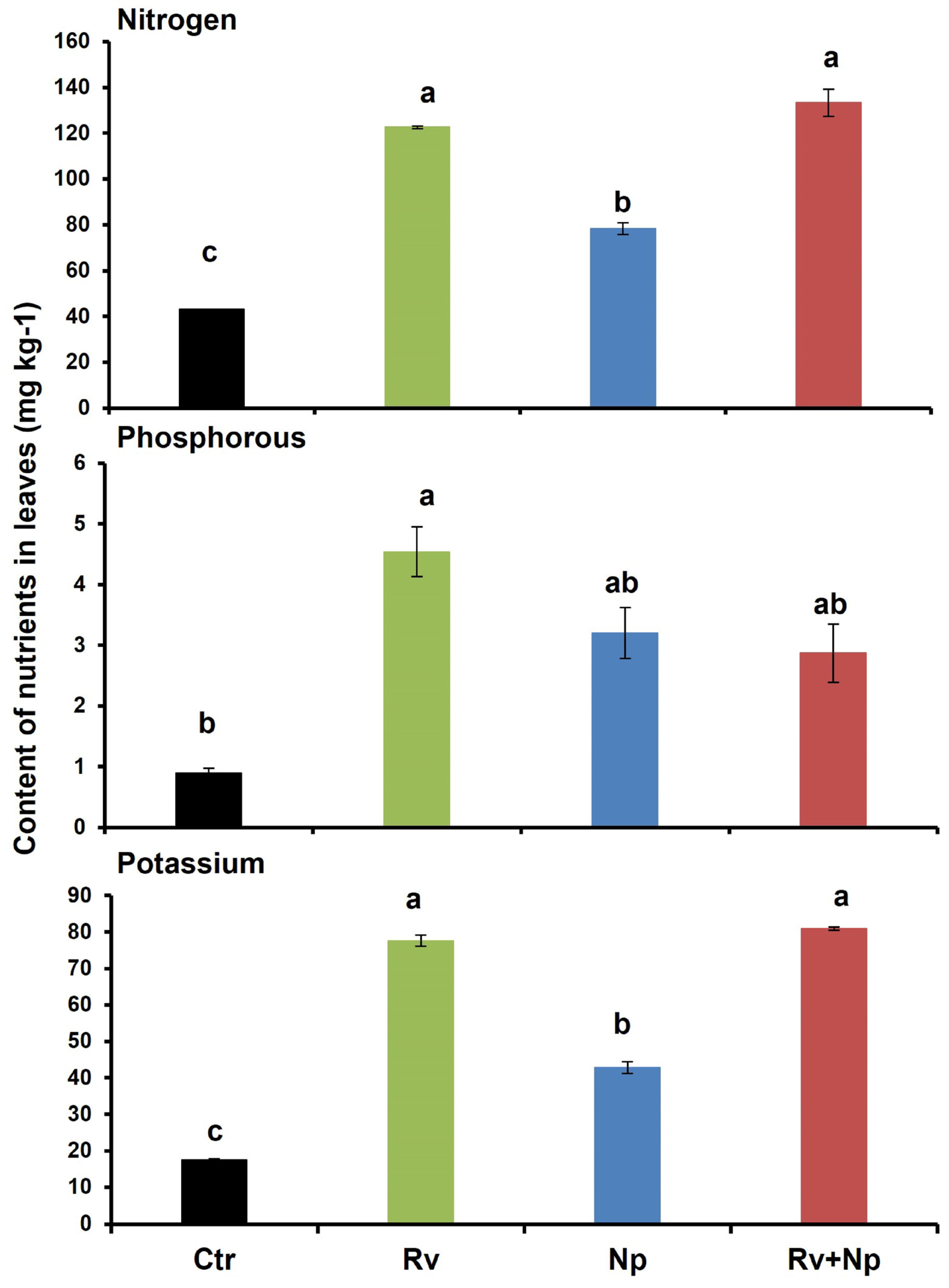
The highest leaf chlorophyll content was recorded following inoculation with both AMF and with R. variabile alone (60.3 and 59.8, respectively), and significantly lower with N. plukenetiae alone (48.9), and the non-mycorrhizal control (27.4 cm2), which was less than half of the dual-inoculated and R. variabile-inoculated plants (Table 4). The leaf N, P and K contents were also significantly higher in plants following AMF inoculation (Figure 5, nitrogen: F = 55.40, p < 0.0001, phosphorus: F = 5.21, p < 0.027 and pottassium: F = 229.80, p < 0.0001) at 2.9, 5.0 and 4.4 times higher, respectively, when inoculated with R. variabile than in the non-mycorrhizal plants. For N. plukenetiae-inoculated plants, the differences were less pronounced than for R. variabile with 1.8, 3.6 and 2.5 times higher N, P and K than in the non-mycorrhizal plants. Following dual-inoculation with both fungi the effects were similar to those with R. variabile for N and K contents, with 3.0 and 4.6 times higher than in the non-mycorrhizal control, while P content was more similar to the P content with N. plukenetiae (3.2 times higher than non-mycorrhizal plants).
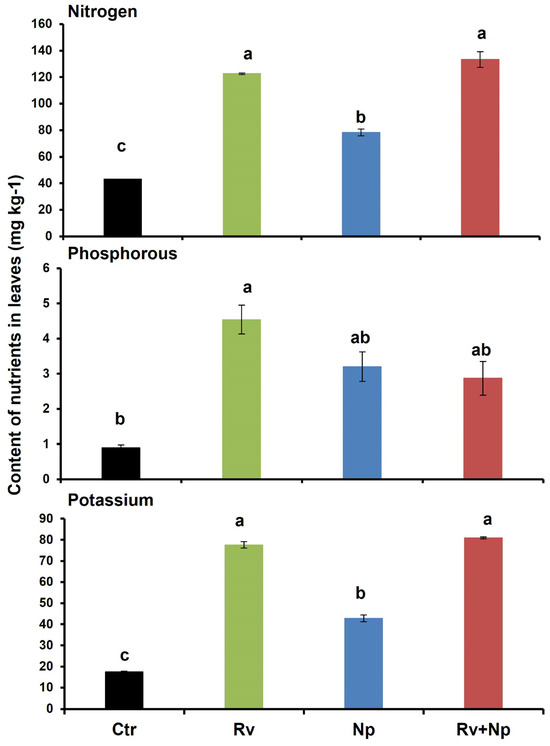
Figure 5.
Nitrogen, phosphorus and potassium contents (mg kg−1) of coffee leaves after 135 days in non-mycorrhizal control plants (Ctr), after single inoculation with Rhizoglomus variabile (Rv), or Nanoglomus plukenetiae (Np), and after dual inoculation with both AMF species (Rv+Np). Mean values per treatment. Error bars indicate standard deviation (±S.D.). Columns sharing the same letter were not significantly different (p < 0.05). Ctr = Non-inoculated, Rv = Inoculation with Rhizoglomus variabile, Np = Inoculation with Nanoglomus plukenetiae.
4. Discussion
AMF species can be identified from field soil samples either by morphological spore or molecular analyses after DNA extraction [86]. Both approaches have their limitations and their advantages as described in Oehl et al. [87], but both are laborious and thus time-consuming and costly. Ideally, they are concomitantly used and should deliver complementary data, but the combination of both has been rarely applied so far, also due to the lack of skills for one or the other methodology [88]. In one of these rare events, morphological AMF identification was even found to be superior to the molecular approach [89]. In this study, AMF taxa were identified on the genus level (e.g., Glomus) and wherever possible to further identify the morphospecies (e.g., Glomus microcarpum, when the morphotype was unequivocally attributable to this species) or to the morphotype level (e.g., Glomus sp. resembling G. spinuliferum, when the species resembled to a known species; or e.g., Glomus sp. 1, when the species most probably is new to science, according to our knowledge).
In the four coffee-growing provinces of Peruvian San Martin State, 35 different morphospecies were detected in 12 coffee plantations, with 14–24 morphospecies recovered per province. The majority of previous studies with coffee report a similar AMF species richness (16–37 species) as found in our study [90,91,92,93,94,95,96], with some reporting higher species richness (43 and 79 species; [52,97]). The large variation in AMF species richness was often explained by the coffee production systems, with a generally higher AMF richness under the shadow (shade-green systems), as these systems allow the interaction of coffee with other plant species of the plantations through the belowground AMF network, as well as by different climatic conditions of the regions and the edaphic soil characteristics [93].
In our study, the species belonged to 13 AMF genera from 6 families, which demonstrates a high diversity, when compared with most studies (already cited within this paragraph). Acaulospora and Glomus genera provided the highest species richness, with 9 and 10 AMF species recorded, respectively. These genera are often the most prevalent in coffee plantations [91,94,98,99]. The genera Rhizoglomus, Sclerocystis, Entrophospora, Dominikia, Ambispora, Diversispora, Gigaspora and Sieverdingia have also been reported from coffee plantations worldwide [90,91,93,94,95,96,97] but represent a lower species richness, as reflected in the current study.
The key important observation of this study was the impressive increase in coffee seedling growth and development, following inoculation with the two indigenous AMF species R. variabile and N. plukenetiae, resulting in up to 5.5 times higher total biomass after 135 days, when compared with non-inoculated plants. This is the first functional study with R. variabile and N. plukenetiae, which were only recently described from coffee plantations in San Martín State, Perú [62,64]. The inoculation of R. variabile alone improved coffee growth to a greater extent than that of N. plukenetiae. This is notable, with now a fourth species within the genus Rhizoglomus, besides the well-known species R. intraradices and R. irregulare [42], and R. invermaium [100], with elevated beneficial effects on an agronomically important crop, underlining the importance of Rhizoglomus species for crop plant nutrition. The results clearly demonstrate the highly mycotrophic nature of coffee, as evidenced by other AMF-coffee associations [48,50,53,56,101] but moreover, highlight the outstanding functions of Rhizoglomus for plant nutrition with now a new ‘super strain candidate’ R. variabile for South American coffee plant establishment. Also, during a study to assess more than 40 AMF strains for their effect on leek (Allium porrum) growth, Rhizoglomus species and the closely related Oehlia diaphana, were the most beneficial species detected [100].
Our results demonstrate that the genus Nanoglomus (N. plukenetiae) from the family Glomeraceae can be added to those that provide strong beneficial effects on plant growth. Nanoglomus plukenetiae belongs to the major Dominikia clade of the Glomeraceae, which has previously not been recognized among the most beneficial clades of AMF [100].
The combination of both AMF inoculants did not lead to increased coffee growth, over inoculation with R. variabile alone. The effects of multiple species inoculations do not necessarily lead to improvements over single inoculations [48,101,102], although Trejo et al. [50] reported significant coffee growth improvement with various combinations of AMF consortia. More research and information is needed if, or in which cases, or for which crop species, single or multiple inoculations with specific AMF species/strains should be recommended. This would also likely depend on crop cultivar, as well as on the prevailing edaphic and climatic conditions, and whether cultivated in mono- or mixed cropping systems. When inoculating Medicago truncatula with three Rhizoglomus species, R. intraradices/irregulare, R. aggregatum and R. custos, Kiers et al. [103] observed that host plants were able to detect, discriminate and select the most beneficial AMF symbionts. Of the three species, R. intraradices/irregulare was the most outstanding, delivering the highest quantities of nutrients to M. truncatula and acquiring the highest amount of carbohydrates itself. Following double or triple combinations of Funneliformis mosseae, R. intraradices/irregulare and Entrophospora claroidea (all belonging to the Order Glomerales), Jansa et al. [36] obtained similar results as in our study: no additional crop growth benefits to M. truncatula were observed following combinations, compared to the inoculation of F. mosseae alone. When A. porrum was inoculated with the same combinations, however, plant biomass was lower than inoculation with F. mosseae alone. These studies consequently demonstrate the complexity of the interactions and associations between host plants and AMF, as well as between AMF species/strains on the same plant. Maherali and Klironomos [104], working with Plantago lanceolata showed that host plant growth benefits were greater when combinations of AMF included species from different families, compared with combinations of species from within a single family. This concept was further supported by Thonar et al. [105] and Yang et al. [40] who indicated that different AMF families have complementary functional capacities in favor of the plant hosts. A number of recorded species of AMF have previously shown beneficial effects on host plants, such as Dioscorea rotundata, e.g., Entrophospora etunicata, E. claroidea, A. scrobiculata and A. spinosa [44]. Many other recorded species have, to date, rarely been cultivated and so their potential benefits remain unknown, for coffee and for any other hosts. From the 35 AMF species detected and the four most abundant and frequently occurring AMF species in our study, just two species multiplied efficiently for inoculum production.
Following inoculation in the current study, AM fungal root colonization for R. variabile or the combined inoculation was similar (~85%) after 135 days, while colonization for N. plukenetiae alone was lower (68%). Similarly, Säle et al. [100] also observed that AMF isolates with the highest root colonization rates provided the greatest growth benefits. Dumbrell et al. [106], further concluded that AMF species with high root colonization rates are more effective at creating symbiotic associations and providing more benefits to host plants than slower-growing fungi. Both R. variabile and N. plukenetiae belong to major clades of the Glomeraceae, which are known for their high root colonization capacity [100,107,108].
Coffee inoculation with AMF resulted in exceptional root length increases, of up to 84%, which is higher than previously reported for coffee under greenhouse conditions [47,48,55,56,104,109]. Such levels of improvement may be an indication of the high AMF dependency of coffee plants, especially in marginal soils with low fertility [45,48,53,69,110,111].
When considering which AMF isolates should be applied, or developed as a product, the high colonization rate is an important criterion, as is the speed of colonization and consequent nutrient assimilation. Voříšková et al. [112], for example, observed the dominance of R. irregulare (92%) in combined inoculations on M. truncatula. Its high capacity to colonize roots enables it to rapidly occupy root niches and possibly exclude other AMF species [43,113].
Our results on N, P and K assimilation appeared dependent on the AMF association. N. plukenetiae had a lower efficiency of P assimilation than R. variabile. Phosphorus can be directly uptaken by the plant through the mycorrhizal networks [114,115,116]. Andrade et al. [55], reported an average three-fold higher P assimilation in mycorrhizal coffee plantlets initially inoculated with an AMF consortium, reflecting results for N. plukenetiae in our study. Smith et al. [117], also showed that R. intraradices/irregulare transferred about 100% of the assimilated P in three plant species, but that Funneliformis caledonius and Gigaspora rosea delivered much less. However, the mycorrhizal symbiosis can also result in the inactivation of direct P uptake by the roots [117,118,119,120]. In other studies, using the split-root system, in which different root areas of the same plant were colonized by different AMF, host plants were able to discriminate between the fungi, delivering Carbon (C) preferentially to the most beneficial AMF species [22,26,121,122,123]. In our study, the lowest quantities of P were recorded in plants in the dual inoculation treatment with R. variabile and N. plukenetiae, indicating that either N. plukenetiae interfered in the transfer of P to the plants, or that there was competition between the two species for P acquisition. In this context, Jansa et al. [36] reported that certain combinations of AMF strains (consortia) reduced the P content in A. porrum leaves when compared to single inoculations. In contrast, Crossay et al. [34] found that combined inoculations with species from different AMF families increased shoot P concentrations, compared to single inoculations with specific AMF species.
Conversely, to P acquisition, N content was superior following inoculation with R. variabile alone or combined with N. plukenetiae than with N. plukenetiae alone. Lower N concentrations in the N. plukenetiae treatment were closely related to the lower biomass of the coffee roots, and the lower AMF colonization of the roots when compared to the single or dual inoculations with R. variabile. Numerous studies have shown that AMFs are able to absorb large amounts of inorganic nitrogen as nitrate-N or ammonium-N [124,125,126,127,128,129] and at least partially transfer it to the plant hosts [25,27,100,130,131,132,133]. Although N is much more easily assimilated by plants than P, N limitation in ecosystems may lead to increased levels of N plant uptake through the mycorrhizal network [124,134] even though some studies have indicated that N assimilation through AMF is not important for the plant [135]. Nevertheless, as shown in our study, N assimilation can be affected by specific AMF species, and that R. intraradices/irregulare is superior to other Glomeraceae species (Glomus/Simiglomus hoi) [130]. Our findings further confirm, therefore, that although the AMF symbiosis is not species-specific, the compatibility and efficiency between the plant host and its AM fungal associates may largely depend on the specific plant-fungal association [136]. The respective differences in N contents for coffee plants inoculated with N. pluneketiae and R. variabile, were similarly reflected in photosynthetic activity and leaf chlorophyll contents (48.9 vs. 59.8 SPAD, respectively), which may have limited the transfer of C to the fungi. Various studies have previously demonstrated the strong correlation between leaf N contents and photosynthesis [137,138,139].
As for P, AMF can also increase the K availability for host plants, which can otherwise be low due to its strong mineral absorption [43,140,141,142,143]. In our study, treatment with AMF led to over four times higher K contents in coffee leaves (42.9–77.6 mg K kg−1) than the non-mycorrhizal control (17.5 mg kg−1), which is much higher than Andrade et al. [55] reported for mycorrhizal coffee seedlings. Similar to our study, Garcia and Zimmermann [142] and Olsson et al. [144,145], also found a clear relationship between the P and K leaf contents during the AM symbiosis.
5. Conclusions
The current study clearly shows the high growth-promoting and biofertilizing potential of two recently described AMFs for coffee seedlings. Both fungi are indigenous to Peruvian coffee plantations but are not restricted to South American ecosystems. Remarkably, Rhizoglomus variabile is now the sixth known Rhizoglomus species, after R. intraradices, R. irregulare, R. fasciculatum, R. clarum and R. invermaium, demonstrating the huge potential to promote crop growth. The potential benefits of these closely related species, consequently, demand greater attention for their benefit to agriculture. Similarly, Nanoglomus also appears to be a genus of interest towards enhancing plant growth and agronomic performance, but the combination of both studied species belonging to the same AMF family had no synergistic effects for the coffee plants. Our study further demonstrates the importance of identifying the most suitable species/strains. Nevertheless, multiple species applications might be most beneficial either for the crop plants or for the whole plant-soil system, especially when AMF strains from other AMF families could be integrated. The results indicate an initial step towards the use of such AMF strains as a profitable and environmentally sustainable strategy for the establishment of coffee plantations, both in traditional and sustainable modern low-input agro-forestry systems. Further steps towards the commercial production of AMF, based on R. variabile, would be to evaluate other relevant species from the Glomeromycetes, alone and in combination with R. variabile, and to select the most beneficial combinations for coffee production in the Peruvian mountains and beyond.
Author Contributions
Conceptualization, M.A.C.-G. and F.O.; methodology, M.A.C.-G., G.R.-C., K.M.D.A. and A.P.-D.; validation, M.A.C.-G., A.C.-M., D.C. and F.O.; formal analysis, M.A.C.-G., A.D.H.-A. and F.O.; investigation, M.A.C.-G., G.R.-C. and K.M.D.A.; data curation, M.A.C.-G., A.D.H.-A. and F.O.; writing—original draft preparation, M.A.C.-G.; writing—review and editing, M.A.C.-G., A.D.H.-A.; D.C and F.O.; supervision, M.A.C.-G., D.C. and F.O.; All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Programa Nacional de Estudios de Investigación Científica y Estudios Avanzados (ProCiencia), a unit of the Consejo Nacional de Ciencia, Tecnología e Innovación Tecnológica (Concytec, Perú), through the project SUBVENTION AGREEMENT N° 163-2020-FONDECYT.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors thank all the members of the Laboratorio de Biología y Genética Molecular for collaborating in the publication of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nieber, K. The Impact of Coffee on Health. Planta Med. 2017, 83, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Donald, P.F. Biodiversity impacts of some agricultural commodity production systems. Conserv. Biol. 2004, 18, 17–38. [Google Scholar] [CrossRef]
- Avelino, J.; Allinne, C.; Cerda, R.; Willocquet, L.; Savary, S. Multiple-Disease System in Coffee: From Crop Loss Assessment to Sustainable Management. Annu. Rev. Phytopathol. 2018, 56, 611–635. [Google Scholar] [CrossRef] [PubMed]
- USDA, United States Department of Agriculture. Foreign Agricultural Service. 2023. Available online: https://apps.fas.usda.gov/psdonline/app/index.html#/app/home/statsByCountry (accessed on 2 August 2023).
- Tulet, J.C. Peru as a New Major Actor in Latin American Coffee Production. Lat. Am. Perspect. 2012, 37, 133–141. [Google Scholar] [CrossRef]
- Nolte, G.E.; Luxbacher, K.W. Peru-Coffee Annual. USDA Foreign Agricultural Services; Global Agricultural Information Network. 2020. Available online: https://www.fas.usda.gov/data/peru-coffee-annual-4 (accessed on 2 August 2023).
- Perfecto, I.; Rice, R.A.; Greenberg, R.; Van der Voort, M.E. Shade coffee: A disappearing refuge for biodiversity: Shade coffee plantations can contain as much biodiversity as forest habitats. BioScience 1996, 46, 598–608. [Google Scholar] [CrossRef]
- Jha, S.; Bacon, C.M.; Philpot, S.M.; Mendez, V.; Laderach, P.; Rice, R.A. Shade coffee: Update on a disappearing refuge for biodiversity. BioScience 2014, 64, 416–428. [Google Scholar] [CrossRef]
- Jezeer, R.E.; Santos, M.J.; Verweij, P.A.; Boot, R.G.A.; Clough, Y. Benefits for multiple ecosystem services in Peruvian coffee agroforestry systems without reducing yield. Ecosyst. Serv. 2019, 40, 101033. [Google Scholar] [CrossRef]
- Muschler, R.G. Shade improves coffee quality in a sub-optimal coffee zone of Costa Rica. Agroforestry Syst. 2001, 51, 131–139. [Google Scholar] [CrossRef]
- Steiman, S. Shade vs. Sun Coffee: A Review P Microsoft Internet Explorer. 2003. Available online: www.geocities.com/RainForest/Canopy/1290/basics.htm (accessed on 2 August 2023).
- Geromel, C.; Ferreira, L.; Davrieux, F.; Guyot, B. Effects of shade on the development and sugar metabolism of coffee fruits. Plant Physiol. Biochem. 2008, 46, 569–579. [Google Scholar] [CrossRef]
- Vaast, P.; Bertrand, B.; Perriot, J.-J.; Guyot, B.; Génard, M. Fruit thinning and shade improve bean characteristics and beverage quality of coffee (Coffea arabica L.) under optimal conditions. J. Sci. Food Agric. 2006, 86, 197–204. [Google Scholar] [CrossRef]
- Jha, S.; Bacon, C.M.; Philpott, S.M.; Rice, R.A.; Méndez, V.; Laderach, P. A Review of Ecosystem Services, Farmer Livelihoods, and Value Chains in Shade Coffee Agroecosystems. In Integrating Agriculture, Conservation and Ecotourism: Examples from the Field; Campbell, B.W., López Ortíz, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 141–208. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Balser, T.C.; Firestone, M.K. Linking microbial community composition to function in a tropical soil. Soil Biol. Biochem. 2000, 32, 1837–1846. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Singh, B.K.; Quince, C.; Macdonald, C.A.; Khachane, A.; Thomas, N.; Al-Soud, W.A.; Søren, J.S.; Zhili, H.; Duncan, W.; Alex, S.; et al. Loss of microbial diversity in soils is coincident with reductions in some specialized functions. Environ. Microbiol. 2014, 16, 2408–2420. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Xiong, W.; Xing, Y.; Sun, Y.; Lin, X.; Dong, Y. Long-Term Coffee Monoculture Alters Soil Chemical Properties and Microbial Communities. Sci. Rep. 2018, 8, 6116. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, M.G.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2007, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Cui, Y.; Li, H.; Kuang, A.; Li, X.; Wei, Y.; Ji, X. Rhizospheric soil and root endogenous fungal diversity and composition in response to continuous Panax notoginseng cropping practices. Microbiol. Res. 2017, 194, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Gu, S.; Xin, Y.; Bello, A.; Sun, W.; Xu, X. Compost Addition Enhanced Hyphal Growth and Sporulation of Arbuscular Mycorrhizal Fungi without Affecting Their Community Composition in the Soil. Front. Microbiol. 2018, 9, 169. [Google Scholar] [CrossRef]
- Brundrett, M.; Tedersoo, L. Misdiagnosis of mycorrhizas and inappropriate recycling of data can lead to false conclusions. New Phytol. 2018, 221, 18–24. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Simbiosis Micorrícica, 3rd ed.; Prensa Académica: London, UK, 2008. [Google Scholar]
- Bücking, H.; Kafle, A. Role of Arbuscular Mycorrhizal Fungi in the Nitrogen Uptake of Plants: Current Knowledge and Research Gaps. Agronomy 2015, 5, 587–612. [Google Scholar] [CrossRef]
- Hodge, A.; Fitter, A.H. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc. Natl. Acad. Sci. USA 2010, 107, 13754–13759. [Google Scholar] [CrossRef]
- Hammer, E.C.; Pallon, J.; Wallander, H.; Olsson, P.A. Tit for tat? A mycorrhizal fungus accumulates phosphorus under low plant carbon availability. FEMS Microbiol. Ecol. 2011, 76, 236–244. [Google Scholar] [CrossRef]
- Fellbaum, C.R.; Gachomo, E.W.; Beesetty, Y.; Choudhari, S.; Strahan, G.D.; Pfeffer, P.E.; Kiers, E.T.; Bucking, H. Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proc. Nat. Acad. Sci. USA 2012, 109, 2666–2671. [Google Scholar] [CrossRef] [PubMed]
- Casieri, L.; Lahmidi, N.; Doidy, J.; Veneault-Fourrey, C.; Migeon, A. Biotrophic transportome in mutualistic plant–fungal interactions. Mycorrhiza 2013, 23, 597–625. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.; Veresoglou, S.D.; Leifheit, E.F.; Rillig, M.C. Arbuscular mycorrhizal influence on zinc nutrition in crop plants a meta-analysis. Soil Biol. Biochem. 2014, 69, 123–131. [Google Scholar] [CrossRef]
- Lehmann, A.; Rillig, M.C. Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops-a meta-analysis. Soil Biol. Biochem. 2015, 81, 147–158. [Google Scholar] [CrossRef]
- García, K.; Doidy, J.; Zimmermann, S.D.; Wipf, D.; Courty, P.-E. Take a Trip Through the Plant and Fungal Transportome of Mycorrhiza. Trends Plant Sci. 2016, 21, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Bukovská, P.; Bonkowski, M.; Konvalinková, T.; Beskid, O.; Hujslová, M.; Püschel, D.; Řezáčová, V.; Gutiérrez-Núñez, M.S.; Gryndler, M.; Jansa, J. Utilization of organic nitrogen by arbuscular mycorrhizal fungi—Is there a specific role for protists and ammonia oxidizers? Mycorrhiza 2018, 28, 465. [Google Scholar] [CrossRef] [PubMed]
- Jansa, J.; Forczek, S.T.; Rozmoš, M.; Püschel, D.; Bukovská, P.; Hršelová, H. Arbuscular mycorrhiza and soil organic nitrogen: Network of players and interactions. Chem. Biol. Tech. Agric. 2019, 6, 10. [Google Scholar] [CrossRef]
- Crossay, T.; Majorel, C.; Redecker, D.; Gensous, S.; Medevielle, V.; Durrieu, G.; Cavaloc, Y.; Amir, H. Is a mixture of arbuscular mycorrhizal fungi better for plant growth than single-species inoculants? Mycorrhiza 2019, 29, 325–339. [Google Scholar] [CrossRef]
- Parvin, S.; Van Geel, M.; Yeasmin, T.; Verbruggen, E.; Honnay, O. Effects of single and multiple species inocula of arbuscular mycorrhizal fungi on the salinity tolerance of a Bangladeshi rice (Oryza sativa L.) cultivar. Mycorrhiza 2020, 30, 431–444. [Google Scholar] [CrossRef]
- Jansa, J.; Smith, F.A.; Smith, S.E. Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol. 2008, 177, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Ortas, I.; Ustuner, O. The effects of single species, dual species and indigenous mycorrhiza inoculation on citrus growth and nutrient uptake. Eur. J. Soil Biol. 2014, 63, 64–69. [Google Scholar] [CrossRef]
- Pellegrino, E.; Bedini, S. Enhancing ecosystem services in sustainable agriculture: Biofertilization and biofortification of chickpea (Cicer arietinum L.) by arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2014, 68, 429–439. [Google Scholar] [CrossRef]
- Walder, F.; van der Heijden, M.G. Regulation of resource exchange in the arbuscular mycorrhizal symbiosis. Nat. Plants 2015, 1, 15159. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, Q.; Koide, R.T.; Hoeksema, J.D.; Tang, J.; Bian, X.; Hu, S.; Chen, X.; Cahill, J. Taxonomic resolution is a determinant of biodiversity effects in arbuscular mycorrhizal fungal communities. J. Ecol. 2016, 105, 219–228. [Google Scholar] [CrossRef]
- Van Geel, M.; De Beenhouwer, M.; Lievens, B.; Honnay, O. Crop-specific and single-species mycorrhizal inoculation is the best approach to improve crop growth in controlled environments. Agron. Sustain. Dev. 2016, 36, 37. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: Let’s benefit from past successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef]
- Öpik, M.; Vanatoa, A.; Vanatoa, E.; Moora, M.; Davison, J.; Kalwij, J.M.; Reier, Ü.; Zobel, M. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 2010, 188, 223–241. [Google Scholar] [CrossRef]
- Tchabi, A.; Coyne, D.; Hountondji, F.; Lawouin, L.; Wiemken, A.; Oehl, F. Efficacy of indigenous arbuscular mycorrhizal fungi for promoting white yam (Dioscorea rotundata) growth in West Africa. Appl. Soil Ecol. 2010, 45, 92–100. [Google Scholar] [CrossRef]
- Janse, J.M. Les endophytes radicaux de quelques plantes javanaises. Ann. Jard. Bot. Buitenzorg 1897, 24, 53–201. (In French) [Google Scholar]
- Posada, R.H.; Sieverding, E. Arbuscular mycorrhiza in Colombian coffee plantations fertilized with coffee pulps as organic manure. J. Appl. Bot. Food Qual 2014, 87, 243–248. [Google Scholar] [CrossRef]
- Siqueira, J.O.; Colozzi-Filho, A.; Saggin-Júnior, O.J.; Guimaraes, P.T.G.; Oliveira, E. Crescimento de mudas e produção do cafeeiro sob influência de fungos micorrízicos e superfosfato. Rev. Bras. Cienc. Solo 1993, 17, 53–60. (In Portuguese) [Google Scholar]
- Siqueira, J.O.; Saggin-Júnior, O.J.; Flores-Aylas, W.W.; Guimarães, P.T.G. Arbuscular mycorrhizal inoculation and superphosphate application influence plant development and yield of coffee in Brazil. Mycorrhiza 1998, 7, 293–300. [Google Scholar] [CrossRef]
- Habte, M.; Bittenbender, H.C. Reactions of coffee to soil solution P concentration and arbuscular mycorrhizal colonization. J. South Pac. Agric. 1999, 6, 29–34. [Google Scholar]
- Trejo, D.; Ferrera-Cerrato, R.; García, R.; Varela, L.; Lara, L.; Alarcón, A. Efectividad de siete consorcios nativos de hongos micorrízicos arbusculares en plantas de café en condiciones de invernadero y campo. Rev. Chil. Hist. Nat. 2011, 84, 23–31. (In Spanish) [Google Scholar] [CrossRef]
- De Beenhouwer, M.; Muleta, D.; Peeters, B.; Van Geel, M.; Lievens, B.; Honnay, O. DNA pyrosequencing evidence for large diversity differences between natural and managed coffee mycorrhizal fungal communities. Agron. Sustain. Dev. 2014, 35, 241–249. [Google Scholar] [CrossRef]
- Prates, P.; Moreira, B.C.; da Silva, M.; Veloso, T.G.R.; Stürmer, S.L.; Fernandes, R.B.A.; Mendonça, E.D.S.; Kasuya, M.C.M. Agroecological coffee management increases arbuscular mycorrhizal fungi diversity. PLoS ONE 2019, 14, e0209093. [Google Scholar] [CrossRef] [PubMed]
- Vaast, P.; Zasoski, R.J.; Bledsoe, C.S. Effects of vesicular-arbuscular mycorrhizal inoculation at different soil P availabilities on growth and nutrient uptake of in vitro propagated coffee (Coffea arabica L.) plants. Mycorrhiza 1997, 6, 493–497. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Bagyaraj, D. Effectiveness of arbuscular mycorrhizal fungal isolates on arabica coffee (Coffea arabica L.). Biol. Agric. Hortic. 2002, 20, 125–131. [Google Scholar] [CrossRef]
- Andrade, S.A.L.; Silveira, A.P.D.; Mazzafera, P. Arbuscular mycorrhiza alters metal uptake and the physiological response of Coffea arabica seedlings to increasing Zn and Cu concentrations in soil. Sci. Total Environ. 2010, 408, 5381–5391. [Google Scholar] [CrossRef]
- Perea-Rojas, Y.C.; Arias, R.M.; Medel-Ortiz, R.; Trejo-Aguilar, D.; Heredia, G.; Rodríguez-Yon, Y. Effects of native arbuscular mycorrhizal and phosphate-solubilizing fungi on coffee plants. Agrofor. Syst. 2018, 93, 961–972. [Google Scholar] [CrossRef]
- Cogo, F.D.; Guimarães, P.T.G.; Rojas, E.P.; Júnior, O.J.S.; Siqueira, J.O.; Caneiro, M.A.C. Arbuscular Mycorrhiza in Coffea arabica L.: Review and Meta-Analysis. Coffee Sci. 2017, 12, 419–443. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sánchez-García, M.; Goto, B.T.; Magurno, F. Outline of fungi and fungus-like taxa—2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- Öpik, M.; Davison, J. Uniting species- and community-oriented approaches to understand arbuscular mycorrhizal fungal diversity. Fungal Ecol. 2016, 24, 106–113. [Google Scholar] [CrossRef]
- Corazon-Guivin, M.A.; Mendoza, A.C.; Guerrero-Abad, J.C.; Vallejos-Tapullima, A.; Carballar-Hernández, S.; Silva, G.A.; Oehl, F. Funneliglomus, gen. nov., and Funneliglomus sanmartinensis, a new arbuscular mycorrhizal fungus from the Amazonia region in Peru. Sydowia 2019, 71, 17–24. [Google Scholar] [CrossRef]
- Corazon-Guivin, M.A.; Cerna-Mendoza, A.; Guerrero-Abad, J.C.; Vallejos-Tapullima, A.; Carballar-Hernández, S.; Silva, G.A.; Oehl, F. Nanoglomus plukenetiae, a new fungus from Peru, and a key to small-spored Glomeraceae species, including three new genera in the “Dominikia complex/clades”. Mycol. Prog. 2019, 18, 1395–1409. [Google Scholar] [CrossRef]
- Corazon-Guivin, M.A.; Cerna-Mendoza, A.; Guerrero-Abad, J.C.; Vallejos-Tapullima, A.; Silva, G.A.; Oehl, F. Acaulospora aspera, a new fungal species in the Glomeromycetes from rhizosphere soils of the inka nut (Plukenetia volubilis L.) in Peru. J. Appl. Bot. Food Qual. 2019, 92, 250–257. [Google Scholar] [CrossRef]
- Corazon-Guivin, M.A.; Cerna-Mendoza, A.C.; Guerrero-Abad, J.C.; Vallejos-Tapullima, A.; Silva, G.A.; Oehl, F. Fungal Systematics and Evolution: FUSE 5. Sydowia 2019, 71, 141–245. [Google Scholar] [CrossRef]
- Corazon-Guivin, M.A.; Vallejos-Tapullima, A.; de la Sota-Ricaldi, A.M.; Vallejos-Torres, G.; Ruíz-Sánchez, M.E.; Santos, V.M.; da Silva, G.A.; Oehl, F. Acaulospora flavopapillosa, a new fungus in the Glomeromycetes from a coffee plantation in Peru, with an updated key for the identification of Acaulosporaceae species. J. Appl. Bot. Food Qual. 2022, 95, 6–16. [Google Scholar] [CrossRef]
- Corazon-Guivin, M.A.; Vallejos-Torres, G.; Vallejos-Tapullima, A.; Tenorio-Cercado, M.; Mendoza Caballero, W.; Marín, C.; Santos, V.M.; da Silva, G.A.; Oehl, F. Rhizoglomus cacao, a new species of the Glomeraceae from the rhizosphere of Theobroma cacao in Peru, with an updated identification key for all species attributed to Rhizoglomus. Nova Hedwigia. 2022, 115, 99–115. [Google Scholar] [CrossRef]
- Álvarez-Solís, J.D.; Ferrera-Cerrato, R. Micorriza Arbuscular y Crecimiento del Café en Vivero. In El Cafetal del Futuro, Realidades y Visiones; Pohlan, J., Soto, L., Barrera, J., Eds.; Shaker Verlag: Aechen, Alemania, 2006; pp. 19–22. (In Spanish) [Google Scholar]
- Gerdemann, J.W.; Nicolson, T.H. Spores of Mycorrhizal Endogone Species Extracted from Soil by Wet Sieving and Decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Sieverding, E. Vesicular-Arbuscular Mycorrhiza Management in Tropical Agrosystems; GTZ: Eschborn, Germany, 1991. [Google Scholar]
- Séry, D.J.; Kouadio, Z.; Voko, B.; Zeze, A. Selecting native arbuscular mycorrhizal fungi to promote cassava growth and increase yield under field conditions. Front. Microbiol. 2016, 7, 2063. [Google Scholar] [CrossRef] [PubMed]
- Koske, R.E.; Tessier, B. A convenient, permanent slide mounting medium. Mycol. Soc. Am. Newsl. 1983, 34, 59. [Google Scholar]
- Brundrett, M.; Melville, L.; Peterson, L. Practical Methods in Mycorrhizal Research; Mycologue Publications: Waterloo, MD, USA, 1994. [Google Scholar]
- Spain, J.L. Arguments for diagnoses based on unaltered wall structures. Mycotaxon 1990, 38, 71–76. [Google Scholar]
- Schenck, N.C.; Perez, Y. Manual for the Identification of VA Mycorrhizal Fungi; Synergistic Publishing: Gainesville, FL, USA, 1990; p. 286. [Google Scholar]
- Błaszkowski, J.; Kovács, G.M.; Gáspár, B.K.; Balázs, T.K.; Buscot, F.; Ryszka, P. The arbuscular mycorrhizal Paraglomus majewskii sp. nov. represents a new distinct basal lineage in Paraglomeraceae (Glomeromycota). Mycologia 2012, 104, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Błaszkowski, J.; Sanchez-Garcia, M.; Niezgoda, P.; Zubek, S.; Fernandez, F.; Vila, A.; Al-Yahya’ei, M.N.; Symanczik, S.; Milczarski, P.; Malinowski, R.; et al. A new order, Entrophosporales, and three new Entrophospora species in Glomeromycota. Front. Microbiol. 2022, 13, 962856. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.A.; Corazon-Guivin, M.A.; de Assis, D.M.A.; Oehl, F. Blaszkowskia, a new genus in Glomeraceae. Mycol. Prog. 2023, 22, 74. [Google Scholar] [CrossRef]
- Hewitt, E.J. Sand and Water Culture Methods Used in the Study of Plant Nutrition; Farnhan Royal, Commonwealth Agricultural Bureau: Farnham, UK, 1966.
- Phillips, D.A.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Brundrett, M.; Bougher, N.; Dell, B.; Grove, T.; Malajczuk, N. Working with Mycorrhizas in Forestry and Agriculture; Australian Centre for International Agricultural Research: Canberra, Australia, 1996. [CrossRef]
- Horneck, D.; Miller, R. Determination of Total Nitrogen in Plant Tissue. In Handbook of Reference Methods for Plant Analysis; Kalra, Y., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 75–83. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Levene, H. Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Olkin, I., Ghurye, S.G., Hoeffding, W., Madow, W.G., Mann, H.B., Eds.; Stanford University Press: Redwood City, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat, Versión 2012.1, Grupo InfoStat, FCA. Universidad Nacional de Córdoba, Argentina. 2012. Available online: https://www.infostat.com.ar/ (accessed on 20 December 2019).
- Baltruschat, H.; Santos, V.M.; Silva, D.K.A.; Schellenberg, I.; Deubel, A.; Sieverding, E.; Oehl, F. Unexpectedly high diversity of arbuscular mycorrhizal fungi in fertile Chernozem croplands in Central Europe. Catena 2019, 182, 104135. [Google Scholar] [CrossRef]
- Oehl, F.; Laczko, E.; Oberholzer, H.-R.; Jansa, J.; Egli, S. Diversity and biogeography of arbuscular mycorrhizal fungi in agricultural soils. Biol. Fertil. Soils 2017, 53, 777–797. [Google Scholar] [CrossRef]
- Njeru, E.M.; Avio, L.; Bocci, G.; Sbrana, C.; Turrini, A.; Bàrberi, P.; Giovannetti, M.; Oehl, F. Contrasting effects of cover crops on ‘hot spot’ arbuscular mycorrhizal fungal communities in organic tomato. Biol. Fertil. Soils 2015, 51, 151–166. [Google Scholar] [CrossRef]
- Wetzel, K.; Silva, G.A.; Matczinski, U.; Oehl, F.; Fester, T. Superior differentiation of arbuscular mycorrhizal fungal communities from till and no-till plots by morphological spore identification when compared to T-RFLP. Soil Biol. Biochem. 2014, 72, 88–96. [Google Scholar] [CrossRef]
- Arias, R.M.; Heredia-Abarca, G.; Sosa, V.J.; Fuentes-Ramirez, L.E. Diversity and abundance of arbuscular mycorrhizal fungi spores under different coffee production systems and in a tropical montane cloud forest patch in Veracruz, Mexico. Agrofor. Syst. 2012, 85, 179–193. [Google Scholar] [CrossRef]
- Dobo, B.; Asefa, F.; Asfaw, Z. Effect of tree-enset-coffee based agro-forestry practices on arbuscular mycorrhizal fungi (AMF) species diversity and spore density. Agrofor. Syst. 2017, 92, 525–540. [Google Scholar] [CrossRef]
- Aldrich-Wolfe, L.; Black, K.L.; Hartmann, E.D.; Shivega, W.G.; Schmaltz, L.C.; McGlynn, R.D.; Johnson, P.G.; Asheim Keller, R.J.; Vink, S.N. Taxonomic shifts in arbuscular mycorrhizal fungal communities with shade and soil nitrogen across conventionally managed and organic coffee agroecosystems. Mycorrhiza 2020, 30, 513–527. [Google Scholar] [CrossRef]
- Belay, Z.; Negash, M.; Kaseva, J.; Vestberg, M.; Kahiluoto, H. Native forests but not agroforestry systems preserve arbuscular mycorrhizal fungal species richness in southern Ethiopia. Mycorrhiza 2020, 30, 749–759. [Google Scholar] [CrossRef]
- Bertolini, V.; Montaño, N.; Salazar-Ortuño, B.; Chimal-Sánchez, E.; Varela, L. Arbuscular mycorrhizal fungi diversity in coffee (Coffea arabica) plantations on the Tacaná volcano, Chiapas, Mexico. Acta Bot. Mex. 2020, 127, e1602. [Google Scholar] [CrossRef]
- Diniz, F.; Saggin, O.; Gontijo, P.; Siquiera, J.; Carbone, M. High rates of agricultural gypsum affect the arbuscular mycorrhiza fungal community and coffee yield. Bragantia 2020, 79, 1–11. [Google Scholar] [CrossRef]
- Lara-Capistran, L.; Zulueta-Rodriguez, R.; Murillo-Amador, B.; Preciado-Rangel, P.; Verdecia-Acosta, D.M.; Hernandez-Montiel, L.G. Biodiversity of AM Fungi in Coffee Cultivated on Eroded Soil. Agronomy 2021, 11, 567. [Google Scholar] [CrossRef]
- Posada, R.H.; de Prager, M.S.; Heredia-Abarca, G.; Sieverding, E. Effects of soil physical and chemical parameters, and farm management practices on arbuscular mycorrhizal fungi communities and diversities in coffee plantations in Colombia and Mexico. Agrofor. Syst. 2016, 92, 555–574. [Google Scholar] [CrossRef]
- Bertolini, V.; Montaño, N.; Chimal-Sánchez, E.; Varela-Fregoso, L.; Gómez-Ruiz, J.; Martínez-Vázquez, J. Abundance and richness of arbuscular mycorrhizal fungi in coffee plantations from Soconusco, Chiapas, Mexico. Rev. Biol. Trop. 2018, 66, 91–105. [Google Scholar] [CrossRef][Green Version]
- Herrera, S.; Castro, R.; Pérez-Moreno, J.; Valdés, E. Endomycorrhizal diversity in coffee plants (Coffea arabica L.) infected with rust (Hemileia vastatrix). Nova Sci. 2019, 11, 102–123. [Google Scholar] [CrossRef]
- Säle, V.; Palenzuela, J.; Azcón-Aguilar, C.; Sánchez-Castro, I.; da Silva, G.A.; Seitz, B.; Sieverding, E.; van der Heijden, M.G.; Oehl, F. Ancient lineages of arbuscular mycorrhizal fungi provide little plant benefit. Mycorrhiza 2021, 3, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Lopes, E.S.; Oliveira, E.; Neptune, A.M.L.; Moraes, F.R.P. Efeito da inoculação do cafeeiro com diferentes espécies de fungos micorrízicos vesicular-arbusculares. Rev. Bras. Cienc. Solo 1983, 7, 137–141. (In Portuguese) [Google Scholar]
- Fonseca, A.J.; Freitas, A.F.; Carvalho, G.R.; Carneiro, M.A.C.; Vilela, D.J.M.; Fassio, L.d.O. Arbuscular mycorrhizal fungus on the initial growth and nutrition of Coffea arabica L. genotypes. Ciência Agrotecnol. 2019, 2019, 43. [Google Scholar] [CrossRef]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A.; et al. Reciprocal Rewards Stabilize Cooperation in the Mycorrhizal Symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef]
- Maherali, H.; Klironomos, J.N. Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 2007, 316, 1746–1748. [Google Scholar] [CrossRef]
- Thonar, C.; Frossard, E.; Šmilauer, P.; Jansa, J. Competition and facilitation in synthetic communities of arbuscular mycorrhizal fungi. Mol. Ecol. 2014, 23, 733–746. [Google Scholar] [CrossRef]
- Dumbrell, A.J.; Nelson, M.; Helgason, T.; Dytham, C.; Fitter, A.H. Idiosyncrasy and overdominance in the structure of natural communities of arbuscular mycorrhizal fungi: Is there a role for stochastic processes? J. Ecol. 2010, 98, 419–428. [Google Scholar] [CrossRef]
- Hart, M.M.; Reader, R.J. Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol. 2002, 153, 335–344. [Google Scholar] [CrossRef]
- Klironomos, J.N.; Hart, M.M. Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza 2002, 12, 181–184. [Google Scholar] [CrossRef]
- Vaast, P.; Zasoski, R.J. Effects of VA-mycorrhizae and nitrogen sources on rhizosphere soil characteristics, growth and nutrient acquisition of coffee seedlings (Coffea arabica L.). Plant Soil. 1992, 147, 31–39. [Google Scholar] [CrossRef]
- Souza, C.; Siqueira, J. Development and nutrient levels of coffee seedlings inoculated with mycorrhizal fungi. Pesqui. Agropecu. Bras. 1991, 26, 1989–2005. [Google Scholar]
- Kahiluoto, H.; Ketoja, E.; Vestberg, M. Plant-available P supply is not the main factor determining the benefit from arbuscular mycorrhizato crop P nutrition and growth in contrasting cropping systems. Plant Soil 2012, 350, 85–98. [Google Scholar] [CrossRef]
- Voříšková, A.; Jansa, J.; Püschel, D.; Vosátka, M.; Šmilauer, P.; Janoušková, M. Abiotic contexts consistently influence mycorrhiza functioning independently of the composition of synthetic arbuscular mycorrhizal fungal communities. Mycorrhiza 2019, 29, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Symanczik, S.; Courty, P.-E.; Boller, T.; Wiemken, A.; Al-Yahyaei, M.N. Impact of water regimes on an experimental community of four desert arbuscular mycorrhizal fungal (AMF) species, as affected by the introduction of a non-native AMF species. Mycorrhiza 2015, 25, 639–647. [Google Scholar] [CrossRef]
- Bucher, M. Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol. 2007, 173, 11–26. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia 2012, 104, 1–13. [Google Scholar] [CrossRef]
- Kumar, S.; Meena, R.S.; Yadav, G.S.; Pandey, A. Response of sesame (Sesamum indicum L.) to sulphur and lime application under soil acidity. Int. J. Plant Soil Sci. 2017, 14, 1–9. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Functional diversity in arbuscular mycorrhizal (AM) symbioses: The contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol. 2004, 162, 511–524. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 2003, 133, 16–20. [Google Scholar] [CrossRef]
- Grunwald, U.; Guo, W.; Fischer, K.; Isayenkov, S.; LudwigMuller, J.; Hause, B.; Yan, X.; Kuster, H.; Franken, P. Overlapping expression patterns and differential transcript levels of phosphate transporter genes in arbuscular mycorrhizal, Pi-fertilised and phytohormonetreated Medicago truncatula roots. Planta 2009, 229, 1023–1034. [Google Scholar] [CrossRef]
- Facelli, E.; Smith, S.E.; Facelli, J.M.; Christophersen, H.M.; Smith, F. Underground friends or enemies: Model plants help to unravel direct and indirect effects of arbuscular mycorrhizal fungi on plant competition. New Phytol. 2010, 185, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Bücking, H.; Shachar-Hill, Y.Y. Phosphate uptake, transport and transfer by the arbuscular mycorrhizal fungus Glomus intraradices is stimulated by increased carbohydrate availability. New Phytol. 2005, 165, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Bever, J.D.; Richardson, S.C.; Lawrence, B.M.; Holmes, J.; Watson, M. Preferential allocation to beneficial symbiont with spatial structure maintains mycorrhizal mutualism. Ecol. Lett. 2009, 12, 13–21. [Google Scholar] [CrossRef]
- Fellbaum, C.R.; Mensah, J.A.; Cloos, A.J.; Strahan, G.E.; Pfeffer, P.E.; Kiers, E.T.; Bücking, H. Fungal nutrient allocation in common mycorrhizal networks is regulated by the carbon source strength of individual host plants. New Phytol. 2014, 203, 646–656. [Google Scholar] [CrossRef]
- Hodge, A.; Robinson, D.; Griffiths, B.S.; Fitter, A.H. Why plants bother: Root proliferation results in increased nitrogen capture from an organic patch when two grasses compete. Plant Cell Environ. 1999, 22, 811–820. [Google Scholar] [CrossRef]
- Hawkins, H.J.; Johansen, A.; George, E. Uptake and transport of organic and inorganic nitrogen by arbuscular mycorrhizal fungi. Plant Soil 2000, 226, 275–285. [Google Scholar] [CrossRef]
- Miller, A.J.; Cramer, M.D. Root nitrogen acquisition and assimilation. Plant Soil 2005, 274, 1–36. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef]
- Fitter, H.A.; Helgason, T.; Hodge, A. Nutritional exchanges in the arbuscular mycorrhizal symbiosis: Implications for sustainable agriculture. Fungal Biol. Rev. 2011, 25, 68–72. [Google Scholar] [CrossRef]
- Nuccio, E.E.; Hodge, A.; Pett-Ridge, J.; Herman, D.J.; Weber, P.K.; Firestone, M.K. An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environ. Microbiol. 2013, 15, 1870–1881. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.; Hodge, A.; Fitter, A.H. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol. 2009, 181, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Nouri, E.; Breuillin-Sessoms, F.; Feller, U.; Reinhardt, D. Phosphorus and nitrogen regulate arbuscular mycorrhizal symbiosis in Petunia hybrida. PLoS ONE 2014, 9, e90841. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.; Storer, K. Arbuscular mycorrhiza and nitrogen: Implications for individual plants through to ecosystems. Plant Soil 2015, 386, 1–19. [Google Scholar] [CrossRef]
- Fellbaum, C.R.; Mensah, J.A.; Pfeffer, P.E.; Kiers, E.T.; Bücking, H. The role of carbon in fungal nutrient uptake and transport: Implications for resource exchange in the arbuscular mycorrhizal symbiosis. Plant Signal. Behav. 2012, 7, 1509–1512. [Google Scholar] [CrossRef]
- Tilman, D. Secondary succession and the pattern of plant dominance along experimental nitrogen gradients. Ecol. Monogr. 1987, 57, 189–214. [Google Scholar] [CrossRef]
- Reynolds, H.L.; Hartley, A.E.; Vogelsang, K.M.; Bever, J.D.; Schultz, P.A. Arbuscular mycorrhizal fungi do not enhance nitrogen acquisition and growth of old-field perennials under low nitrogen supply in glasshouse culture. New Phytol. 2005, 167, 869–880. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Oguchi, R.; Hikosaka, K.; Hirose, T. Leaf anatomy as a constraint for photosynthetic acclimation: Differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell Environ. 2005, 28, 916–927. [Google Scholar] [CrossRef]
- Konstantopoulou, E.; Kapotis, G.; Salachas, G.; Petropoulos, S.A.; Chatzieustratiou, E.; Karapanos, I.C.; Passam, H.C. Effect of nitrogen application on growth parameters, yield and leaf nitrate content of greenhouse lettuce cultivated during three seasons. J. Plant Nutr. 2012, 35, 1246–1254. [Google Scholar] [CrossRef]
- Kaldorf, M.; Kuhn, A.J.; Schroder, W.H.; Hildebrandt, U.; Bothe, H. Selective element deposits in maize colonized by a heavy metal tolerance conferring arbuscular mycorrhizal fungus. J. Plant Physiol. 1999, 154, 718–728. [Google Scholar] [CrossRef]
- Perner, H.; Schwarz, D.; Bruns, C.; Mäder, P.; George, E. Effect of arbuscular mycorrhizal colonization and two levels of compost supply on nutrient uptake and flowering of pelargonium plants. Mycorrhiza 2007, 17, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Garcia, K.; Zimmermann, S.D. The role of mycorrhizal associations in plant potassium nutrition. Front. Plant Sci. 2014, 5, 337. [Google Scholar] [CrossRef]
- Garcia, K.; Chasman, D.; Roy, S.; Ané, J.-M. Physiological Responses and Gene Co-Expression Network of Mycorrhizal Roots under K + Deprivation. Plant Physiol. 2017, 173, 1811–1823. [Google Scholar] [CrossRef]
- Olsson, P.A.; Hammer, E.C.; Wallander, H.; Pallon, J. Phosphorus availability influences elemental uptake in the mycorrhizal fungus Glomus intraradices, as revealed by particle-induced X-ray emission analysis. Appl. Environ. Microbiol. 2008, 74, 4144–4148. [Google Scholar] [CrossRef]
- Olsson, P.A.; Hammer, E.C.; Pallon, J.; van Aarle, I.M.; Wallander, H. Elemental composition in vesicles of an arbuscular mycorrhizal fungus, as revealed by PIXE analysis. Fungal Biol. 2011, 115, 643–648. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).