Abstract
Plant pomaces in suitable forms (powders, extracts) can be used in foods of animal origin to increase the nutritional value and safety of these foods. In the present study, water extracts of apple, black currant, rhubarb and tomato pomaces were used in fish marinade solutions to evaluate their effect on the growth dynamics of microorganisms and the growth potential of Listeria monocytogenes by challenge testing. The results showed that mesophilic aerobic microorganisms, Pseudomonas spp., yeasts and moulds remained at acceptable levels throughout the predetermined storage period. The challenge test results showed that the overall growth potential of L. monocytogenes in all marinated rainbow trout samples remained at ≤0.5 log10 cfu/g during the study period, and none of the marinated fish samples supported the growth of L. monocytogenes. In addition, the effect of fruit and berry pomaces on the sensory properties of marinated rainbow trout samples was evaluated. The results revealed that it is possible to effectively use fruit and berry pomaces in marinated fish products, ensuring food safety, high microbiological quality, acceptable sensory characteristics and a sufficiently long shelf life of the products.
1. Introduction
Improved management of by-products from food production is an essential step in the transition towards a circular and bio-economy [1]. The production of plant-based food produces large amounts of by-products, e.g., pomaces, which are natural sources of biologically active compounds. Therefore, it is utmost importance to find new ways to valorise these valuable natural food materials.
Marination is used to preserve food products, and the use of natural antioxidants in order to improve the quality and nutritional value of fish products is increasing [2,3,4]. Fish and fish products are highly susceptible to microbial spoilage, which reduces shelf life and increases the risk of foodborne infections. Microbiological spoilage is associated with many different microorganisms including Pseudomonas spp., yeasts and moulds [5].
There are many ways foodborne pathogens can be transmitted into the food production chain, which can ultimately lead to the contamination of the end products, e.g., ready-to-eat (RTE) foods [6]. Studies by Kramarenko et al. [7] and Koskar et al. [8] have shown a high prevalence and concentration of Listeria monocytogenes in certain RTE fish products, which poses serious food safety risks, especially for consumers belonging to risk groups. In addition, RTE fish products have been linked to a prolonged multicountry listeriosis outbreak [9]. According to the European Union Reference Laboratory for L. monocytogenes (EURL Lm), RTE foods are susceptible to L. monocytogenes contamination, but only those in which the pathogen can survive and/or grow are potential causes of listeriosis [10]. Food business operators need to prove that food safety is ensured throughout the food’s shelf life, and challenge testing is one of the options to verify food safety.
Fruits and berries and their processing residues are known as natural sources of polyphenolic antioxidants, which may possess antimicrobial activity [11,12,13,14]. Some studies have demonstrated the antimicrobial effect of fruit and berry processing by-products in food matrices [15,16]. In the study of Tamkute et al. [17], cranberry pomace extracts were shown to efficiently inhibit meat spoilage and the growth of foodborne pathogens including pathogenic L. monocytogenes in pork slurry, burgers and cooked ham.
The antimicrobial activity of fruits and berries is related to flavonoids and organic acids [18]. Of the organic acids, malic, fumaric, succinic, citric and tartaric acids predominate in apples [19]. The most common organic acids in black currants are malic acid, citric acid, quinic acid and ascorbic acid [20]. Citric, oxalic, malic, succinic and tartaric acids are the main organic acids in rhubarb stems [21]. Mostly citric and malic acids are present in tomatoes [22]. It has been found that at the same pH, organic acids are more effective than inorganic acids at bacterial growth inhibition [23]. This has led to their use as preservatives in food production [24].
However, according to our knowledge, studies of the effects of fruit and berry pomace extracts on the growth of bacteria and some other quality indicators in marinated raw fish products are rather scarce. Therefore, this study aimed to evaluate the effect of marinades containing water extracts of apple, black currant, rhubarb or tomato pomaces on the growth dynamics of microorganisms in raw rainbow trout samples within a 22-day study period as well as on the sensory properties of marinated fish. Also, the growth potential of Listeria monocytogenes in marinated fish samples was assessed.
2. Materials and Methods
2.1. Raw Material and Marinade Solutions
Fresh rainbow trout (Oncorhynchus mykiss) fillets (Trim B) were purchased from a local fish farm in Jõgeva County, Estonia. The fillets were kept on ice at a temperature of −1…+3 °C in cold storage. The day after the fish were caught, the fillets were cut into 2 × 4 cm pieces, and the cutting area was selected to ensure that the pieces were of equal thickness.
Pomaces obtained from the juicing of apples (Malus domestica Borkh.), black currants (Ribes nigrum L.), rhubarb (Rheum rhaponticum L.) and tomato (Lycopersicon esculentum Mill.) were used as raw material for powder preparation at the laboratories of the Polli Horticultural Research Center of Estonian University of Life Sciences. The marinating solutions (Table 1) consisted of aqueous extracts of the apple, black currant, rhubarb or tomato pomaces and additionally contained 3% sugar, 3% salt, 1% acetic and 0.25% citric acid. The content of the phenolic compounds in the pomace powders is given in Figure S1.

Table 1.
Composition and pH of the marinades.
Pomace extracts were prepared in a ratio of 1:10 (w/v), calculated for a solids content of 10%. Mixtures of distilled water and pomace powders were heated in a water bath at 70–80 °C for 30 min.
The marinating solutions (marinades) were bottled in sterile glass bottles, pasteurized at 70–80 °C for 20 min, sealed airtight and cooled. Then, sugar, salt, acetic and citric acid were added to the marinades. The pH of the prepared solutions was measured (Table 1). The marinades were stored in the dark under refrigerated conditions until further use.
2.2. Sample Preparation
The fillet pieces were weighed into plastic boxes (one box for one sampling day) and covered with the marinating solution at a ratio of 1:1. A lid was placed on the plastic boxes, and the boxes were stored in a refrigerator at a temperature of 6 ± 1 °C. The fillet pieces were marinated for 22 days, and samples were taken on days 1, 4, 8, 11, 15, 18 and 22. The challenge test of the marinated fish samples was carried out for up to 15 days. All analyses were performed in triplicate.
2.3. pH Determination
Homogenates made up of 10 g of sample and 100 mL of distilled water were used to measure the pH values of the samples. A digital HandyLab680 pH meter (SI Analytics GmbH, Mainz, Germany) was used to take the readings at room temperature. The pH meter calibration was regularly checked.
2.4. Water Activity (aw) Determination
Water activity (aw) was determined at 25 °C, using an Aqualab Decagon 3TE water activity meter (Decagon Devices Inc., Pullman, WA, USA) following the manufacturer’s instructions.
The water activity and pH were measured on the same measurement day in parallel.
2.5. Enumeration of Microorganisms
For the enumeration of total microorganisms [25], yeasts and moulds [26], presumptive Pseudomonas spp. [27] and for the detection [28] and enumeration [29] of L. monocytogenes standard methods were followed. Briefly, a Kern KB 2000-2N scale (Kern & SOHN GmbH, Balingen, Germany) was used to weigh 10 g of the material into a sterile Stomacher bag; the sample was then diluted with 90 mL of sterile buffered peptone water (LAB204, Lab M, Lancashire, UK) to obtain an initial 10-fold dilution. Samples were blended for one minute at 230 rpm using a Stomacher™ 400 Circulator (Seward, UK).
Plate Count Agar (PCA, LAB010, Lab M, Lancashire, UK) was used for the enumeration of microorganisms, DRBC Agar (ISO) (LAB217, Lab M, Lancashire, UK) was used for the enumeration of yeasts and moulds, Pseudomonas Agar Base (CFC, Biolife, Italiana S.r.l. Viale Monza, Milano, Italia) was used for the enumeration of presumptive Pseudomonas spp. and Agar Listeria Ottaviani Agosti (ALOA) was used for the enumeration of L. monocytogenes (Biolife, Italiana S.r.l. Viale Monza, Milano, Italia). For the analyses, the surface plating technique was used. For small and large agar plates, 100 µL or 1000 µL of the initial dilution was spread onto the agar surfaces, respectively. Plates for counting total microorganisms were incubated under aerobic conditions at 30 °C for 72 ± 3 h, yeast and mould agar plates (DRBC) were incubated at 25 °C for 5 days, agar plates for Pseudomonas spp. (CFC) were incubated at 25 °C for 44 ± 4 h and plates for Listeria (ALOA) were incubated at 37 °C for 24–48 h. After incubation, the colonies were enumerated, and the results were presented as log10 colony-forming units per gram (cfu/g).
2.6. Challenge Test
The suspension used for challenge testing contained a mixture of two L. monocytogenes strains (12MOB101LM, genoserotype II and 12MOB102LM, genoserotype IV) originating from the EURL Lm strain collection. Both strains were isolated from fish. The challenge test was carried out in accordance with the technical guidance document (version 4) of the EURL Lm. Samples without pomaces contaminated or not contaminated with L. monocytogenes were used as positive and negative controls, respectively.
Three samples for each day of analysis were artificially contaminated with the microbial suspension using a syringe. The volume of the inoculum introduced into the food matrix did not exceed 1% of the whole mass. Different points of the sample were injected with the total of 100 μL of the mixture at an approximate concentration of 100 cfu/g. The inoculated samples were placed separately into sterile cups, and the marinade was added after the inoculation. Samples were stored in an incubator at 6 ± 1 °C and analysed on days 1, 4, 8, 11 and 15. The total microbial count, pH and water activity (aw) were also determined on each day of analysis in triplicate. Based on the enumeration of L. monocytogenes, the growth potential of the pathogen was determined.
2.7. Sensory Evaluation
The sensory properties of the samples were assessed using the hedonic and just-about-right (JAR) scales of the Fizz by Biosystems (version 3.7.3) software (Couternon, France). Twelve panellists from the Chair of Veterinary Biomedicine and Food Hygiene and Chair of Food Science and Technology evaluated the sensory properties of the marinated rainbow trout on day 4 of marination. Pieces of fish from each treatment were presented to the panellists on a tray labelled with a three-digit random number. White bread and warm black tea were provided to neutralize the mouth between the samples.
The panellists were asked to rate the samples for the appearance of the marinade and the flesh, odour, flavour, aftertaste, texture and overall acceptance on a 9-point hedonic scale (1—“dislike extremely” and 9—“like extremely”); intensity levels of saltiness, acidity and consistency were evaluated on a 5-point JAR scale (“0”—just about right). For evaluation, panellists used individual booths.
2.8. Statistical Analyses
Microsoft Excel 365 (Microsoft Corporation; Redmond, WA, USA) was used to record the results, and R v4.2.3 was used for statistical analyses [30]. A two-factor analysis of variance was performed to compare L. monocytogenes logarithmic counts in samples with different marinated fish samples and on different study days within the challenge test. Tukey’s post hoc test was used for pairwise comparison analysis. Additionally, correlation analysis was performed separately on different samples using the sensory panel evaluation data regarding appearance, smell, taste, juiciness, aftertaste and overall rating. Results were considered statistically significant at p < 0.05.
3. Results
3.1. pH and aw in Marinated Fish
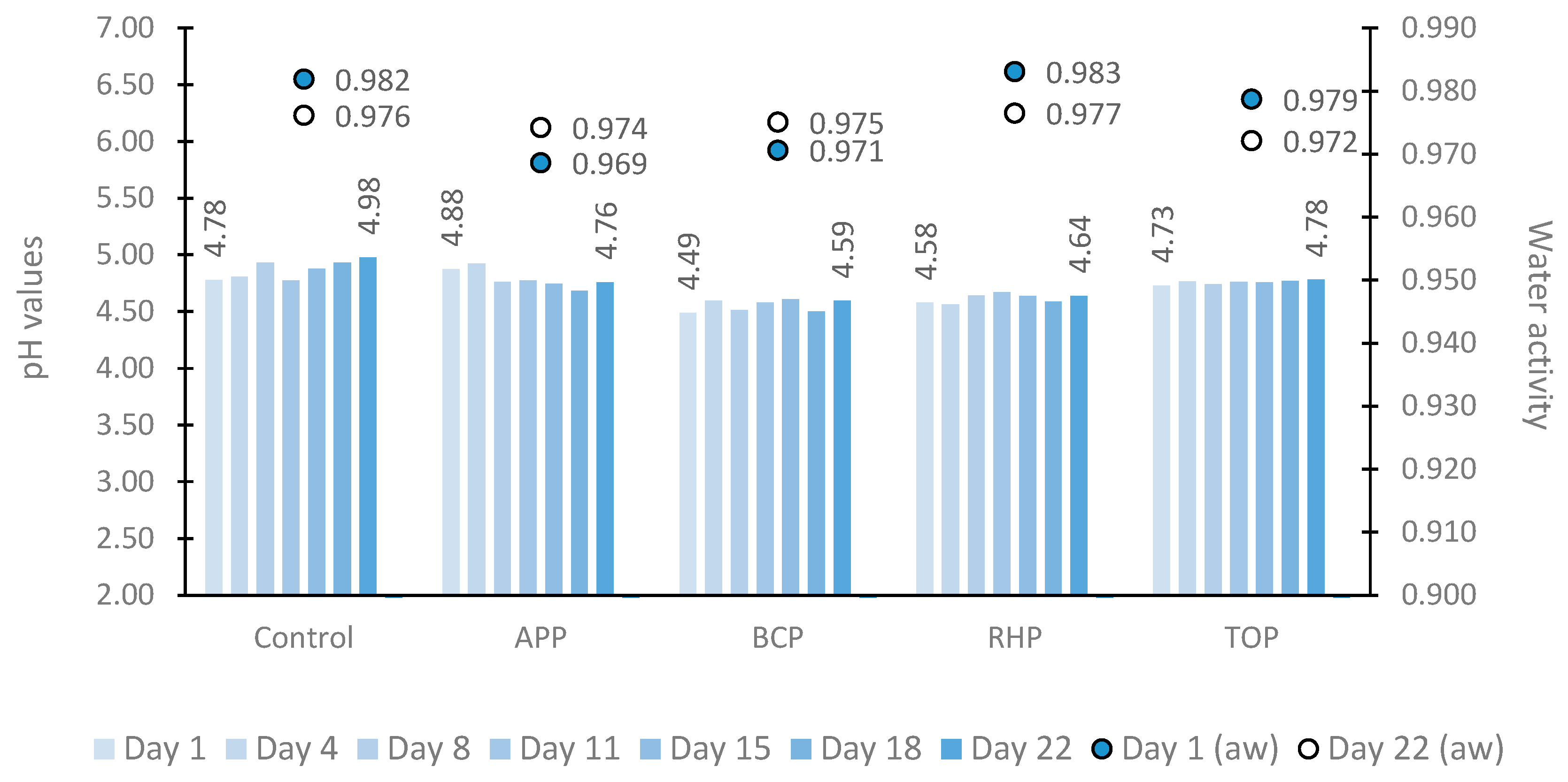
The dynamics of pH and water activity in marinated fish products are shown in Figure 1. The pH values varied greatly between different samples at each time point tested depending on whether or which plant pomace the sample contained in this study. However, throughout the 22-day study period, the pH of the control sample remained higher (4.78 to 4.98) than those of the other tested samples as follows: apple pomace (APP) (4.88 to 4.76) and tomato pomace (TOP) (4.73 to 4.78) samples. The lowest pH values were observed in the fish samples marinated in rhubarb pomace (RHP) (4.58 to 4.64) followed by black currant pomace (BCP) (4.49 to 4.59).
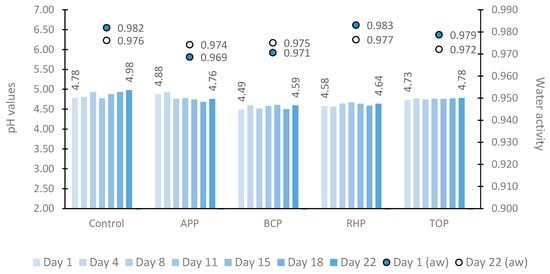
Figure 1.
The pH (bar chart) and water activity (point chart) in marinated fish samples on storage days 1, 4, 8, 11, 15, 18 and 22. Abbreviations: APP, apple pomace; BCP, black currant pomace; RHP, rhubarb pomace; and TOP, tomato pomace.
Within the storage period, the lowest and highest water activity values were 0.969 and 0.983, respectively. Throughout the study period, higher water activity values were observed in the control (a decrease from 0.982 to 0.976) and RHP (0.983 to 0.977) samples. Water activity was lower in the APP (an increase from 0.969 to 0.974) and BCP (0.971 to 0.975) samples.
3.2. Microorganisms in Marinated Fish
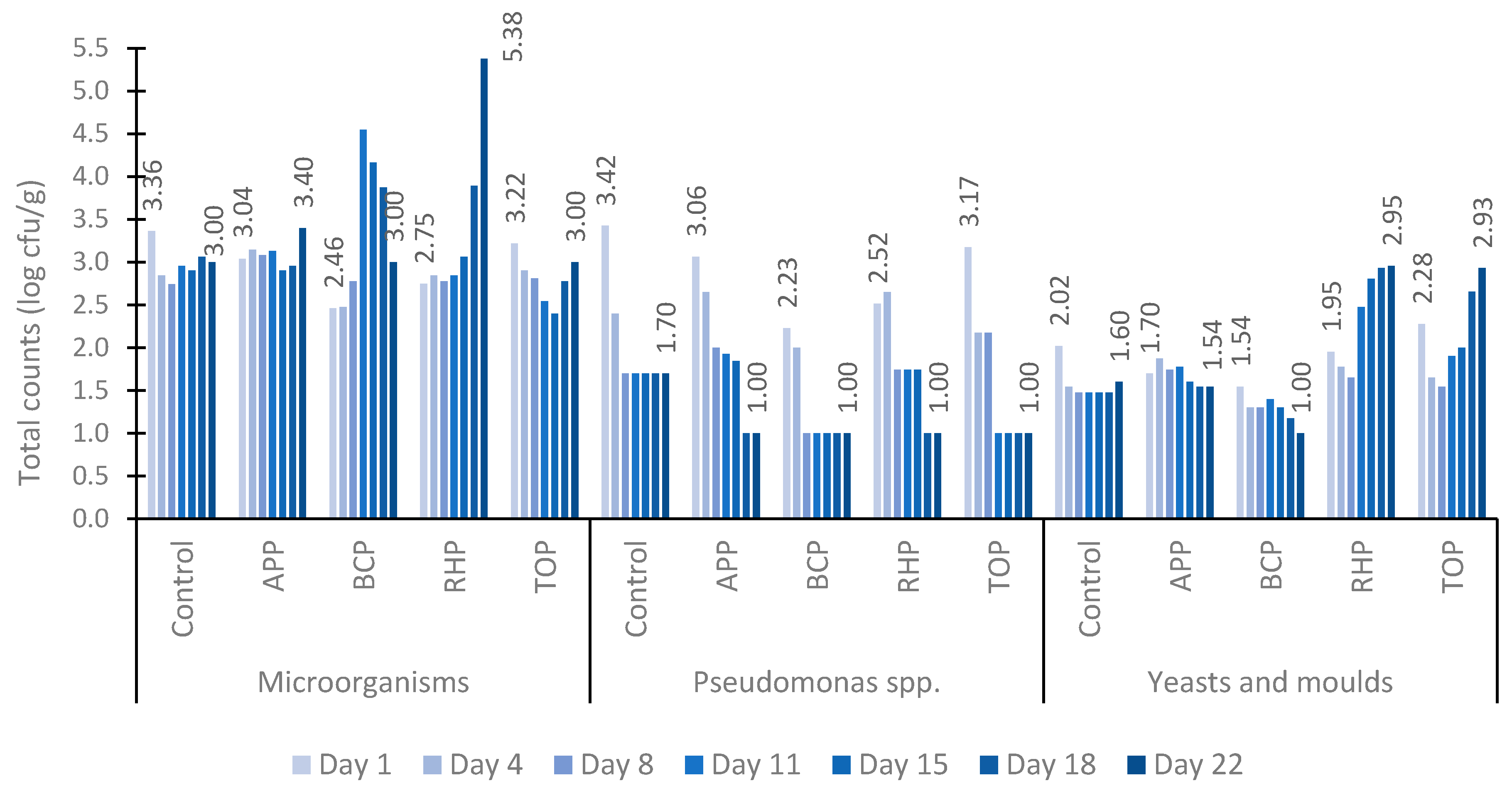
The dynamics of the counts of total microorganisms, Pseudomonas spp., yeasts and moulds in marinated fish products are presented in Figure 2. In all tested samples, the total number of microorganisms was higher than the counts for Pseudomonas spp. and yeasts and moulds during the 22-day study period. Initially, the total number of microorganisms averaged between 2.46 and 3.36 log10 cfu/g on the first day and 3.00 and 3.40 log10 cfu/g on the last day of the experiment, except for the RHP sample, which contained 5.38 log10 cfu/g on day 22. However, the total microbial counts remained at an acceptable level in all tested samples.
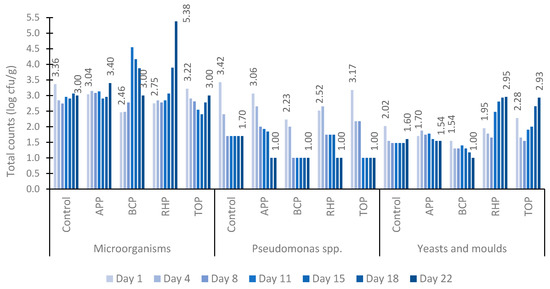
Figure 2.
The counts of total microorganisms, Pseudomonas spp., yeasts and moulds in marinated fish samples on days 1, 4, 8, 11, 15, 18 and 22. Abbreviations: APP, apple pomace; BCP, black currant pomace; RHP, rhubarb pomace; and TOP, tomato pomace.
In contrast to the increase in the counts of microorganisms, there was a clear decrease in the number of Pseudomonas spp. from 3.42 log10 cfu/g to 1.00 log10 cfu/g in all samples during the 22-day testing period. Similar dynamics of the counts were observed in both the control sample and the samples containing pomaces.
Similarly to those of Pseudomonas spp., the numbers of moulds and yeasts were also low in all tested samples throughout the study period remaining between 1.00 and 2.95 log10 cfu/g. However, while the APP and BCP samples showed a decrease in the numbers of moulds and yeasts from 1.70 to 1.54 log10 cfu/g and 1.54 to 1.00 log10 cfu/g, respectively, the total numbers in the RHP and TOP samples increased up to tenfold by the end of the study period.
3.3. Challenge Testing
For the control of L. monocytogenes in raw material, the standard protocol [28] was followed. All samples were negative for L. monocytogenes. The results of the L. monocytogenes challenge tests in the marinated fish samples are presented in Table 2. The growth potential of L. monocytogenes was lower than 0.5 log10 cfu/g in all tested samples during the 15-day challenge period. The lowest growth potential (δ = 0.169 log10 cfu/g) was found in the control sample followed by the TOP (δ = 0.209 log10 cfu/g) and APP (δ = 0.293 log10 cfu/g) samples. Although the RHP sample had a higher growth potential of 0.464 log10 cfu/g, it did not exceed 0.5 log10 cfu/g. The RHP fish sample had a lower pH (4.58 ± 0.17 to 4.64 ± 0.06) than the other samples (on average 4.769 ± 0.07), but the water activity (average 0.969 ± 0.003) was similar to the other samples (average 0.972 ± 0.003).

Table 2.
Results of the L. monocytogenes challenge tests in the marinated fish samples.
The total mesophilic aerobic counts in the challenge tests of the marinated fish samples increased over 15 days in the BCP (from 2.46 to 4.16 log10 cfu/g) and RHP (from 2.75 to 2.90 log10 cfu/g) samples but decreased less than tenfold in the control, APP and TOP samples. The total microbial counts remained at acceptable levels in all tested samples. In this study, the tolerable value for the total microbial count was 6.0 log10 cfu/g.
The average counts of L. monocytogenes on different days and in the tested marinated fish samples are shown in Table 3. The initial counts of L. monocytogenes in all tested samples on day 1 of storage were similar, averaging 2.41 ± 0.084 log10 cfu/g. Also, on the following days of storage, all samples contained similar numbers of L. monocytogenes on average, namely, 2.43 ± 0.414 log10 cfu/g on day 4, 2.38 ± 0.148 log10 cfu/g on day 8 and 2.38 ± 0.189 log10 cfu/g on day 11. However, on the last day of the challenge experiment, significantly lower (p < 0.05) average numbers of L. monocytogenes (on average 2.17 ± 0.498 log10 cfu/g) were detected than on the previous storage days.

Table 3.
Statistically significant differences (p < 0.05, Tukey post hoc test) between the L. monocytogenes challenge test average counts (log10 cfu/g) ± standard deviation on different storage days and in the tested marinated fish samples *.
Comparing the initial and final results, a tenfold decrease in the average counts of L. monocytogenes was found for the BCP samples, followed by the APP and RHP fish samples. Throughout the study period, the lowest average numbers (p < 0.05) of L. monocytogenes were observed in the BCP fish samples (on average 2.35 ± 0.235 log10 cfu/g) compared to the control samples.
L. monocytogenes growth potential dynamics between tested marinated samples on different storage days are shown in Table 4. The highest overall positive growth potential occurred on day 4 both in the APP (δ = 0.293) and in the RHP (δ = 0.464) samples, on day 8 in the TOP (δ = 0.209) samples, on day 11 in the BCP samples (δ = 0.321) and on day 15 in the control samples (δ = 0.169). The growth dynamics of L. monocytogenes in the marinated fish samples showed that for the last day of the experiment, the numbers of L. monocytogenes remained lower compared to the control. On day 15, the lowest growth potential of L. monocytogenes was found for the BCP and APP fish samples.

Table 4.
L. monocytogenes growth potential dynamics between tested marinated samples on different storage days.
3.4. Sensory Evaluation
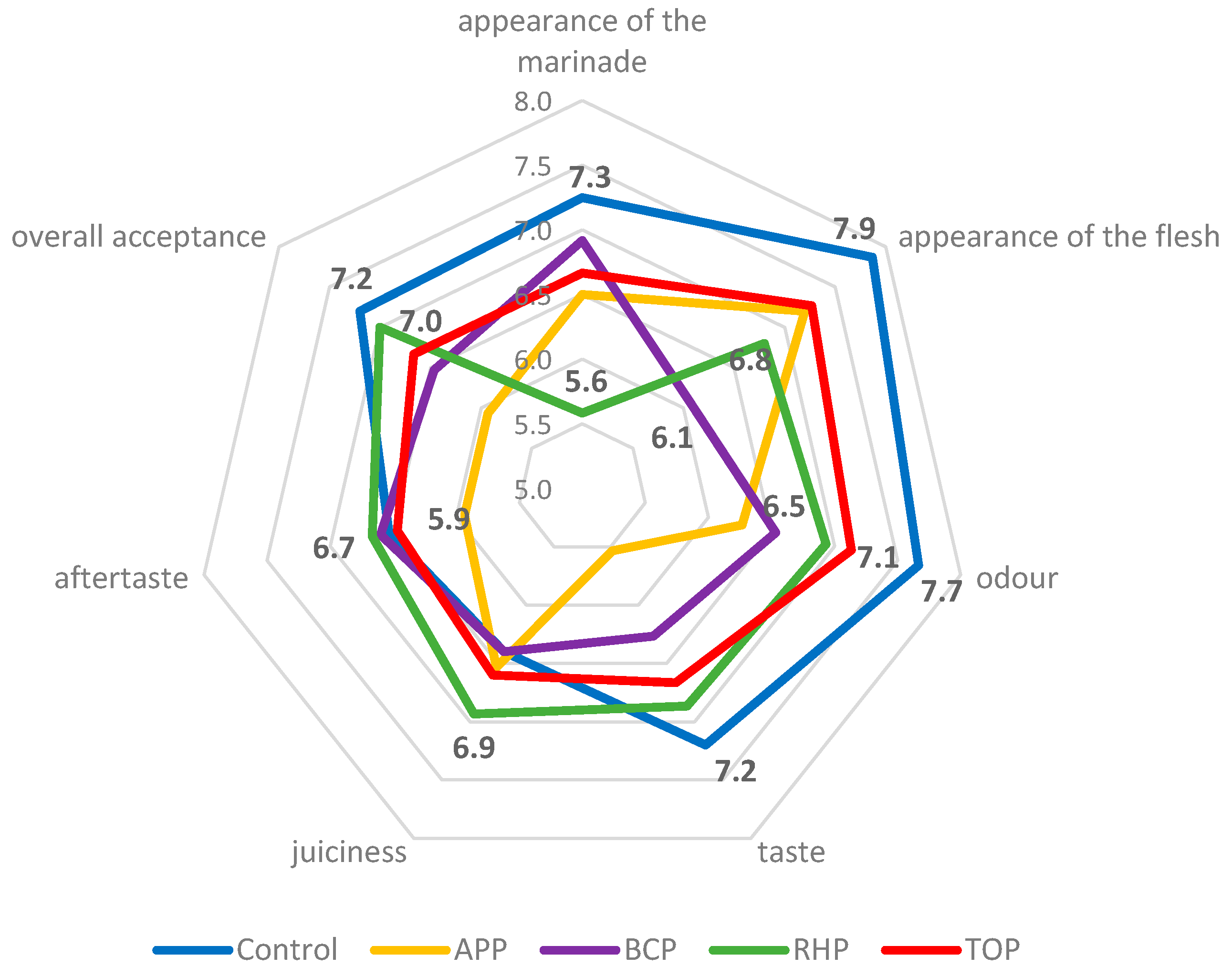
The results of the sensory evaluations are shown in Figure 3. The appearance of the acetic acid marinade as the control was scored with the highest points (7.3), and the marinade with RHP with the lowest (5.6). The appearance of the flesh in the control sample was also scored with the highest points (7.9), but the unusual purple colour of the flesh in the marinade with BCP resulted in this sample scoring the lowest (6.1). The odour, taste and overall acceptance of the fish in the acetic acid marinade were evaluated with the highest points, namely, 7.7, 7.2 and 7.2, respectively. The highest points for juiciness and aftertaste, namely, 6.9 and 6.7, respectively, were given to the fish in the marinade with RHP.
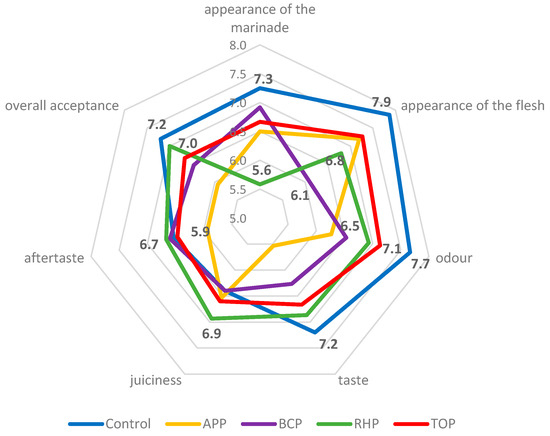
Figure 3.
The sensory attributes of the marinated rainbow trout samples. Abbreviations: APP, apple pomace; BCP, black currant pomace; RHP, rhubarb pomace; and TOP, tomato pomace. The bold numbers in the figure are the scores, which are reflected in the results and discussion of the article.
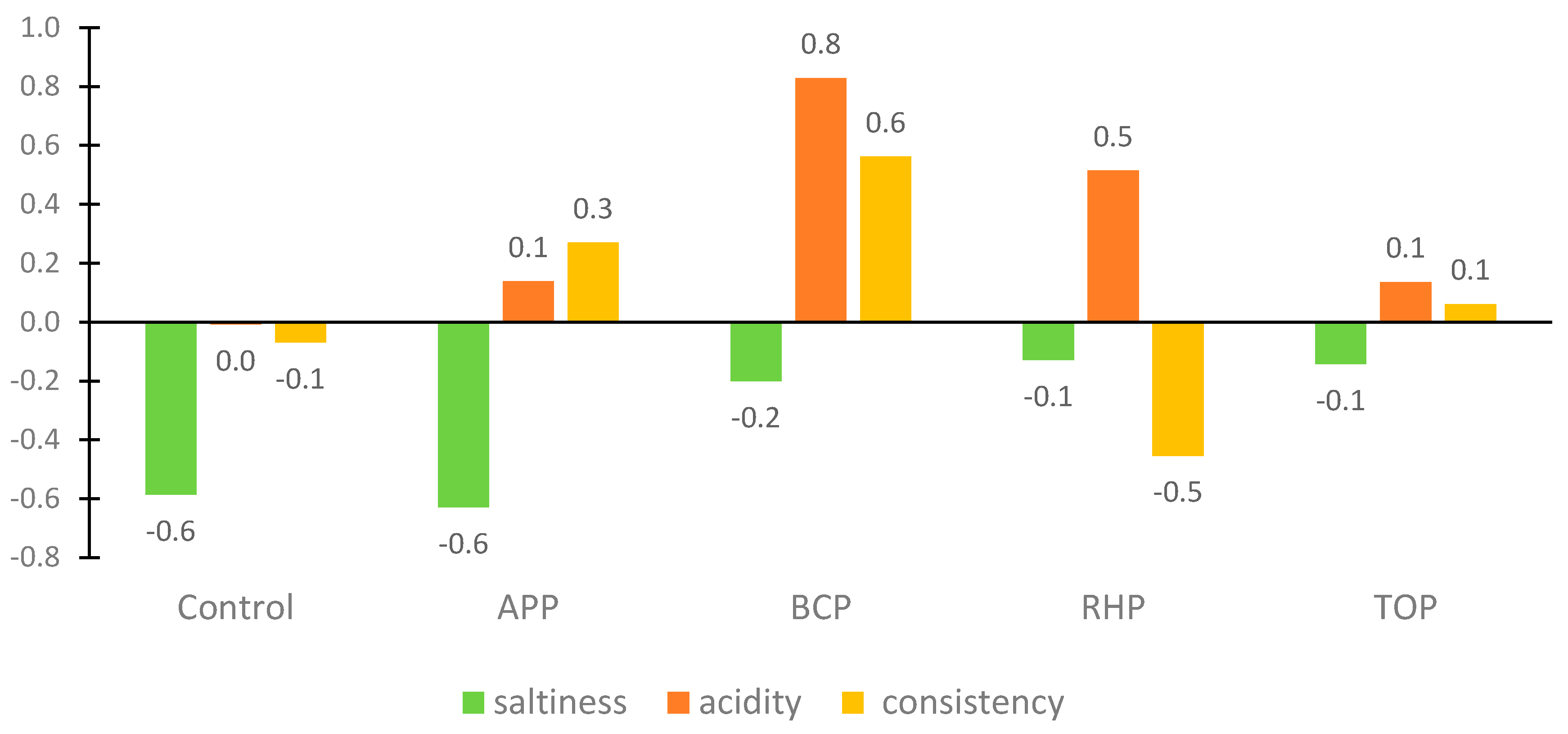
The appearance, colour, odour and taste of the flesh depended on the pomaces used in the marinade. The APP gave a strong apple smell and taste, which some panellists considered unusual, peculiar and too sweet, while others liked it. The BCP turned the fish an unusual dark purple colour, adding acidity and a peculiar taste that the panellists found surprisingly good. The RHP gave the marinade an orange-pinkish colour, added acidity, made the flesh consistency quite soft and gave the product a delicate sour smell. The appearance of the marinade with the TOP was slightly more orange than the others. The flesh had a soft consistency, was slightly sour and had a good tomato flavour and taste that most panellists liked; however, some did not, and some would have preferred even more salt. Results of the just-about-right (JAR) assessment are shown in Figure 4.
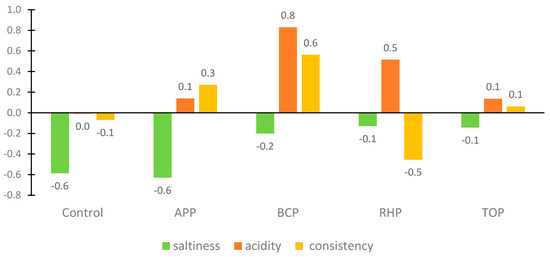
Figure 4.
Results of the just-about-right (JAR) evaluation of the marinated rainbow trout samples. Abbreviations: APP, apple pomace; BCP, black currant pomace; RHP, rhubarb pomace; and TOP, tomato pomace.
Consistency and taste intensity, such as saltiness and acidity, were assessed on a 5-point JAR rating scale ranging from “too little’’ to “too much”, with “0” indicating “just about right”. The JAR results showed a positive acceptance of the products. However, the JAR scores showed that the panellists were not satisfied with the consistency of the flesh in the RHB marinade sample and would have liked a bit more saltiness and less acidity in the BCP and RHB marinade samples. Correlation analyses showed a statistically significant (p < 0.05) positive correlation between the overall rating and the appearance of the fish samples in the marinades with the apple and tomato pomaces.
4. Discussion
Food business operators are tasked with producing a safe food product that has a sufficiently long shelf life and is acceptable to the consumer. For the inactivation or inhibition of pathogenic and spoilage microorganisms or the suppression of the chemical deterioration of food, a variety of processing and preservation methods are used including marinating fish [31].
The shelf life of a food product is determined by several food quality and/or food safety factors. Food quality can be affected by organoleptic, (bio)chemical and microbiological changes [32]. Food safety of ready-to-eat foods can be compromised by the presence or growth of L. monocytogenes or other pathogenic microorganisms to which food safety criteria are applied according to Commission Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs. Non-legislative food quality and safety criteria can also be established by the food business operators themselves within the self-control system.
In the present study, the general numbers of microorganisms such as mesophilic microorganisms and yeasts and moulds provide a quantitative assessment of the microbiological quality of the marinated fish products within a defined time period of the durability study. While the number of microorganisms provides a quantitative assessment of the microbiological quality of marinated fish products, the total count of Pseudomonas spp. indicates the presence of specific spoilage microbes, and the L. monocytogenes count is the food safety indicator in RTE foods. For acidified and marinated fishery products, within the shelf life of the product, the target and tolerance level of the aerobic count can be as high as 3 × 105 and 3 × 106 cfu/g [33]. In the present study, within the determined shelf life, the number of mesophilic aerobic microorganisms remained at acceptable levels in all tested samples including the control. The total numbers of mesophilic aerobic microorganisms in the tested samples remained below 5 log10 cfu/g, except for that in the RHP fish samples in which the number was 5.38 log10 cfu/g (Figure 2). However, all tested marinated fish products can be characterized as having good microbiological quality up to the “use by” date of 22 days. The same applies to the count of yeasts and moulds, for which the tolerance can be up to 3 × 104 [33], but in the present study, the highest value (2.95 log10 cfu/g) was determined for fish in RHP containing marinade (Figure 2). The counts of moulds and yeasts and Pseudomonas spp. remained acceptable in all tested product samples. Psychrotrophic Pseudomonas spp. can be a dominant bacterial species in skin samples of farmed fish [34]. In the present study, the total count of presumptive Pseudomonas spp. indicates the levels of specific spoilage bacteria. However, according to Nixon et al. [35], many Pseudomonas species are also known as opportunistic pathogens of humans, animals and farmed fish. A total of 90 Pseudomonas strains belonging to 20 species were isolated from aquaculture farm water and fish in a recent study by Duman et al. [36].
The present study showed that marinades enriched with fruit and berry pomaces were able to suppress the growth of Pseudomonas bacteria as well as to kill them as compared to the initial concentrations; the number of Pseudomonas was decreased by about 2 log10 cfu/g (100 times) on the last day (day 22) of the determined shelf life. This can be explained by the fact that Pseudomonas bacteria are not acid-tolerant and rarely grow below pH 5.0–6.0 [37]. Membré and Burlot’s [38] study has shown that no growth of P. marginalis was detected at 4 °C with 5% salt and a pH of 6. In the present study, the pH of the fish samples at 6 °C was below 5 throughout the 22-day study period, namely, 4.78–4.98 for the control samples and 4.49–4.78 for the other tested fish samples. The decrease in the total counts of Pseudomonas spp. is probably due to the acidic environment that prolonged the lag phase of Pseudomonas spp. for the entire durability study. Gonçalves et al. [39] also observed a long lag phase in the growth of P. fluorescens at more acidic pH values. The relationship between pH and the antibacterial effect of organic acids has been previously demonstrated [40,41]. Organic acids are, at the same pH, more effective than inorganic acids in inhibiting bacterial growth [23]. The mechanism is related to the ability of organic acids in the dissociated state to cross the membrane and then ionize in the bacterial cytoplasm [40]. This may cause inhibition of bacterial cell growth because of the lowered cytoplasmic pH, causing osmotic stress, and toxicity of organic acid anions [23,24,41].
Similar to Pseudomonas spp., the same phenomenon was observed for L. monocytogenes in challenge testing, where the growth potential of the pathogen was decreased in all samples, especially in those enriched with fruit and berry pomaces. Recently, Koskar et al. [42] demonstrated that several fruit and berry pomaces were able to inhibit the growth of microorganisms in raw minced pork samples. However, the same study found that plant powders alone were not able to inhibit the growth of L. monocytogenes in minced meat samples.
Regulation (EC) No 2073/2005 establishes the food safety criteria for L. monocytogenes in RTE foods. The quantitative limit of 100 cfu/g applies to RTE foods, which are not able to support the growth of L. monocytogenes or if the food business operator is able to demonstrate to the competent authority that the number of pathogens in the product will not exceed the limit of 100 cfu/g throughout the shelf life of the food. In challenge testing, the growth potential of L. monocytogenes below the criterion of 0.5 log10 cfu/g indicates whether the RTE food is able to support the growth of this foodborne pathogen or not [10]. In the present study, the growth potential of L. monocytogenes in all samples was below the aforementioned criterion. Furthermore, the average numbers were lowest (p < 0.05) on the last day (day 15) of challenge testing. This is a very good indicator of food safety because L. monocytogenes is known to be resistant to many environmental stressors including refrigeration and modified atmosphere packaging [9]. Therefore, Listeria can grow to high numbers in chilled foods with a long shelf life, especially in certain RTE fish and meat products [7,43,44]. Raw RTE fish products belong to the high-risk food category, due to the high prevalence and counts of L. monocytogenes found in these products [8]. Previous studies have shown that some plant materials can be effective in the growth inhibition of foodborne pathogens both in vitro and in foods [17,45,46]. Plants that contain phenolic acids, flavonoids, tannins, alkaloids and glycosides as well as their derivates have been found to have antimicrobial properties [47]. Natural preservatives can also be derived from pomaces obtained from juice pressing, which makes using natural additives both economically and environmentally reasonable. Fruit and berry pomaces consist of skins, seeds and stems with a high concentration of phytochemicals that may have good antimicrobial properties, thus able to prolong food spoilage [48]. This is in good agreement with the results of the current study because the strongest overall microbial growth inhibition effect was found in the BCP fish samples which also had the highest content of polyphenols, including anthocyanins (Figure S1).
There are only a few studies that have analysed the antimicrobial effect of marinades with plant-derived ingredients on food. Wine-marinated beef, which contained essential oils of Juniperus communis and Satureja montana, had antibacterial, including a listericidal effect in a study by Vasilijević et al. [49]. In a study by Testa et al. [50], olive leaf extracts in marinated anchovy fillets showed an inhibitory effect against all tested pathogenic bacteria, including Listeria). Urbonavičiūtė et al. [15] showed that herring coated with films containing cranberry pomace and grape seeds inhibited the growth of L. monocytogenes and Pseudomonas aeruginosa at 4 °C for 18 days. Also, a study by Zavistanaviciute et al. [16] found that the dairy, berry and fruit by-products used in marinades and their combinations can suppress the growth of pathogenic and opportunistic microorganisms to improve the safety and quality characteristics of lamb meat. The low counts of aerobic mesophilic microorganisms and decreased numbers of L. monocytogenes found in this study indicate that raw rainbow trout in fruit and berry pomaces containing marinades can be considered microbiologically safe and of high microbiological quality. Therefore, these products may have the potential for industrial food production.
Another important task of this study was to evaluate the effect of the used fruit and berry pomace ingredients on the sensory quality of marinated rainbow trout. Healthier products are preferred, but also sensory characteristics have a particular significance for consumer preference. It is essential that added plant-based ingredients are in agreement with consumers’ preferences and willingness to buy. The 9-point hedonic scale is a widely used method in terms of comparison purposes, particularly when using novel ingredients, in addition to JAR scaling in product development and determining whether attribute intensity is at an optimal level [51,52].
The control samples, followed by the RHP samples showed good overall acceptance with scores of 7.2 and 7.0, respectively, on a 9-point hedonic scale (Figure 3). For the control samples, the good taste of the marinated fish and the soft texture were highlighted; the RHP samples had an interesting pink marinade, good taste and quite soft consistency. The lowest scores (5.9) for overall acceptance were given to the APP samples. The assessors pointed out that these samples were evenly brownish and had an unusual apple taste, which is foreign to fish. Apple pomace is very prone to polyphenol oxidation during fruit processing [53]; therefore, the colour of the pomace powder tends to become brown eventually, and it may affect the colour of the food products to which it is added. The colour and appearance of a product are noticed as the first and very important sensory characteristics for consumers, and these features determine the purchase decision. In the current study, statistical analyses revealed that the overall sensory rating was positively correlated (p < 0.05) with the appearance of the fish samples marinated in the APP and TOP solutions. This was not the case for the control and other samples. The appearance of the marinade and flesh were rated high with scores of 7.3 and 7.9, respectively, for the control samples. The assessors least liked the appearance of the RHP marinade (5.6), although the appearance of the flesh was acceptable (6.8). Black currants are known for their intensive dark-purple, almost black, colour, and the marinade with BCP strongly affected the appearance of the flesh, which obtained the lowest score (6.1) compared to the other samples. Black currants contain a high amount of natural pigments (anthocyanins), and the colour stability depends on acid, salt and sugar concentrations in the existing matrix [54]. In the case of BCP, the anthocyanins present the darkest red colour with the lowest pH and blend with an increase in pH levels, thereby again affecting the appearance of the food if applied as an ingredient. The panellists noted the different appearance compared to the other samples and indicated the uniformly purple colour as the main reason for the low score (do not like the beetroot colour); nevertheless, the BCP samples were reported to have a surprisingly good sour berry taste and smell, and the panellists highlighted these samples as being an “interesting” product.
In marinated products, the specific product characteristics are a synergistic effect of salt and acid content. The best or “just about right” result in the JAR assessment for acidity was awarded to the control samples containing citric acid and acetic acid; for saltiness, these samples were indicated to be a little salty, although some assessors commented that saltiness and sourness were in balance; consistency was evaluated as soft and good (−0.1 on JAR scale). In the case of the addition of the RHB, TOP and BCP ingredients, it was observed that their natural sourness affected positively the “saltiness” level being almost “just about right” (−0.1, −0.1 and −0.2, respectively). It may be hypothesized that by adding sour plant-based ingredients to the marinade, there is no need to add as much salt. In the case of consistency, the results differed among the marinades from −0.5 for RHP to 0.6 for BCP being most of the cases close to “just about right”.
It can be summarised that the results of this study present a basis for using these fruit and berry pomace ingredients in marinades for raw trout with fully acceptable sensory and safety characteristics.
5. Conclusions
Valorisation of by-products generated from fruit and berry juice production is in good agreement with the concepts of a circular bio-economy and zero waste. The results revealed that the use of apple, black currant, rhubarb and tomato pomaces in marinades for fish products can ensure a sufficiently long microbiological shelf life of the products. The appearance, consistency, colour, odour and taste intensities of the fish marinades depend on the pomaces and salt content used in the marinade but still have acceptable sensory properties to the consumer. Our findings for the L. monocytogenes challenge tests showed that the marinated rainbow trout samples with apple, black currant, rhubarb and tomato pomaces can be classified as “ready-to-eat food unable to support the growth of L. monocytogenes” according to Commission Regulation (EC) No 2005/2073 and therefore may have good potential for industrial food production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11122960/s1, Figure S1: The content of phenolic compounds.
Author Contributions
Conceptualization, M.R., T.P. and K.M.; methodology, M.R., M.M. and K.M.; validation, M.R. and K.M.; formal analysis, M.R., K.M., M.M., T.E., K.K. (Karmen Kapp), M.T. and K.K. (Kristi Kerner); investigation, M.R., K.M., S.J., M.T. and M.P.; resources, M.R. and R.R.; data curation, M.R., K.M., M.M., K.K. (Kristi Kerner) and T.E.; writing—original draft preparation, K.M., M.R., M.M., D.A., M.P., M.T. and K.K. (Kristi Kerner); writing—review and editing, T.P., R.R., T.E., S.J., K.K. (Karmen Kapp) and K.M.; visualization, K.M., M.M. and D.A.; supervision, M.R. and K.M.; project administration, M.R. and R.R.; and funding acquisition, M.R. and R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Estonian Research Council, grant number PRG1441, and by the European Regional Development Fund and Estonian Research Council, project RESTA14.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sharma, P.; Gaur, V.K.; Sirohi, R.; Varjani, S.; Kim, S.H.; Wong, J.W.C. Sustainable processing of food waste for production of bio-based products for circular bioeconomy. Bioresource Technol. 2021, 325, 124684. [Google Scholar] [CrossRef] [PubMed]
- Origbemisoye, B.A.; Ifesan, B.O.T. Chemical composition of ‘Kiaat’ (Pteropcarpus angolensis) bark and the effect of herb pastes on the quality changes in marinated cat fish during chilled storage. Food Biol. 2019, 8, 7–12. [Google Scholar] [CrossRef][Green Version]
- Essid, I.; Tajine, S.; Gharbi, S.; Bellagha, S. Use of pomegranate peel and artichoke leaf extracts to improve the quality of marinated sardine (Sardinella aurita) fillets. J. Food Sci. Technol. 2020, 57, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, N.; Nalbone, L.; Di Rosa, A.R.; Ed-Dra, A.; Nait-Mohamed, S.; Mhamdi, R.; Giuffrida, A.; Giarratana, F. Marinated anchovies (Engraulis encrasicolus) prepared with flavored olive oils (Chétoui cv.): Anisakicidal effect, microbiological, and sensory evaluation. Sustainability 2021, 13, 5310. [Google Scholar] [CrossRef]
- Tahiluddin, A.B.; Maribao, I.; Amlani, M.; Sarri, J.H. A review on spoilage microorganisms in fresh and processed aquatic food products. Food Bull. 2022, 1, 21–36. [Google Scholar] [CrossRef]
- Jami, M.; Ghanbari, M.; Zunabovic, M.; Domig, K.J.; Kneifel, W. Listeria monocytogenes in aquatic food products—A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 798–813. [Google Scholar] [CrossRef]
- Kramarenko, T.; Roasto, M.; Keto-Timonen, R.; Mäesaar, M.; Meremäe, K.; Kuningas, M.; Korkeala, H. Listeria monocytogenes in ready-to-eat vacuum and modified atmosphere packaged meat and fish products of Estonian origin at retail level. Food Control 2016, 67, 48–52. [Google Scholar] [CrossRef]
- Koskar, J.; Kramarenko, T.; Meremäe, K.; Kuningas, M.; Sõgel, J.; Mäesaar, M.; Anton, D.; Lillenberg, M.; Roasto, M. Prevalence and numbers of Listeria monocytogenes in various ready-to-eat foods over a 5-year period in Estonia. J. Food Protect. 2019, 4, 597–604. [Google Scholar] [CrossRef]
- Mäesaar, M.; Mamede, R.; Elias, T.; Roasto, M. Retrospective use of Whole-Genome Sequencing expands the multicountry outbreak cluster of Listeria monocytogenes ST1247. Int. J. Genom. 2021, 1, 6636138. [Google Scholar] [CrossRef]
- European Union Reference Laboratory for Listeria monocytogenes (EURL Lm). EURL Lm Technical Guidance Document on Challenge Tests and Durability Studies for Assessing Shelf-Life of Ready-to-Eat Foods Related to Listeria monocytogenes, Version 4 of 1 July 2021. ANSES Laboratory for Food Safety. 2021. Available online: https://food.ec.europa.eu/system/files/2021-07/biosafety_fh_mc_tech-guide-doc_listeria-in-rte-foods_en_0.pdf (accessed on 11 April 2023).
- Häkkinen, S.; Heinonen, M.; Kärenlampi, S.; Mykkänen, H.; Ruuskanen, J.; Törrönen, R. Screening of selected flavonoids and phenolic acids in 19 berries. Food Res. Int. 1999, 32, 345–353. [Google Scholar] [CrossRef]
- Heinonen, M. Antioxidant activity and antimicrobial effect of berry phenolics—A Finnish perspective. Mol. Nutr. Food Res. 2007, 51, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Raudsepp, P.; Koskar, J.; Anton, D.; Meremäe, K.; Kapp, K.; Laurson, P.; Bleive, U.; Kaldmäe, H.; Roasto, M.; Püssa, T. Antibacterial and antioxidative properties of different parts of garden rhubarb, black currant, chokeberry and blue honeysuckle. J. Sci. Food Agric. 2019, 99, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Anton, D.; Koskar, J.; Raudsepp, P.; Meremäe, K.; Kaart, T.; Püssa, T.; Roasto, M. Antimicrobial and antioxidative effects of plant powders in raw and cooked minced pork. Foods 2019, 8, 661. [Google Scholar] [CrossRef] [PubMed]
- Urbonavičiūtė, G.; Dyglė, G.; Černauskas, D.; Šipailienė, A.; Venskutonis, P.R.; Leskauskaitė, D. Alginate/pectin film containing extracts isolated from cranberry pomace and grape seeds for the preservation of herring. Foods 2023, 12, 1678. [Google Scholar] [CrossRef]
- Zavistanaviciute, P.; Klementaviciute, J.; Klupsaite, D.; Zokaityte, E.; Ruzauskas, M.; Buckiuniene, V.; Viskelis, P.; Bartkiene, E. Effects of marinades prepared from food industry by-products on quality and biosafety parameters of lamb meat. Foods 2023, 12, 1391. [Google Scholar] [CrossRef] [PubMed]
- Tamkutė, L.; Gil, B.M.; Carballido, J.R.; Pukalskienė, M.; Venskutonis, P.R. Effect of cranberry pomace extracts isolated by pressurized ethanol and water on the inhibition of food pathogenic/spoilage bacteria and the quality of pork products. Food Res. Int. 2019, 120, 38–51. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef]
- Celic, F.; Gundogdu, M.; Ercisli, S.; Kaki, B. Variation in organic acid, sugar and phenolic compounds in fruits of historical apple cultivars. Not. Bot. Horti Agrobot. 2018, 46, 622–629. [Google Scholar] [CrossRef]
- Tian, Y.; Laaksonen, O.; Haikonen, H.; Vanag, A.; Ejaz, H.; Linderborg, K.; Karhu, S.; Yang, B. Compositional diversity among blackcurrant (Ribes nigrum) cultivars originating from European Countries. J. Agric. Food Chem. 2019, 67, 5621–5633. [Google Scholar] [CrossRef]
- Golubkina, N.; Kharchenko, V.; Bogachuk, M.; Koshevarov, A.; Sheshnitsan, S.; Kosheleva, O.; Pirogov, N.; Caruso, G. Biochemical characteristics and elemental composition peculiarities of Rheum tataricum L. in semi-desert conditions and of European garden rhubarb. Int. J. Plant Biol. 2022, 13, 368–380. [Google Scholar] [CrossRef]
- Agius, C.; von Tucher, S.; Poppenberger, B.; Rozhon, W. Quantification of sugars and organic acids in tomato fruits. Methods X 2018, 5, 537–550. [Google Scholar] [CrossRef]
- Hirshfield, I.N.; Terzulli, S.; O’Byrne, C. Weak organic acids: A panoply of effects on bacteria. Sci. Prog. 2003, 86 Pt 4, 245–269. [Google Scholar] [CrossRef] [PubMed]
- Brul, S.; Coote, P. Preservative agents in foods. Mode of action and microbial resistance mechanisms. Int. J. Food Microbiol. 1999, 15, 1–17. [Google Scholar] [CrossRef]
- EVS-EN ISO 4833-2:2013; Horizontal Method for the Enumeration of Microorganisms. Part 2: Colony Count at 30 °C by the Surface Plating Technique. ISO: Geneva, Switzerland, 2013.
- EVS-ISO 21527-1:2009; Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. ISO: Geneva, Switzerland, 2009.
- EVS-EN ISO 13720:2010; Meat and Meat Products—Enumeration of Presumptive Pseudomonas spp. ISO: Geneva, Switzerland, 2010.
- EVS-EN ISO 11290-1:2017; Horizontal Method for the Detection and Enumeration of L. monocytogenes and of Listeria spp. Part 1: Detection Method. ISO: Geneva, Switzerland, 2017.
- EVS-EN ISO 11290-2:2017; Horizontal Method for the Detection and Enumeration of L. monocytogenes and of Listeria spp. Part 2: Enumeration Method. ISO: Geneva, Switzerland, 2017.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 21 June 2023).
- Lado, B.H.; Yousef, A.E. Characteristics of Listeria monocytogenes important to food processors. In Listeria, Listeriosis, and Food Safety, 3rd ed.; Ryser, E.T., Marth, E.H., Eds.; CRC Press: Boca Raton, FL, USA, 2007; p. 157213. [Google Scholar]
- European Food Safety Authority (EFSA). Scientific opinion. Guidance on date marking and related food information: Part 1 (date marking). EFSA J. 2020, 18, 6306. [Google Scholar] [CrossRef]
- Uyttendaele, M.; De Loy-Hendrickx, A.; Vermeulen, A.; Jacxsens, L.; Debevere, J.; Devlieghere, F. Microbiological Guidelines: Support for Interpretation of Microbiological Test Results in Foods; Uyttendaele, M., Ed.; Universiteit Gent, Die Keure Professional Publishing: Brugge, Belgium, 2018; pp. 312–313. ISBN 9782874035036. [Google Scholar]
- Aydin, A.; Sudagidan, M.; Mamatova, Z.; Yurt, M.N.Z.; Ozalp, V.C.; Zornu, J.; Tavornpanich, S.; Brun, E. Bacterial skin microbiota of seabass from Aegean fish farms and antibiotic susceptibility of psychrotrophic Pseudomonas. Foods 2023, 12, 1956. [Google Scholar] [CrossRef]
- Nixon, G.M.; Armstrong, D.S.; Carzino, R.; Carlin, J.B.; Olinsky, A.; Robertson, C.F.; Grimwood, K. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J. Pediatr. 2001, 138, 699–704. [Google Scholar] [CrossRef]
- Duman, M.; Mulet, M.; Altun, S.; Saticioglu, I.B.; Ozdemir, B.; Ajmi, N.; Lalucat, J.; Elena García-Valdés, E. The diversity of Pseudomonas species isolated from fish farms in Turkey. Aquaculture 2021, 535, 736369. [Google Scholar] [CrossRef]
- Cousin, M.A. Pseudomonas. In Encyclopedia of Food Microbiology; Robinson, R.K., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 1864–1867. ISBN 9780122270703. [Google Scholar] [CrossRef]
- Membré, J.M.; Burlot, P.M. Effects of temperature, pH, and NaCl on growth and pectinolytic activity of Pseudomonas marginalis. Appl. Environ. Microbiol. 1994, 60, 2017–2022. [Google Scholar] [CrossRef]
- Gonçalves, L.D.d.A.; Piccoli, R.H.; Peres, A.d.P.; Saúde, A.V. Predictive modeling of Pseudomonas fluorescens growth under different temperature and pH values. Braz. J. Microbiol. 2017, 48, 352–358. [Google Scholar] [CrossRef]
- Bushell, F.M.L.; Tonner, P.D.; Jabbari, S.; Schmid, A.K.; Lund, P.A. Synergistic impacts of organic acids and pH on growth of Pseudomonas aeruginosa: A comparison of parametric and bayesian non-parametric methods to model growth. Front. Microbiol. 2019, 9, 3196. [Google Scholar] [CrossRef]
- Carpenter, C.E.; Broadbent, J.R. External concentration of organic acid anions and pH: Key independent variables for studying how organic acids inhibit growth of bacteria in mildly acidic foods. J. Food Sci. 2009, 74, 12–15. [Google Scholar] [CrossRef]
- Koskar, J.; Meremäe, K.; Püssa, T.; Anton, D.; Elias, T.; Rätsep, R.; Mäesaar, M.; Kapp, K.; Roasto, M. Microbial growth dynamics in minced meat enriched with plant powders. Appl. Sci. 2022, 12, 11292. [Google Scholar] [CrossRef]
- Bērzinš, A.; Hörman, A.; Lunden, J.; Korkeala, H. Factors associated with Listeria monocytogenes contamination of cold-smoked pork products produced in Latvia and Lithuania. Int. J. Food Microbiol. 2007, 115, 173–179. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific opinion. Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018, 16, 5134. [Google Scholar] [CrossRef]
- Raudsepp, P.; Anton, D.; Roasto, M.; Meremäe, K.; Pedastsaar, P.; Mäesaar, M.; Raal, A.; Laikoja, K.; Püssa, T. The antioxidative and antimicrobial properties of the blue honeysuckle (Lonicera caerulea L.), Siberian rhubarb (Rheum rhaponticum L.) and some other plants, compared to ascorbic acid and sodium nitrite. Food Control 2013, 31, 129–135. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Zhang, L.-F.; Xu, J.-G. Chemical composition, antibacterial activity and action mechanism of different extracts from hawthorn (Crataegus pinnatifida Bge.). Sci. Rep. 2020, 10, 8876. [Google Scholar] [CrossRef]
- Rathod, N.B.; Ranveer, R.C.; Benjakul, S.; Kim, S.K.; Pagarkar, A.U.; Patange, S.; Ozogul, F. Recent developments of natural antimicrobials and antioxidants on fish and fishery food products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4182–4210. [Google Scholar] [CrossRef]
- Babaoğlu, A.S.; Unal, K.; Dilek, N.M.; Poçan, H.B.; Karakaya, M. Antioxidant and antimicrobial effects of blackberry, black chokeberry, blueberry, and red currant pomace extracts on beef patties subject to refrigerated storage. Meat Sci. 2022, 187, 108765. [Google Scholar] [CrossRef]
- Vasilijević, B.; Mitić-Ćulafić, D.; Djekic, I.; Marković, T.; Knežević-Vukčević, J.; Tomasevic, I.; Velebit, B.; Nikolić, B. Antibacterial effect of Juniperus communis and Satureja montana essential oils against Listeria monocytogenes in vitro and in wine marinated beef. Food Control 2019, 100, 247–256. [Google Scholar] [CrossRef]
- Testa, B.; Lombardi, S.J.; Macciola, E.; Succi, M.; Tremonte, P.; Iorizzo, M. Efficacy of olive leaf extract (Olea europaea L. cv Gentile di Larino) in marinated anchovies (Engraulis encrasicolus, L.) process. Heliyon 2019, 5, e01727. [Google Scholar] [CrossRef] [PubMed]
- Popper, R.; Gibes, K. Workshop summary: Data analysis workshop: Getting the most out of just-about-right data. Food Qual. Prefer. 2004, 15, 891–899. [Google Scholar]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices, 2nd ed.; Springer: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Guyot, S.; Renard, C.M.G.C. Interactions between apple (Malus × domestica Borkh.) polyphenols and cell walls modulate the extractability of polysaccharides. Carbohydr. Polym. 2009, 75, 251–261. [Google Scholar] [CrossRef]
- Hubbermann, E.M.; Heins, A.; Stöckmann, H.; Schwarz, K. Influence of acids, salt, sugars and hydrocolloids on the colour stability of anthocyanin rich black currant and elderberry concentrates. Eur. Food Res. Technol. 2006, 223, 83–90. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).