Diatom–Bacteria Interactions in the Marine Environment: Complexity, Heterogeneity, and Potential for Biotechnological Applications
Abstract
:1. Introduction
2. Diatom–Bacteria Niches of Interactions
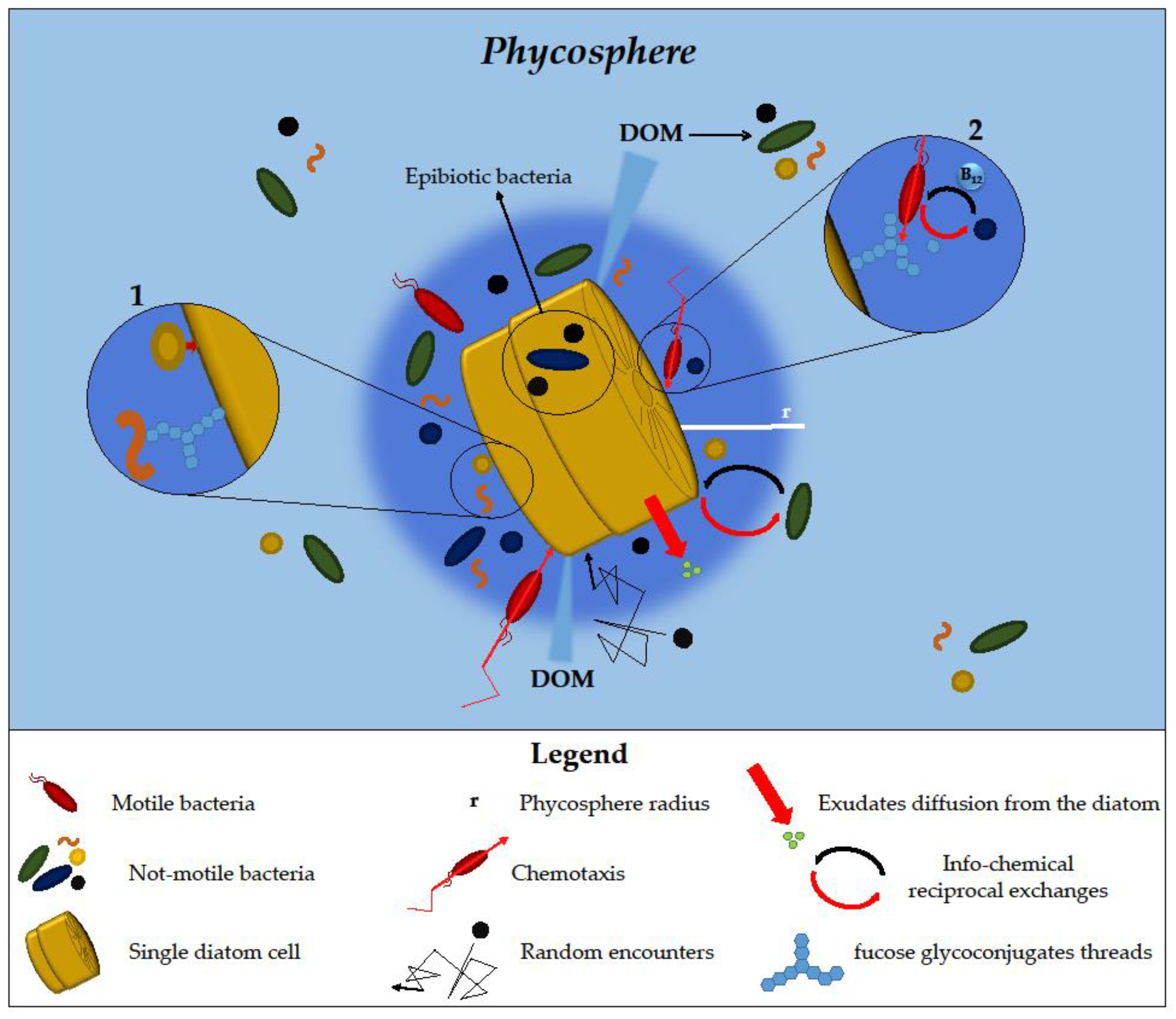
2.1. Phycosphere
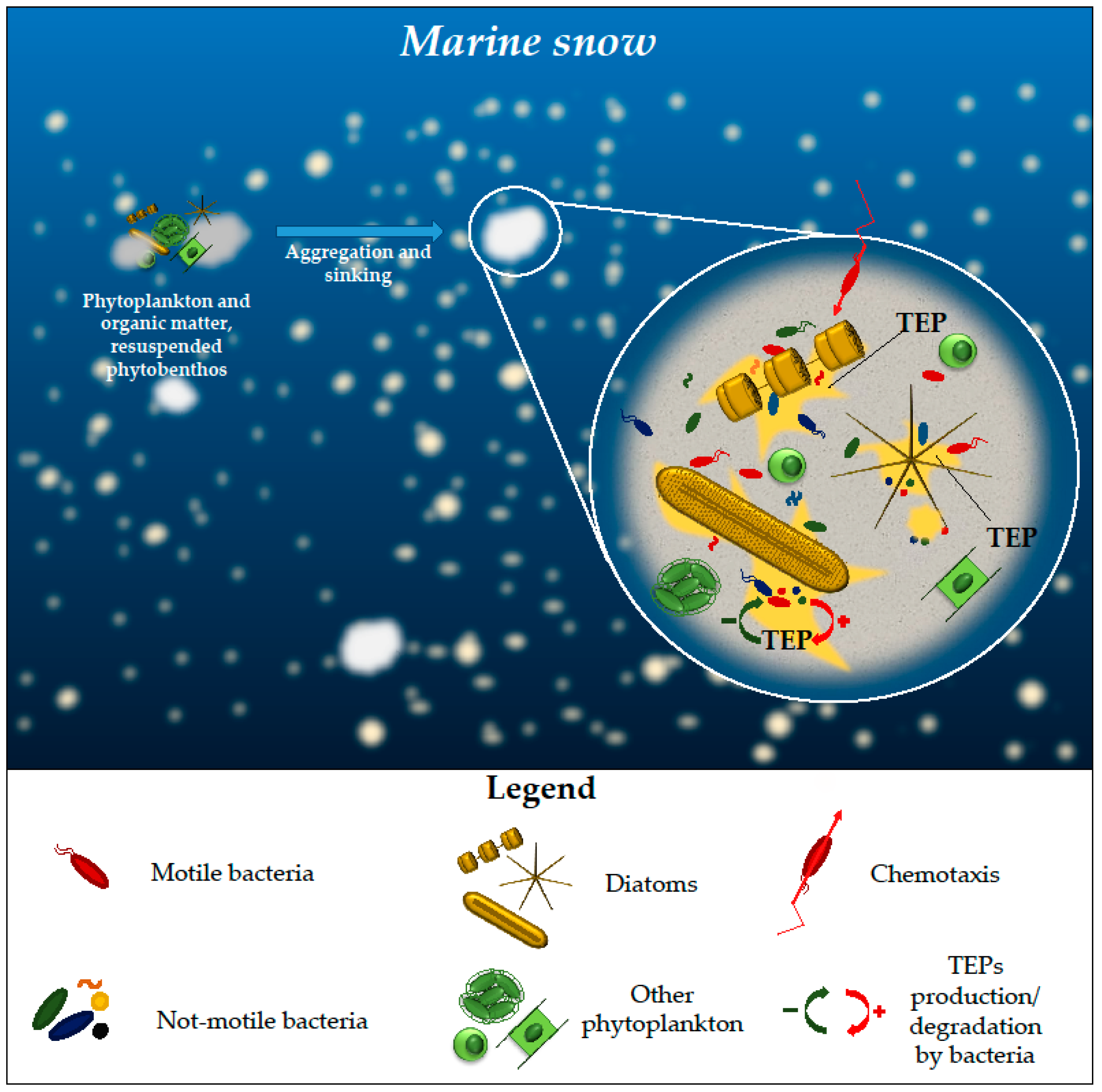
2.2. Marine Snow
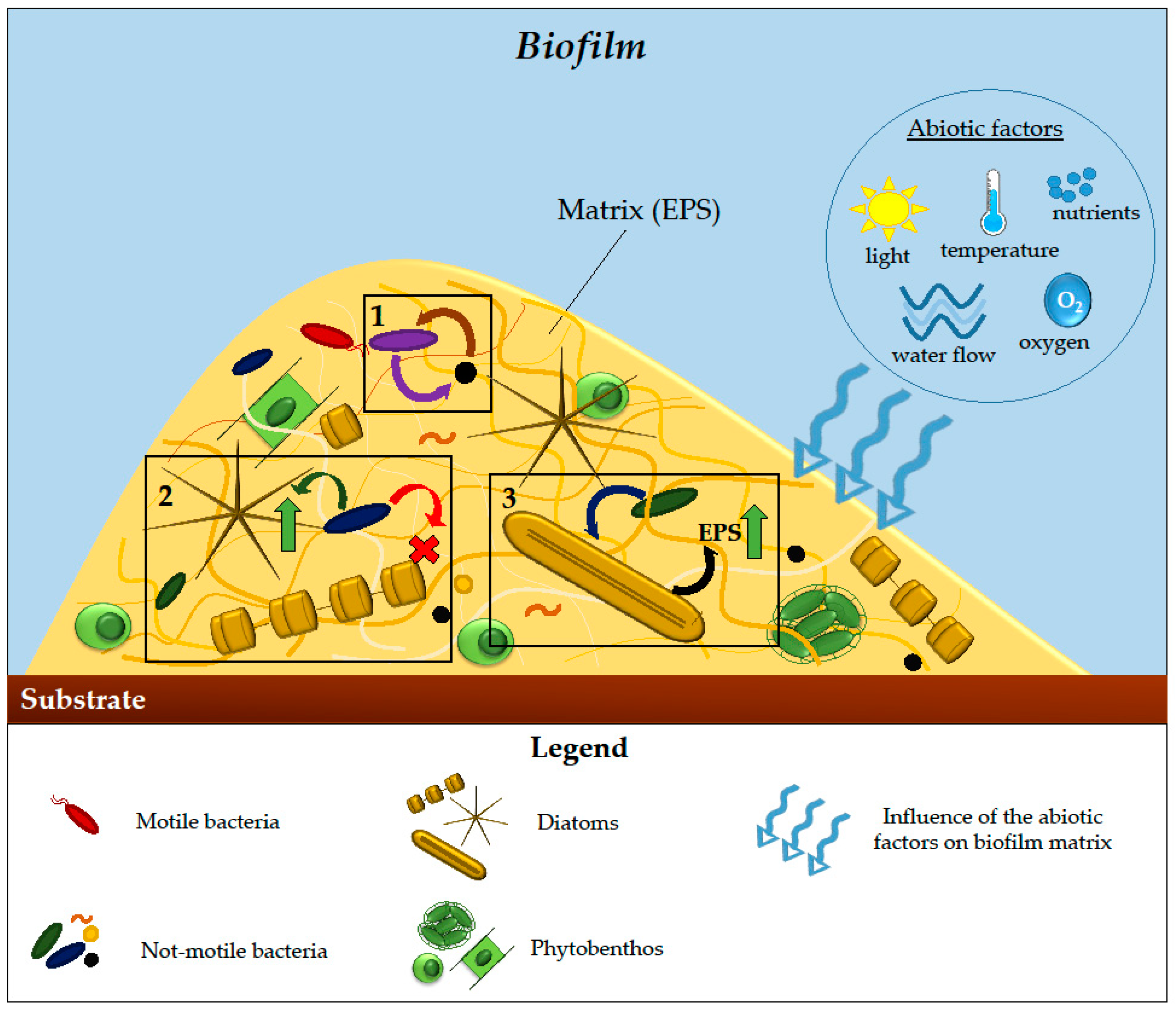
2.3. Biofilms
3. Diatom–Bacteria Types of Interactions
3.1. Mutualistic Interactions
3.2. Facilitative Interactions
3.3. Antagonistic Interactions
3.3.1. Inhibitory Effects of Bacteria on Diatoms
3.3.2. Algicidal Bacteria
3.3.3. Inhibitory Effects of Diatoms on Bacterial Growth
4. Diatom-Associated Microbiomes
5. Most Used Approaches to Study the Bacterial Communities’ Diversity
6. Potential Biotechnological Applications of Diatom–Bacteria Consortia
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vincent, F.; Bowler, C. An integrated view of diatom interactions. In The Molecular Life of Diatoms; Falciatore, A., Mock, T., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 59–86. ISBN 978-3-030-92499-7. [Google Scholar]
- Ianora, A.; Miralto, A. Toxigenic effects of diatoms on grazers, phytoplankton and other microbes: A review. Ecotoxicology 2010, 19, 493–511. [Google Scholar] [CrossRef] [PubMed]
- Di Dato, V.; Di Costanzo, F.; Barbarinaldi, R.; Perna, A.; Ianora, A.; Romano, G. Unveiling the presence of biosynthetic pathways for bioactive compounds in the Thalassiosira rotula transcriptome. Sci. Rep. 2019, 9, 9893. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.A.; Parker, M.S.; Armbrust, E.V. Interactions between Diatoms and Bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 667–684. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Fenchel, T.; Field, J.; Gray, J.; Meyer-Reil, L.; Thingstad, F. The Ecological Role of Water-Column Microbes in the Sea. Mar. Ecol. Prog. Ser. 1983, 10, 257–263. [Google Scholar] [CrossRef]
- Behringer, G.; Ochsenkühn, M.A.; Fei, C.; Fanning, J.; Koester, J.A.; Amin, S.A. Bacterial Communities of Diatoms Display Strong Conservation across Strains and Time. Front. Microbiol. 2018, 9, 659. [Google Scholar] [CrossRef]
- Cirri, E.; Pohnert, G. Algae−bacteria Interactions That Balance the Planktonic Microbiome. New Phytol. 2019, 223, 100–106. [Google Scholar] [CrossRef]
- Seymour, J.R.; Amin, S.A.; Raina, J.-B.; Stocker, R. Zooming in on the Phycosphere: The Ecological Interface for Phytoplankton–Bacteria Relationships. Nat. Microbiol. 2017, 2, 17065. [Google Scholar] [CrossRef]
- Cirri, E.; De Decker, S.; Bilcke, G.; Werner, M.; Osuna-Cruz, C.M.; De Veylder, L.; Vandepoele, K.; Werz, O.; Vyverman, W.; Pohnert, G. Associated Bacteria Affect Sexual Reproduction by Altering Gene Expression and Metabolic Processes in a Biofilm Inhabiting Diatom. Front. Microbiol. 2019, 10, 1790. [Google Scholar] [CrossRef]
- Kaur-Kahlon, G.; Kumar, B.K.; Ruwandeepika, H.A.D.; Defoirdt, T.; Karunasagar, I. Quorum Sensing Regulation of Virulence Gene Expression in Vibrio Harveyi during Its Interaction with Marine Diatom Skeletonema marinoi. J. Pure Appl. Microbiol. 2021, 15, 2507–2519. [Google Scholar] [CrossRef]
- Sison-Mangus, M.P.; Jiang, S.; Tran, K.N.; Kudela, R.M. Host-Specific Adaptation Governs the Interaction of the Marine Diatom, Pseudo-Nitzschia and Their Microbiota. ISME J. 2014, 8, 63–76. [Google Scholar] [CrossRef]
- Stocker, R.; Seymour, J.R. Ecology and Physics of Bacterial Chemotaxis in the Ocean. Microbiol. Mol. Biol. Rev. 2012, 76, 792–812. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Lovell, C.R. Microbial Surface Colonization and Biofilm Development in Marine Environments. Microbiol. Mol. Biol. Rev. 2015, 80, 91–138. [Google Scholar] [CrossRef] [PubMed]
- Wahl, M.; Goecke, F.; Labes, A.; Dobretsov, S.; Weinberger, F. The Second Skin: Ecological Role of Epibiotic Biofilms on Marine Organisms. Front. Microbiol. 2012, 3, 292. [Google Scholar] [CrossRef] [PubMed]
- Fei, C.; Ochsenkühn, M.A.; Shibl, A.A.; Isaac, A.; Wang, C.; Amin, S.A. Quorum Sensing Regulates ‘Swim-or-stick’ Lifestyle in the Phycosphere. Environ. Microbiol. 2020, 22, 4761–4778. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, K.E.; Shibl, A.A.; Amin, S.A. The Diatom Microbiome: New Perspectives for Diatom-Bacteria Symbioses. In The Molecular Life of Diatoms; Falciatore, A., Mock, T., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 679–712. ISBN 978-3-030-92499-7. [Google Scholar]
- Torres-Monroy, I.; Ullrich, M.S. Identification of Bacterial Genes Expressed during Diatom-Bacteria Interactions Using an in Vivo Expression Technology Approach. Front. Mar. Sci. 2018, 5, 200. [Google Scholar] [CrossRef]
- Gärdes, A.; Iversen, M.H.; Grossart, H.-P.; Passow, U.; Ullrich, M.S. Diatom-Associated Bacteria Are Required for Aggregation of Thalassiosira weissflogii. ISME J. 2011, 5, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Cheah, Y.T.; Chan, D.J.C. Physiology of Microalgal Biofilm: A Review on Prediction of Adhesion on Substrates. Bioengineered 2021, 12, 7577–7599. [Google Scholar] [CrossRef]
- Johansson, O.N.; Pinder, M.I.M.; Ohlsson, F.; Egardt, J.; Töpel, M.; Clarke, A.K. Friends with Benefits: Exploring the Phycosphere of the Marine Diatom Skeletonema marinoi. Front. Microbiol. 2019, 10, 1828. [Google Scholar] [CrossRef]
- van Tol, H.M.; Amin, S.A.; Armbrust, E.V. Ubiquitous Marine Bacterium Inhibits Diatom Cell Division. ISME J. 2017, 11, 31–42. [Google Scholar] [CrossRef]
- Long, R.; Azam, F. Microscale Patchiness of Bacterioplankton Assemblage Richness in Seawater. Aquat. Microb. Ecol. 2001, 26, 103–113. [Google Scholar] [CrossRef]
- Mönnich, J.; Tebben, J.; Bergemann, J.; Case, R.; Wohlrab, S.; Harder, T. Niche-Based Assembly of Bacterial Consortia on the Diatom Thalassiosira rotula Is Stable and Reproducible. ISME J. 2020, 14, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Daly, G.; Decorosi, F.; Viti, C.; Adessi, A. Shaping the Phycosphere: Analysis of the EPS in Diatom-Bacterial Co-Cultures. J. Phycol. 2023, 59, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.D.; Neu, T.R.; Sultana, S.; Giebel, H.-A.; Simon, M.; Billerbeck, S. Distinct Glycoconjugate Cell Surface Structures Make the Pelagic Diatom Thalassiosira rotula an Attractive Habitat for Bacteria. J. Phycol. 2023, 59, 309–322. [Google Scholar] [CrossRef]
- Kaczmarska, I.; Ehrman, J.M.; Bates, S.S.; Green, D.H.; Léger, C.; Harris, J. Diversity and Distribution of Epibiotic Bacteria on Pseudo-nitzschia multiseries (Bacillariophyceae) in Culture, and Comparison with Those on Diatoms in Native Seawater. Harmful Algae 2005, 4, 725–741. [Google Scholar] [CrossRef]
- Isaac, A.; Francis, B.; Amann, R.I.; Amin, S.A. Tight Adherence (Tad) Pilus Genes Indicate Putative Niche Differentiation in Phytoplankton Bloom Associated Rhodobacterales. Front. Microbiol. 2021, 12, 718297. [Google Scholar] [CrossRef] [PubMed]
- Shibl, A.A.; Isaac, A.; Ochsenkühn, M.A.; Cárdenas, A.; Fei, C.; Behringer, G.; Arnoux, M.; Drou, N.; Santos, M.P.; Gunsalus, K.C.; et al. Diatom Modulation of Select Bacteria through Use of Two Unique Secondary Metabolites. Proc. Natl. Acad. Sci. USA 2020, 117, 27445–27455. [Google Scholar] [CrossRef] [PubMed]
- Shibl, A.; Ochsenkuhn, M.; Mohamed, A.; Coe, L.; Yun, Y.; Amin, S. Molecular Mechanisms of Microbiome Modulation by the Diatom Secondary Metabolite Azelaic Acid. bioRXiv 2022. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going Back to the Roots: The Microbial Ecology of the Rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Borrego, E.J.; Kolomiets, M.V. Synthesis and Functions of Jasmonates in Maize. Plants 2016, 5, 41. [Google Scholar] [CrossRef]
- Prost, I.; Dhondt, S.; Rothe, G.; Vicente, J.; Rodriguez, M.J.; Kift, N.; Carbonne, F.; Griffiths, G.; Esquerré-Tugayé, M.-T.; Rosahl, S.; et al. Evaluation of the Antimicrobial Activities of Plant Oxylipins Supports Their Involvement in Defense against Pathogens. Plant Physiol. 2005, 139, 1902–1913. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Dennis, P.G.; Badri, D.V.; Kidd, B.N.; Vivanco, J.M.; Schenk, P.M. Linking Jasmonic Acid Signaling, Root Exudates, and Rhizosphere Microbiomes. Mol. Plant Microbe Interact. 2015, 28, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Pohnert, G. Interactions of the Algicidal Bacterium Kordia algicida with Diatoms: Regulated Protease Excretion for Specific Algal Lysis. PLoS ONE 2011, 6, e21032. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Rettner, J.; Werner, M.; Werz, O.; Pohnert, G. Algal Oxylipins Mediate the Resistance of Diatoms against Algicidal Bacteria. Mar. Drugs 2018, 16, 486. [Google Scholar] [CrossRef] [PubMed]
- Di Dato, V.; Orefice, I.; Amato, A.; Fontanarosa, C.; Amoresano, A.; Cutignano, A.; Ianora, A.; Romano, G. Animal-like Prostaglandins in Marine Microalgae. ISME J. 2017, 11, 1722–1726. [Google Scholar] [CrossRef]
- Di Dato, V.; Barbarinaldi, R.; Amato, A.; Di Costanzo, F.; Fontanarosa, C.; Perna, A.; Amoresano, A.; Esposito, F.; Cutignano, A.; Ianora, A.; et al. Variation in Prostaglandin Metabolism during Growth of the Diatom Thalassiosira rotula. Sci. Rep. 2020, 10, 5374. [Google Scholar] [CrossRef] [PubMed]
- Thornton, D. Diatom Aggregation in the Sea: Mechanisms and Ecological Implications. Eur. J. Phycol. 2002, 37, 149–161. [Google Scholar] [CrossRef]
- Azam, F.; Long, R.A. Sea Snow Microcosms. Nature 2001, 414, 495, 497–498. [Google Scholar] [CrossRef]
- Arandia-Gorostidi, N.; Krabberød, A.K.; Logares, R.; Deutschmann, I.M.; Scharek, R.; Morán, X.A.G.; González, F.; Alonso-Sáez, L. Novel Interactions between Phytoplankton and Bacteria Shape Microbial Seasonal Dynamics in Coastal Ocean Waters. Front. Mar. Sci. 2022, 9, 901201. [Google Scholar] [CrossRef]
- Ortega-Retuerta, E.; Duarte, C.M.; Reche, I. Significance of Bacterial Activity for the Distribution and Dynamics of Transparent Exopolymer Particles in the Mediterranean Sea. Microb. Ecol. 2010, 59, 808–818. [Google Scholar] [CrossRef]
- Bhaskar, P.V.; Bhosle, N.B. Microbial Extracellular Polymeric Substances in Marine Biogeochemical Processes. Curr. Sci. 2005, 88, 45–53. [Google Scholar]
- Paul, C.; Reunamo, A.; Lindehoff, E.; Bergkvist, J.; Mausz, M.A.; Larsson, H.; Richter, H.; Wängberg, S.-Å.; Leskinen, P.; Båmstedt, U.; et al. Diatom Derived Polyunsaturated Aldehydes Do Not Structure the Planktonic Microbial Community in a Mesocosm Study. Mar. Drugs 2012, 10, 775–792. [Google Scholar] [CrossRef] [PubMed]
- Bartual, A.; Vicente Cera, I.; Flecha, S.; Prieto, L. Effect of Dissolved Polyunsaturated Aldehydes on the Size Distribution of Transparent Exopolymeric Particles in an Experimental Diatom Bloom. Mar. Biol. 2017, 164, 120. [Google Scholar] [CrossRef]
- Edwards, B.R.; Bidle, K.D.; Van Mooy, B.A.S. Dose-Dependent Regulation of Microbial Activity on Sinking Particles by Polyunsaturated Aldehydes: Implications for the Carbon Cycle. Proc. Natl. Acad. Sci. USA 2015, 112, 5909–5914. [Google Scholar] [CrossRef] [PubMed]
- Grossart, H.-P.; Kiørboe, T.; Tang, K.; Allagier, M.; Yam, M.; Ploug, H. Interactions between Marine Snow and Heterotrophic Bacteria: Aggregate Formation and Microbial Dynamics. Aquat. Microb. Ecol. 2005, 42, 19–26. [Google Scholar] [CrossRef]
- Grossart, H.-P.; Czub, G.; Simon, M. Algae-Bacteria Interactions and Their Effects on Aggregation and Organic Matter Flux in the Sea. Environ. Microbiol. 2006, 8, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.S.; Anil, A.C. Quantification of Diatoms in Biofilms: Standardisation of Methods. Biofouling 2005, 21, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.; Anil, A. Biofilm Diatom Community Structure: Influence of Temporal and Substratum Variability. Biofouling 2005, 21, 189–206. [Google Scholar] [CrossRef]
- Buhmann, M.T.; Schulze, B.; Förderer, A.; Schleheck, D.; Kroth, P.G. Bacteria May Induce the Secretion of Mucin-like Proteins by the Diatom Phaeodactylum tricornutum. J. Phycol. 2016, 52, 463–474. [Google Scholar] [CrossRef]
- Khan, M.J.; Singh, R.; Shewani, K.; Shukla, P.; Bhaskar, P.V.; Joshi, K.B.; Vinayak, V. Exopolysaccharides Directed Embellishment of Diatoms Triggered on Plastics and Other Marine Litter. Sci. Rep. 2020, 10, 18448. [Google Scholar] [CrossRef]
- Bohórquez, J.; McGenity, T.J.; Papaspyrou, S.; García-Robledo, E.; Corzo, A.; Underwood, G.J.C. Different Types of Diatom-Derived Extracellular Polymeric Substances Drive Changes in Heterotrophic Bacterial Communities from Intertidal Sediments. Front. Microbiol. 2017, 8, 245. [Google Scholar] [CrossRef]
- Steele, D.J.; Franklin, D.J.; Underwood, G.J.C. Protection of Cells from Salinity Stress by Extracellular Polymeric Substances in Diatom Biofilms. Biofouling 2014, 30, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, P.V.; Bhosle, N.B. Bacterial Extracellular Polymeric Substance (EPS): A Carrier of Heavy Metals in the Marine Food-Chain. Environ. Int. 2006, 32, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Mühlenbruch, M.; Grossart, H.-P.; Eigemann, F.; Voss, M. Mini-Review: Phytoplankton-Derived Polysaccharides in the Marine Environment and Their Interactions with Heterotrophic Bacteria. Environ. Microbiol. 2018, 20, 2671–2685. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, C.G.; Bahulikar, R.; Rahalkar, M.; Schink, B.; Kroth, P.G. Bacteria Associated with Benthic Diatoms from Lake Constance: Phylogeny and Influences on Diatom Growth and Secretion of Extracellular Polymeric Substances. Appl. Environ. Microbiol. 2008, 74, 7740–7749. [Google Scholar] [CrossRef] [PubMed]
- Chiovitti, A.; Bacic, A.; Burke, J.; Wetherbee, R. Heterogeneous Xylose-Rich Glycans Are Associated with Extracellular Glycoproteins from the Biofouling Diatom Craspedostauros australis (Bacillariphyceae). Eur. J. Phycol. 2003, 38, 351–360. [Google Scholar] [CrossRef]
- Staats, N.; de Winder, B.; Stal, L.J.; Mur, L.R. Isolation and Characterization of Extracellular Polysaccharides from the Epipelic Diatoms Cylindrotheca Closterium and Navicula Salinarum. Eur. J. Phycol. 1999, 34, 161–169. [Google Scholar] [CrossRef]
- Underwood, G.; Boulcott, M.; Raines, C.; Waldron, K. Environmental Effects on Exopolymer Production by Marine Benthic Diatoms: Dynamics, Changes in Composition, and Pathways of Production. J. Phycol. 2004, 40, 293–304. [Google Scholar] [CrossRef]
- Kamalanathan, M.; Chiu, M.-H.; Bacosa, H.; Schwehr, K.; Tsai, S.-M.; Doyle, S.; Yard, A.; Mapes, S.; Vasequez, C.; Bretherton, L.; et al. Role of Polysaccharides in Diatom Thalassiosira Pseudonana and Its Associated Bacteria in Hydrocarbon Presence. Plant Physiol. 2019, 180, 1898–1911. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Son, J.; Howard, L.; Yu, X.-Y. Revealing the Bacterial Quorum-Sensing Effect on the Biofilm Formation of Diatom Cylindrotheca sp. Using Multimodal Imaging. Microorganisms 2023, 11, 1841. [Google Scholar] [CrossRef]
- Bruckner, C.G.; Rehm, C.; Grossart, H.-P.; Kroth, P.G. Growth and Release of Extracellular Organic Compounds by Benthic Diatoms Depend on Interactions with Bacteria. Environ. Microbiol. 2011, 13, 1052–1063. [Google Scholar] [CrossRef]
- Yang, C.; Fang, S.; Chen, D.; Wang, J.; Liu, F.; Xia, C. The Possible Role of Bacterial Signal Molecules N-Acyl Homoserine Lactones in the Formation of Diatom-Biofilm (Cylindrotheca sp.). Mar. Pollut. Bull. 2016, 107, 118–124. [Google Scholar] [CrossRef]
- Koedooder, C.; Stock, W.; Willems, A.; Mangelinckx, S.; De Troch, M.; Vyverman, W.; Sabbe, K. Diatom-Bacteria Interactions Modulate the Composition and Productivity of Benthic Diatom Biofilms. Front. Microbiol. 2019, 10, 1255. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G. Microbial Colonization in Marine Environments: Overview of Current Knowledge and Emerging Research Topics. J. Mar. Sci. Eng. 2020, 8, 78. [Google Scholar] [CrossRef]
- Schmidt, H.; Thom, M.; Wieprecht, S.; Manz, W.; Gerbersdorf, S. The Effect of Light Intensity and Shear Stress on Microbial Biostabilization and the Community Composition of Natural Biofilms. Res. Rep. Biol. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Paul, C.; Mausz, M.A.; Pohnert, G. A Co-Culturing/Metabolomics Approach to Investigate Chemically Mediated Interactions of Planktonic Organisms Reveals Influence of Bacteria on Diatom Metabolism. Metabolomics 2012, 9, 349–359. [Google Scholar] [CrossRef]
- Fuentes, J.L.; Garbayo, I.; Cuaresma, M.; Montero, Z.; González-del-Valle, M.; Vílchez, C. Impact of Microalgae-Bacteria Interactions on the Production of Algal Biomass and Associated Compounds. Mar. Drugs 2016, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.S.; Hubbard, K.A.; Lundholm, N.; Montresor, M.; Leaw, C.P. Pseudo-nitzschia, Nitzschia, and Domoic Acid: New Research since 2011. Harmful Algae 2018, 79, 3–43. [Google Scholar] [CrossRef]
- Pan, Y.; Bates, S.S.; Cembella, A.D. Environmental Stress and Domoic Acid Production by Pseudo-nitzschia: A Physiological Perspective. Nat. Toxins 1998, 6, 127–135. [Google Scholar] [CrossRef]
- Sun, J.; Hutchins, D.A.; Feng, Y.; Seubert, E.L.; Caron, D.A.; Fu, F.-X. Effects of Changing p CO 2 and Phosphate Availability on Domoic Acid Production and Physiology of the Marine Harmful Bloom Diatom Pseudo-nitzschia multiseries. Limnol. Oceanogr. 2011, 56, 829–840. [Google Scholar] [CrossRef]
- Maldonado, M.; Hughes, M.; Rue, E.; Wells, M. The Effect of Fe and Cu on Growth and Domoic Acid Production by Pseudo-nitzschia multiseries and Pseudo-nitzschia australis. Limnol. Oceanogr. 2002, 47, 515–526. [Google Scholar] [CrossRef]
- Wells, M.; Trick, C.; Cochlan, W.; Hughes, M.; Trainer, V. Domoic Acid: The Synergy of Iron, Copper, and the Toxicity of Diatoms. Limnol. Oceanogr. 2005, 50, 1908–1917. [Google Scholar] [CrossRef]
- Bates, S.S.; Douglas, D.J.; Doucette, G.J.; Léger, C. Enhancement of Domoic Acid Production by Reintroducing Bacteria to Axenic Cultures of the Diatom Pseudo-nitzschia multiseries. Nat. Toxins 1995, 3, 428–435. [Google Scholar] [CrossRef]
- Stewart, J. Bacterial Involvement in Determining Domoic Acid Levels in Pseudo-nitzschia multiseries Cultures. Aquat. Microb. Ecol. 2008, 50, 135–144. [Google Scholar] [CrossRef]
- Lelong, A.; Hégaret, H.; Soudant, P. Link between Domoic Acid Production and Cell Physiology after Exchange of Bacterial Communities between Toxic Pseudo-nitzschia multiseries and Non-Toxic Pseudo-nitzschia delicatissima. Mar. Drugs 2014, 12, 3587–3607. [Google Scholar] [CrossRef] [PubMed]
- Bates, S. Ecophysiology and Metabolism of ASP Toxin Production. NATO ASI Ser. G Ecol. Sci. 1998, 41, 405–426. [Google Scholar]
- Kobayashi, K.; Takata, Y.; Kodama, M. Direct Contact between Pseudo-nitzschia multiseries and Bacteria Is Necessary for the Diatom to Produce a High Level of Domoic Acid. Fish. Sci. 2009, 75, 771–776. [Google Scholar] [CrossRef]
- Guannel, M.; Horner-Devine, M.C.; Rocap, G. Bacterial Community Composition Differs with Species and Toxigenicity of the Diatom Pseudo-nitzschia. Aquat. Microb. Ecol. 2011, 64, 117–133. [Google Scholar] [CrossRef]
- Suleiman, M.; Zecher, K.; Yücel, O.; Jagmann, N.; Philipp, B. Interkingdom Cross-Feeding of Ammonium from Marine Methylamine-Degrading Bacteria to the Diatom Phaeodactylum tricornutum. Appl. Environ. Microbiol. 2016, 82, 7113–7122. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Mauri, M.; Vallet, M.; Staudinger, M.; Allen, R.J.; Pohnert, G. Dynamic Diatom-Bacteria Consortia in Synthetic Plankton Communities. Appl. Environ. Microbiol. 2022, 88, e01619-22. [Google Scholar] [CrossRef]
- Diner, R.E.; Schwenck, S.M.; McCrow, J.P.; Zheng, H.; Allen, A.E. Genetic Manipulation of Competition for Nitrate between Heterotrophic Bacteria and Diatoms. Front. Microbiol. 2016, 7, 880. [Google Scholar] [CrossRef]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae Acquire Vitamin B12 through a Symbiotic Relationship with Bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Bruns, S.; Wilkes, H.; Simon, M.; Wienhausen, G. Vitamin B12 Is Not Shared by All Marine Prototrophic Bacteria with Their Environment. ISME J. 2023, 17, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Durham, B.P.; Sharma, S.; Luo, H.; Smith, C.B.; Amin, S.A.; Bender, S.J.; Dearth, S.P.; Van Mooy, B.A.S.; Campagna, S.R.; Kujawinski, E.B.; et al. Cryptic Carbon and Sulfur Cycling between Surface Ocean Plankton. Proc. Natl. Acad. Sci. USA 2015, 112, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Durham, B.P.; Dearth, S.P.; Sharma, S.; Amin, S.A.; Smith, C.B.; Campagna, S.R.; Armbrust, E.V.; Moran, M.A. Recognition Cascade and Metabolite Transfer in a Marine Bacteria-Phytoplankton Model System. Environ. Microbiol. 2017, 19, 3500–3513. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, E.M.; McCrow, J.P.; Moustafa, A.; Zheng, H.; McQuaid, J.B.; Delmont, T.O.; Post, A.F.; Sipler, R.E.; Spackeen, J.L.; Xu, K.; et al. Phytoplankton–Bacterial Interactions Mediate Micronutrient Colimitation at the Coastal Antarctic Sea Ice Edge. Proc. Natl. Acad. Sci. USA 2015, 112, 9938–9943. [Google Scholar] [CrossRef]
- Andrew, S.; Wilson, T.; Smith, S.; Marchetti, A.; Septer, A.N. A Tripartite Model System for Southern Ocean Diatom-Bacterial Interactions Reveals the Coexistence of Competing Symbiotic Strategies. ISME Commun. 2022, 2, 97. [Google Scholar] [CrossRef]
- Foster, R.A.; Kuypers, M.M.M.; Vagner, T.; Paerl, R.W.; Musat, N.; Zehr, J.P. Nitrogen Fixation and Transfer in Open Ocean Diatom–Cyanobacterial Symbioses. ISME J. 2011, 5, 1484–1493. [Google Scholar] [CrossRef]
- Villareal, T.A. Marine Nitrogen-Fixing Diatom-Cyanobacteria Symbioses. In Marine Pelagic Cyanobacteria: Trichodesmium and Other Diazotrophs; Carpenter, E.J., Capone, D.G., Rueter, J.G., Eds.; NATO ASI Series; Springer Netherlands: Dordrecht, The Netherlands, 1992; pp. 163–175. ISBN 978-94-015-7977-3. [Google Scholar]
- Foster, R.; Goebel, N.; Zehr, J. Isolation of Calothrix rhizosoleniae (Cyanobacteria) Strain SC01 from Chaetoceros (Bacillariophyta) spp. Diatoms of the Subtropical North Pacific Ocean. J. Phycol. 2010, 46, 1028–1037. [Google Scholar] [CrossRef]
- Zecher, K.; Hayes, K.R.; Philipp, B. Evidence of Interdomain Ammonium Cross-Feeding from Methylamine- and Glycine Betaine-Degrading Rhodobacteraceae to Diatoms as a Widespread Interaction in the Marine Phycosphere. Front. Microbiol. 2020, 11, 533894. [Google Scholar] [CrossRef]
- Amin, S.A.; Hmelo, L.R.; van Tol, H.M.; Durham, B.P.; Carlson, L.T.; Heal, K.R.; Morales, R.L.; Berthiaume, C.T.; Parker, M.S.; Djunaedi, B.; et al. Interaction and Signalling between a Cosmopolitan Phytoplankton and Associated Bacteria. Nature 2015, 522, 98–101. [Google Scholar] [CrossRef]
- Seymour, J.R.; Simó, R.; Ahmed, T.; Stocker, R. Chemoattraction to Dimethylsulfoniopropionate throughout the Marine Microbial Food Web. Science 2010, 329, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.A.; Reisch, C.R.; Kiene, R.P.; Whitman, W.B. Genomic Insights into Bacterial DMSP Transformations. Ann. Rev. Mar. Sci. 2012, 4, 523–542. [Google Scholar] [CrossRef] [PubMed]
- Vuong, T.T.; Kwon, B.-R.; Eom, J.-I.; Shin, B.-K.; Kim, S.M. Interaction between Marine Bacterium Stappia sp. K01 and Diatom Phaeodactylum tricornutum through Extracellular Fatty Acids. J. Appl. Phycol. 2020, 32, 71–82. [Google Scholar] [CrossRef]
- Heal, K.R.; Qin, W.; Ribalet, F.; Bertagnolli, A.D.; Coyote-Maestas, W.; Hmelo, L.R.; Moffett, J.W.; Devol, A.H.; Armbrust, E.V.; Stahl, D.A.; et al. Two Distinct Pools of B12 Analogs Reveal Community Interdependencies in the Ocean. Proc. Natl. Acad. Sci. USA 2017, 114, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Sittmann, J.; Bae, M.; Mevers, E.; Li, M.; Quinn, A.; Sriram, G.; Clardy, J.; Liu, Z. Bacterial Diketopiperazines Stimulate Diatom Growth and Lipid Accumulation. Plant Physiol. 2021, 186, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Jauffrais, T.; Agogué, H.; Gemin, M.-P.; Beaugeard, L.; Martin-Jézéquel, V. Effect of Bacteria on Growth and Biochemical Composition of Two Benthic Diatoms Halamphora coffeaeformis and Entomoneis paludosa. J. Exp. Mar. Biol. Ecol. 2017, 495, 65–74. [Google Scholar] [CrossRef]
- Kimura, K.; Tomaru, Y. Coculture with Marine Bacteria Confers Resistance to Complete Viral Lysis of Diatom Cultures. Aquat. Microb. Ecol. 2014, 73, 69–80. [Google Scholar] [CrossRef]
- Fenizia, S.; Thume, K.; Wirgenings, M.; Pohnert, G. Ectoine from Bacterial and Algal Origin Is a Compatible Solute in Microalgae. Mar. Drugs 2020, 18, 42. [Google Scholar] [CrossRef]
- Sison-Mangus, M.P.; Kempnich, M.W.; Appiano, M.; Mehic, S.; Yazzie, T. Specific Bacterial Microbiome Enhances the Sexual Reproduction and Auxospore Production of the Marine Diatom, Odontella. PLoS ONE 2022, 17, e0276305. [Google Scholar] [CrossRef]
- Di Costanzo, F.; Di Dato, V.; van Zyl, L.J.; Cutignano, A.; Esposito, F.; Trindade, M.; Romano, G. Three Novel Bacteria Associated with Two Centric Diatom Species from the Mediterranean Sea, Thalassiosira rotula and Skeletonema marinoi. Int. J. Mol. Sci. 2021, 22, 13199. [Google Scholar] [CrossRef]
- Bartolek, Z.; van Creveld, S.G.; Coesel, S.; Cain, K.R.; Schatz, M.; Morales, R.; Virginia Armbrust, E. Flavobacterial Exudates Disrupt Cell Cycle Progression and Metabolism of the Diatom Thalassiosira pseudonana. ISME J. 2022, 16, 2741–2751. [Google Scholar] [CrossRef] [PubMed]
- Cirri, E.; Vyverman, W.; Pohnert, G. Biofilm Interactions—Bacteria Modulate Sexual Reproduction Success of the Diatom Seminavis robusta. FEMS Microbiol. Ecol. 2018, 94, fiy161. [Google Scholar] [CrossRef] [PubMed]
- Stock, F.; Syrpas, M.; Graff van Creveld, S.; Backx, S.; Blommaert, L.; Dow, L.; Stock, W.; Ruysbergh, E.; Lepetit, B.; Bailleul, B.; et al. N-Acyl Homoserine Lactone Derived Tetramic Acids Impair Photosynthesis in Phaeodactylum tricornutum. ACS Chem. Biol. 2019, 14, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Dow, L.; Stock, F.; Peltekis, A.; Szamosvári, D.; Prothiwa, M.; Lapointe, A.; Böttcher, T.; Bailleul, B.; Vyverman, W.; Kroth, P.G.; et al. The Multifaceted Inhibitory Effects of an Alkylquinolone on the Diatom Phaeodactylum tricornutum. Chembiochem 2020, 21, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Long, R.A.; Qureshi, A.; Faulkner, D.J.; Azam, F. 2-n-Pentyl-4-Quinolinol Produced by a Marine Alteromonas sp. and Its Potential Ecological and Biogeochemical Roles. Appl. Environ. Microbiol. 2003, 69, 568–576. [Google Scholar] [CrossRef]
- Wigglesworth-Cooksey, B.; Cooksey, K.E.; Long, R. Antibiotic from the Marine Environment with Antimicrobial Fouling Activity. Environ. Toxicol. 2007, 22, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Syrpas, M.; Ruysbergh, E.; Blommaert, L.; Vanelslander, B.; Sabbe, K.; Vyverman, W.; De Kimpe, N.; Mangelinckx, S. Haloperoxidase Mediated Quorum Quenching by Nitzschia Cf Pellucida: Study of the Metabolization of n-Acyl Homoserine Lactones by a Benthic Diatom. Mar. Drugs 2014, 12, 352–367. [Google Scholar] [CrossRef]
- Bigalke, A.; Meyer, N.; Papanikolopoulou, L.A.; Wiltshire, K.H.; Pohnert, G. The Algicidal Bacterium Kordia algicida Shapes a Natural Plankton Community. Appl. Environ. Microbiol. 2019, 85, e02779-18. [Google Scholar] [CrossRef]
- Meyer, N.; Bigalke, A.; Kaulfuß, A.; Pohnert, G. Strategies and Ecological Roles of Algicidal Bacteria. FEMS Microbiol. Rev. 2017, 41, 880–899. [Google Scholar] [CrossRef]
- Sohn, J.H.; Lee, J.-H.; Yi, H.; Chun, J.; Bae, K.S.; Ahn, T.-Y.; Kim, S.-J. Kordia algicida Gen. Nov., sp. Nov., an Algicidal Bacterium Isolated from Red Tide. Int. J. Syst. Evol. Microbiol. 2004, 54, 675–680. [Google Scholar] [CrossRef]
- Lee, S.; Kato, J.; Takiguchi, N.; Kuroda, A.; Ikeda, T.; Mitsutani, A.; Ohtake, H. Involvement of an Extracellular Protease in Algicidal Activity of the Marine Bacterium Pseudoalteromonas sp. Strain A28. Appl. Environ. Microbiol. 2000, 66, 4334–4339. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Yoshikawa, T.; Nishitarumizu, S. Algicidal Activity and Identification of an Algicidal Substance Produced by Marine Pseudomonas sp. C55a-2. Fish. Sci. 2011, 77, 397–402. [Google Scholar] [CrossRef]
- Li, Y.; Lei, X.; Zhu, H.; Zhang, H.; Guan, C.; Chen, Z.; Zheng, W.; Fu, L.; Zheng, T. Chitinase Producing Bacteria with Direct Algicidal Activity on Marine Diatoms. Sci. Rep. 2016, 6, 21984. [Google Scholar] [CrossRef] [PubMed]
- Imai, I.; Ishida, Y.; Hata, Y. Killing of Marine Phytoplankton by a Gliding Bacterium Cytophaga sp., Isolated from the Coastal Sea of Japan. Mar. Biol. 1993, 116, 527–532. [Google Scholar] [CrossRef]
- Furusawa, G.; Yoshikawa, T.; Yasuda, A.; Sakata, T. Algicidal Activity and Gliding Motility of Saprospira sp. SS98-5. Can. J. Microbiol. 2003, 49, 92–100. [Google Scholar] [CrossRef]
- Molina-Cárdenas, C.A.; del Pilar Sánchez-Saavedra, M. Inhibitory Effect of Benthic Diatom Species on Three Aquaculture Pathogenic Vibrios. Algal. Res. 2017, 27, 131–139. [Google Scholar] [CrossRef]
- Desbois, A.P.; Mearns-Spragg, A.; Smith, V.J. A Fatty Acid from the Diatom Phaeodactylum tricornutum Is Antibacterial against Diverse Bacteria Including Multi-Resistant Staphylococcus aureus (MRSA). Mar. Biotechnol. 2009, 11, 45–52. [Google Scholar] [CrossRef]
- Desbois, A.P.; Lebl, T.; Yan, L.; Smith, V.J. Isolation and Structural Characterisation of Two Antibacterial Free Fatty Acids from the Marine Diatom, Phaeodactylum tricornutum. Appl. Microbiol. Biotechnol. 2008, 81, 755–764. [Google Scholar] [CrossRef]
- Desbois, A.; Walton, M.; Smith, V. Differential Antibacterial Activities of Fusiform and Oval Morphotypes of Phaeodactylum tricornutum (Bacillariophyceae). J. Mar. Biol. Assoc. UK 2010, 90, 769–774. [Google Scholar] [CrossRef]
- Ribalet, F.; Intertaglia, L.; Lebaron, P.; Casotti, R. Differential Effect of Three Polyunsaturated Aldehydes on Marine Bacterial Isolates. Aquat. Toxicol. 2008, 86, 249–255. [Google Scholar] [CrossRef]
- Balestra, C.; Alonso-Sáez, L.; Gasol, J.; Casotti, R. Group-Specific Effects on Coastal Bacterioplankton of Polyunsaturated Aldehydes Produced by Diatoms. Aquat. Microb. Ecol. 2011, 63, 123–131. [Google Scholar] [CrossRef]
- Paul, C.; Pohnert, G. Induction of Protease Release of the Resistant Diatom Chaetoceros didymus in Response to Lytic Enzymes from an Algicidal Bacterium. PLoS ONE 2013, 8, e57577. [Google Scholar] [CrossRef] [PubMed]
- Sushanth, V.; Rajashekhar, M. Antioxidant and Antimicrobial Activities in the Four Species of Marine Microalgae Isolated from Arabian Sea of Karnataka Coast. Indian J. Geo-Mar. Sci. 2015, 44, 69–75. [Google Scholar]
- Qin, J.G.; D’Antignana, T.; Zhang, W.; Franco, C. Discovery of Antimicrobial Activities of a Marine Diatom Thalassiosira rotula. Afr. J. Microbiol. Res. 2013, 7, 5687–5696. [Google Scholar] [CrossRef]
- Majzoub, M.E.; Beyersmann, P.G.; Simon, M.; Thomas, T.; Brinkhoff, T.; Egan, S. Phaeobacter inhibens Controls Bacterial Community Assembly on a Marine Diatom. FEMS Microbiol. Ecol. 2019, 95, fiz060. [Google Scholar] [CrossRef] [PubMed]
- Ajani, P.A.; Kahlke, T.; Siboni, N.; Carney, R.; Murray, S.A.; Seymour, J.R. The Microbiome of the Cosmopolitan Diatom Leptocylindrus Reveals Significant Spatial and Temporal Variability. Front. Microbiol. 2018, 9, 2758. [Google Scholar] [CrossRef]
- Le Reun, N.; Bramucci, A.; O’Brien, J.; Ostrowski, M.; Brown, M.V.; Van de Kamp, J.; Bodrossy, L.; Raina, J.-B.; Ajani, P.; Seymour, J. Diatom Biogeography, Temporal Dynamics, and Links to Bacterioplankton across Seven Oceanographic Time-Series Sites Spanning the Australian Continent. Microorganisms 2022, 10, 338. [Google Scholar] [CrossRef]
- Stock, W.; Willems, A.; Mangelinckx, S.; Vyverman, W.; Sabbe, K. Selection Constrains Lottery Assembly in the Microbiomes of Closely Related Diatom Species. ISME Commun. 2022, 2, 11. [Google Scholar] [CrossRef]
- Landa, M.; Blain, S.; Christaki, U.; Monchy, S.; Obernosterer, I. Shifts in Bacterial Community Composition Associated with Increased Carbon Cycling in a Mosaic of Phytoplankton Blooms. ISME J. 2016, 10, 39–50. [Google Scholar] [CrossRef]
- Brisson, V.; Swink, C.; Kimbrel, J.; Mayali, X.; Samo, T.; Kosina, S.M.; Thelen, M.; Northen, T.R.; Stuart, R.K. Dynamic Phaeodactylum tricornutum Exometabolites Shape Surrounding Bacterial Communities. New Phytol. 2023, 239, 1420–1433. [Google Scholar] [CrossRef]
- Steinrücken, P.; Jackson, S.; Müller, O.; Puntervoll, P.; Kleinegris, D.M.M. A Closer Look into the Microbiome of Microalgal Cultures. Front. Microbiol. 2023, 14, 1108018. [Google Scholar] [CrossRef] [PubMed]
- Barreto Filho, M.M.; Walker, M.; Ashworth, M.P.; Morris, J.J. Structure and Long-Term Stability of the Microbiome in Diverse Diatom Cultures. Microbiol. Spectr. 2021, 9, e00269-21. [Google Scholar] [CrossRef] [PubMed]
- Crenn, K.; Duffieux, D.; Jeanthon, C. Bacterial Epibiotic Communities of Ubiquitous and Abundant Marine Diatoms Are Distinct in Short- and Long-Term Associations. Front. Microbiol. 2018, 9, 2879. [Google Scholar] [CrossRef]
- Ahern, O. Marine Microbial Interactions: Diversity and Function of the Thalassiosira rotula Phycosphere Community; University of Rhode Island: Kingston, RI, USA, 2021. [Google Scholar] [CrossRef]
- Baker, L.J.; Kemp, P.F. Bacterial Inoculations Can Perturb the Growth Trajectory of Diatoms with an Existing Microbiome. PeerJ 2020, 8, e8352. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lim, K.M.K.; Chng, K.R.; Nagarajan, N. Predicting Microbial Interactions through Computational Approaches. Methods 2016, 102, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Song, L. A Multiomics Approach to Study the Microbiome Response to Phytoplankton Blooms. Appl. Microbiol. Biotechnol. 2017, 101, 4863–4870. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Le Roux, X.; Niklaus, P.A.; Van Bodegom, P.M.; Lennon, J.T.; Bertilsson, S.; Grossart, H.-P.; Philippot, L.; Bodelier, P.L.E. Trait-Based Approaches for Understanding Microbial Biodiversity and Ecosystem Functioning. Front. Microbiol. 2014, 5, 251. [Google Scholar] [CrossRef]
- Zoccarato, L.; Sher, D.; Miki, T.; Segrè, D.; Grossart, H.-P. A Comparative Whole-Genome Approach Identifies Bacterial Traits for Marine Microbial Interactions. Commun. Biol. 2022, 5, 276. [Google Scholar] [CrossRef]
- Clark, C.M.; Costa, M.S.; Conley, E.; Li, E.; Sanchez, L.M.; Murphy, B.T. Using the Open-Source MALDI TOF-MS IDBac Pipeline for Analysis of Microbial Protein and Specialized Metabolite Data. J. Vis. Exp. 2019, 147, e59219. [Google Scholar] [CrossRef]
- Baker, L.J.; Kemp, P.F. Exploring Bacteria-Diatom Associations Using Single-Cell Whole Genome Amplification. Aquat. Microb. Ecol. 2014, 72, 73–88. [Google Scholar] [CrossRef]
- Pruesse, E.; Peplies, J.; Glöckner, F. SINA: Accurate High-Throughput Multiple Sequence Alignment of Ribosomal RNA Genes. Bioinformatics 2012, 28, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, W.; Strunk, O.; Westram, R.; Richter, L.; Meier, H.; Yadhukumar; Buchner, A.; Lai, T.; Steppi, S.; Jobb, G.; et al. ARB: A Software Environment for Sequence Data. Nucleic. Acids. Res. 2004, 32, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.; Westcott, S.; Ryabin, T.; Hall, J.; Hartmann, M.; Hollister, E.; Lesniewski, R.; Oakley, B.; Parks, D.; Robinson, C.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A Fast, Scalable and User-Friendly Tool for Maximum Likelihood Phylogenetic Inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.; Vázquez-Baeza, Y.; Koslicki, D.; McClelland, J.; Reeve, N.; Xu, Z.; Gonzalez, A.; Knight, R. Striped UniFrac: Enabling Microbiome Analysis at Unprecedented Scale. Nat. Methods 2018, 15, 847–848. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid Annotations Using Subsystems Technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Skinnider, M.A.; Merwin, N.J.; Johnston, C.W.; Magarvey, N.A. PRISM 3: Expanded Prediction of Natural Product Chemical Structures from Microbial Genomes. Nucleic. Acids. Res. 2017, 45, W49–W54. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and Improved Predictions for Detection, Regulation, Chemical Structures and Visualisation. Nucleic. Acids. Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef]
- Yao, S.; Lyu, S.; An, Y.; Lu, J.; Gjermansen, C.; Schramm, A. Microalgae-Bacteria Symbiosis in Microalgal Growth and Biofuel Production: A Review. J. Appl. Microbiol. 2019, 126, 359–368. [Google Scholar] [CrossRef]
- Labeeuw, L.; Bramucci, A.; Case, R. Bioactive Small Molecules Mediate Microalgal-Bacterial Interactions. In Systems Biology of Marine Ecosystems; Springer: Berlin/Heidelberg, Germany, 2017; pp. 279–300. ISBN 978-3-319-62092-3. [Google Scholar]
- Liu, J.; Wu, Y.; Wu, C.; Muylaert, K.; Vyverman, W.; Yu, H.-Q.; Muñoz, R.; Rittmann, B. Advanced Nutrient Removal from Surface Water by a Consortium of Attached Microalgae and Bacteria: A Review. Bioresour. Technol. 2017, 241, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Higa, L.; Barela, A.; Lee, C.; Chen, Y.; Du, Z.-Y. Microalgal Consortia for Waste Treatment and Valuable Bioproducts. Energies 2023, 16, 884. [Google Scholar] [CrossRef]
- Kahla, O.; Garali, S.; Karray, F.; Ben Abdallah, M.; Kallel, N.; Mhiri, N.; Zaghden, H.; Barhoumi, B.; Pringault, O.; Quéméneur, M.; et al. Efficiency of Benthic Diatom-Associated Bacteria in the Removal of Benzo(a)Pyrene and Fluoranthene. Sci. Total Environ. 2021, 751, 141399. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, B.; Zhang, J.; Lei, X.; Zhang, H.; Li, Y.; Yang, L.; Zheng, W.; Tian, Y.; Boughner, L.A.; et al. A Lytic Bacterium’s Potential Application in Biofuel Production through Directly Lysing the Diatom Phaeodactylum tricornutum Cell. Algal. Res. 2015, 12, 197–205. [Google Scholar] [CrossRef]
- Chorazyczewski, A.M.; Huang, I.-S.; Abdulla, H.; Mayali, X.; Zimba, P.V. The Influence of Bacteria on the Growth, Lipid Production, and Extracellular Metabolite Accumulation by Phaeodactylum tricornutum (Bacillariophyceae). J. Phycol. 2021, 57, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Mata, M.T.; Luza, M.F.; Riquelme, C.E. Production of Diatom–Bacteria Biofilm Isolated from Seriola lalandi Cultures for Aquaculture Application. Aquac. Res. 2017, 48, 4308–4320. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Cui, F.; Lens, P.N.L.; Tay, J.H. Microalgal-Bacterial Consortia: From Interspecies Interactions to Biotechnological Applications. Renew. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
- Fouilland, E.; Galès, A.; Beaugelin, I.; Lanouguère, E.; Pringault, O.; Leboulanger, C. Influence of Bacteria on the Response of Microalgae to Contaminant Mixtures. Chemosphere 2018, 211, 449–455. [Google Scholar] [CrossRef]
- Malik, S.; Khan, F.; Atta, Z.; Habib, N.; Haider, M.N.; Wang, N.; Alam, A.; Jambi, E.J.; Gull, M.; Mehmood, M.A.; et al. Microalgal Flocculation: Global Research Progress and Prospects for Algal Biorefinery. Biotechnol. Appl. Biochem. 2020, 67, 52–60. [Google Scholar] [CrossRef]
- Hu, Y.; Xiao, Y.; Liao, K.; Leng, Y.; Lu, Q. Development of Microalgal Biofilm for Wastewater Remediation: From Mechanism to Practical Application. J. Chem. Tech. Biotechnol. 2021, 96, 2993–3008. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating Micro-Algae into Wastewater Treatment: A Review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef] [PubMed]
- Coyne, K.J.; Wang, Y.; Johnson, G. Algicidal Bacteria: A Review of Current Knowledge and Applications to Control Harmful Algal Blooms. Front. Microbiol. 2022, 13, 871177. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pohnert, G.; Wei, D. Extracellular Metabolites from Industrial Microalgae and Their Biotechnological Potential. Mar. Drugs 2016, 14, 191. [Google Scholar] [CrossRef] [PubMed]
| Bacterial Species | Diatom Species | Compounds Involved | Mutual Behoof | References | |
|---|---|---|---|---|---|
| Bacteria | Diatom | ||||
| Ruegeria pomeroyi | Thalassiosira pseudonana | Vitamin B12 | DHPS | nutrients exchange | [85,86] |
| Oceanospirillaceae ASP10-02a | phytoplankton communities | Vitamin B12 | Organic carbon | nutrients exchange | [87] |
| Sulfitobacter sp. SA1 | Pseudo-nitzschia subcurvata | Biotin, thiamine, and vitamin B12 | Organic carbon | nutrients exchange | [88] |
| Richelia intracellularis | Rhizosolenia and Hemiaulus | Fixed nitrogen | Amino acids and organic carbon | nutrients exchange | [89,90,91] |
| Calothrix rhizosoleniae | Chaetoceros spp. | Fixed nitrogen | Amino acids and organic carbon | nutrients exchange | [89,90,91] |
| KarMa strain of Donghicola sp. | Phaeodactylum tricornutum, Amphora coffeaeformis and Thalassiosira pseudonana | Ammonium | Organic carbon | Nutrient exchange based on the bacterial metabolization of environmental MMA | [80,92] |
| Rhodobacteraceae | Phaeodactylum tricornutum, Amphora coffeaeformis and Thalassiosira pseudonana | Ammonium | Organic carbon | Nutrient exchange based on the bacterial metabolization of MAs and GBT | [80,92] |
| Sulfitobacter, strain SA11 | Pseudo-nitzschia multiseries | Ammonium | Organic carbon and DMSP | Nutrient exchanges based on IAA production by bacteria | [93,94,95] |
| Stappia sp. K01 | Phaeodactylum tricornutum | NA | FAs | Nutrient exchange | [96] |
| Bacterial Species | Diatom Species | Bacterial Compounds | Diatom Behoof | References |
|---|---|---|---|---|
| Dinoroseobacter shibae | Thalassiosira pseudonana | NA | increase in cell abundance and metabolic activity | [67] |
| Bacillus thuringiensis | Phaeodactylum tricornutum | DKPs | increase in growth and lipid content | [98] |
| Associated microbiome | Halamphora coffeaeformis and Entomoneis paludosa | NA | increased growth rates and cellular division but lower cellular amount of proteins, lipids and carbon | [99] |
| Nautella sp., Sulfitobacter sp., and Polaribacter sp. | Chaetoceros tenuissimum | NA | protection against the infection of the RNA virus CtenRNAV | [100] |
| Associated microbiome | Thalassiosira weissflogii/Thalassiosira rotula | Ectoine | survival to osmotic stress | [101,103] |
| Polaribacter sp. and Cellulophaga sp. | Odontella sp. | NA | increase in sexual cells and auxospores | [102] |
| Bacterial Species | Diatom Species | Bacterial Compounds | Effects on Diatoms | References |
|---|---|---|---|---|
| Croceibacter atlanticus | Pseudo-nitzschia multistriata | NA | induction of DNA fragmentation | [21] |
| Methylophaga | phytoplankton communities | NA | competition for vitamin B12 | [87] |
| Olleya sp. A30 | Pseudo-nitzschia subcurvata | NA | growth impairment | [88] |
| Croceibacter atlanticus | Thalassiosira pseudonana | extracellular metabolites | inhibition of cell division, alteration of cell morphology, increase in organic matter release | [104] |
| Maribacter sp. and Marinobacter sp. | Seminavis robusta | NA | negative influence on sexual reproduction rate by affecting diproline production | [9,105] |
| marine Proteobacteria | Phaeodactylum tricornutum | OXO12 and TA12 | inhibition of growth | [106] |
| Pseudoalteromonas sp. and Alteromonas sp. | Phaeodactylum tricornutum | HHQ | Growth impairment by inhibition of photosynthetic electron transport and respiration | [107] |
| Thalassiosira weissflogii and Cylindrotheca fusiformis | PHQ | inhibition of growth | [108] | |
| Amphora coffeaeformis, Navicula sp., and Auricula sp. | PQ | inhibition of motility | [109] |
| Bacterial Species | Diatom Species | Bacterial Mode of Action | Effects on Diatoms | References |
|---|---|---|---|---|
| Kordia algicida | Skeletonema costatum, Thalassiosira weissflogii, Chaetoceros socialis, and Phaeodactylum tricornutum | Release of diffusible proteases | death | [34,111,113] |
| Pseudoalteromonas sp. | Chaetoceros didymus, Thalassiosira sp., and Eucampia zodiacs | Release of diffusible factors with protease and DNase activities | death | [114] |
| Pseudomonas, C55a-2 strain | Chaetoceros ceratosporum | Release of 2,3-indolinedione | death | [115] |
| Chitiniomonas prasina, LY03 strain | Thalassiosira pseudonana | Release of chitinase | death | [116] |
| Cytophaga sp. J18/M01 | Ditylum brightwellii, Chaetoceros didymus, Skeletonema costatum, and Thalassiosira sp. | direct contact with diatoms | death | [117] |
| Saprospira sp., SS98-5 strain | Chaetoceros ceratosporum | direct contact with diatoms | induction of aggregation and cells lysis | [118] |
| Bacterial Species | Diatom Species | Diatoms Compounds | Effects on Bacteria | References |
|---|---|---|---|---|
| Kordia algicida | Chaetoceros didymus | 15-HEPE | inhibition of growth | [35] |
| Vibrio alginolyticus, V. campbellii, and V. harveyi | Nitzschia laevis, two Nitzschia frustulum strains, Navicula incerta, Navicula cf. incerta, and Navicula biskanterae | NA | inhibition of growth | [119] |
| marine and not-marine gram-positive and negative bacteria | Phaeodactylum tricornutum | EPA, PA, and HTA | death | [120,121,122] |
| Software | Description | Website | References |
|---|---|---|---|
| IDBac | A MALDI Protein and Small Molecule Bioinformatics Platform | https://chasemc.github.io/IDBac/ | [143] |
| Geneious® | Comprehensive suite of molecular biology and sequence analysis tools | https://www.geneious.com/ | [144] |
| SILVA INcremental Aligner tool (SINA) | Web tool for multiple sequence alignment (MSA) specifically designed for the multiple alignment of ribosomal RNA genes (rRNA). SINA is also able to taxonomically classify the sequences. | https://bioinformaticshome.com/tools/msa/descriptions/SINA.html#gsc.tab=0 | [145] |
| ARB | Graphically oriented package comprising various tools for sequence database handling and data analysis | http://www.arb-home.de/ | [146] |
| Mothur | Single piece of open-source, expandable software to fill the bioinformatics needs of the microbial ecology community | https://mothur.org/ | [147] |
| NodeXL | Makes it easy to explore, analyse, and visualize network graphs in Microsoft Office Excel | https://nodexl.com/ | NA |
| RAxML | Popular maximum likelihood (ML) tree inference tool | https://raxml-ng.vital-it.ch/#/ | [148] |
| UniFrac | Repository for high-performance phylogenetic diversity calculations repository for high-performance phylogenetic diversity calculations | https://pypi.org/project/unifrac/ | [149] |
| Prokka | Software tool to annotate bacterial, archaeal and viral genomes quickly and produce standards-compliant output files | https://github.com/tseemann/prokka | [150] |
| RAST | Rapid Annotation using Subsystem Technology is a fully-automated service for annotating complete or nearly complete bacterial and archaeal genomes. It provides high quality genome annotations for these genomes across the whole phylogenetic tree. | https://rast.nmpdr.org/ | [151] |
| PRISM | Combinatorial approach to chemical structure prediction for genetically encoded non-ribosomal peptides and type I and II polyketides | http://magarveylab.ca/prism/ | [152] |
| antiSMASH | Tool for rapid genome-wide identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genomes. It integrates and cross-links with a large number of in silico secondary metabolite analysis tools that have been published earlier. | https://antismash.secondarymetabolites.org/#!/start | [153] |
| Potential Biotechnological Applications | Diatom | Bacteria | Reference |
|---|---|---|---|
| Increase in biomass yield | Thalassiosira pseudonana | Dinoroseobacter shibae | [67] |
| Increase in pigments and biomass production | Phaeodactylum tricornutum | Stappia sp. K01 | [96] |
| Increase in biomass yield | Odontella sp. | associated bacteria | [102] |
| Possible exploitation of NRPS/T1PKS, ectoine, bacteriocins, terpenes, and putative β-lactamase/cephalosporinases genes present in diatom-associated bacteria | Thalassiosira rotula and Skeletonema marinoi | Cluster 1, 2 and 8 bacteria | [103] |
| Increase in diatom-produced proteases by algicidal bacteria | Chaetoceros didymus | Kordia algicida | [125] |
| Bioremediation of PAHs in wastewater treatment | Nitzschia sp. | Marivita, Erythrobacter, and Alcaligenes | [158] |
| Diatoms disruption for biofuel production | Phaeodactylum tricornutum | Labrenzia sp. KD531 | [159] |
| Increase in biomass and lipid yield | Phaeodactylum tricornutum | Marinobacter sp. | [160] |
| Increase in Seriola lalandi larval growth and viability in aquaculture | Navicula phyllepta | bacteria from the Rhodobacteraceae family | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Costanzo, F.; Di Dato, V.; Romano, G. Diatom–Bacteria Interactions in the Marine Environment: Complexity, Heterogeneity, and Potential for Biotechnological Applications. Microorganisms 2023, 11, 2967. https://doi.org/10.3390/microorganisms11122967
Di Costanzo F, Di Dato V, Romano G. Diatom–Bacteria Interactions in the Marine Environment: Complexity, Heterogeneity, and Potential for Biotechnological Applications. Microorganisms. 2023; 11(12):2967. https://doi.org/10.3390/microorganisms11122967
Chicago/Turabian StyleDi Costanzo, Federica, Valeria Di Dato, and Giovanna Romano. 2023. "Diatom–Bacteria Interactions in the Marine Environment: Complexity, Heterogeneity, and Potential for Biotechnological Applications" Microorganisms 11, no. 12: 2967. https://doi.org/10.3390/microorganisms11122967
APA StyleDi Costanzo, F., Di Dato, V., & Romano, G. (2023). Diatom–Bacteria Interactions in the Marine Environment: Complexity, Heterogeneity, and Potential for Biotechnological Applications. Microorganisms, 11(12), 2967. https://doi.org/10.3390/microorganisms11122967








