Mathematical Modeling of the Lethal Synergism of Coinfecting Pathogens in Respiratory Viral Infections: A Review
Abstract
1. Introduction
2. Virus–Bacteria Coinfections: Immune Dysregulation and Mathematical Models
2.1. Changes and Dysregulation of the Immune Response after Virus–Bacteria Coinfection
2.1.1. Dysregulation of Cytokine Responses
2.1.2. Changes to Effector Cells’ Activation and Function
2.1.3. Effect of Damage to the Epithelium
2.1.4. Effect of Timing and Sequence of Inocula
2.2. Mathematical Modeling of Dynamics of Respiratory Virus–Bacteria Coinfection
2.2.1. Within-Host Ordinary Differential Equation (ODE) Models of Coinfection
2.2.2. Population-Level Dynamics of Virus–Bacteria Coinfection
3. Virus–Virus Coinfection: Viral Competition and Mathematical Models
3.1. Changes and Dysregulation of the Immune Response after Virus–Virus Coinfection
3.1.1. Interferon Stimulation and Antiviral Immunity
3.1.2. Resource Limitation and Competition
3.2. Mathematical Modeling of Dynamics of Virus–Virus Coinfection
3.2.1. Within-Host ODE Models of Virus–Virus Coinfection
3.2.2. Population-Level Models of Virus–Virus Coinfection Dynamics
4. Current Limitations in Coinfection Modeling
5. Conclusions
Funding
Conflicts of Interest
References
- Zambon, M.C. The Pathogenesis of Influenza in Humans. Rev. Med. Virol. 2001, 11, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. Predominant Role of Bacterial Pneumonia as a Cause of Death in Pandemic Influenza: Implications for Pandemic Influenza Preparedness. J. Infect. Dis. 2008, 198, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.-W.; Klugman, K.P.; Morens, D.M. Bacterial Pathogens and Death during the 1918 Influenza Pandemic. N. Engl. J. Med. 2009, 361, 2582–2583. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.R.; Sheng, Z.-M.; Ely, S.F.; Guinee, D.G., Jr.; Beasley, M.B.; Suh, J.; Deshpande, C.; Mollura, D.J.; Morens, D.M.; Bray, M.; et al. Pulmonary Pathologic Findings of Fatal 2009 Pandemic Influenza A/H1N1 Viral Infections. Arch. Pathol. Lab. Med. 2010, 134, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.J.; Robinson, J.O.; White, J.N.; Hughes, F.; Coombs, G.W.; Pearson, J.C.; Tan, H.-L.; Chidlow, G.; Williams, S.; Christiansen, K.J.; et al. Community-Acquired Pneumonia Due to Pandemic A(H1N1)2009 Influenzavirus and Methicillin Resistant Staphylococcus aureus Co-Infection. PLoS ONE 2010, 5, e8705. [Google Scholar] [CrossRef] [PubMed]
- Podewils, L.J.; Liedtke, L.A.; McDonald, L.C.; Hageman, J.C.; Straustaaugh, L.J.; Fischer, T.K.; Jernigan, D.B.; Uyeki, T.M.; Kuehnert, M.J. A National Survey of Severe Influenza-Associated Complications among Children and Adults, 2003–2004. Clin. Infect. Dis. 2005, 40, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Randolph, A.G.; Vaughn, F.; Sullivan, R.; Rubinson, L.; Thompson, B.T.; Yoon, G.; Smoot, E.; Rice, T.W.; Loftis, L.L.; Helfaer, M.; et al. Critically Ill Children during the 2009-2010 Influenza Pandemic in the United States. Pediatrics 2011, 128, e1450–e1458. [Google Scholar] [CrossRef]
- Brundage, J.F.; Shanks, G.D. Deaths from Bacterial Pneumonia during 1918-19 Influenza Pandemic. Emerg. Infect. Dis. 2008, 14, 1193–1199. [Google Scholar] [CrossRef]
- Klugman, K.P.; Chien, Y.-W.; Madhi, S.A. Pneumococcal Pneumonia and Influenza: A Deadly Combination. Vaccine 2009, 27 (Suppl. S3), C9–C14. [Google Scholar] [CrossRef]
- Avadhanula, V.; Rodriguez, C.A.; Devincenzo, J.P.; Wang, Y.; Webby, R.J.; Ulett, G.C.; Adderson, E.E. Respiratory Viruses Augment the Adhesion of Bacterial Pathogens to Respiratory Epithelium in a Viral Species- and Cell Type-Dependent Manner. J. Virol. 2006, 80, 1629–1636. [Google Scholar] [CrossRef]
- Manna, S.; Baindara, P.; Mandal, S.M. Molecular Pathogenesis of Secondary Bacterial Infection Associated to Viral Infections Including SARS-CoV-2. J. Infect. Public Health 2020, 13, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Nickol, M.E.; Ciric, J.; Falcinelli, S.D.; Chertow, D.S.; Kindrachuk, J. Characterization of Host and Bacterial Contributions to Lung Barrier Dysfunction Following Co-Infection with 2009 Pandemic Influenza and Methicillin Resistant Staphylococcus aureus. Viruses 2019, 11, 116. [Google Scholar] [CrossRef]
- Siegel, S.J.; Roche, A.M.; Weiser, J.N. Influenza Promotes Pneumococcal Growth during Coinfection by Providing Host Sialylated Substrates as a Nutrient Source. Cell Host Microbe 2014, 16, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.E.; Cleary, D.W.; Clarke, S.C. Secondary Bacterial Infections Associated with Influenza Pandemics. Front. Microbiol. 2017, 8, 1041. [Google Scholar] [CrossRef]
- Hoque, M.N.; Akter, S.; Mishu, I.D.; Islam, M.R.; Rahman, M.S.; Akhter, M.; Islam, I.; Hasan, M.M.; Rahaman, M.M.; Sultana, M.; et al. Microbial Co-Infections in COVID-19: Associated Microbiota and Underlying Mechanisms of Pathogenesis. Microb. Pathog. 2021, 156, 104941. [Google Scholar] [CrossRef]
- Asner, S.A.; Rose, W.; Petrich, A.; Tran, S.; Richardson, D.J. Is Virus Coinfection a Predictor of Severity in Children with Viral Respiratory Infections? Clin. Microbiol. Infect. 2020, 21, 264.e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Goka, E.A.; Vallely, P.J.; Mutton, K.J.; Klapper, P.E. Single, Dual and Multiple Respiratory Virus Infections and Risk of Hospitalization and Mortality. Epidemiol. Infect. 2015, 143, 37–47. [Google Scholar] [CrossRef]
- Martin, E.T.; Kuypers, J.; Wald, A.; Englund, J.A. Multiple versus Single Virus Respiratory Infections: Viral Load and Clinical Disease Severity in Hospitalized Children. Influ. Other Respir. Viruses 2012, 6, 71–77. [Google Scholar] [CrossRef]
- Martin, E.T.; Fairchok, M.P.; Stednick, Z.J.; Kuypers, J.; Englund, J.A. Epidemiology of Multiple Respiratory Viruses in Childcare Attendees. J. Infect. Dis. 2013, 207, 982–989. [Google Scholar] [CrossRef]
- Ratnamohan, V.M.; Taylor, J.; Zeng, F.; McPhie, K.; Blyth, C.C.; Adamson, S.; Kok, J.; Dwyer, D.E. Pandemic Clinical Case Definitions Are Non-Specific: Multiple Respiratory Viruses Circulating in the Early Phases of the 2009 Influenza Pandemic in New South Wales, Australia. Virol. J. 2014, 11, 113. [Google Scholar] [CrossRef]
- De Paulis, M.; Gilio, A.E.; Ferraro, A.A.; Ferronato, A.E.; Rossi do Sacramento, P.; Botosso, V.F.; Bruna Leal de Oliveira, D.; Marinheiro, J.C.; Hársi, C.M.; Durigon, E.L.; et al. Severity of Viral Coinfection in Hospitalized Infants with Respiratory Syncytial Virus Infection. J. Pediatr. (Rio J.) 2011, 87, 307–313. [Google Scholar] [PubMed]
- Meskill, S.D.; O’Bryant, S.C. Respiratory Virus Co-Infection in Acute Respiratory Infections in Children. Curr. Infect. Dis. Rep. 2020, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Goodarzi, P.; Asadi, M.; Soltani, A.; Aljanabi, H.A.A.; Jeda, A.S.; Dashtbin, S.; Jalalifar, S.; Mohammadzadeh, R.; Teimoori, A.; et al. Bacterial Co-Infections with SARS-CoV-2. IUBMB Life 2020, 72, 2097–2111. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Xie, J.; Zhao, J.; Cao, D.; Liang, Y.; Hou, X.; Wang, L.; Li, Z. Mechanisms of Severe Mortality-Associated Bacterial Co-Infections Following Influenza Virus Infection. Front. Cell. Infect. Microbiol. 2017, 7, 338. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhao, J.; Yang, C.; Liang, Y.; Long, P.; Liu, X.; Qiu, S.; Wang, L.; Xie, J.; Li, H.; et al. Severe Pneumonia Caused by Coinfection With Influenza Virus Followed by Methicillin-Resistant Staphylococcus aureus Induces Higher Mortality in Mice. Front. Immunol. 2018, 9, 3189. [Google Scholar] [CrossRef] [PubMed]
- Quah, J.; Jiang, B.; Tan, P.C.; Siau, C.; Tan, T.Y. Impact of Microbial Aetiology on Mortality in Severe Community-Acquired Pneumonia. BMC Infect. Dis. 2018, 18, 451. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; Olsen, R.J.; LaCasse, R.A.; Safronetz, D.; Ashraf, M.; Porter, A.R.; Braughton, K.R.; Feldmann, F.; Clifton, D.R.; Kash, J.C.; et al. Seasonal H3N2 Influenza A Virus Fails to Enhance Staphylococcus aureus Co-Infection in a Non-Human Primate Respiratory Tract Infection Model. Virulence 2013, 4, 707–715. [Google Scholar] [CrossRef]
- Tosche, A.; Aranz, S.; von Kries, R.; Puppe, W.; Weigl, J.; Hohle, M.; Heininger, U. No Temporal Association between Influenza Outbreaks and Invasive Pneumococcal Infections. Arch. Dis. Child. 2007, 93, 218–220. [Google Scholar] [CrossRef][Green Version]
- Small, C.-L.; McCormick, S.; Gill, N.; Kugathasan, K.; Santosuosso, M.; Donaldson, N.; Heinrichs, D.E.; Ashkar, A.; Xing, Z.; Donaldson, N. NK Cells Play a Critical Protective Role in Host Defense against Acute Extracellular Staphylococcus aureus Bacterial Infection in the Lung. J. Immunol. 2013, 180, 5558–5568. [Google Scholar] [CrossRef]
- Sun, K.; Metzger, D.W. Inhibition of Pulmonary Antibacterial Defense by Interferon-γ during Recovery from Influenza Infection. Nat. Med. 2008, 14, 558–564. [Google Scholar] [CrossRef]
- Lee, M.-H.; Arrecubieta, C.; Martin, F.J.; Prince, A.; Borczuk, A.C.; Lowy, F.D. A Postinfluenza Model of Staphylococcus aureus Pneumonia. J. Infect. Dis. 2010, 201, 508–515. [Google Scholar] [CrossRef] [PubMed]
- McCullers, J.A.; Rehg, J.E. Lethal Synergism between Influenza Virus and Streptococcus pneumoniae: Characterization of a Mouse Model and the Role of Platelet-Activating Factor Receptor. J. Infect. Dis. 2002, 186, 341–350. [Google Scholar] [CrossRef] [PubMed]
- McCullers, J.A.; Bartmess, K.C. Role of Neuraminidase in Lethal Synergism between Influenza Virus and Streptococcus pneumoniae. J. Infect. Dis. 2003, 187, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Tuvim, M.J.; Gilbert, B.E.; Dickey, B.F.; Evans, S.E. Synergistic TLR2/6 and TLR9 Activation Protects Mice against Lethal Influenza Pneumonia. PLoS ONE 2012, 7, e30596. [Google Scholar] [CrossRef] [PubMed]
- Iverson, A.R.; Boyd, K.L.; McAuley, J.L.; Plano, L.R.; Hart, M.E.; McCullers, J.A. Influenza Virus Primes Mice for Pneumonia from Staphylococcus aureus. J. Infect. Dis. 2011, 203, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Khader, S.A.; Gaffen, S.L.; Kolls, J.K. Th17 Cells at the Crossroads of Innate and Adaptive Immunity against Infectious Diseases at the Mucosa. Mucosal Immunol. 2009, 2, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Kudva, A.; Scheller, E.V.; Robinson, K.M.; Crowe, C.R.; Choi, S.M.; Slight, S.R.; Khader, S.A.; Dubin, P.J.; Enelow, R.I.; Kolls, J.K.; et al. Influenza A Inhibits Th17-Mediated Host Defense against Bacterial Pneumonia in Mice. J. Immunol. 2011, 186, 1666–1674. [Google Scholar] [CrossRef]
- van der Sluijs, K.F.; van Elden, L.J.R.; Nijhuis, M.; Schuurman, R.; Pater, J.M.; Florquin, S.; Goldman, M.; Jansen, H.M.; Lutter, R.; van der Poll, T. IL-10 Is an Important Mediator of the Enhanced Susceptibility to Pneumococcal Pneumonia after Influenza Infection. J. Immunol. 2004, 172, 7603–7609. [Google Scholar] [CrossRef]
- Bakaletz, L.O. Viral-Bacterial Co-Infections in the Respiratory Tract. Curr. Opin. Microbiol. 2017, 35, 30–35. [Google Scholar] [CrossRef]
- Seki, M.; Yanagihara, K.; Higashiyama, Y.; Fukuda, Y.; Kaneko, Y.; Ohno, H.; Miyazaki, Y.; Hirakata, Y.; Tomono, K.; Kadota, J.; et al. Immunokinetics in Severe Pneumonia Due to Influenza Virus and Bacteria Coinfection in Mice. Eur. Respir. J. 2004, 24, 143–149. [Google Scholar] [CrossRef]
- Verma, A.K.; Bauer, C.; Palani, S.; Metzger, D.W.; Sun, K. IFN-γ Drives TNF-α Hyperproduction and Lethal Lung Inflammation during Antibiotic Treatment of Postinfluenza Staphylococcus aureus Pneumonia. J. Immunol. 2021, 207, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Duvigneau, S.; Sharma-Chawla, N.; Boianelli, A.; Stegemann-Koniszewski, S.; Nguyen, V.K.; Bruder, D.; Hernandez-Vargas, E.A. Hierarchical Effects of Pro-Inflammatory Cytokines on the Post-Influenza Susceptibility to Pneumococcal Coinfection. Sci. Rep. 2016, 6, 37045. [Google Scholar] [CrossRef] [PubMed]
- Shahangian, A.; Chow, E.K.; Tian, X.; Kang, J.R.; Ghaffari, A.; Liu, S.Y.; Belperio, J.A.; Cheng, G.; Deng, J.C. Type I IFNs Mediate Development of Postinfluenza Bacterial Pneumonia in Mice. J. Clin. Investig. 2009, 119, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Sharma-Chawla, N.; Stegemann-Koniszewski, S.; Christen, H.; Boehme, J.D.; Kershaw, O.; Schreiber, J.; Guzmán, C.A.; Bruder, D.; Hernandez-Vargas, E.A. In Vivo Neutralization of Pro-Inflammatory Cytokines During Secondary Streptococcus pneumoniae Infection Post Influenza A Virus Infection. Front. Immunol. 2019, 10, 1864. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Davis, K.M.; Weiser, J.N. Synergistic Stimulation of Type I Interferons during Influenza Virus Coinfection Promotes Streptococcus pneumoniae Colonization in Mice. J. Clin. Investig. 2011, 121, 3657–3665. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.; Planet, P.J.; Soong, G.; Narechania, A.; Prince, A. Induction of Type I Interferon Signaling Determines the Relative Pathogenicity of Staphylococcus aureus Strains. PLoS Pathog. 2014, 10, e1003951. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Robinson, K.M.; McHugh, K.J.; Scheller, E.V.; Mandalapu, S.; Chen, C.; Di, Y.P.; Clay, M.E.; Enelow, R.I.; Dubin, P.J.; et al. Influenza-Induced Type I Interferon Enhances Susceptibility to Gram-Negative and Gram-Positive Bacterial Pneumonia in Mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L158–L167. [Google Scholar] [CrossRef]
- Schmit, T.; Guo, K.; Tripathi, J.K.; Wang, Z.; McGregor, B.; Klomp, M.; Ambigapathy, G.; Mathur, R.; Hur, J.; Pichichero, M.; et al. Interferon-γ Promotes Monocyte-Mediated Lung Injury during Influenza Infection. Cell Rep. 2022, 38, 110456. [Google Scholar] [CrossRef]
- Li, N.; Fan, X.; Xu, M.; Zhou, Y.; Wang, B. Flu Virus Attenuates Memory Clearance of Pneumococcus via IFN-γ-Dependent Th17 and Independent Antibody Mechanisms. iScience 2020, 23, 101767. [Google Scholar] [CrossRef]
- Palani, S.; Uddin, M.B.; McKelvey, M.; Shao, S.; Sun, K. Immune Predisposition Drives Susceptibility to Pneumococcal Pneumonia after Mild Influenza A Virus Infection in Mice. Front. Immunol. 2023, 14, 1272920. [Google Scholar] [CrossRef]
- Rich, H.E.; McCourt, C.C.; Zheng, W.Q.; McHugh, K.J.; Robinson, K.M.; Wang, J.; Alcorn, J.F. Interferon Lambda Inhibits Bacterial Uptake during Influenza Superinfection. Infect. Immun. 2019, 87, e00114-19. [Google Scholar] [CrossRef] [PubMed]
- Broggi, A.; Ghosh, S.; Sposito, B.; Spreafico, R.; Balzarini, F.; Lo Cascio, A.; Clementi, N.; De Santis, M.; Mancini, N.; Granucci, F.; et al. Type III Interferons Disrupt the Lung Epithelial Barrier upon Viral Recognition. Science 2020, 369, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Schultz-Cherry, S.; Hinshaw, V.S. Influenza Virus Neuraminidase Activates Latent Transforming Growth Factor Beta. J. Virol. 1996, 70, 8624–8629. [Google Scholar] [CrossRef] [PubMed]
- Aleith, J.; Brendel, M.; Weipert, E.; Müller, M.; Schultz, D.; Ko-Infekt Study Group; Müller-Hilke, B. Influenza A Virus Exacerbates Group A Streptococcus Infection and Thwarts Anti-Bacterial Inflammatory Responses in Murine Macrophages. Pathogens 2022, 11, 1320. [Google Scholar] [CrossRef] [PubMed]
- Barman, T.K.; Metzger, D.W. Disease Tolerance during Viral-Bacterial Co-Infections. Viruses 2021, 13, 2362. [Google Scholar] [CrossRef] [PubMed]
- LeVine, A.M.; Koeningsknecht, V.; Stark, J.M. Decreased Pulmonary Clearance of S. pneumoniae Following Influenza A Infection in Mice. J. Virol. Methods 2001, 94, 173–186. [Google Scholar] [CrossRef]
- Smith, M.W.; Schmidt, J.E.; Rehg, J.E.; Orihuela, C.J.; McCullers, J.A. Induction of Pro- and Anti-Inflammatory Molecules in a Mouse Model of Pneumococcal Pneumonia after Influenza. Comp. Med. 2007, 57, 82–89. [Google Scholar]
- Wilden, J.J.; Jacob, J.C.; Ehrhardt, C.; Ludwig, S.; Boergeling, Y. Altered Signal Transduction in the Immune Response to Influenza Virus and S. pneumoniae or S. aureus Co-Infections. Int. J. Mol. Sci. 2021, 22, 5486. [Google Scholar] [CrossRef]
- McNamee, L.A.; Harmsen, A.G. Both Influenza-Induced Neutrophil Dysfunction and Neutrophil-Independent Mechanisms Contribute to Increased Susceptibility to a Secondary Streptococcus pneumoniae Infection. Infect. Immun. 2006, 74, 6707–6721. [Google Scholar] [CrossRef]
- Klomp, M.; Ghosh, S.; Mohammed, S.; Nadeem Khan, M. From Virus to Inflammation, How Influenza Promotes Lung Damage. J. Leukoc. Biol. 2021, 110, 115–122. [Google Scholar] [CrossRef]
- Ellis, G.T.; Davidson, S.; Crotta, S.; Branzk, N.; Papayannopoulos, V.; Wack, A. TRAIL Monocytes and Monocyte-related Cells Cause Lung Damage and Thereby Increase. EMBO Rep. 2015, 16, 1051–1232. [Google Scholar] [CrossRef] [PubMed]
- LeMessurier, K.S.; Tiwary, M.; Morin, N.P.; Samarasinghe, A.E. Respiratory Barrier as a Safeguard and Regulator of Defense Against Influenza A Virus and Streptococcus pneumoniae. Front. Immunol. 2020, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Plotkowski, M.-C.; Puchelle, E.; Beck, G.; Jacquot, J.; Hannoun, C. Adherence of Type I Streptococcus pneumoniae to Tracheal Epithelium of Mice Infected with Influenza A/PR8 Virus. Am. Rev. Respir. Dis. 1985, 134, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Sohail, I.; Ghosh, S.; Mukundan, S.; Zelewski, S.; Khan, M.N. Role of Inflammatory Risk Factors in the Pathogenesis of Streptococcus pneumoniae. Front. Immunol. 2018, 9, 2275. [Google Scholar] [CrossRef]
- Kash, J.C.; Walters, K.-A.; Davis, A.S.; Sandouk, A.; Schwartzman, L.M.; Jagger, B.W.; Chertow, D.S.; Qi, L.; Kuestner, R.E.; Ozinsky, A.; et al. Lethal Synergism of 2009 Pandemic H1N1 Influenza Virus and Streptococcus pneumoniae Coinfection Is Associated with Loss of Murine Lung Repair Responses. MBio 2011, 2, e00172-11. [Google Scholar] [CrossRef] [PubMed]
- Mongodin, E.; Bajolet, O.; Cutrona, J.; Bonnet, N.; Dupuit, F.; Puchelle, E.; Bentzmann, S.D. Fibronectin-Binding Proteins of Staphylococcus aureus Are Involved in Adherence to Human Airway Epithelium. Infect. Immun. 2002, 70, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Pittet, L.A.; Hall-Stoodley, L.; Rutkowski, M.R.; Harmsen, A.G. Influenza Virus Infection Decreases Tracheal Mucociliary Velocity and Clearance of Streptococcus pneumoniae. Am. J. Respir. Cell Mol. Biol. 2010, 42, 450–460. [Google Scholar] [CrossRef]
- Kuek, L.E.; Lee, R.J. First Contact: The Role of Respiratory Cilia in Host-Pathogen Interactions in the Airways. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L603–L619. [Google Scholar] [CrossRef]
- Joma, B.H.; Siwapornchai, N.; Vanguri, V.K.; Shrestha, A.; Roggensack, S.E.; Davidson, B.A.; Tai, A.K.; Hakansson, A.P.; Meydani, S.N.; Leong, J.M.; et al. A Murine Model for Enhancement of Streptococcus pneumoniae Pathogenicity upon Viral Infection and Advanced Age. Infect. Immun. 2021, 89, e0047120. [Google Scholar] [CrossRef]
- Baccam, P.; Beauchemin, C.; Macken, C.A.; Hayden, F.G.; Perelson, A.S. Kinetics of Influenza A Virus Infection in Humans. J. Virol. 2006, 80, 7590–7599. [Google Scholar] [CrossRef]
- Hancioglu, B.; Swigon, D.; Clermont, G. A Dynamical Model of Human Immune Response to Influenza A Virus Infection. J. Theor. Biol. 2007, 246, 70–86. [Google Scholar] [CrossRef]
- Saenz, R.A.; Quinlivan, M.; Elton, D.; Macrae, S.; Blunden, A.S.; Mumford, J.A.; Daly, J.M.; Digard, P.; Cullinane, A.; Grenfell, B.T.; et al. Dynamics of Influenza Virus Infection and Pathology. J. Virol. 2010, 84, 3974–3983. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Hollenbaugh, J.A.; Zand, M.S.; Holden-Wiltse, J.; Mosmann, T.R.; Perelson, A.S.; Wu, H.; Topham, D.J. Quantifying the Early Immune Response and Adaptive Immune Response Kinetics in Mice Infected with Influenza A Virus. J. Virol. 2010, 84, 6687–6698. [Google Scholar] [CrossRef]
- Canini, L.; Carrat, F. Population Modeling of Influenza A/H1N1 Virus Kinetics and Symptom Dynamics. J. Virol. 2011, 85, 2764–2770. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Adler, F.R.; McAuley, J.L.; Gutenkunst, R.N.; Ribeiro, R.M.; McCullers, J.A.; Perelson, A.S. Effect of 1918 PB1-F2 Expression on Influenza A Virus Infection Kinetics. PLoS Comput. Biol. 2011, 7, e1001081. [Google Scholar] [CrossRef]
- Pawelek, K.A.; Huynh, G.T.; Quinlivan, M.; Cullinane, A.; Rong, L.; Perelson, A.S. Modeling Within-Host Dynamics of Influenza Virus Infection Including Immune Responses. PLoS Comput. Biol. 2012, 8, e1002588. [Google Scholar] [CrossRef]
- Price, I.; Mochan-Keef, E.D.; Swigon, D.; Ermentrout, G.B.B.; Lukens, S.; Toapanta, F.R.F.R.; Ross, T.M.T.M.; Clermont, G. The Inflammatory Response to Influenza A Virus (H1N1): An Experimental and Mathematical Study. J. Theor. Biol. 2015, 374, 83–93. [Google Scholar] [CrossRef]
- Sachak-Patwa, R.; Lafferty, E.I.; Schmit, C.J.; Thompson, R.N.; Byrne, H.M. A Target-Cell Limited Model Can Reproduce Influenza Infection Dynamics in Hosts with Differing Immune Responses. J. Theor. Biol. 2023, 567, 111491. [Google Scholar] [CrossRef]
- Mochan, E.; Swigon, D.; Ermentrout, G.B.B.; Lukens, S.; Clermont, G. A Mathematical Model of Intrahost Pneumococcal Pneumonia Infection Dynamics in Murine Strains. J. Theor. Biol. 2014, 353, 44–54. [Google Scholar] [CrossRef]
- Mochan-Keef, E.; Swigon, D.; Ermentrout, G.B.B.; Clermont, G. A Three-Tiered Study of Differences in Murine Intrahost Immune Response to Multiple Pneumococcal Strains. PLoS ONE 2015, 10, e0134012. [Google Scholar] [CrossRef][Green Version]
- Diep, J.K.; Russo, T.A.; Rao, G.G. Mechanism-Based Disease Progression Model Describing Host-Pathogen Interactions During the Pathogenesis of Acinetobacter baumannii Pneumonia. CPT Pharmacomet. Syst. Pharmacol. 2018, 7, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Schirm, S.; Ahnert, P.; Wienhold, S.; Mueller-Redetzky, H.; Nouailles-Kursar, G.; Loeffler, M.; Witzenrath, M.; Scholz, M. A Biomathematical Model of Pneumococcal Lung Infection and Antibiotic Treatment in Mice. PLoS ONE 2016, 11, e0156047. [Google Scholar] [CrossRef] [PubMed]
- Schirm, S.; Ahnert, P.; Berger, S.; Nouailles, G.; Wienhold, S.-M.; Müller-Redetzky, H.; Suttorp, N.; Loeffler, M.; Witzenrath, M.; Scholz, M. A Biomathematical Model of Immune Response and Barrier Function in Mice with Pneumococcal Lung Infection. PLoS ONE 2020, 15, e0243147. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Adler, F.R.; Ribeiro, R.M.; Gutenkunst, R.N.; McAuley, J.L.; McCullers, J.A.; Perelson, A.S. Kinetics of Coinfection with Influenza A Virus and Streptococcus pneumoniae. PLoS Pathog. 2013, 9, e1003238. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Smith, A.P. A Critical, Nonlinear Threshold Dictates Bacterial Invasion and Initial Kinetics during Influenza. Sci. Rep. 2016, 6, 38703. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; You, S.-H.; Lin, Y.-J.; Chen, S.-C.; Chen, W.-Y.; Chou, W.-C.; Hsieh, N.-H.; Liao, C.-M. Mathematical Modeling of Postcoinfection with Influenza A Virus and Streptococcus pneumoniae, with Implications for Pneumonia and COPD-Risk Assessment. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 1973–1988. [Google Scholar] [CrossRef]
- Shrestha, S.; Foxman, B.; Weinberger, D.M.; Steiner, C.; Viboud, C.; Rohani, P. Identifying the Interaction between Influenza and Pneumococcal Pneumonia Using Incidence Data. Sci. Transl. Med. 2013, 5, 191ra84. [Google Scholar] [CrossRef]
- Shrestha, S.; Foxman, B.; Dawid, S.; Aiello, A.E.; Davis, B.M.; Berus, J.; Rohani, P. Time and Dose-Dependent Risk of Pneumococcal Pneumonia Following Influenza: A Model for within-Host Interaction between Influenza and Streptococcus pneumoniae. J. R. Soc. Interface 2013, 10, 20130233. [Google Scholar] [CrossRef]
- Boianelli, A.; Nguyen, V.K.; Ebensen, T.; Schulze, K.; Wilk, E.; Sharma, N.; Stegemann-Koniszewski, S.; Bruder, D.; Toapanta, F.R.; Guzmán, C.A.; et al. Modeling Influenza Virus Infection: A Roadmap for Influenza Research. Viruses 2015, 7, 5274–5304. [Google Scholar] [CrossRef]
- Handel, A.; Longini, I.M.; Antia, R. Towards a Quantitative Understanding of the Within-Host Dynamics of Influenza A Infections. J. R. Soc. Interface 2010, 7, 35–47. [Google Scholar] [CrossRef]
- Shrestha, S.; Foxman, B.; Berus, J.; van Panhuis, W.G.; Steiner, C.; Viboud, C.; Rohani, P. The Role of Influenza in the Epidemiology of Pneumonia. Sci. Rep. 2015, 5, 15314. [Google Scholar] [CrossRef] [PubMed]
- Handel, A.; Longini, I.M.; Antia, R. Intervention Strategies for an Influenza Pandemic Taking into Account Secondary Bacterial Infections. Epidemics 2009, 1, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Kanyiri, C.W.; Luboobi, L.; Kimathi, M. Application of Optimal Control to Influenza Pneumonia Coinfection with Antiviral Resistance. Comput. Math. Methods Med. 2020, 2020, 5984095. [Google Scholar] [CrossRef] [PubMed]
- Crowe, S.; Utley, M.; Walker, G.; Grove, P.; Pagel, C. A Model to Evaluate Mass Vaccination against Pneumococcus as a Countermeasure against Pandemic Influenza. Vaccine 2011, 29, 5065–5077. [Google Scholar] [CrossRef] [PubMed]
- Calvo, C.; Garcia-Garcia, M.L.; Pozo, F.; Paula, G.; Molinero, M.; Calderon, A.; Gonzalez-Esguevillas, M.; Casas, I. Respiratory Syncytial Virus Coinfections with Rhinovirus and Human Bocavirus in Hospitalized Children. Medicine 2015, 94, e1788. [Google Scholar] [CrossRef] [PubMed]
- Karppinen, S.; Toivonen, L.; Schuez-Havupalo, L.; Waris, M.; Peltola, V. Interference between Respiratory Syncytial Virus and Rhinovirus in Respiratory Tract Infections in Children. Clin. Microbiol. Infect. 2016, 22, 208.e1–208.e6. [Google Scholar] [CrossRef] [PubMed]
- Brand, H.K.; De Groot, R.; Galama, J.M.D.; Brouwer, M.L.; Teuwen, K.; Hermans, P.W.M.; Melchers, W.J.G.; Warris, A. Infection with Multiple Viruses Is Not Associated with Increased Disease Severity in Children with Bronchiolitis. Pediatr. Pulmonol. 2012, 47, 393–400. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, Y.; Wang, H.; Zhang, L.; Bao, Y.; Zhou, X. High Incidence of Multiple Viral Infections Identified in Upper Respiratory Tract Infected Children under Three Years of Age in Shanghai, China. PLoS ONE 2012, 7, e44568. [Google Scholar] [CrossRef]
- Williams, B.G.; Gouws, E.; Boschi-Pinto, C.; Bryce, J.; Dye, C. Estimates of World-Wide Distribution of Child Deaths from Acute Respiratory Infections. Lancet Infect. Dis. 2002, 2, 25–32. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Kim, T.; Park, K.-H.; Choi, S.-H.; Kwak, Y.G.; Choo, E.J.; Chung, J.-W.; Lee, M.S. Dual Respiratory Virus Detection in Adult Patients with Acute Respiratory Illness. BMC Infect. Dis. 2021, 21, 997. [Google Scholar] [CrossRef]
- Goka, E.; Vallely, P.; Mutton, K.; Klapper, P. Influenza A Viruses Dual and Multiple Infections with Other Respiratory Viruses and Risk of Hospitalisation and Mortality. Influenza Other Respir. Viruses 2013, 7, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Glezen, W.P.; Taber, L.H.; Frank, A.L.; Kasel, J.A. Risk of Primary Infection and Reinfection With Respiratory Syncytial Virus. Am. J. Dis. Child. 1986, 140, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Jartti, T.; Jartti, L.; Peltola, V.; Waris, M.; Ruuskanen, O. Identification of Respiratory Viruses in Asymptomatic Subjects: Asymptomatic Respiratory Viral Infections. Pediatr. Infect. Dis. J. 2008, 27, 1103–1107. [Google Scholar] [CrossRef]
- Drori, Y.; Jacob-Hirsch, J.; Pando, R.; Glatman-Freedman, A.; Friedman, N.; Mendelson, E.; Mandelboim, M. Influenza A Virus Inhibits RSV Infection via a Two-Wave Expression of IFIT Proteins. Viruses 2020, 12, 1171. [Google Scholar] [CrossRef] [PubMed]
- Van Leuven, J.T.; Gonzalez, A.J.; Ijezie, E.C.; Wixom, A.Q.; Clary, J.L.; Naranjo, M.N.; Ridenhour, B.J.; Miller, C.R.; Miura, T.A. Rhinovirus Reduces the Severity of Subsequent Respiratory Viral Infections by Interferon-Dependent and -Independent Mechanisms. mSphere 2021, 6, e00479-21. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.; Gonzalez, A.J.; Ijezie, E.C.; Rodriguez, A.; Miller, C.R.; Van Leuven, J.T.; Miura, T.A. Priming With Rhinovirus Protects Mice Against a Lethal Pulmonary Coronavirus Infection. Front. Immunol. 2022, 13, 886611. [Google Scholar] [CrossRef] [PubMed]
- Vanderwall, E.R.; Barrow, K.A.; Rich, L.M.; Read, D.F.; Trapnell, C.; Okoloko, O.; Ziegler, S.F.; Hallstrand, T.S.; White, M.P.; Debley, J.S. Airway Epithelial Interferon Response to SARS-CoV-2 Is Inferior to Rhinovirus and Heterologous Rhinovirus Infection Suppresses SARS-CoV-2 Replication. Sci. Rep. 2022, 12, 6972. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Viral Interference between Respiratory Viruses. Emerg. Infect. Dis. 2022, 28, 273–281. [Google Scholar] [CrossRef]
- Laurie, K.L.; Guarnaccia, T.A.; Carolan, L.A.; Yan, A.W.C.; Aban, M.; Petrie, S.; Cao, P.; Heffernan, J.M.; McVernon, J.; Mosse, J.; et al. Interval between Infections and Viral Hierarchy Are Determinants of Viral Interference Following Influenza Virus Infection in a Ferret Model. J. Infect. Dis. 2015, 212, 1701–1710. [Google Scholar] [CrossRef]
- Nickbakhsh, S.; Mair, C.; Matthews, L.; Reeve, R.; Johnson, P.C.D.; Thorburn, F.; von Wissmann, B.; Reynolds, A.; McMenamin, J.; Gunson, R.N.; et al. Virus-Virus Interactions Impact the Population Dynamics of Influenza and the Common Cold. Proc. Natl. Acad. Sci. USA 2019, 116, 27142–27150. [Google Scholar] [CrossRef]
- Anestad, G.; Nordbo, S.A. Interference between Outbreaks of Respiratory Viruses. Eurosurveillance 2009, 14, 19359. [Google Scholar] [CrossRef] [PubMed]
- Price, O.H.; Sullivan, S.G.; Sutterby, C.; Druce, J.; Carville, K.S. Using Routine Testing Data to Understand Circulation Patterns of Influenza A, Respiratory Syncytial Virus and Other Respiratory Viruses in Victoria, Australia. Epidemiol. Infect. 2019, 147, e221. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.F.; Carolan, L.A.; Korenkov, D.; Druce, J.; Mccaw, J.; Reading, P.C.; Barr, I.G.; Laurie, K.L. Investigating Viral Interference between Influenza a Virus and Human Respiratory Syncytial Virus in a Ferret Model of Infection. J. Infect. Dis. 2018, 218, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Greer, R.M.; McErlean, P.; Arden, K.E.; Faux, C.E.; Nitsche, A.; Lambert, S.B.; Nissen, M.D.; Sloots, T.P.; Mackay, I.M. Do Rhinoviruses Reduce the Probability of Viral Co-Detection during Acute Respiratory Tract Infections? J. Clin. Virol. 2009, 45, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Ånestad, G.; Nordbø, S.A. Virus Interference. Did Rhinoviruses Activity Hamper the Progress of the 2009 Influenza A (H1N1) Pandemic in Norway? Med. Hypotheses 2011, 77, 1132–1134. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.-C.; Li, W.; Sui, J.; Marasco, W.; Choe, H.; Farzan, M. Influenza A Virus Neuraminidase Limits Viral Superinfection. J. Virol. 2008, 82, 4834–4843. [Google Scholar] [CrossRef]
- Shinjoh, M.; Omoe, K.; Saito, N.; Matsuo, N.; Nerome, K. In Vitro Growth Profiles of Respiratory Syncytial Virus in the Presence of Influenza Virus. Acta Virol. 2000, 44, 91–97. [Google Scholar]
- Wu, A.; Mihaylova, V.T.; Landry, M.L.; Foxman, E.F. Interference between Rhinovirus and Influenza A Virus: A Clinical Data Analysis and Experimental Infection Study. Lancet Microbe 2020, 1, e254–e262. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e19. [Google Scholar] [CrossRef]
- Bai, L.; Zhao, Y.; Dong, J.; Liang, S.; Guo, M.; Liu, X.; Wang, X.; Huang, Z.; Sun, X.; Zhang, Z.; et al. Coinfection with Influenza A Virus Enhances SARS-CoV-2 Infectivity. Cell Res. 2021, 31, 395–403. [Google Scholar] [CrossRef]
- Pinky, L.; Dobrovolny, H.M. Coinfections of the Respiratory Tract: Viral Competition for Resources. PLoS ONE 2016, 11, e0155589. [Google Scholar] [CrossRef] [PubMed]
- Pinky, L.; Dobrovolny, H.M. The Impact of Cell Regeneration on the Dynamics of Viral Coinfection. Chaos 2017, 27, 63109. [Google Scholar] [CrossRef] [PubMed]
- Pinky, L.; Gonzalez-Parra, G.; Dobrovolny, H.M. Effect of Stochasticity on Coinfection Dynamics of Respiratory Viruses. BMC Bioinform. 2019, 20, 191. [Google Scholar] [CrossRef] [PubMed]
- Pinky, L.; González-Parra, G.; Dobrovolny, H.M. Superinfection and Cell Regeneration Can Lead to Chronic Viral Coinfections. J. Theor. Biol. 2019, 466, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Yan, A.W.C.; Heffernan, J.M.; Petrie, S.; Moss, R.G.; Carolan, L.A.; Guarnaccia, T.A.; Kelso, A.; Barr, I.G.; McVernon, J.; et al. Innate Immunity and the Inter-Exposure Interval Determine the Dynamics of Secondary Influenza Virus Infection and Explain Observed Viral Hierarchies. PLoS Comput. Biol. 2015, 11, e1004334. [Google Scholar] [CrossRef]
- Pinky, L.; Burke, C.W.; Russell, C.J.; Smith, A.M. Quantifying Dose-, Strain-, and Tissue-Specific Kinetics of Parainfluenza Virus Infection. PLoS Comput. Biol. 2021, 17, e1009299. [Google Scholar] [CrossRef]
- Cao, P.; McCaw, J.M. The Mechanisms for Within-Host Influenza Virus Control Affect Model-Based Assessment and Prediction of Antiviral Treatment. Viruses 2017, 9, 197. [Google Scholar] [CrossRef]
- Merler, S.; Poletti, P.; Ajelli, M.; Caprile, B.; Manfredi, P. Coinfection Can Trigger Multiple Pandemic Waves. J. Theor. Biol. 2008, 254, 499–507. [Google Scholar] [CrossRef]
- Chen, S.; Ran, Y.; Huang, H.; Wang, Z.; Shang, K. Epidemic Dynamics of Two-Pathogen Spreading for Pairwise Models. Mathematics 2022, 10, 1906. [Google Scholar] [CrossRef]
- Palsson, S.; Hickling, T.P.; Bradshaw-Pierce, E.L.; Zager, M.; Jooss, K.; O’Brien, P.J.; Spilker, M.E.; Palsson, B.O.; Vicini, P. The Development of a Fully-Integrated Immune Response Model (FIRM) Simulator of the Immune Response through Integration of Multiple Subset Models. BMC Syst. Biol. 2013, 7, 95. [Google Scholar] [CrossRef]
- Pienaar, E.; Cilfone, N.A.; Lin, P.L.; Dartois, V.; Mattila, J.T.; Butler, J.R.; Flynn, J.L.; Kirschner, D.E.; Linderman, J.J. A Computational Tool Integrating Host Immunity with Antibiotic Dynamics to Study Tuberculosis Treatment. J. Theor. Biol. 2015, 367, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Baldazzi, V.; Castiglione, F.; Bernaschi, M. An Enhanced Agent Based Model of the Immune System Response. Cell. Immunol. 2006, 244, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, F.; Duca, K.; Jarrah, A.; Laubenbacher, R.; Hochberg, D.; Thorley-Lawson, D. Simulating Epstein-Barr Virus Infection with C-ImmSim. Bioinformatics 2007, 23, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Vafadar, S.; Shahdoust, M.; Kalirad, A.; Zakeri, P.; Sadeghi, M. Competitive Exclusion during Co-Infection as a Strategy to Prevent the Spread of a Virus: A Computational Perspective. PLoS ONE 2021, 16, e0247200. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, M.E.; Brooke, C.B.; Ke, R.; Koelle, K. Causes and Consequences of Spatial Within-Host Viral Spread. Viruses 2018, 10, 627. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ghanbarnejad, F.; Brockmann, D. Fundamental Properties of Cooperative Contagion Processes. New J. Phys. 2017, 19, 103041. [Google Scholar] [CrossRef]
- Kumberger, P.; Durso-Cain, K.; Uprichard, S.L.; Dahari, H.; Graw, F. Accounting for Space—Quantification of Cell-To-Cell Transmission Kinetics Using Virus Dynamics Models. Viruses 2018, 10, 200. [Google Scholar] [CrossRef]
- Moses, M.E.; Hofmeyr, S.; Cannon, J.L.; Andrews, A.; Gridley, R.; Hinga, M.; Leyba, K.; Pribisova, A.; Surjadidjaja, V.; Tasnim, H.; et al. Spatially Distributed Infection Increases Viral Load in a Computational Model of SARS-CoV-2 Lung Infection. PLoS Comput. Biol. 2021, 17, e1009735. [Google Scholar] [CrossRef]
- Sego, T.J.; Aponte-Serrano, J.O.; Gianlupi, J.F.; Glazier, J.A. Generation of Multicellular Spatiotemporal Models of Population Dynamics from Ordinary Differential Equations, with Applications in Viral Infection. BMC Biol. 2021, 19, 196. [Google Scholar] [CrossRef]
- Swat, M.H.; Thomas, G.L.; Belmonte, J.M.; Shirinifard, A.; Hmeljak, D.; Glazier, J.A. Chapter 13—Multi-Scale Modeling of Tissues Using CompuCell3D. In Computational Methods in Cell Biology; Asthagiri, A.R., Arkin, A.P., Eds.; Academic Press: Cambridge, MA, USA, 2012; Volume 110, pp. 325–366. ISBN 0091-679X. [Google Scholar]
- Sego, T.J.; Aponte-Serrano, J.O.; Ferrari Gianlupi, J.; Heaps, S.R.; Breithaupt, K.; Brusch, L.; Crawshaw, J.; Osborne, J.M.; Quardokus, E.M.; Plemper, R.K.; et al. A Modular Framework for Multiscale, Multicellular, Spatiotemporal Modeling of Acute Primary Viral Infection and Immune Response in Epithelial Tissues and Its Application to Drug Therapy Timing and Effectiveness. PLoS Comput. Biol. 2020, 16, e1008451. [Google Scholar] [CrossRef]
- Aponte-Serrano, J.O.; Weaver, J.J.A.; Sego, T.J.; Glazier, J.A.; Shoemaker, J.E. Multicellular Spatial Model of RNA Virus Replication and Interferon Responses Reveals Factors Controlling Plaque Growth Dynamics. PLoS Comput. Biol. 2021, 17, e1008874. [Google Scholar] [CrossRef] [PubMed]
- Ferrari Gianlupi, J.; Mapder, T.; Sego, T.J.; Sluka, J.P.; Quinney, S.K.; Craig, M.; Stratford, R.E.; Glazier, J.A. Multiscale Model of Antiviral Timing, Potency, and Heterogeneity Effects on an Epithelial Tissue Patch Infected by SARS-CoV-2. Viruses 2022, 14, 605. [Google Scholar] [CrossRef] [PubMed]
- Sego, T.J.; Mochan, E.D.; Ermentrout, G.B.; Glazier, J.A. A Multiscale Multicellular Spatiotemporal Model of Local Influenza Infection and Immune Response. J. Theor. Biol. 2022, 532, 110918. [Google Scholar] [CrossRef] [PubMed]
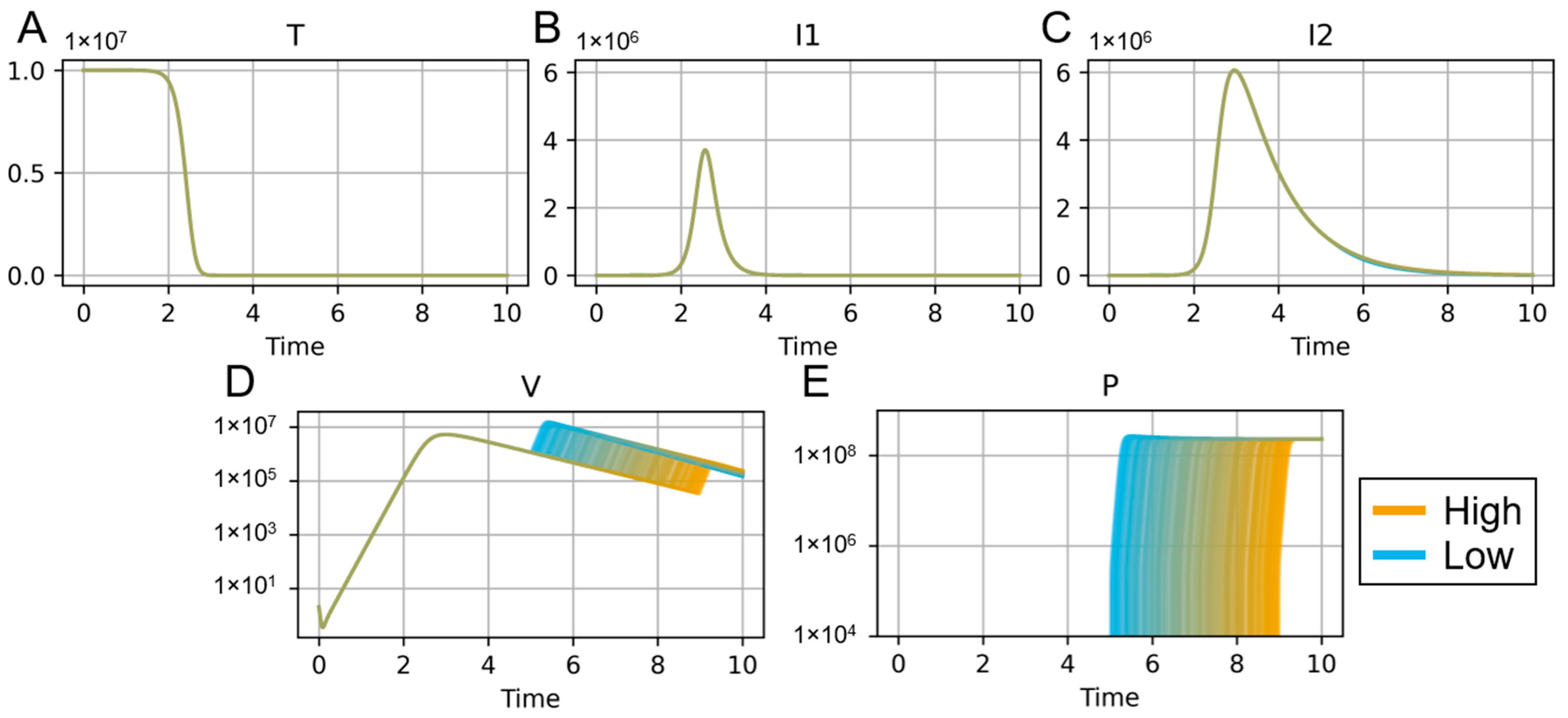
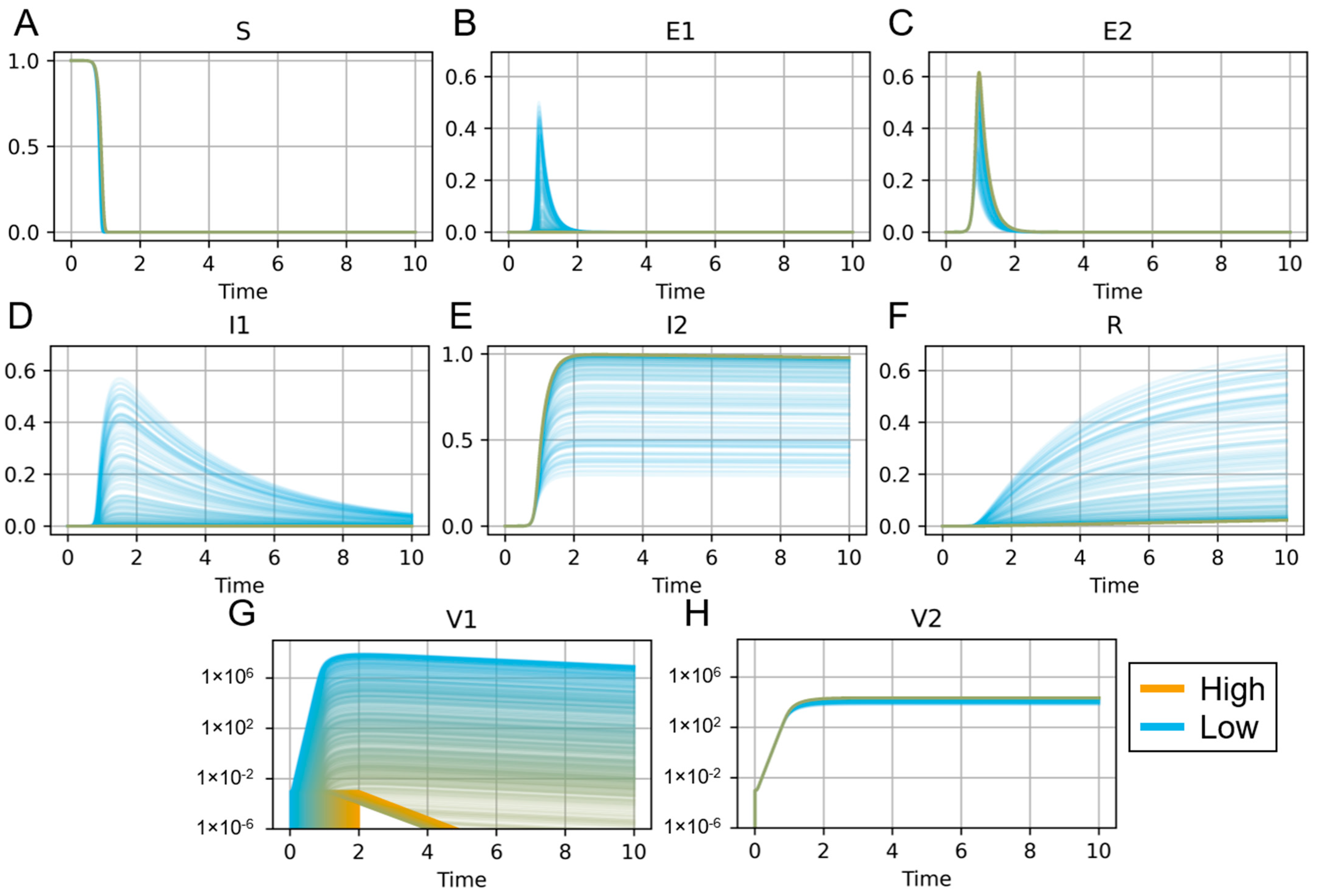

| Reference | Notable Variables and Parameters | Primary Results and Conclusions |
|---|---|---|
| Duvigneau et al. [42] | Interferon-γ | IFN-γ weakens bacterial clearance, allowing for increased post-influenza bacterial replication. |
| Sharma-Chawla et al. [44] | Interferon-γ IL-6 | Neutralizing IFN-γ improves bacterial clearance. Neutralizing both IFN-γ and IL-6 further improves bacterial clearance after influenza infection. |
| Smith et al. [84] | φ (decreased rate of macrophage phagocytosis) ψ (increased bacterial carrying capacity) μ (increased bacterial adherence to epithelial cells) | Viral titers increased in the presence of bacteria, and post-influenza macrophage impairment allows bacteria to grow at a faster rate. |
| Smith and Smith [85] | Φ (percent of alveolar macrophage depletion) | Macrophage depletion, bacterial growth rates, and bacterial inoculum are interconnected, and balancing them is key to survival of the coinfection. |
| Cheng et al. [86] | TNF-α | TNF-α levels can reflect the overall level of inflammatory response, providing an early warning against possible cytokine storm. |
| Shrestha et al. [88] | Time between influenza and bacterial inoculation Bacterial inoculum size | Bacteria administered 4–6 days post-influenza produce the most severe infections and require a lower inoculum size than coinfections started outside of this window. |
| Reference | Notable Variables and Parameters | Primary Results and Conclusions |
|---|---|---|
| Pinky and Dobrovolny [121] | Size and timing of secondary viral inoculum | Primary viruses can block secondary viruses by infecting host cells without viral interference. |
| Pinky and Dobrovolny [122] | Cell regeneration rate | Chronic coinfection was not possible for the considered coinfection models with cellular regeneration. Only a single-virus infection could produce chronic infection. |
| Pinky et al. [123] | Relative viral production rate | Stochasticity allows a slower-growing virus to outcompete a faster-growing virus. |
| Pinky et al. [124] | Infection rate of superinfected cells Cell regeneration rate | Chronic viral coinfection required both cell superinfection and regeneration. |
| Cao et al. [125] | Rate of IFN-induced conversion from target cells to virus-resistant cells Viral production rate sensitivity to IFN Killing rate of infected cells by IFN-activated NK cells | Viral hierarchy could be reproduced with IFN inhibition of viral production and IFN-mediated killing of infected cells by NK cells. Viral hierarchy and interactions between competing viruses are highly dependent on the timing of secondary infection. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mochan, E.; Sego, T.J. Mathematical Modeling of the Lethal Synergism of Coinfecting Pathogens in Respiratory Viral Infections: A Review. Microorganisms 2023, 11, 2974. https://doi.org/10.3390/microorganisms11122974
Mochan E, Sego TJ. Mathematical Modeling of the Lethal Synergism of Coinfecting Pathogens in Respiratory Viral Infections: A Review. Microorganisms. 2023; 11(12):2974. https://doi.org/10.3390/microorganisms11122974
Chicago/Turabian StyleMochan, Ericka, and T. J. Sego. 2023. "Mathematical Modeling of the Lethal Synergism of Coinfecting Pathogens in Respiratory Viral Infections: A Review" Microorganisms 11, no. 12: 2974. https://doi.org/10.3390/microorganisms11122974
APA StyleMochan, E., & Sego, T. J. (2023). Mathematical Modeling of the Lethal Synergism of Coinfecting Pathogens in Respiratory Viral Infections: A Review. Microorganisms, 11(12), 2974. https://doi.org/10.3390/microorganisms11122974







