The Cell Wall, Cell Membrane and Virulence Factors of Staphylococcus aureus and Their Role in Antibiotic Resistance
Abstract
:1. Introduction
2. Antibiotics
3. Antibiotic Resistance
4. Staphylococcus aureus
Methicillin Resistant Staphylococcus aureus
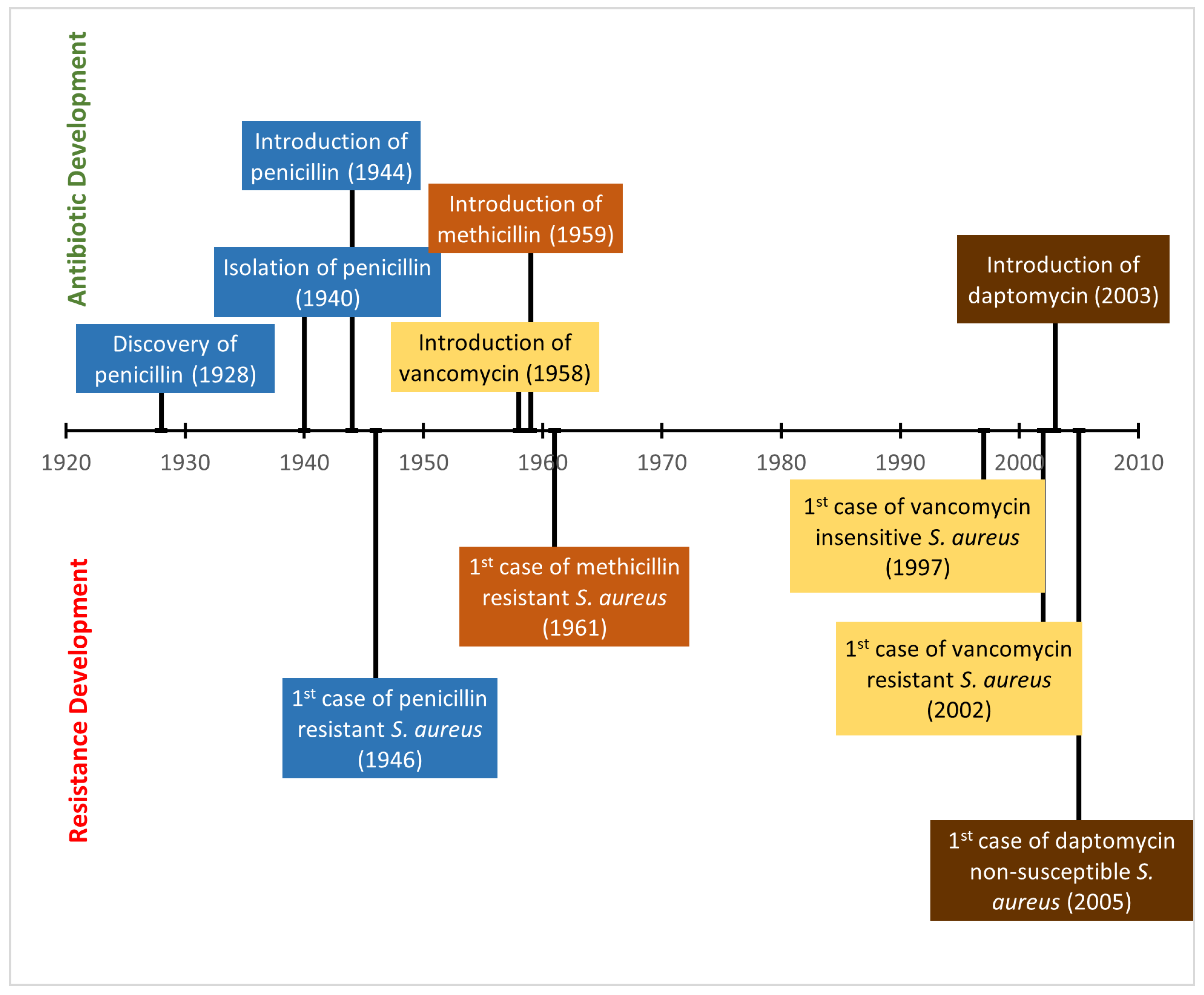
5. Development of Antibiotic Resistance in Staphylococcus aureus
6. Bacterial Cell Components
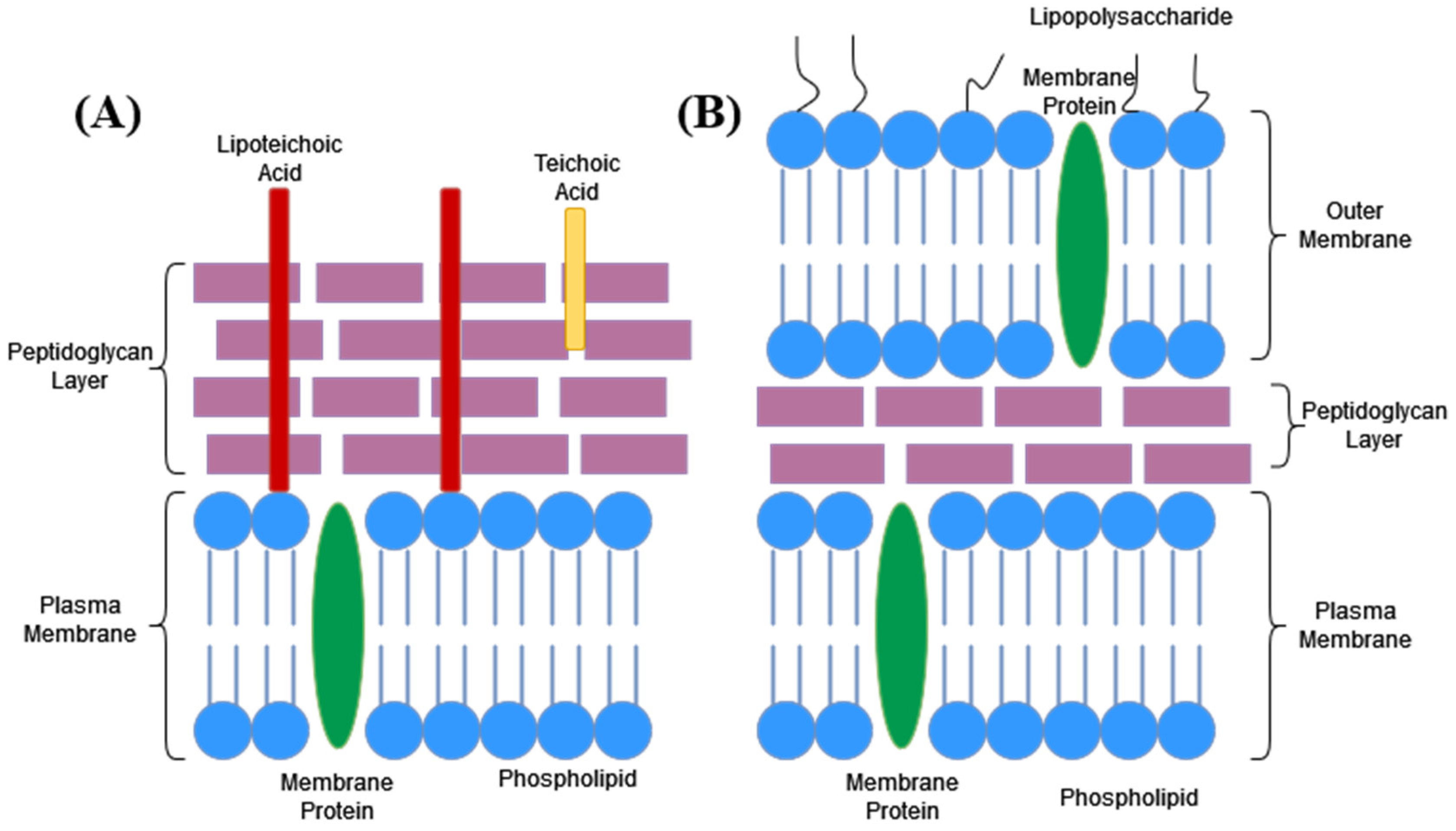
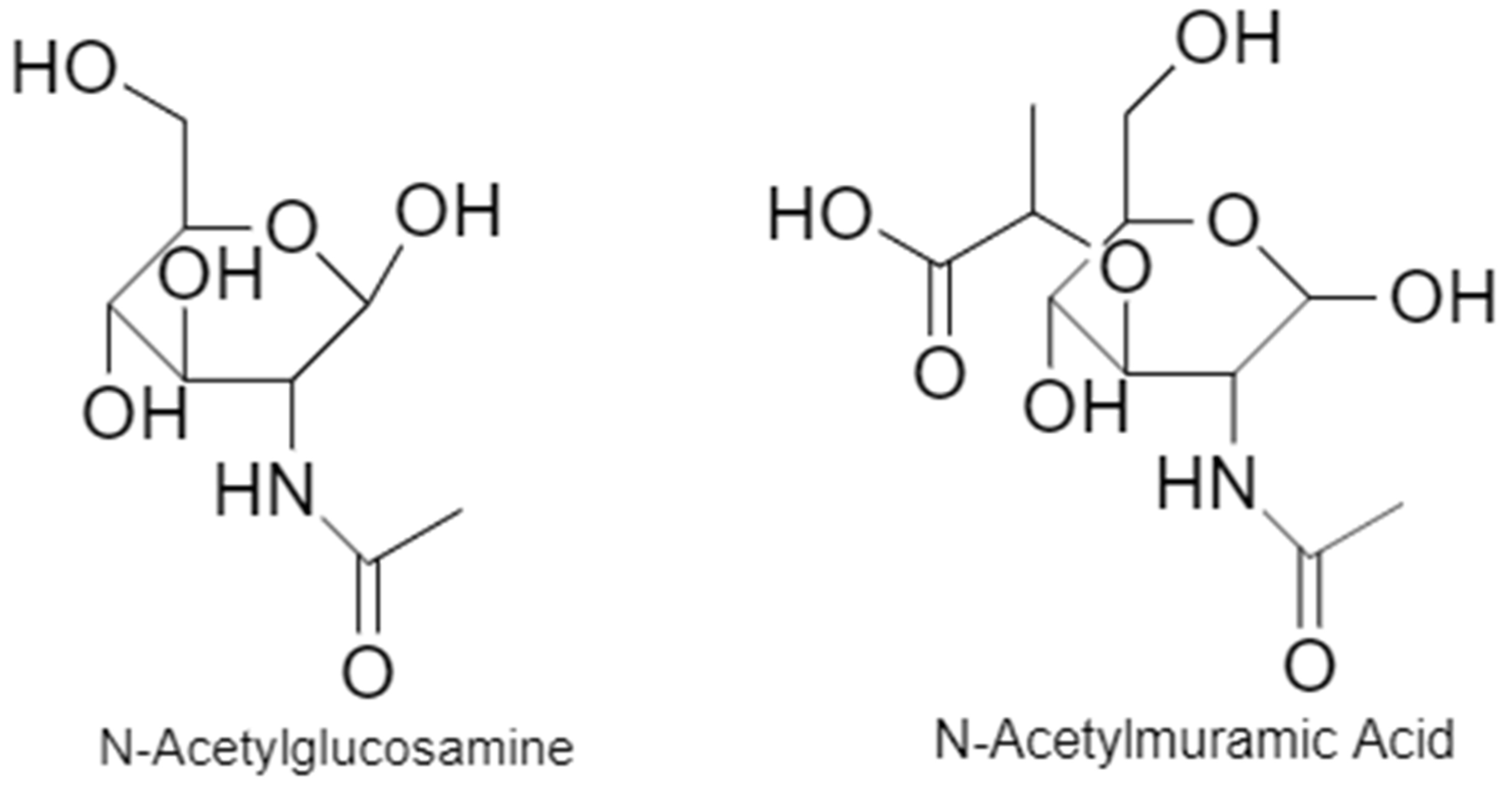
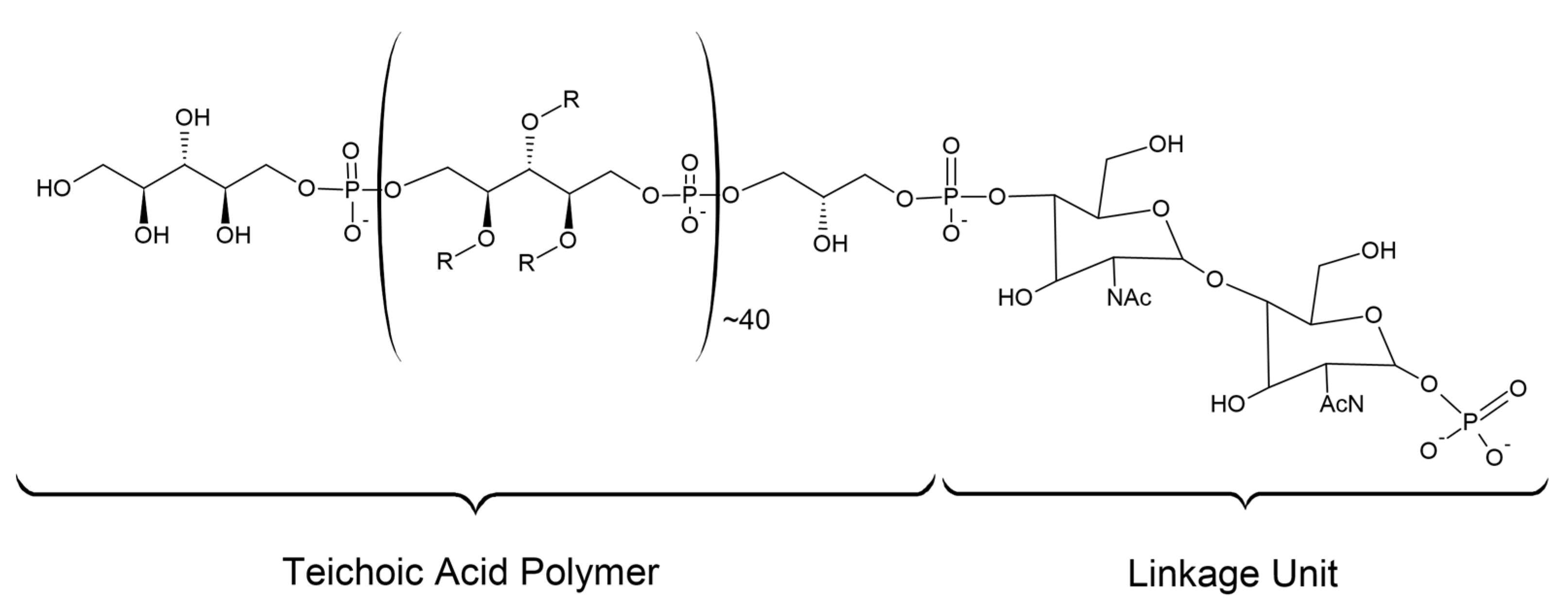
6.1. Cell Wall
6.2. Cell Membrane
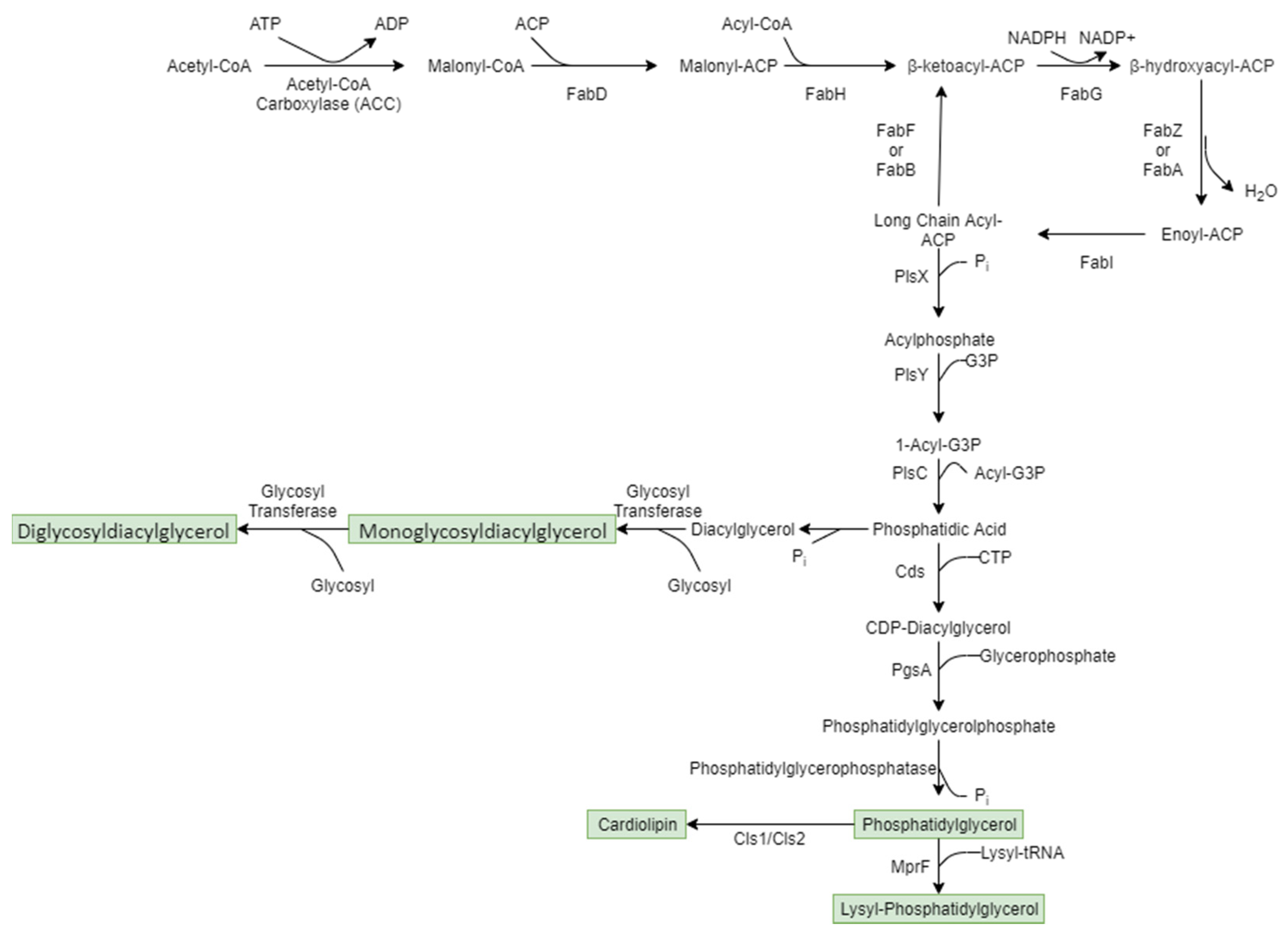
6.2.1. Membrane Lipid Synthesis
6.2.2. Role of Membrane Lipid Species
6.3. Virulence Factors
| Virulence Factor | Group | Function | Reference |
|---|---|---|---|
| Panton–Valentine Leucocidin | Toxin | Pore forming toxin that targets neutrophils and monocytes. | [69] |
| Phenol-Soluble Modulin | Toxin | Cytolytic activity against neutrophils and erythrocytes. | [15] |
| Staphylococcal Enterotoxin | Toxin | Binds to MHC class II receptors on T-cells causing rapid proliferation and massive cytokine release. | [72] |
| Toxic Shock Syndrome Toxin-1 | Toxin | Major virulence factor in toxic shock syndrome. | [71] |
| Exfoliative Toxin | Toxin | Protease that cleaves desmoglein 1 that connects epidermal cells. | [71,73] |
| Haemolysin | Toxin | Pore forming toxin that causes destruction of epithelial cells, erythrocytes, monocytes, fibroblasts, neutrophils and macrophages. | [74,75] |
| Protein A | Immunomodulator | Cell wall anchored protein that binds to IgG antibodies, impairing host response in phagocytosis. | [15,69] |
| Staphyloxanthin | Immunomodulator | Protects cell from reactive oxygen species produced by the host immune system. | [76] |
| Arginine Catabolic Mobile Element | Immunomodulator | Contributes to colonisation by ammonification of the acidic skin environment. | [15,77] |
| Coagulase | Immunomodulator | Triggers cleavage of fibrinogen to fibrin that coats cell surface allowing evasion of opsonophagocytic clearance by the host immune system. | [78,79] |
Therapy Targeting Virulence Factors in Staphylococcus aureus
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Casanova, G.N.; Ruiz, S.M.; Bellido, M.J.L. Mechanisms of Resistance to Daptomycin in Staphylococcus aureus. Rev. Española Quimioter. 2017, 30, 391–396. [Google Scholar]
- Sirichoat, A.; Lulitanond, A.; Kanlaya, R.; Tavichakorntrakool, R.; Chanawong, A.; Wongthong, S.; Thongboonkerd, V. Phenotypic Characteristics and Comparative Proteomics of Staphylococcus aureus Strains with Different Vancomycin-Resistance Levels. Diagn. Microbiol. Infect. Dis. 2016, 86, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Romaniuk, J.A.; Cegelski, L. Bacterial Cell Wall Composition and the Influence of Antibiotics by Cell-Wall and Whole-Cell NMR. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef]
- Hurdle, J.G.; O’Neill, A.J.; Chopra, I.; Lee, R.E. Targeting Bacterial Membrane Function: An Underexploited Mechanism for Treating Persistent Infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, J.; Rudkin, J.; Recker, M.; Pozzi, C.; O’Gara, J.P.; Massey, R.C. Offsetting Virulence and Antibiotic Resistance Costs by MRSA. Isme J. 2010, 4, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Zaffiri, L.; Gardner, J.; Toledo-Pereyra, L.H. History of Antibiotics. From Salvarsan to Cephalosporins. J. Investig. Surg. 2012, 25, 67–77. [Google Scholar] [CrossRef]
- Gould, K. Antibiotics: From Prehistory to the Present Day. J. Antimicrob. Chemother. 2016, 71, 572–575. [Google Scholar] [CrossRef]
- Foster, T.J. Antibiotic Resistance in Staphylococcus aureus. Current Status and Future Prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef]
- Turnidge, J.; Paterson, D.L. Setting and Revising Antibacterial Susceptibility Breakpoints. Clin. Microbiol. Rev. 2007, 20, 391–408. [Google Scholar] [CrossRef] [Green Version]
- Ashkenazi, S. Beginning and Possibly the End of the Antibiotic Era. J. Paediatr. Child Health 2013, 49, E179–E182. [Google Scholar] [CrossRef]
- Perry, J.; Waglechner, N.; Wright, G. The Prehistory of Antibiotic Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025197. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, J.; Rodríguez-Beltrán, J.; Matic, I. Antibiotic-Induced Genetic Variation: How It Arises and How It Can Be Prevented. Annu. Rev. Microbiol. 2018, 72, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Song, P.I.; Seo, C.H.; Cheong, H.; Park, Y. Colonization and Infection of the Skin by S. aureus: Immune System Evasion and the Response to Cationic Antimicrobial Peptides. Int. J. Mol. Sci. 2014, 15, 8753–8772. [Google Scholar] [CrossRef] [Green Version]
- Rojo, P.; Barrios, M.; Palacios, A.; Gomez, C.; Chaves, F. Community-Associated Staphylococcus aureus Infections in Children. Expert Rev. Anti-Infect. Ther. 2010, 8, 541–554. [Google Scholar] [CrossRef]
- Kale, P.; Dhawan, B. The Changing Face of Community-Acquired Methicillin-Resistant Staphylococcus aureus. Indian J. Med. Microbiol. 2016, 34, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Uhlemann, A.C.; Otto, M.; Lowy, F.D.; DeLeo, F.R. Evolution of Community- and Healthcare-Associated Methicillin-Resistant Staphylococcus aureus. Infect. Genet. Evol. 2014, 21, 563–574. [Google Scholar] [CrossRef] [Green Version]
- Kong, E.F.; Johnson, J.K.; Jabra-Rizk, M.A. Community-Associated Methicillin-Resistant Staphylococcus aureus: An Enemy Amidst Us. PLoS Pathog. 2016, 12, e1005837. [Google Scholar] [CrossRef] [Green Version]
- Crespo-Piazuelo, D.; Lawlor, P.G. Livestock-Associated Methicillin-Resistant Staphylococcus aureus (LA-MRSA) Prevalence in Humans in Close Contact with Animals and Measures to Reduce On-Farm Colonisation. Ir. Vet. J. 2021, 74, 21. [Google Scholar] [CrossRef]
- Antimicrobial Use and Resistance in Australia. Second Australian Report on Antimicrobial Use and Resistance in Human Health; Australian Commission on Safety and Quality in Health Care: Sydney, Australia, 2017. [Google Scholar]
- Cuny, C.; Köck, R.; Witte, W. Livestock Associated MRSA (LA-MRSA) and Its Relevance for Humans in Germany. Int. J. Med. Microbiol. 2013, 303, 331–337. [Google Scholar] [CrossRef]
- Cuny, C.; Wieler, L.H.; Witte, W. Livestock-Associated MRSA: The Impact on Humans. Antibiotics 2015, 4, 521–543. [Google Scholar] [CrossRef] [Green Version]
- Enright, M.C.; Robinson, D.A.; Randle, G.; Feil, E.J.; Grundmann, H.; Spratt, B.G. The Evolutionary History of Methicillin-Resistant Staphylococcus aureus (MRSA). Proc. Natl. Acad. Sci. USA 2002, 99, 7687–7692. [Google Scholar] [CrossRef] [PubMed]
- Enright, M.C. The Evolution of a Resistant Pathogen—The Case of MRSA. Curr. Opin. Pharmacol. 2003, 3, 474–479. [Google Scholar] [CrossRef]
- Rubin, J.E.; Ball, K.R.; Chirino-Trejo, M. Antimicrobial Susceptibility of Staphylococcus aureus and Staphylococcus pseudintermedius Isolated from Various Animals. Can. Vet. J. 2011, 52, 153–157. [Google Scholar] [PubMed]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-Resistant Staphylococcus aureus: An Overview of Basic and Clinical Research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Steffy, K.; Chatterjee, M.; Sugumar, M.; Dinesh, K.R.; Manoharan, A.; Karim, S.; Biswas, R. Detection of Oxacillin-Susceptible mecA-Positive Staphylococcus aureus Isolates by Use of Chromogenic Medium MRSA ID. J. Clin. Microbiol. 2013, 51, 318–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Hu, Y.; Baker, M.; Dottorini, T.; Li, H.; Dong, Y.; Bai, Y.; Fanning, S.; Li, F. Novel SCCmec Type XV (7A) and Two Pseudo-SCCmec Variants in Foodborne MRSA in China. J. Antimicrob. Chemother. 2022, 77, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Urushibara, N.; Aung, M.S.; Kawaguchiya, M.; Kobayashi, N. Novel Staphylococcal Cassette Chromosome mec (SCCmec) Type XIV (5A) and a Truncated SCCmec Element in SCC Composite Islands Carrying speG in ST5 MRSA in Japan. J. Antimicrob. Chemother. 2019, 75, 46–50. [Google Scholar] [CrossRef]
- Peacock, S.J.; Paterson, G.K. Mechanisms of Methicillin Resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef]
- Brown, S.; Xia, G.; Luhachack, L.G.; Campbell, J.; Meredith, T.C.; Chen, C.; Winstel, V.; Gekeler, C.; Irazoqui, J.E.; Peschel, A.; et al. Methicillin Resistance in Staphylococcus aureus Requires Glycosylated Wall Teichoic Acids. Proc. Natl. Acad. Sci. USA 2012, 109, 18909–18914. [Google Scholar] [CrossRef] [Green Version]
- Sastalla, I.; Datta, S.K. Antibiotic Education: Not Just Another Brick in the Cell Wall. Cell Host Microbe 2015, 18, 520–522. [Google Scholar] [CrossRef] [Green Version]
- Deurenberg, R.H.; Stobberingh, E.E. The Evolution of Staphylococcus aureus. Infect. Genet. Evol. 2008, 8, 747–763. [Google Scholar] [CrossRef]
- Tsubakishita, S.; Kuwahara-Arai, K.; Sasaki, T.; Hiramatsu, K. Origin and Molecular Evolution of the Determinant of Methicillin Resistance in Staphylococci. Antimicrob. Agents Chemother. 2010, 54, 4352–4359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cong, Y.; Yang, S.; Rao, X. Vancomycin Resistant Staphylococcus aureus Infections: A Review of Case Updating and Clinical Features. J. Adv. Res. 2020, 21, 169–176. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Vancomycin Resistance in Staphylococcus aureus. Yale J. Biol. Med. 2017, 90, 269–281. [Google Scholar]
- Walters, M.S.; Eggers, P.; Albrecht, V.; Travis, T.; Lonsway, D.; Hovan, G.; Taylor, D.; Rasheed, K.; Limbago, B.; Kallen, A. Vancomycin-Resistant Staphylococcus aureus - Delaware, 2015. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrisette, T.; Alosaimy, S.; Abdul-Mutakabbir, J.C.; Kebriaei, R.; Rybak, M.J. The Evolving Reduction of Vancomycin and Daptomycin Susceptibility in MRSA-Salvaging the Gold Standards with Combination Therapy. Antibiotics 2020, 9, 762. [Google Scholar] [CrossRef]
- Egan, A.J.F.; Cleverley, R.M.; Peters, K.; Lewis, R.J.; Vollmer, W. Regulation of Bacterial Cell Wall Growth. FEBS J. 2017, 284, 851–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Sauvage, E.; Kerff, F.; Terrak, M.; Ayala, J.A.; Charlier, P. The Penicillin-Binding Proteins: Structure and Role in Peptidoglycan Biosynthesis. FEMS Microbiol. Rev. 2008, 32, 234–258. [Google Scholar] [CrossRef] [Green Version]
- Sharif, S.; Singh, M.; Kim, S.J.; Schaefer, J. Staphylococcus aureus Peptidoglycan Tertiary Structure from Carbon-13 Spin Diffusion. J. Am. Chem. Soc. 2009, 131, 7023–7030. [Google Scholar] [CrossRef] [Green Version]
- Vollmer, W. Structural Variation in the Glycan Strands of Bacterial Peptidoglycan. FEMS Microbiol. Rev. 2008, 32, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.A.F.; Carnell, O.T.; Lafage, L.; Gray, J.; Biboy, J.; Gibson, J.F.; Pollitt, E.J.G.; Tazoll, S.C.; Turnbull, W.; Hajdamowicz, N.H.; et al. Staphylococcus aureus Cell Wall Structure and Dynamics During Host-Pathogen Interaction. PLOS Pathog. 2021, 17, e1009468. [Google Scholar] [CrossRef]
- Dmitriev, B.A.; Toukach, F.V.; Holst, O.; Rietschel, E.T.; Ehlers, S. Tertiary Structure of Staphylococcus aureus Cell Wall Murein. J. Bacteriol. 2004, 186, 7141–7148. [Google Scholar] [CrossRef] [Green Version]
- Nikolic, P.; Mudgil, P.; Harman, D.G.; Whitehall, J. Untargeted Lipidomic Differences Between Clinical Strains of Methicillin-Sensitive and Methicillin-Resistant Staphylococcus aureus. Infect. Dis. 2022, 54, 497–507. [Google Scholar] [CrossRef]
- Vollmer, W.; Blanot, D.; de Pedro, M.A. Peptidoglycan Structure and Architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef] [Green Version]
- Malanovic, N.; Lohner, K. Gram-Positive Bacterial Cell Envelopes: The Impact on the Activity of Antimicrobial peptides. Biochim. Biochim. Biophys. Acta 2016, 1858, 936–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasquina, L.W.; Santa Maria, J.P.; Walker, S. Teichoic Acid Biosynthesis as an Antibiotic Target. Curr. Opin. Microbiol. 2013, 16, 531–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.; Santa Maria, J.P., Jr.; Walker, S. Wall Teichoic Acids of Gram-Positive Bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef] [Green Version]
- Swoboda, J.G.; Campbell, J.; Meredith, T.C.; Walker, S. Wall Teichoic Acid Function, Biosynthesis, and Inhibition. Chembiochem 2010, 11, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Sewell, E.W.; Brown, E.D. Taking Aim at Wall Teichoic Acid Synthesis: New Biology and New Leads for Antibiotics. J. Antibiot. 2014, 67, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Strahl, H.; Errington, J. Bacterial Membranes: Structure, Domains, and Function. Annu. Rev. Microbiol. 2017, 71, 519–538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Rock, C.O. Membrane Lipid Homeostasis in Bacteria. Nat. Rev. Microbiol. 2008, 6, 222–233. [Google Scholar] [CrossRef]
- Kuhn, S.; Slavetinsky, C.J.; Peschel, A. Synthesis and Function of Phospholipids in Staphylococcus aureus. Int. J. Med. Microbiol. 2015, 305, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Koprivnjak, T.; Zhang, D.; Ernst, C.M.; Peschel, A.; Nauseef, W.M.; Weiss, J.P. Characterization of Staphylococcus aureus Cardiolipin Synthases 1 and 2 and Their Contribution to Accumulation of Cardiolipin in Stationary Phase and Within Phagocytes. J. Bacteriol. 2011, 193, 4134–4142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrés, E.; Biarnés, X.; Faijes, M.; Planas, A. Bacterial Glycoglycerolipid Synthases: Processive and Non-Processive Glycosyltransferases in Mycoplasma. Biocatal. Biotransform. 2012, 30, 274–287. [Google Scholar] [CrossRef]
- Andrés, E.; Martínez, N.; Planas, A. Expression and Characterization of a Mycoplasma genitalium Glycosyltransferase in Membrane Glycolipid Biosynthesis: Potential Target Against Mycoplasma Infections. J. Biol. Chem. 2011, 286, 35367–35379. [Google Scholar] [CrossRef] [Green Version]
- Hölzl, G.; Dörmann, P. Structure and Function of Glycoglycerolipids in Plants and Bacteria. Prog. Lipid Res. 2007, 46, 225–243. [Google Scholar] [CrossRef]
- Yeagle, P. Chapter 12—Lipid–Protein Interactions in Membranes. In The Membranes of Cells; Academic Press: Boston, MA, USA, 2016; pp. 291–334. [Google Scholar]
- Zhao, W.; Róg, T.; Gurtovenko, A.A.; Vattulainen, I.; Karttunen, M. Role of Phosphatidylglycerols in the Stability of Bacterial Membranes. Biochimie 2008, 90, 930–938. [Google Scholar] [CrossRef]
- Ichihashi, N.; Kurokawa, K.; Matsuo, M.; Kaito, C.; Sekimizu, K. Inhibitory Effects of Basic or Neutral Phospholipid on Acidic Phospholipid-Mediated Dissociation of Adenine Nucleotide Bound to DnaA Protein, the Initiator of Chromosomal DNA Replication. J. Biol. Chem. 2003, 278, 28778–28786. [Google Scholar] [CrossRef] [Green Version]
- Řezanka, T.; Kolouchová, I.; Gharwalová, L.; Palyzová, A.; Sigler, K. Lipidomic Analysis: From Archaea to Mammals. Lipids 2018, 53, 5–25. [Google Scholar] [CrossRef]
- Ohniwa, R.L.; Kitabayashi, K.; Morikawa, K. Alternative Cardiolipin Synthase Cls1 Compensates for Stalled Cls2 Function in Staphylococcus aureus Under Conditions of Acute Acid Stress. FEMS Microbiol. Lett. 2013, 338, 141–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Muraih, J.K.; Tishbi, N.; Herskowitz, J.; Victor, R.L.; Silverman, J.; Uwumarenogie, S.; Taylor, S.D.; Palmer, M.; Mintzer, E. Cardiolipin Prevents Membrane Translocation and Permeabilization by Daptomycin. J. Biol. Chem. 2014, 289, 11584–11591. [Google Scholar] [CrossRef] [PubMed]
- Dennison, S.R.; Morton, L.H.; Harris, F.; Phoenix, D.A. Low pH Enhances the Action of Maximin H5 against Staphylococcus aureus and Helps Mediate Lysylated Phosphatidylglycerol-Induced Resistance. Biochemistry 2016, 55, 3735–3751. [Google Scholar] [CrossRef] [Green Version]
- Band, V.I.; Weiss, D.S. Mechanisms of Antimicrobial Peptide Resistance in Gram-Negative Bacteria. Antibiotics 2015, 4, 18–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavetinsky, C.; Kuhn, S.; Peschel, A. Bacterial Aminoacyl Phospholipids—Biosynthesis and Role in Basic Cellular Processes and Pathogenicity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.J.; Lowy, F.D. Pathogenesis of Methicillin-Resistant Staphylococcus aureus Infection. Clin. Infect Dis. 2008, 46 (Suppl. S5), S350–S359. [Google Scholar] [CrossRef] [Green Version]
- Kane, T.L.; Carothers, K.E.; Lee, S.W. Virulence Factor Targeting of the Bacterial Pathogen Staphylococcus aureus for Vaccine and Therapeutics. Curr. Drug Targets 2018, 19, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, D.; Blanco-Valle, K.; Hennekinne, J.A.; Simon, S.; Fenaille, F.; Becher, F.; Nia, Y. Multiplex Detection of 24 Staphylococcal Enterotoxins in Culture Supernatant Using Liquid Chromatography Coupled to High-Resolution Mass Spectrometry. Toxins 2022, 14, 249. [Google Scholar] [CrossRef]
- Tristan, A.; Ferry, T.; Durand, G.; Dauwalder, O.; Bes, M.; Lina, G.; Vandenesch, F.; Etienne, J. Virulence Determinants in Community and Hospital Meticillin-Resistant Staphylococcus aureus. J. Hosp. Infect. 2007, 65 (Suppl. S2), 105–109. [Google Scholar] [CrossRef]
- Holtfreter, S.; Kolata, J.; Bröker, B.M. Towards the Immune Proteome of Staphylococcus aureus—The Anti-S. aureus Antibody Response. Int. J. Med. Microbiol. 2010, 300, 176–192. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Z.J.; Sun, Z.; Feng, X.; Zou, M.; Cao, W.; Wang, S.; Zeng, J.; Wang, Y.; Sun, M. Molecular Characteristics and Virulence Factors in Methicillin-Susceptible, Resistant, and Heterogeneous Vancomycin-Intermediate Staphylococcus aureus from Central-Southern China. J. Microbiol. Immunol. Infect. 2015, 48, 490–496. [Google Scholar] [CrossRef] [Green Version]
- Chambers, H.F.; Deleo, F.R. Waves of Resistance: Staphylococcus aureus in the Antibiotic Era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Vandenesch, F.; Lina, G.; Henry, T. Staphylococcus aureus Hemolysins, Bi-Component Leukocidins, and Cytolytic Peptides: A Redundant Arsenal of Membrane-Damaging Virulence Factors? Front. Cell. Infect. Microbiol. 2012, 2, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.I.; Liu, G.Y.; Song, Y.; Yin, F.; Hensler, M.E.; Jeng, W.Y.; Nizet, V.; Wang, A.H.; Oldfield, E. A Cholesterol Biosynthesis Inhibitor Blocks Staphylococcus aureus Virulence. Science 2008, 319, 1391–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shore, A.C.; Rossney, A.S.; Brennan, O.M.; Kinnevey, P.M.; Humphreys, H.; Sullivan, D.J.; Goering, R.V.; Ehricht, R.; Monecke, S.; Coleman, D.C. Characterization of a Novel Arginine Catabolic Mobile Element (ACME) and Staphylococcal Chromosomal Cassette mec Composite Island with Significant Homology to Staphylococcus epidermidis ACME Type II in Methicillin-Resistant Staphylococcus aureus Genotype ST22-MRSA-IV. Antimicrob. Agents Chemother. 2011, 55, 1896–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, A.G.; McAdow, M.; Kim, H.K.; Bae, T.; Missiakas, D.M.; Schneewind, O. Contribution of Coagulases Towards Staphylococcus aureus Disease and Protective Immunity. PLoS Pathog. 2010, 6, e1001036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAdow, M.; Missiakas, D.M.; Schneewind, O. Staphylococcus aureus Secretes Coagulase and von Willebrand Factor Binding Protein to Modify the Coagulation Cascade and Establish Host Infections. J. Innate Immun. 2012, 4, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Durand, G.A.; Raoult, D.; Dubourg, G. Antibiotic Discovery: History, Methods and Perspectives. Int. J. Antimicrob. Agents 2019, 53, 371–382. [Google Scholar] [CrossRef]
- Dickey, S.W.; Cheung, G.Y.C.; Otto, M. Different Drugs for Bad Bugs: Antivirulence Strategies in the Age of Antibiotic Resistance. Nat. Rev. Drug Discov. 2017, 16, 457–471. [Google Scholar] [CrossRef]
- Ford, C.A.; Hurford, I.M.; Cassat, J.E. Antivirulence Strategies for the Treatment of Staphylococcus aureus Infections: A Mini Review. Front. Microbiol. 2021, 11, 632706. [Google Scholar] [CrossRef]
- Gao, P.; Wei, Y.; Tai, S.S.C.; Halebeedu Prakash, P.; Iu, H.T.V.; Li, Y.; Yam, H.C.B.; Chen, J.H.K.; Ho, P.L.; Davies, J.; et al. Antivirulence Agent as an Adjuvant of β-Lactam Antibiotics in Treating Staphylococcal Infections. Antibiotics 2022, 11, 819. [Google Scholar] [CrossRef] [PubMed]
- Khodaverdian, V.; Pesho, M.; Truitt, B.; Bollinger, L.; Patel, P.; Nithianantham, S.; Yu, G.; Delaney, E.; Jankowsky, E.; Shoham, M. Discovery of Antivirulence Agents Against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 3645–3652. [Google Scholar] [CrossRef] [PubMed]
- Rasko, D.A.; Sperandio, V. Anti-Virulence Strategies to Combat Bacteria-Mediated Disease. Nat. Rev. Drug Discov. 2010, 9, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Sully, E.K.; Malachowa, N.; Elmore, B.O.; Alexander, S.M.; Femling, J.K.; Gray, B.M.; DeLeo, F.R.; Otto, M.; Cheung, A.L.; Edwards, B.S.; et al. Selective Chemical inhibition of agr Quorum Sensing in Staphylococcus aureus Promotes Host Defense with Minimal Impact on Resistance. PLoS Pathog 2014, 10, e1004174. [Google Scholar] [CrossRef] [PubMed]
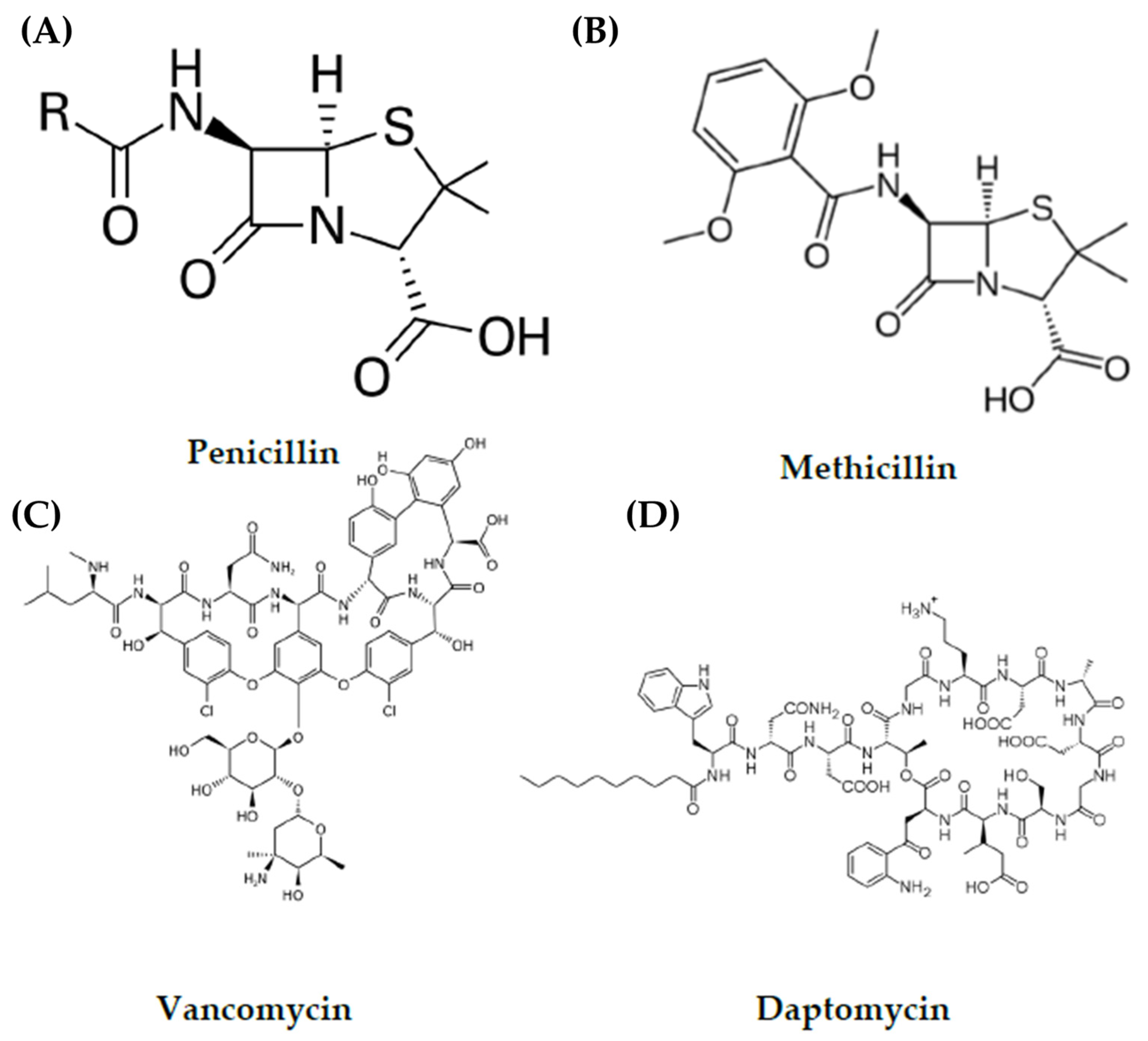

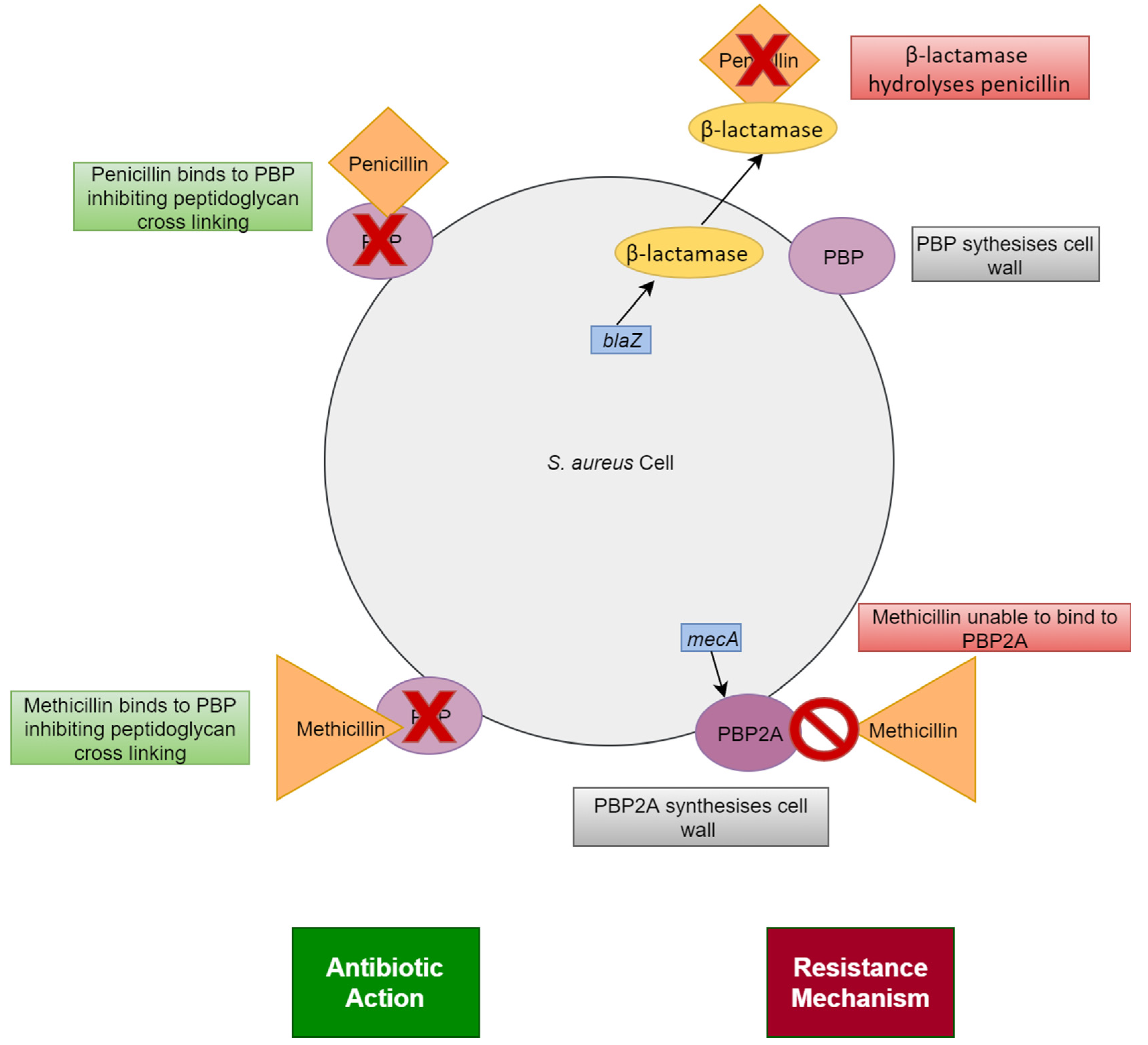
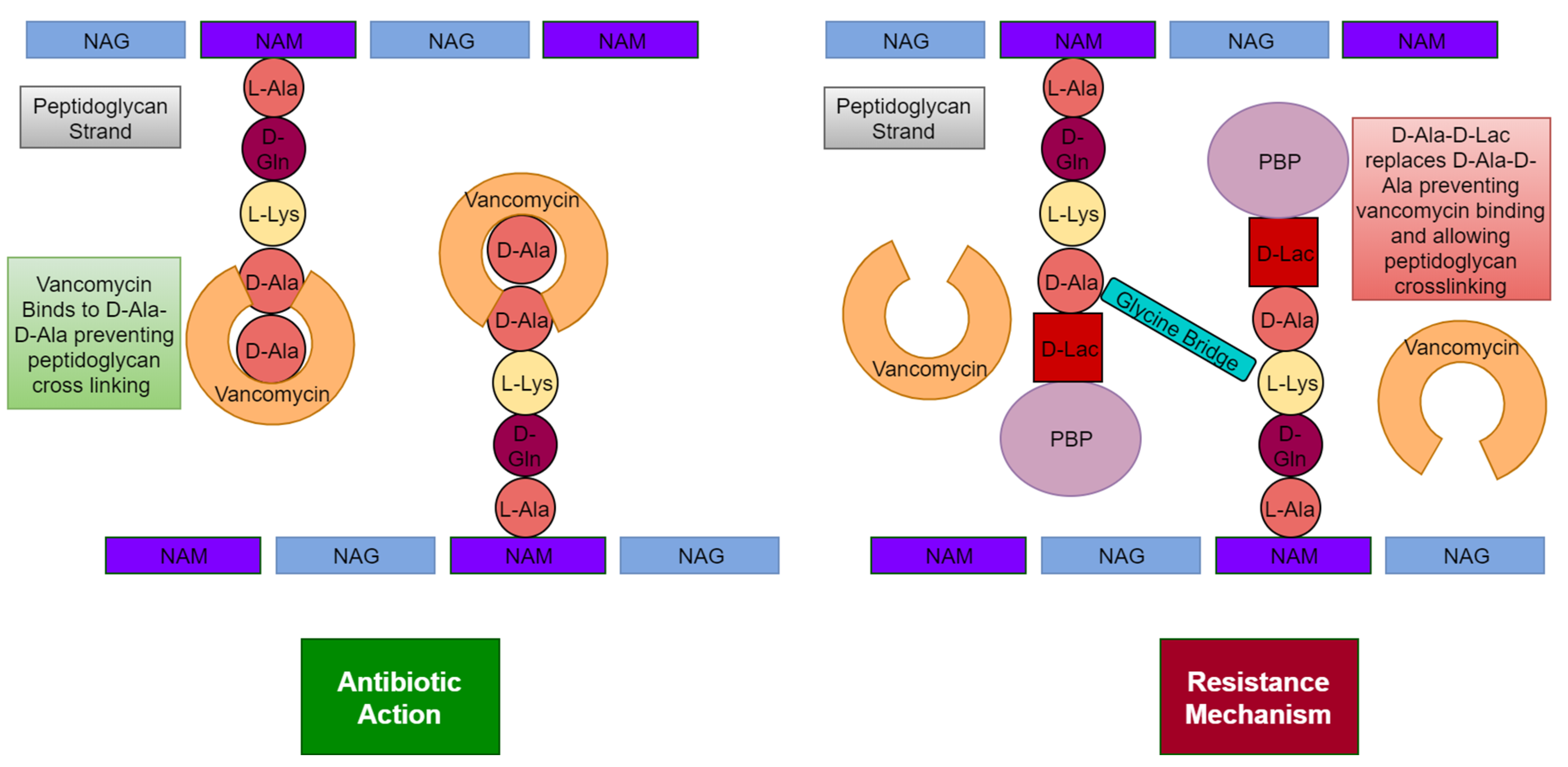
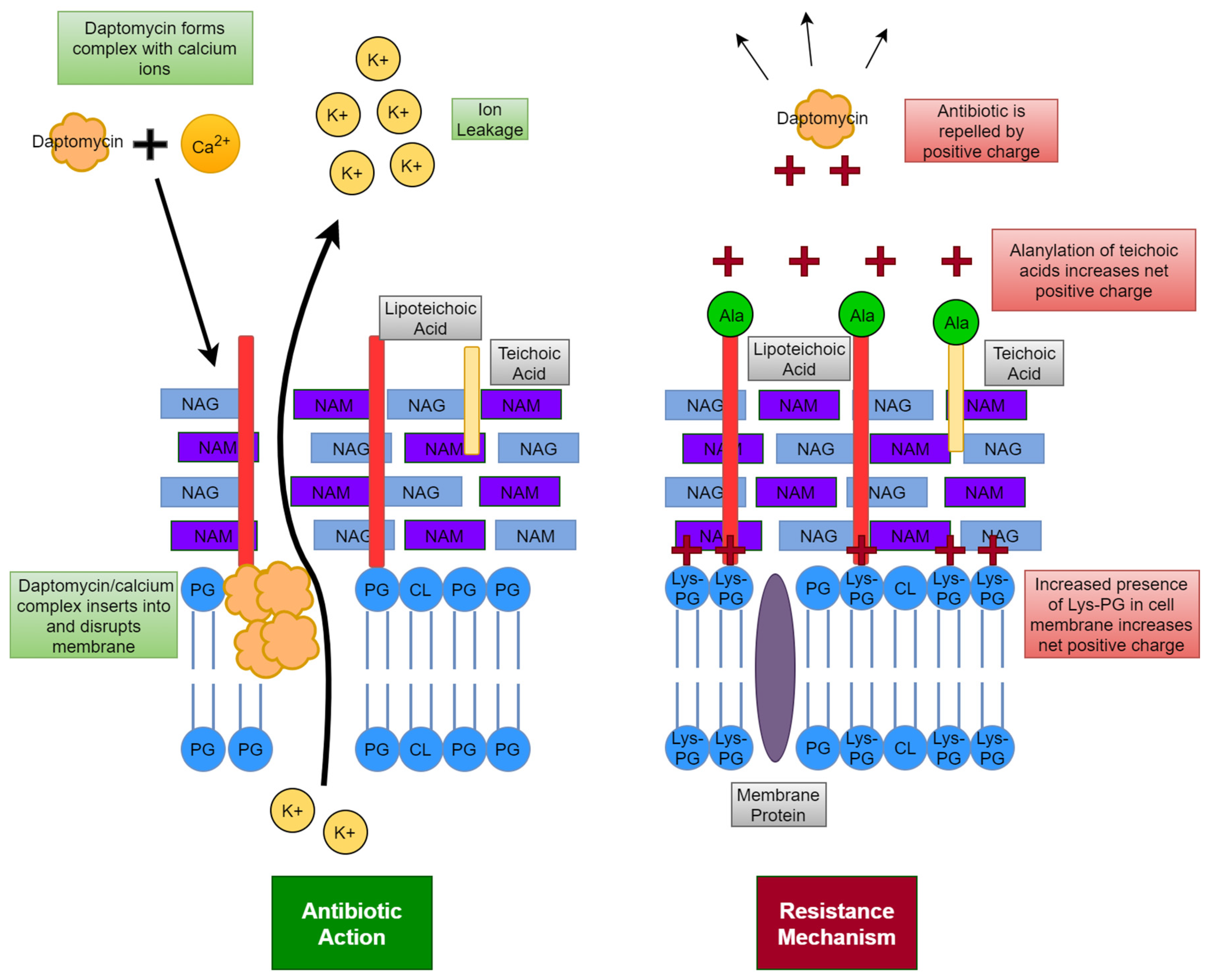
| Type of MRSA | Location Acquired | Associated Diseases |
|---|---|---|
| Healthcare-Associated | Obtained in healthcare settings including nursing homes and hospitals | Bloodstream infections, pneumonia |
| Community-Associated | Obtained in the wider community or within 48 h of admission to a hospital | Skin and soft tissue infections |
| Livestock-Associated | Obtained from close contact with livestock such as cows, pigs and chickens | Mainly skin and soft tissue infections |
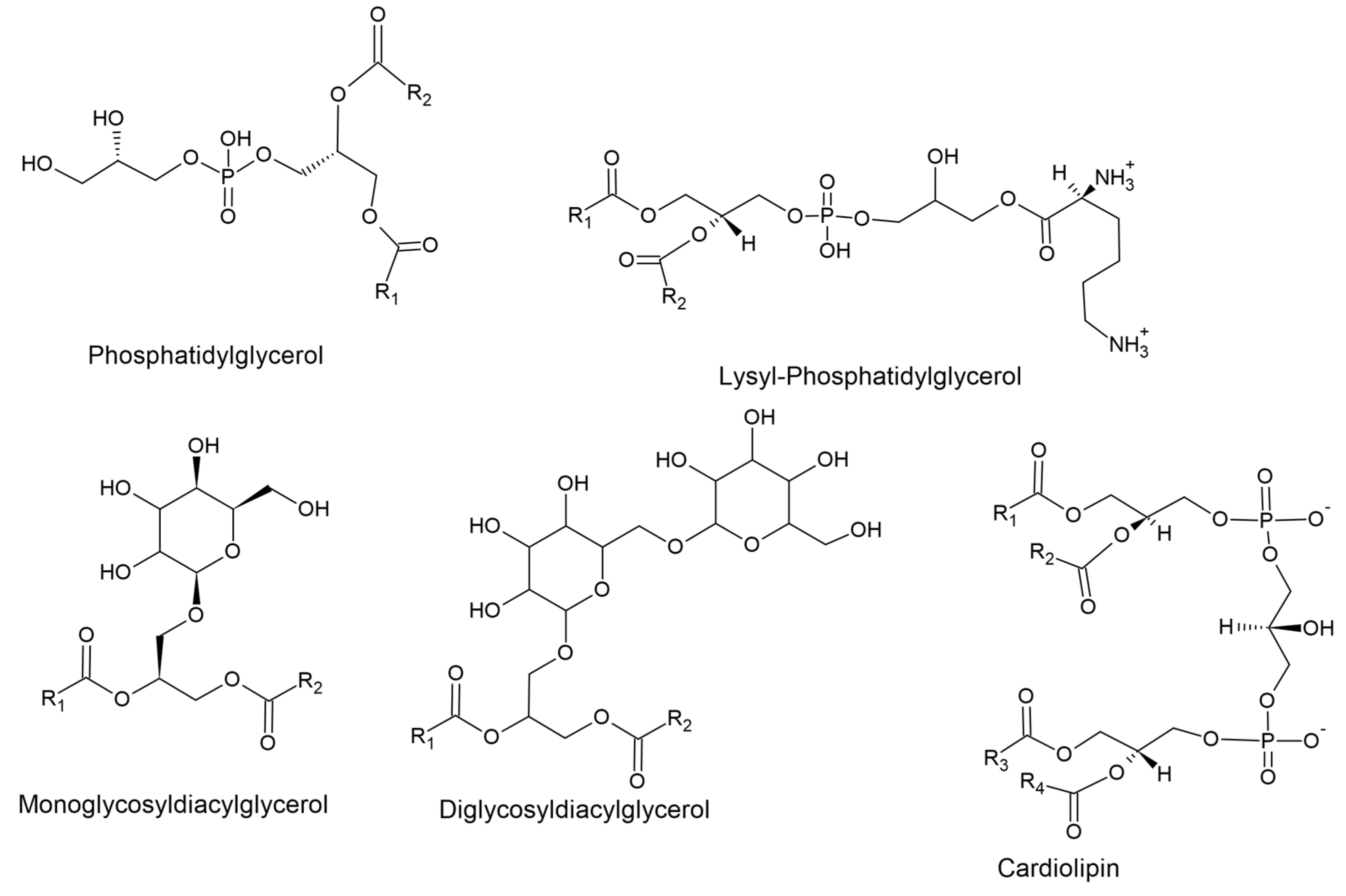
| Lipid Class | Role in Cell Functioning | Role in Antibiotic Resistance | Reference |
|---|---|---|---|
| Phosphatidylglycerol | Contributes negative charge to membrane. Functions as stabiliser and destabiliser by electrostatic interactions between charged species. | Increases susceptibility to positively charged molecules such as CAMPs. | [59,60] |
| Lysyl-Phosphatidylglycerol | Contributes positive charge to membrane. Regulates DNA replication initiation through DNA replication initiator protein DnaA. | Decreases susceptibility to positively charged molecules such as CAMPs. | [54,61] |
| Cardiolipin | Stabilises liposomes against osmotic stress. Essential for long term survival in high salt conditions. | Contributes to daptomycin resistance by preventing membrane permeabilisation | [62,63,64] |
| Monoglycosyldiacylglycerol | Responsible for the stability and fluidity of the cell membrane. Non-bilayer forming. | May play a role in affecting the permeability of the cell to antibiotics. | [57] |
| Diglycosyldiacylglycerol | Responsible for the stability and fluidity of the cell membrane. Bilayer forming. | May play a role in affecting the permeability of the cell to antibiotics. | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolic, P.; Mudgil, P. The Cell Wall, Cell Membrane and Virulence Factors of Staphylococcus aureus and Their Role in Antibiotic Resistance. Microorganisms 2023, 11, 259. https://doi.org/10.3390/microorganisms11020259
Nikolic P, Mudgil P. The Cell Wall, Cell Membrane and Virulence Factors of Staphylococcus aureus and Their Role in Antibiotic Resistance. Microorganisms. 2023; 11(2):259. https://doi.org/10.3390/microorganisms11020259
Chicago/Turabian StyleNikolic, Philip, and Poonam Mudgil. 2023. "The Cell Wall, Cell Membrane and Virulence Factors of Staphylococcus aureus and Their Role in Antibiotic Resistance" Microorganisms 11, no. 2: 259. https://doi.org/10.3390/microorganisms11020259
APA StyleNikolic, P., & Mudgil, P. (2023). The Cell Wall, Cell Membrane and Virulence Factors of Staphylococcus aureus and Their Role in Antibiotic Resistance. Microorganisms, 11(2), 259. https://doi.org/10.3390/microorganisms11020259






