In Vitro Fermentation of Edible Mushrooms: Effects on Faecal Microbiota Characteristics of Autistic and Neurotypical Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fermentation Substrates
2.2. Faecal Donors’ Characteristics
2.3. Ethical Standards
2.4. Fecal Sample Collection and In Vitro Static Batch Culture Fermentations
2.5. Quantitative Polymerase Chain Reaction (qPCR) Enumeration of Gut Microbiota In Vitro
2.6. Prebiotic Indexes
2.7. Measurement of SCFAs
2.8. Statistical Analysis
3. Results
3.1. Descriptive Characteristics of Faecal Donors
3.2. Faecal Microbiota Analysis
3.2.1. Bacterial Enumeration in Neurotypical Children’s Faeces after 24 h of Fermentation
3.2.2. Bacterial Enumeration in ASD Children’s Faeces after 24 h of Fermentation
3.2.3. Alterations in Faecal Bacterial Populations between ASD and Neurotypical Children after 24 h of Fermentation
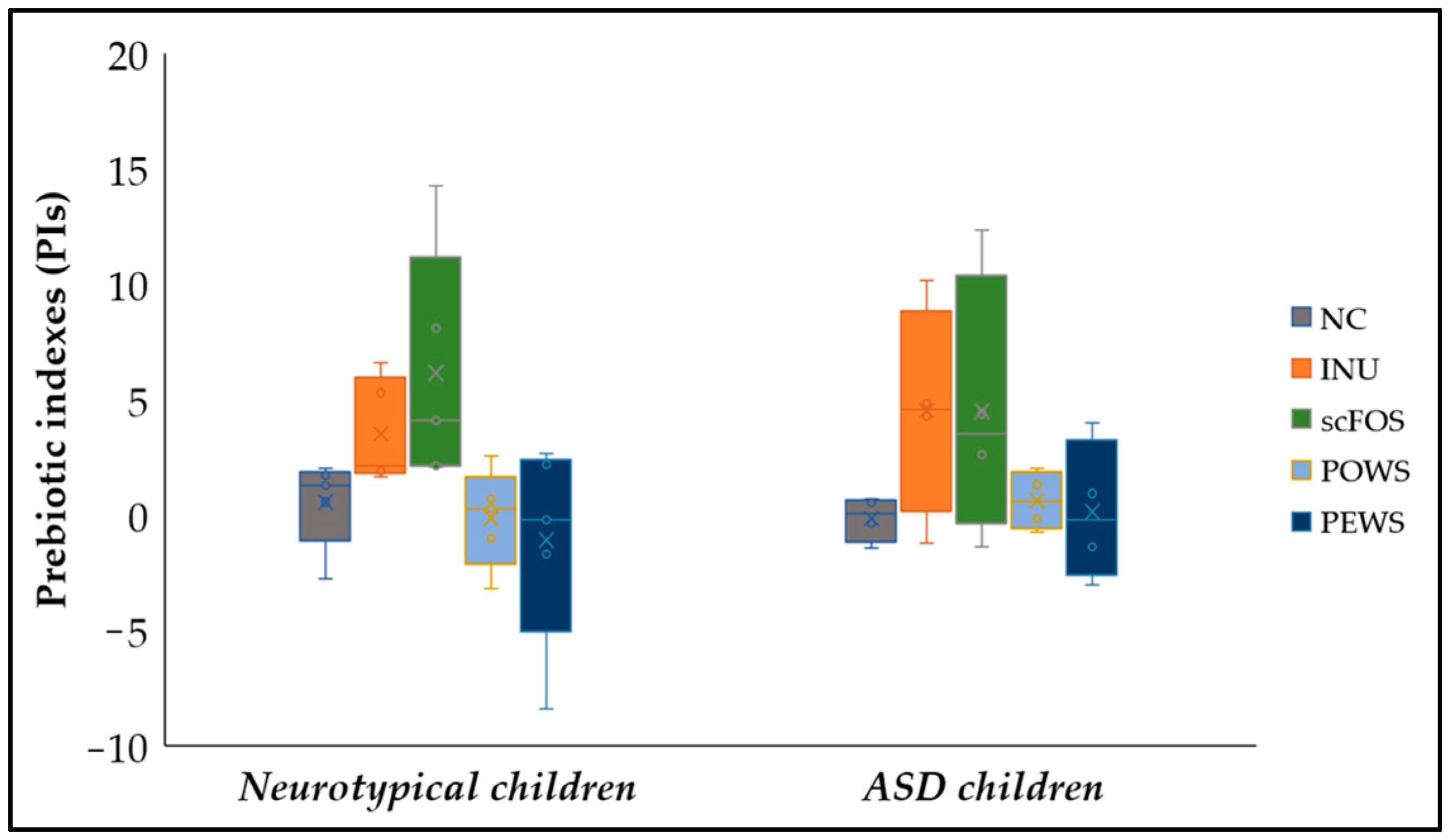
3.3. Prebiotic Indexes (PIs)
3.4. SCFA Production
3.4.1. SCFA Production after 8 and 24 h of Fermentation for the Neurotypical Children Group
3.4.2. SCFA Production after 8 and 24 h of Fermentation for the Autistic Children Group
3.4.3. Differences in Metabolic Products between ASD and Neurotypical Children’ Faeces after 24 h of Fermentation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.; Guo, M.; Yang, T.; Lai, X.; Tang, T.; Chen, J.; Li, L.; Li, T. Nutritional Status and Symptoms in Preschool Children With Autism Spectrum Disorder: A Two-Center Comparative Study in Chongqing and Hainan Province, China. Front. Pediatr. 2020, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Bougeard, C.; Picarel-Blanchot, F.; Schmid, R.; Campbell, R.; Buitelaar, J. Prevalence of Autism Spectrum Disorder and Co-Morbidities in Children and Adolescents: A Systematic Literature Review. Front. Psychiatry 2021, 12, 744709. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gutierrez, E.; Narbad, A.; Rodríguez, J.M. Autism Spectrum Disorder Associated With Gut Microbiota at Immune, Metabolomic, and Neuroactive Level. Front. Neurosci. 2020, 14, 578666. [Google Scholar] [CrossRef] [PubMed]
- Laue, H.E.; Coker, M.O.; Madan, J.C. The Developing Microbiome From Birth to 3 Years: The Gut-Brain Axis and Neurodevelopmental Outcomes. Front. Pediatr. 2022, 10, 815885. [Google Scholar] [CrossRef]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The Role of Microbiota-Gut-Brain Axis in Neuropsychiatric and Neurological Disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Beopoulos, A.; Gea, M.; Fasano, A.; Iris, F. Autonomic Nervous System Neuroanatomical Alterations Could Provoke and Maintain Gastrointestinal Dysbiosis in Autism Spectrum Disorder (ASD): A Novel Microbiome–Host Interaction Mechanistic Hypothesis. Nutrients 2022, 14, 65. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Yitik Tonkaz, G.; Esin, I.S.; Turan, B.; Uslu, H.; Dursun, O.B. Determinants of Leaky Gut and Gut Microbiota Differences in Children With Autism Spectrum Disorder and Their Siblings. J. Autism Dev. Disord. 2022. [Google Scholar] [CrossRef]
- Andreo-Martínez, P.; García-Martínez, N.; Sánchez-Samper, E.; González, A. An Approach to Gut Microbiota Profile in Children with Autism Spectrum Disorder. Environ. Microbiol. Rep. 2019, 12, 115–135. [Google Scholar] [CrossRef]
- Chakraborty, P.; Carpenter, K.L.H.; Major, S.; Deaver, M.; Vermeer, S.; Herold, B.; Franz, L.; Howard, J.; Dawson, G. Gastrointestinal Problems Are Associated with Increased Repetitive Behaviors but Not Social Communication Difficulties in Young Children with Autism Spectrum Disorders. Autism 2021, 25, 405–415. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Jin, Y.; Chen, G.; Ma, X.; Zhang, L. Gut Microbiota Dysbiosis Drives the Development of Colorectal Cancer. Digestion 2021, 102, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, A.; Alhazmi, S.; Alburae, N.; Bahieldin, A. The Human Gut Microbiome as a Potential Factor in Autism Spectrum Disorder. Int. J. Mol. Sci. 2022, 23, 1363. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Vázquez, L.; Van Ginkel Riba, G.; Arija, V.; Canals, J. Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 792. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Grimaldi, R.; Cela, D.; Swann, J.R.; Vulevic, J.; Gibson, G.R.; Tzortzis, G.; Costabile, A. In Vitro Fermentation of B-GOS: Impact on Faecal Bacterial Populations and Metabolic Activity in Autistic and Non-Autistic Children. FEMS Microbiol. Ecol. 2017, 93, fiw233. [Google Scholar] [CrossRef]
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder Not Otherwise Specified. PLoS ONE 2013, 8, e76993. [Google Scholar] [CrossRef]
- Ng, Q.X.; Loke, W.; Venkatanarayanan, N.; Lim, D.Y.; Soh, A.Y.; Yeo, W.S. A Systematic Review of the Role of Prebiotics and Probiotics in Autism Spectrum Disorders. Medicina 2019, 55, 129. [Google Scholar] [CrossRef]
- Saxami, G.; Kerezoudi, E.N.; Mitsou, E.K.; Koutrotsios, G.; Zervakis, G.I.; Pletsa, V.; Kyriacou, A. Fermentation Supernatants of Pleurotus eryngii Mushroom Ameliorate Intestinal Epithelial Barrier Dysfunction in Lipopolysaccharide-Induced Caco-2 Cells via Upregulation of Tight Junctions. Microorganisms 2021, 9, 2071. [Google Scholar] [CrossRef]
- Han, Y.; Xiao, H. Whole Food–Based Approaches to Modulating Gut Microbiota and Associated Diseases. Annu. Rev. Food Sci. Technol. 2020, 11, 119–143. [Google Scholar] [CrossRef] [Green Version]
- Mitsou, E.K.; Saxami, G.; Stamoulou, E.; Kerezoudi, E.; Terzi, E.; Koutrotsios, G.; Bekiaris, G.; Zervakis, G.I.; Mountzouris, K.C.; Pletsa, V.; et al. Effects of Rich in Β-Glucans Edible Mushrooms on Aging Gut Microbiota Characteristics: An In Vitro Study. Molecules 2020, 25, 2806. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.; Mohajeri, H. The Application of in Vitro Human Intestinal Models on the Screening and Development of Pre-And Probiotics. Benef. Microbes 2018, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Koutrotsios, G.; Mountzouris, K.C.; Chatzipavlidis, I.; Zervakis, G.I. Bioconversion of Lignocellulosic Residues by Agrocybe Cylindracea and Pleurotus Ostreatus Mushroom Fungi—Assessment of Their Effect on the Final Product and Spent Substrate Properties. Food Chem. 2014, 161, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Olano-Martin, E.; Gibson, G.R.; Rastell, R.A. Comparison of the in Vitro Bifidogenic Properties of Pectins and Pectic-Oligosaccharides. J. Appl. Microbiol. 2002, 93, 505–511. [Google Scholar] [CrossRef]
- Rycroft, C.; Jones, M.R.; Gibson, G.R.; Rastall, R.A. A Comparative in Vitro Evaluation of the Fermentation Properties of Prebiotic Oligosacharides. J. Appl. Microbiol. 2001, 91, 878–887. [Google Scholar] [CrossRef]
- Palframan, R.; Gibson, G.; Rastall, R. Effect of PH and Dose on the Growth of Gut Bacteria on Prebiotic Carbohydrates in Vitro. Anaerobe 2002, 8, 287–292. [Google Scholar] [CrossRef]
- Duque, A.L.R.F.; Demarqui, F.M.; Santoni, M.M.; Zanelli, C.F.; Adorno, M.A.T.; Milenkovic, D.; Mesa, V.; Sivieri, K. Effect of Probiotic, Prebiotic, and Synbiotic on the Gut Microbiota of Autistic Children Using an in Vitro Gut Microbiome Model. Food Res. Int. 2021, 149, 110657. [Google Scholar] [CrossRef]
- Nadkarni, M.; Martin, F.; Jacques, N.; Hunter, N.; Nadkarni, M.A.; Martin, F.E.; Jacques, N.A.; Hunter, N. Determination of Bacterial Load by Real-Time PCR Using a Broad-Range (Universal) Probe and Primers Set. Microbiol. (Read. Engl.) 2002, 148, 257–266. [Google Scholar] [CrossRef]
- Rinttilä, T.; Lyra (former Kassinen), A.; Malinen, E.; Krogius, L.; Palva, A. Development of an Extensive Set of 16S RRNA-Targeted Primers for Quantification of Pathogenic and Indigenous Bacteria in Fecal Samples by Real-Time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef]
- Feng, J.; Tang, H.; Li, M.; Pang, X.; Wang, L.; Zhang, M.; Zhao, Y.; Zhang, X.; Shen, J. The Abundance of Fecal Faecalibacterium prausnitzii in Relation to Obesity and Gender in Chinese Adults. Arch. Microbiol. 2013, 196, 73–77. [Google Scholar] [CrossRef]
- Suau, A.; Rochet, V.; Sghir, A.; Gramet, G.; Brewaeys, S.; Sutren, M.; Rigottier-Gois, L.; Dore, J. Fusobacterium prausnitzii and Related Species Represent a Dominant Group Within the Human Fecal Flora. Syst. Appl. Microbiol. 2001, 24, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.F.; Cao, W.W.; Cerniglia, C.E. PCR Detection and Quantitation of Predominant Anaerobic Bacteria in Human and Animal Fecal Samples. Appl. Environ. Microbiol. 1996, 62, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Derrien, M.; Isolauri, E.; De Vos, W.; Salminen, S. Intestinal Integrity and Akkermansia muciniphila, a Mucin-Degrading Member of the Intestinal Microbiota Present in Infants, Adults, and the Elderly. Appl. Environ. Microbiol. 2007, 73, 7767–7770. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Distinct Composition of Gut Microbiota during Pregnancy in Overweight and Normal-Weight Women. Am. J. Clin. Nutr. 2008, 88, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Phong, S.F.; Shanmugavelu, S.; Thayalini, K.; Noraini, S.; Wong, H.K. Detection of Lactobacillus, Bacteroides and Clostridium perfringens in the Gastrointestinal Contents of Chicken Fed Different Diets by Real-Time PCR. J. Trop. Agric. Food Sci. 2010, 38, 81–87. [Google Scholar]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Miyamoto, Y.; Takada, T.; Matsumoto, K.; Oyaizu, H.; Tanaka, R. Development of 16S RRNA-Gene-Targeted Group-Specific Primers for the Detection and Identification of Predominant Bacteria in Human Feces. Appl. Environ. Microbiol. 2002, 68, 5445–5451. [Google Scholar] [CrossRef]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of Inulin on the Human Gut Microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2008, 101, 541–550. [Google Scholar] [CrossRef]
- Palframan, R.; Gibson, G.R.; Rastall, R.A. Development of a Quantitative Tool for the Comparison of the Prebiotic Effect of Dietary Oligosaccharides. Lett. Appl. Microbiol. 2003, 37, 281–284. [Google Scholar] [CrossRef]
- Mountzouris, K.; Balaskas, C.; Fava, F.; Tuohy, K.; Gibson, G.; Kostas, F. Profiling of Composition and Metabolic Activities of the Colonic Microflora of Growing Pigs Fed Diets Supplemented with Prebiotic Oligosaccharides. Anaerobe 2006, 12, 178–185. [Google Scholar] [CrossRef]
- StataCorp Stata Statistical Software: Release 15; StataCorp LLC.: College Station, TX, USA, 2017.
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered Gut Microbiota and Short Chain Fatty Acids in Chinese Children with Autism Spectrum Disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Rong, J.; Fu, C.; Wang, W.; Xu, J.; Ju, X.-D. Overall Rebalancing of Gut Microbiota Is Key to Autism Intervention. Front. Psychol. 2022, 13, 862719. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, E.; Bordenstein, S.R.; Quinn, J.L.; Dinan, T.G.; Cryan, J.F. Microbiota and the Social Brain. Science 2019, 366, eaar2016. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.E.; Yano, J.M.; Fung, T.C.; Hsiao, E.Y. The Microbiome and Host Behavior. Annu. Rev. Neurosci. 2017, 40, 21–49. [Google Scholar] [CrossRef]
- Grimaldi, R.; Gibson, G.R.; Vulevic, J.; Giallourou, N.; Castro-Mejía, J.L.; Hansen, L.H.; Leigh Gibson, E.; Nielsen, D.S.; Costabile, A. A Prebiotic Intervention Study in Children with Autism Spectrum Disorders (ASDs). Microbiome 2018, 6, 133. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 2019, 101, 246–259.e6. [Google Scholar] [CrossRef]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal Microbiota in Children with Autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef]
- Inoue, R.; Sakaue, Y.; Kawada, Y.; Tamaki, R.; Yasukawa, Z.; Ozeki, M.; Ueba, S.; Sawai, C.; Nonomura, K.; Tsukahara, T.; et al. Dietary Supplementation with Partially Hydrolyzed Guar Gum Helps Improve Constipation and Gut Dysbiosis Symptoms and Behavioral Irritability in Children with Autism Spectrum Disorder. J. Clin. Biochem. Nutr. 2019, 64, 217–223. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Yang, J.-J.; Zhao, D.-M.; Chen, B.; Zhang, G.-Q.; Chen, S.; Cao, R.-F.; Yu, H.; Zhao, C.-Y.; et al. Probiotics and Fructo-Oligosaccharide Intervention Modulate the Microbiota-Gut Brain Axis to Improve Autism Spectrum Reducing Also the Hyper-Serotonergic State and the Dopamine Metabolism Disorder. Pharmacol. Res. 2020, 157, 104784. [Google Scholar] [CrossRef]
- Kerezoudi, E.N.; Mitsou, E.K.; Gioti, K.; Terzi, E.; Avgousti, I.; Panagiotou, A.; Koutrotsios, G.; Zervakis, G.I.; Mountzouris, K.C.; Tenta, R.; et al. Fermentation of Pleurotus ostreatus and Ganoderma lucidum Mushrooms and Their Extracts by the Gut Microbiota of Healthy and Osteopenic Women: Potential Prebiotic Effect and Impact of Mushroom Fermentation Products on Human Osteoblasts. Food Funct. 2021, 12, 1529–1546. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Audhya, T.; McDonough-Means, S.; Rubin, R.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Nutritional and Metabolic Status of Children with Autism vs. Neurotypical Children, and the Association with Autism Severity. Nutr. Metab. 2011, 8, 34. [Google Scholar] [CrossRef]
- Jendraszak, M.; Gałęcka, M.; Kotwicka, M.; Regdos, A.; Pazgrat-Patan, M.; Andrusiewicz, M. Commercial Microbiota Test Revealed Differences in the Composition of Intestinal Microorganisms between Children with Autism Spectrum Disorders and Neurotypical Peers. Sci. Rep. 2021, 11, 24274. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Elhefnawy, A.M.; Azouz, H.G.; Roshdy, Y.S.; Ashry, M.H.; Ibrahim, A.E.; Meheissen, M.A. Study of the Gut Microbiome Profile in Children with Autism Spectrum Disorder: A Single Tertiary Hospital Experience. J. Mol. Neurosci. 2020, 70, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Bin-Khattaf, R.M.; Alonazi, M.A.; Al-Dbass, A.M.; Almnaizel, A.T.; Aloudah, H.S.; Soliman, D.A.; El-Ansary, A.K. Probiotic Ameliorating Effects of Altered GABA/Glutamate Signaling in a Rodent Model of Autism. Metabolites 2022, 12, 720. [Google Scholar] [CrossRef] [PubMed]
- Pokusaeva, K.; Johnson, C.U.; Luk, B.; Uribe, G.U.; Fu, Y.; Oezguen, N.; Matsunami, R.K.; Lugo, M.; Major, A.M.; Mori-Akiyama, Y.; et al. GABA-producing Bifidobacterium Dentium Modulates Visceral Sensitivity in the Intestine. Neurogastroenterol. Motil. 2017, 29, 147–189. [Google Scholar] [CrossRef] [PubMed]
- Puts, N.; Wodka, E.; Harris, A.; Crocetti, D.; Tommerdahl, M.; Mostofsky, S.; Edden, R. Reduced GABA and Altered Somatosensory Function in Children with Autism Spectrum Disorder. Autism Res. Off. J. Int. Soc. Autism Res. 2016, 10, 1691. [Google Scholar] [CrossRef]
- El-Ansary, A. GABA and Glutamate Imbalance in Autism and Their Reversal as Novel Hypothesis for Effective Treatment Strategy. Autism Dev. Disord. 2020, 18, 46–63. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, W.; Pei, F.; Zhao, L.; Hu, Q. In Vitro Fermentation of Six Kinds of Edible Mushrooms and Its Effects on Fecal Microbiota Composition. LWT 2018, 96, 627–635. [Google Scholar] [CrossRef]
- Russo, P.; López, P.; Capozzi, V.; De Palencia, P.F.; Dueñas, M.T.; Spano, G.; Fiocco, D. Beta-Glucans Improve Growth, Viability and Colonization of Probiotic Microorganisms. Int. J. Mol. Sci. 2012, 13, 6026–6039. [Google Scholar] [CrossRef]
- Muthuramalingam, K.; Singh, V.; Choi, C.; Choi, S.I.; Kim, Y.M.; Unno, T.; Cho, M. Dietary Intervention Using (1,3)/(1,6)-β-Glucan, a Fungus-Derived Soluble Prebiotic Ameliorates High-Fat Diet-Induced Metabolic Distress and Alters Beneficially the Gut Microbiota in Mice Model. Eur. J. Nutr. 2020, 59, 2617–2629. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii Is an Anti-Inflammatory Commensal Bacterium Identified by Gut Microbiota Analysis of Crohn Disease Patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, R.R.; Bourgonje, A.R.; Saeed, A.; Vich Vila, A.; Plomp, N.; Blokzijl, T.; Sadaghian Sadabad, M.; von Martels, J.Z.H.; van Leeuwen, S.S.; Weersma, R.K.; et al. Inulin-Grown Faecalibacterium prausnitzii Cross-Feeds Fructose to the Human Intestinal Epithelium. Gut Microbes 2021, 13, 1993582. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of Microbiota-Derived Short-Chain Fatty Acids in Nervous System Disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef] [PubMed]
- MacFabe, D. Enteric Short-Chain Fatty Acids: Microbial Messengers of Metabolism, Mitochondria, and Mind: Implications in Autism Spectrum Disorders. Microb. Ecol. Health Dis. 2015, 26, 28177. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Kang, D.-W.; Ilhan, Z.E.; Isern, N.G.; Hoyt, D.W.; Howsmon, D.P.; Shaffer, M.; Lozupone, C.A.; Hahn, J.; Adams, J.B.; Krajmalnik-Brown, R. Differences in Fecal Microbial Metabolites and Microbiota of Children with Autism Spectrum Disorders. Anaerobe 2018, 49, 121–131. [Google Scholar] [CrossRef]
- Srikantha, P.; Mohajeri, M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef]
- Oh, D.; Cheon, K.-A. Alteration of Gut Microbiota in Autism Spectrum Disorder: An Overview. J. Korean Acad. Child Adolesc. Psychiatry 2020, 31, 131–145. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of Inflammation by Short Chain Fatty Acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shultz, S.R.; MacFabe, D.F. Propionic Acid Animal Model of Autism. In Comprehensive Guide to Autism; Patel, V., Preedy, V., Martin, C., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
| Description | Abbreviation |
|---|---|
| Pleurotus eryngii LGAM 216 | PE |
| Pleurotus ostreatus IK 1123 | PO |
| Inulin | INU |
| Short-chain fructooligosaccharides | scFOS |
| Negative control (basal medium with no carbohydrate source) | NC |
| Target | Primer | Primer Sequence (5′-3′) | Reference Strains | References |
|---|---|---|---|---|
| Total Bacteria | Forward | TCCTACGGGAGGCAGCAGT | Bacteroides fragilis MM44 (ATCC 25285) | [28] |
| Reverse | GGACTACCAGGGTATCTAATCC TGTT | |||
| Lactobacillus group | Forward | AGCAGTAGGGAATCTTCCA | L. gasseri DSM 20243 | [29] |
| Reverse | CACCGCTACACATGGAG | |||
| Bifidobacterium spp. | Forward | TCGCGTCYGGTGTGAAAG | B. bifidum DSM 20456 | [29] |
| Reverse | CCACATCCAGCRTCCAC | |||
| F. prausnitzii | FPR-2F | GGAGGAAGAAGGTCTTCGG | F. prausnitzii DSM 17677 | [30,31,32] |
| Fprau645R | AATTCCGCCTACCTCTGCACT | |||
| A. muciniphila | AM1 | CAGCACGTGAAGGTGGGGAC | A. muciniphila DSM 22959 | [33,34] |
| AM2 | CCTTGCGGTTGGCTTCAGAT | |||
| C. perfringens group | CPF | ATGCAAGTCGAGCGATG | C. perfringens ATCC 13124 | [35] |
| CPR | TATGCGGTATTAATCTCCCTTT | |||
| Prevotella spp. | g-Prevo-F | CACRGTAAACGATGGATGC | Prevotella copri DSM 18205 | [36] |
| g-Prevo-R | GGTCGGGTTGCAGACC | |||
| Bacteroides spp. | Bac303F | GAAGGTCCCCCACATTG | B. fragilis MM44 (ATCC 25285) | [37] |
| Bfr-Fmrev | CGCKACTTGGCTGGTTCAG |
| ASD Subjects (n = 5) | NT Subjects (n = 5) | p-Value | |
|---|---|---|---|
| Sociodemographic parameters | |||
| Sex (no. of males/females), n (%) | 5/0 (100%, 0%) | 5/0 (100%, 0%) | 1.000 |
| Age (years) | 5.5 (5.5, 6) | 7 (5, 7.5) | 0.461 |
| Stool characteristics | |||
| Evacuation frequency (times d−1) | 2 (1.5, 2) | 1 (1, 1.5) | 0.072 |
| Stool moisture (%) | 75.97 (68, 31, 85, 18) | 63.19 (59.51, 78.40) | 0.175 |
| Stool pH | 5.74 (5.66, 6.25) | 7.04 (6.32, 7.41) | 0.016 * |
| Bristol Stool Scale | 4 (3, 4.5) | 2 (1.5, 4.5) | 0.167 |
| Anthropometric measurements | |||
| Body weight (kg) | 25 (21, 26) | 26 (14.6, 28) | 0.917 |
| Height (cm) | 110 (110, 125) | 124 (112, 129) | 0.462 |
| BMI (kg m−2) | 17.3 (16, 17.4) | 15.6 (15.3, 16.8) | 0.465 |
| Nutritional analysis | |||
| Energy intake (kcal d−1) | 1742.5 (1645, 1909.2) | 1435.5 (1092.6, 1694.3) | 0.463 |
| Carbohydrate (% of energy) | 40.2 (39.4, 40.2) | 45.4 (44.5, 48.5) | 0.075 |
| Carbohydrate (g d−1) | 170.5 (163.9, 191.7) | 146 (134.3, 192.4) | 0.600 |
| Protein (% of energy) | 19.6 (17, 22.9) | 17.6 (17.1, 23.9) | 0.600 |
| Protein (g d−1) | 80.7 (62.9, 109) | 66.2 (57.2, 74.4) | 0.600 |
| Fat (% of energy) | 36.9 (36.9, 41) | 30.7 (27.6, 37.1) | 0.463 |
| Fat (g d−1) | 78.3 (75.1, 78.3) | 69.8 (34, 70.5) | 0.075 |
| Fibers (g d−1) | 10.7 (9.7, 11.1) | 9.6 (8.4, 13.6) | 0.917 |
| NC | INU | scFOS | PO | PE | Overall p | |
|---|---|---|---|---|---|---|
| Total bacteria | 9.83 (9.77–9.99) | 10.34 (10.26–10.37) a | 10.33 (10.27–10.41) a | 10.40 (10.27–10.47) *,a | 10.41 (10.30–10.60) *,a | 0.013 |
| Bifidobacterium spp. | 8.96 (8.60–9.47) | 10.17 (9.88–10.30) *,a | 10.23 (10.15–10.33) *,a | 9.90 (9.34–10.00) a | 9.72 (9.23–10.18) a | 0.004 |
| Lactobacillus group | 5.09 (4.76–6.31) | 5.43 (4.75–6.40) | 5.31 (4.61–6.35) | 5.35 (4.80–6.29) | 5.21 (4.88–6.52) | 0.998 |
| C. perfringens group | 6.99 (6.54–7.21) | 7.27 (6.74–7.93) | 7.18 (6.70–7.79) | 7.25 (6.69–7.56) | 7.42 (6.79–8.03) | 0.563 |
| Bacteroides spp. | 9.16 (9.04–9.44) | 9.85 (9.73–10.10) a | 9.67 (9.59–10.08) a | 10.00 (9.60–10.28) a | 9.82 (9.51–10.21) a | 0.033 |
| F. prausnitzii | 8.24 (6.97–8.49) a | 8.67 (8.11–8.88) | 8.62 (8.07–8.86) | 9.08 (8.36–9.20) a | 8.80 (8.52–9.25) | 0.050 |
| Prevotella spp. | 7.69 (7.44–8.52) | 9.61 (7.87–10.05) | 9.39 (7.83–9.92) | 9.35 (7.12–10.09) | 9.30 (8.25–9.98) | 0.431 |
| A. muciniphila | 4.80 (4.65–8.23) | 4.81 (4.25–7.99) | 4.76 (4.18–8.20) | 4.74 (4.55–8.26) | 4.67 (4.45–8.32) a | 0.981 |
| NC | INU | scFOS | PO | PE | Overall p | |
|---|---|---|---|---|---|---|
| Total bacteria | 10.14 (10.05–10.18) † | 10.34 (10.33–10.37) a | 10.42 (10.33–10.48) a | 10.39 (10.31–10.54) | 10.66 (10.42–10.75) * | 0.005 |
| Bifidobacterium spp. | 8.83 (7.50–9.28) a | 10.11 (8.21–10.25) a | 10.15 (8.22–9.26) a | 9.72 (7.89–9.93) a | 9.56 (7.95–10.20) a | 0.132 |
| Lactobacillus group | 5.11 (4.75–5.71) | 5.46 (5.05–5.64) | 5.45 (5.06–5.56) | 5.38 (5.11–5.57) | 5.58 (5.28–5.76) | 0.832 |
| C. perfringens group | 6.65 (5.45–7.21) | 6.82 (5.53–7.66) | 6.94 (5.53–7.73) | 6.97 (5.71–7.60) | 7.30 (5.86–8.54) | 0.715 |
| Bacteroides spp. | 9.32 (9.14–9.48) | 9.74 (9.53–9.89) a | 9.74 (9.47-9.89) a | 9.82 (9.50–9.86) | 10.04 (9.75–10.07) * | 0.029 |
| F. prausnitzii | 8.44 (8.40–8.70) | 9.03 (8.80–9.20) † | 8.92 (8.82–9.16) † | 9.02 (8.82–9.35) | 9.23 (8.92–9.60) * | 0.045 |
| Prevotella spp. | 7.04 (6.59–7.53) | 7.16 (6.93–8.33) a | 7.24 (7.05–8.47) a | 7.08 (7.00–8.33) a | 7.32 (7.15–8.63) a | 0.450 |
| A. muciniphila | 8.59 (6.32–8.99) | 7.02 (5.55–8.97) | 6.74 (5.63–8.97) | 6.93 (5.80–8.97) | 7.20 (5.88–9.00) | 0.979 |
| Concentration μmol/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | INU | scFOS | PO | PE | Overall p Value | |||||||
| 8 h | 24 h | 8 h | 24 h | 8 h | 24 h | 8 h | 24 h | 8 h | 24 h | 8 h | 24 h | |
| Total VFAs | 11.88 (7.24–14.31) a | 22.11 (20.39–24.34) a,b | 35.62 (19.25–46.16) a | 56.00 (51.03–82.07) a,b | 34.76 (21.03–48.66) a | 73.51 (71.48–79.49) a,b | 33.79 (22.03–46.65) a | 71.03 (57.36–74.97) a,b | 39.78 (22.24–62.09) *,a | 81.38 (76.27–84.40) *,a,b | 0.017 | 0.005 |
| AA | 6.86 (4.54–8.70) a | 13.06 (11.46–13.61) a,b | 22.86 (14.13–29.03) a | 42.41 (28.33–63.88) a,b | 25.57 (15.70–35.04) *,a | 67.79 (52.66–68.99) *,a,b | 23.28 (16.41–27.21) a | 38.90 (33.21–39.80) a,b | 25.46 (16.73–32.49) *,a | 44.82 (36.25–49.76) a,b | 0.018 | 0.002 |
| PPA | 1.92 (1.28–3.11) a | 2.77 (2.47–5.05) a,b | 4.24 (1.65–15.09) a | 7.04 (5.62–19.73) a,b | 3.93 (1.76–11.68) a | 5.18 (3.85–14.23) a,b | 4.69 (2.28–12.45) a | 11.19 (8.76–19.98) *,a,b | 4.94 (2.31–22.79) a | 16.53 (9.92–27.63) *,a,b | 0.327 | 0.010 |
| BA | 1.14 (0.64–1.97) | 2.70 (1.95–3.59) a,b | 3.83 (2.05-4.61) | 4.97 (3.95–10.85) a,b | 3.10 (1.20–4.38) a | 3.58 (1.52–7.11) a | 4.79 (2.34–6.98) a | 14.15 (10.39–17.52) a b | 4.78 (2.55–8.44) a | 16.45 (14.34–21.18) *,†,a,b | 0.109 | 0.001 |
| BSCFAs | 0.46 (0.29–0.71) a | 2.39 (2.11–2.50)a,b | 0.13 (0.10–0.31) | 0.21 (0.16–0.41) b | 0.11 (0.09–0.27) | 0.13 (0.09–0.30) * | 0.25 (0.15–0.35) | 0.38 (0.29–2.34) a,b | 0.19 (0.16–0.31) | 0.28 (0.21–0.92) a,b | 0.056 | 0.006 |
| Other | 0.47 (0.24–0.57) a | 1.17 (0.74–1.25) a,b | 0.29 (0.20–0.38) a | 0.33 (0.24–0.46) a | 0.21 (0.17–0.31) | 0.24 (0.19–0.33) *,b | 0.29 (0.22–0.51) a | 0.82 (0.55–2.29) †,a,b | 0.27 (0.23–0.40) a | 0.60 (0.48–1.33) a,b | 0.297 | 0.002 |
| Concentration (μmol/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NC | INU | scFOS | PO | PE | Overall p Value | |||||||
| 8 h | 24 h | 8 h | 24 h | 8 h | 24 h | 8 h | 24 h | 8 h | 24 h | 8 h | 24 h | |
| TSCFAs | 11.67 (11.44–18.27) a | 23.44 (22.94–28.34) a,b | 28.21 (22.80–41.49) a | 47.78 (39.33–66.32) a,b | 31.11 (26.93–47.15) a | 63.78 (46.38–79.41) a,b | 36.74 (30.63–44.75) a | 60.81 (51.83–69.15) a,b | 47.21 (36.06–52.26) *,a | 78.61 (60.99–84.45) *,a,b | 0.007 | 0.005 |
| AA | 8.03 (6.45–11.69) a | 14.58 (12.07–17.25) a,b | 17.74 (15.76–31.10) a | 33.82 (23.50–52.40) a,b | 22.96 (18.35–38.53) a | 52.91 (34.03–70.19) *,a,b | 26.35 (19.85–27.67) a | 30.12 (27.82–37.95) a,b | 26.58 (23.52–37.06) *,a | 40.53 (30.59–52.39) a | 0.013 | 0.012 |
| PPA | 2.21 (1.74–2.63) a | 3.80 (3.18–4.12) a,b | 4.37 (2.28–4.65) a | 5.80 (3.20–6.22) a,b | 3.51 (2.03–5.24) a | 3.87 (2.39–6.24) a,b | 6.94 (3.64–7.40) a | 9.69 (9.29–12.13) *,a,b | 7.88 (3.33–11.22) *,a | 12.63 (9.79–17.90) *,†,a, b | 0.029 | 0.001 |
| BA | 1.80 (1.62–2.56) | 3.34 (2.81–4.91) a,b | 5.04 (3.09–6.68) a | 9.35 (5.61–11.71) a,b | 4.99 (2.19–6.50) a | 6.10 (2.54–9.75) a | 7.39 (3.29–10.28) a | 17.86 (10.18–20.37) *,a,b | 6.99 (2.73–11.61) a | 19.97 (7.35–26.23) *,a,b | 0.042 | 0.010 |
| BSCFAs | 0.53 (0.37–1.27) a | 2.25 (2.24–2.30) a,b | 0.22 (0.19–0.34) a | 0.48 (0.43–0.92) a,b | 0.20 (0.15–0.31) *,a | 0.24 (0.18–0.28) * | 0.25 (0.21–0.35) a | 0.59 (0.31–2.32) a,b | 0.29 (0.24–0.63) a | 0.91 (0.29–1.55) a,b | 0.030 | 0.003 |
| Other | 0.32 (0.24–0.54) a | 0.84 (0.78–0.94) a,b | 0.23 (0.18–0.32) | 0.28 (0.26–0.45) a,b | 0.17 (0.15–0.27) | 0.19 (0.16–0.29) * | 0.17 (0.15–0.37) | 0.32 (0.24–1.48) b | 0.15 (0.13–0.51) | 0.37 (0.20–1.25) a,b | 0.257 | 0.029 |
| Other | 2.72 (1.79–3.52) | 3.41 (3.00–3.89) | 0.71 (0.63–1.04) a | 0.62 (0.41–1.01) a | 0.54 (0.41–0.82) *,a | 0.30 (0.22–0.58) *,a | 0.55 (0.47–0.86) a | 0.53 (0.46–2.22) a | 0.39 (0.32–1.05) *,a | 0.51 (0.34–1.49) a | 0.007 | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saxami, G.; Mitsou, E.K.; Kerezoudi, E.N.; Mavrouli, I.; Vlassopoulou, M.; Koutrotsios, G.; Mountzouris, K.C.; Zervakis, G.I.; Kyriacou, A. In Vitro Fermentation of Edible Mushrooms: Effects on Faecal Microbiota Characteristics of Autistic and Neurotypical Children. Microorganisms 2023, 11, 414. https://doi.org/10.3390/microorganisms11020414
Saxami G, Mitsou EK, Kerezoudi EN, Mavrouli I, Vlassopoulou M, Koutrotsios G, Mountzouris KC, Zervakis GI, Kyriacou A. In Vitro Fermentation of Edible Mushrooms: Effects on Faecal Microbiota Characteristics of Autistic and Neurotypical Children. Microorganisms. 2023; 11(2):414. https://doi.org/10.3390/microorganisms11020414
Chicago/Turabian StyleSaxami, Georgia, Evdokia K. Mitsou, Evangelia N. Kerezoudi, Ioanna Mavrouli, Marigoula Vlassopoulou, Georgios Koutrotsios, Konstantinos C. Mountzouris, Georgios I. Zervakis, and Adamantini Kyriacou. 2023. "In Vitro Fermentation of Edible Mushrooms: Effects on Faecal Microbiota Characteristics of Autistic and Neurotypical Children" Microorganisms 11, no. 2: 414. https://doi.org/10.3390/microorganisms11020414







