High-Molecular-Weight Plasmids Carrying Carbapenemase Genes blaNDM-1, blaKPC-2, and blaOXA-48 Coexisting in Clinical Klebsiella pneumoniae Strains of ST39
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioethical Requirements
2.2. Bacterial Strains, Growing, and Identification
2.3. Susceptibility to Antimicrobials
2.4. Whole Genome Sequencing, Assembly and Annotation
2.5. Whole Genome Analysis
2.6. Nucleotide Sequences Submitted into GenBank Database and Reference Plasmids
3. Results
3.1. Characteristics of K. pneumoniae Strains
3.2. Characteristics of the Chromosoms
- -
- the lysophospholipid acyltransferase family protein gene was functional in the strain SCPM-O-B-8922 (KIF64_00650), but disrupted by insertion of an IS5-like element ISKpn26 family transposase in the strains SCPM-O-B-8912, SCPM-O-B-8919, and SCPM-O-B-8923 (KFX87_000655, KF986_000655, and KIF80_000655, respectively);
- -
- the O-antigen ligase family protein gene in the strain SCPM-O-B-8922 (KIF64_00770) was disrupted by insertion of an IS5-like element ISKpn26 family transposase (KIF64_00765), unlike this gene in the other three strains;
- -
- the gene glpT encoding glycerol-3-phosphate transporter in the strain SCPM-O-B-8923 (KIF80_007475) was disrupted by the insertion of an IS4-like element by an ISVsa5 family transposase;
- -
- the insertion of an IS5-like element ISKpn26 family transposase (KIF64_08135) was detected in the intergenic space between the phosphatase PAP2 family protein gene (KIF64_08130) and hypothetical protein gene (KIF64_08140) in the strain SCPM-O-B-8922, in contrast to other three strains;
- -
- four genes, homologues to gmlABC (providing the structural modification of D-galactan I) and kfoC (unknown function), in the rfb cluster were deleted and replaced by the insertion of an IS5-like element ISKpn26 family transposase in the strain SCPM-O-B-8922; the rfb cluster in the chromosomes of the other three strains was complete and homologous to the K. pneumoniae type O1/O2-antigen gene cluster;
- -
- the IV secretion system protein gene was disrupted by the insertion of an IS5-like element, ISKpn26 family transposase (KFX87_008725), in the strain SCPM-O-B-8912;
- -
- the aspartate/glutamate racemase family protein gene was functional in the strain SCPM-O-B-8922 (KIF64_08860), while it was disrupted by the insertion of an IS1-like element by the IS1B family transposase in the strains SCPM-O-B-8912, SCPM-O-B-8919, and SCPM-O-B-8923 (KFX87_008890, KF986_008880, and KIF80_008890, respectively);
- -
- the PhoP/PhoQ regulator membrane protein MgrB gene was disrupted by the insertion of an IS5-like element ISKpn26 family transposase (KIF64_09410) in the strain SCPM-O-B-8922;
- -
- the inversion of three genes, KF986_013800, KF986_013805, and KF986_013810, encoding hypothetical proteins, has been detected in the strain SCPM-O-B-8919.
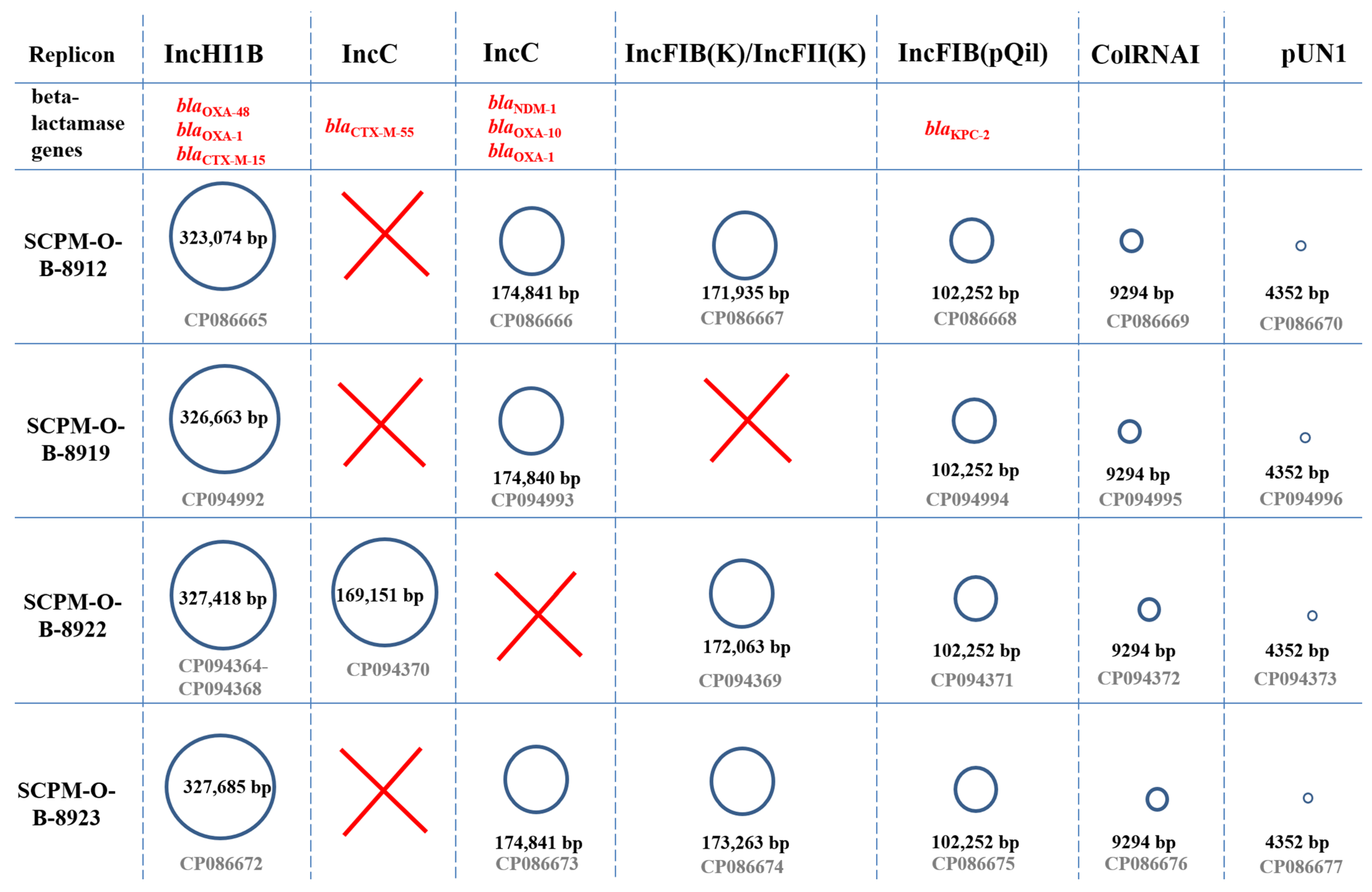
3.3. Characteristics of the Plasmids
3.3.1. Characteristics of IncHI1B Plasmids Carrying the blaOXA-48 Carbapenemase Gene
3.3.2. Characteristics of IncC plasmids Carrying the blaNDM-1 Carbapenemase Gene
3.3.3. Characteristics of IncFIB(pQil) Plasmids Carrying the blaKPC-2 Carbapenemase Gene
3.3.4. Characteristics of IncC Plasmids Carrying the blaCTX-M-55 Cephalosporinase Gene
3.3.5. Characteristics of IncFIB(K)/IncFII(K) Plasmids
3.3.6. Characteristics of Low-Molecular-Weight Plasmid pColRNAI
3.3.7. Characteristics of Low-Molecular-Weight Plasmid pUN1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoo, G.S.R.; Cai, Y.; Quek, Y.C.; Teo, J.Q.; Choudhury, S.; Koh, T.H.; Lim, T.P.; Marimuthu, K.; Ng, O.T.; Kwa, A.L. Predictors and outcomes of healthcare-associated infections caused by carbapenem-nonsusceptible Enterobacterales: A parallel matched case-control study. Front. Cell Infect. Microbiol. 2022, 12, 719421. [Google Scholar] [CrossRef] [PubMed]
- Iordanou, S.; Papathanassoglou, E.; Middleton, N.; Palazis, L.; Timiliotou-Matsentidou, C.; Raftopoulos, V. Device-associated health care-associated infections: The effectiveness of a 3-year prevention and control program in the Republic of Cyprus. Nurs. Crit. Care. 2022, 27, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Effah, C.Y.; Sun, T.; Liu, S.; Wu, Y. Klebsiella pneumoniae: An increasing threat to public health. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1–9. [Google Scholar] [CrossRef]
- Suleyman, G.; Alangaden, G.; Bardossy, A.C. The role of environmental contamination in the transmission of nosocomial pathogens and healthcare-associated infections. Curr. Infect. Dis. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Dhingra, S.; Rahman, N.A.A.; Peile, E.; Rahman, M.; Sartelli, M.; Hassali, M.A.; Islam, T.; Islam, S.; Haque, M. Microbial resistance movements: An overview of global public health threats posed by antimicrobial resistance, and how best to counter. Front. public health. 2020, 8, 535668. [Google Scholar] [CrossRef] [PubMed]
- Abadi, A.T.B.; Rizvanov, A.A.; Haertlé, T.; Blatt, N.L. World Health Organization report: Current crisis of antibiotic resistance. BioNanoSci 2019, 9, 778–788. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Gorrie, C.L.; Mirčeta, M.; Wick, R.R.; Judd, L.M.; Lam, M.M.C.; Gomi, R.; Abbott, I.J.; Thomson, N.R.; Strugnell, R.A.; Pratt, N.F.; et al. Genomic dissection of Klebsiella pneumoniae infections in hospital patients reveals insights into an opportunistic pathogen. Nat. Commun. 2022, 13, 3017. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Aurilio, C.; Sansone, P.; Barbarisi, M.; Pota, V.; Giaccari, L.G.; Coppolino, F.; Barbarisi, A.; Passavanti, M.B.; Pace, M.C. Mechanisms of action of carbapenem resistance. Antibiotics 2022, 11, 421. [Google Scholar] [CrossRef]
- Jeon, J.H.; Lee, J.H.; Lee, J.J.; Park, K.S.; Karim, A.M.; Lee, C.R.; Jeong, B.C.; Lee, S.H. Structural basis for carbapenem-hydrolyzing mechanisms of carbapenemases conferring antibiotic resistance. Int. J. Mol. Sci. 2015, 16, 9654–9692. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Y.; Wang, R.; Wang, Q.; Jin, L.; Wang, H. The transferability and evolution of NDM-1 and KPC-2 co-producing Klebsiella pneumoniae from clinical settings. EBioMedicine 2020, 51, 102599. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Zhang, B.; Deng, J.; Wei, Y.; Xiao, X.; Liu, J. Emergence of a hypervirulent tigecycline-resistant Klebsiella pneumoniae strain co-producing blaNDM-1 and blaKPC-2 with an uncommon sequence type ST464 in Southwestern China. Front. Microbiol. 2022, 13, 868705. [Google Scholar] [CrossRef] [PubMed]
- Protonotariou, E.; Meletis, G.; Chatzopoulou, F.; Malousi, A.; Chatzidimitriou, D.; Skoura, L. Emergence of Klebsiella pneumoniae ST11 co-producing NDM-1 and OXA-48 carbapenemases in Greece. J. Glob. Antimicrob. Resist. 2019, 19, 81–82. [Google Scholar] [CrossRef]
- Lorenzin, G.; Gona, F.; Battaglia, S.; Spitaleri, A.; Saluzzo, F.; Trovato, A.; di Marco, F.; Cichero, P.; Biancardi, A.; Nizzero, P.; et al. Detection of NDM-1/5 and OXA-48 co-producing extensively drug-resistant hypervirulent Klebsiella pneumoniae in Northern Italy. J. Glob. Antimicrob. Resist. 2022, 28, 146–150. [Google Scholar] [CrossRef]
- Fursova, N.K.; Astashkin, E.I.; Ershova, O.N.; Aleksandrova, I.A.; Savin, I.A.; Novikova, T.S.; Fedyukina, G.N.; Kislichkina, A.A.; Fursov, M.V.; Kuzina, E.S.; et al. Multidrug-resistant Klebsiella pneumoniae causing severe infections in the neuro-ICU. Antibiotics 2021, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.E.E.; Yang, Y.; Yang, Y.; Yan, B.; Chen, G.; Hassan, R.M.; Zhong, L.L.; Chen, Y.; Roberts, A.P.; Wu, Y.; et al. Emergence of hypervirulent carbapenem-resistant Klebsiella pneumoniae coharboring a blaNDM-1-carrying virulent plasmid and a blaKPC-2-carrying plasmid in an Egyptian hospital. mSphere 2021, 6, e00088-21. [Google Scholar] [CrossRef]
- Yang, X.; Dong, N.; Chan, E.W.; Zhang, R.; Chen, S. Carbapenem resistance-encoding and virulence-encoding conjugative plasmids in Klebsiella pneumoniae. Trends Microbiol. 2021, 29, 65–83. [Google Scholar] [CrossRef]
- Khan, M.A.; Mohamed, A.M.; Faiz, A.; Ahmad, J. Enterobacterial infection in Saudi Arabia: First record of Klebsiella pneumoniae with triple carbapenemase genes resistance. J. Infect. Dev. Ctries 2019, 13, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Mataseje, L.F.; Chen, L.; Peirano, G.; Fakharuddin, K.; Kreiswith, B.; Mulvey, M.; Pitout, J.D.D. Klebsiella pneumoniae ST147: And then there were three carbapenemases. Eur. J. Clin. Microbiol. Infect Dis. 2022, 41, 1467–1472. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhao, Z.; Ye, C.; Zhou, S.; Wu, S.; Han, L.; Han, Z.; Ye, H. Molecular characterization of carbapenem-resistant Klebsiella pneumoniae isolates with focus on antimicrobial resistance. BMC Genom. 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Russo, T.A.; Marr, C.M. Hypervirulent Klebsiella pneumoniae. Clin. Microbiol. Rev. 2019, 32, e00001-19. [Google Scholar] [CrossRef]
- Han, Y.L.; Wen, X.H.; Zhao, W.; Cao, X.S.; Wen, J.X.; Wang, J.R.; Hu, Z.D.; Zheng, W.Q. Epidemiological characteristics and molecular evolution mechanisms of carbapenem-resistant hypervirulent Klebsiella pneumoniae. Front. Microbiol. 2022, 13, 1003783. [Google Scholar] [CrossRef] [PubMed]
- Starkova, P.; Lazareva, I.; Avdeeva, A.; Sulian, O.; Likholetova, D.; Ageevets, V.; Lebedeva, M.; Gostev, V.; Sopova, J.; Sidorenko, S. Emergence of hybrid resistance and virulence plasmids harboring New Delhi Metallo-β-Lactamase in Klebsiella pneumoniae in Russia. Antibiotics 2021, 10, 691. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, Y.; Ahmed, M.A.E.E.; Qin, M.; He, R.; Wu, Y.; Liang, X.; Zhong, L.L.; Chen, P.; Deng, B.; et al. Carriage of distinct blaKPC-2 and blaOXA-48 plasmids in a single ST11 hypervirulent Klebsiella pneumoniae isolate in Egypt. BMC Genom. 2022, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Kuzina, E.S.; Novikova, T.S.; Astashkin, E.I.; Fedyukina, G.N.; Kislichkina, A.A.; Kurdyumova, N.V.; Savin, I.A.; Ershova, O.N.; Fursova, N.K. Rectal and tracheal carriage of carbapenemase genes and class 1 and 2 integrons in patients in neurosurgery Intensive Care Unit. Antibiotics 2022, 11, 886. [Google Scholar] [CrossRef]
- Wilson, K. Preparation of genomic DNA from bacteria. Curr. Protoc. Mol. Biol. 2001, 56, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Oxford Nanopore Technologies. Available online: https://nanoporetech.com/ (accessed on 12 April 2022).
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic. Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Falush, D.; Lan, R.; Colles, F.; Mensa, P.; Wieler, L.H.; Karch, H.; Reeves, P.R.; Maiden, M.C.; Ochman, H.; et al. Sex and virulence in Escherichia coli: An evolutionary perspective. Mol. Microbiol. 2006, 60, 1136–1151. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Kevin Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Darling, A.E.; Mau, B.; Perna, N.T. progressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 2010, 5, e11147. [Google Scholar] [CrossRef]
- Snippy. Available online: https://github.com/tseemann/snippy (accessed on 15 November 2022).
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic. Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef]
- Shelenkov, A.; Mikhaylova, Y.; Yanushevich, Y.; Samoilov, A.; Petrova, L.; Fomina, V.; Gusarov, V.; Zamyatin, M.; Shagin, D.; Akimkin, V. Molecular typing, characterization of antimicrobial resistance, virulence profiling and analysis of whole-genome sequence of clinical Klebsiella pneumoniae isolates. Antibiotics 2020, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Bonnin, R.A.; Nordmann, P. Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob. Agents Chemother. 2011, 56, 559–562. [Google Scholar] [CrossRef]
- Carattoli, A. Resistance plasmid families in Enterobacteriaceae. Antimicrob. Agents Chemother. 2009, 53, 2227–2238. [Google Scholar] [CrossRef]
- Shaidullina, E.; Shelenkov, A.; Yanushevich, Y.; Mikhaylova, Y.; Shagin, D.; Alexandrova, I.; Ershova, O.; Akimkin, V.; Kozlov, R.; Edelstein, M. Antimicrobial resistance and genomic characterization of OXA-48- and CTX-M-15-co-producing hypervirulent Klebsiella pneumoniae ST23 recovered from nosocomial outbreak. Antibiotics 2020, 9, 862. [Google Scholar] [CrossRef]
- Fursova, N.K.; Astashkin, E.I.; Gabrielyan, N.I.; Novikova, T.S.; Fedyukina, G.N.; Kubanova, M.K.; Esenova, N.M.; Sharapchenko, S.O.; Volozhantsev, N.V. Emergence of five genetic lines ST395NDM−1, ST13OXA−48, ST3346OXA−48, ST39CTX-M−14, and Novel ST3551OXA−48 of multidrug-resistant clinical Klebsiella pneumoniae in Russia. Microb. Drug Resist. 2020, 26, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Tartor, Y.H.; Abd El-Aziz, N.K.; Gharieb, R.M.A.; El Damaty, H.M.; Enany, S.; Soliman, E.A.; Abdellatif, S.S.; Attia, A.S.A.; Bahnass, M.M.; El-Shazly, Y.A.; et al. Whole-Genome Sequencing of Gram-Negative Bacteria Isolated From Bovine Mastitis and Raw Milk: The First Emergence of Colistin mcr-10 and Fosfomycin fosA5 Resistance Genes in Klebsiella pneumoniae in Middle East. Front. Microbiol. 2021, 12, 770813. [Google Scholar] [CrossRef]
- Callejón Fernández, M.; Madueño Alonso, A.; Abreu Rodríguez, R.; Aguirre-Jaime, A.; Castro Hernández, M.B.; Ramos-Real, M.J.; Pedroso-Fernández, Y.; Lecuona Fernández, M. Risk factors for colonization by carbapenemase-producing bacteria in Spanish long-term care facilities: A multicentre point-prevalence study. Antimicrob. Resist. Infect. Control 2022, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Silago, V.; Mshana, S.E. Whole genome sequencing reveals presence of high-risk global clones of Klebsiella pneumoniae harboring multiple antibiotic resistance genes in multiple plasmids in Mwanza, Tanzania. Microorganisms 2022, 10, 2396. [Google Scholar]
- Campos-Madueno, E.I.; Moser, A.I.; Jost, G.; Maffioli, C.; Bodmer, T.; Perreten, V.; Endimiani, A. Carbapenemase-Producing Klebsiella pneumoniae strains in Switzerland: Human and non-human settings may share high-risk clones. J. Glob. Antimicrob. Resist. 2022, 28, 206–215, Erratum in J. Glob. Antimicrob. Resist. 2022, 31, 394. [Google Scholar] [CrossRef] [PubMed]




| Information | SCPM-O-B-8912 | SCPM-O-B-8919 | SCPM-O-B-8922 | SCPM-O-B-8923 |
|---|---|---|---|---|
| Patient (gender, age) | Male, 36 | Male, 67 | Male, 59 | Female, 64 |
| ICU state, days | 42 | 23 | 95 | 96 |
| Outcome | Discharged | Discharged | Fatal | Fatal |
| Isolation source | rectal swab | rectal swab | rectal swab | tracheal swab |
| Collection date | 18 October 2019 | 21 October 2019 | 21 October 2019 | 21 November 2019 |
| Patient’s diagnosis | Diffuse cerebral and cerebellar injury, unspecified | Secondary malignant neoplasm of the brain and cerebral meninges | Nontraumatic intracerebral hemorrhage in the hemisphere, unspecified | Benign neoplasm of cranial nerves |
| Infectious manifestation | GD | No | GD | GD, RTI |
| Antimicrobials | SCPM-O-B-8912 | SCPM-O-B-8919 | SCPM-O-B-8922 | SCPM-O-B-8923 |
|---|---|---|---|---|
| Ampicillin | >16 | >16 | >16 | >16 |
| Amoxicillin-clavulanic acid | >128 | >128 | >128 | >128 |
| Cefoperazone | 64 | 64 | 32 | 64 |
| Cefotaxime | >32 | >32 | >32 | >32 |
| Ceftazidime | >256 | >256 | >256 | >256 |
| Cefepime | >16 | >16 | >16 | >16 |
| Aztreonam | 32 | 64 | 64 | 32 |
| Imipenem | >64 | >64 | >64 | >64 |
| Meropenem | 128 | >256 | >256 | 256 |
| Ertapenem | >4 | >4 | >4 | >4 |
| Tigecycline | >4 | >4 | 4 | >4 |
| Ciprofloxacin | 256 | 256 | 256 | >256 |
| Chloramphenicol | 8 | 8 | 4 | 16 |
| Gentamicin | >256 | >256 | >256 | >256 |
| Netilmicin | >16 | >16 | >16 | >16 |
| Amikacin | >32 | >32 | >32 | >32 |
| Trimethoprim-sulfamethoxazole | >160 | >160 | >160 | >160 |
| Fosfomycin | 128 | 128 | >128 | 128 |
| Colistin | ≤0.5 | ≤0.5 | >8 | ≤0.5 |
| Features | SCPM-O-B-8912 | SCPM-O-B-8919 | SCPM-O-B-8922 | SCPM-O-B-8923 |
|---|---|---|---|---|
| GenBank chromosome | CP086664 | CP094991 | CP094363 | CP086671 |
| Chromosome size, bp | 5,351,360 | 5,350,432 | 5,348,898 | 5,351,820 |
| Genes (total) | 6079 | 5902 | 6079 | 6091 |
| Genes (coding) | 5788 | 5626 | 5790 | 5801 |
| Genes (RNA) | 126 | 126 | 126 | 126 |
| rRNA genes (5S, 16S, 23S) | 9, 8, 8 | 9, 8, 8 | 9, 8, 8 | 9, 8, 8 |
| tRNA genes | 88 | 88 | 88 | 88 |
| Pseudo Genes (total) | 165 | 150 | 163 | 164 |
| - frameshifted | 67 | 61 | 65 | 66 |
| - incomplete | 102 | 88 | 101 | 102 |
| - internal stop | 25 | 22 | 25 | 26 |
| - multiple problems | 24 | 18 | 23 | 25 |
| Plasmids | 6 | 5 | 6 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzina, E.S.; Kislichkina, A.A.; Sizova, A.A.; Skryabin, Y.P.; Novikova, T.S.; Ershova, O.N.; Savin, I.A.; Khokhlova, O.E.; Bogun, A.G.; Fursova, N.K. High-Molecular-Weight Plasmids Carrying Carbapenemase Genes blaNDM-1, blaKPC-2, and blaOXA-48 Coexisting in Clinical Klebsiella pneumoniae Strains of ST39. Microorganisms 2023, 11, 459. https://doi.org/10.3390/microorganisms11020459
Kuzina ES, Kislichkina AA, Sizova AA, Skryabin YP, Novikova TS, Ershova ON, Savin IA, Khokhlova OE, Bogun AG, Fursova NK. High-Molecular-Weight Plasmids Carrying Carbapenemase Genes blaNDM-1, blaKPC-2, and blaOXA-48 Coexisting in Clinical Klebsiella pneumoniae Strains of ST39. Microorganisms. 2023; 11(2):459. https://doi.org/10.3390/microorganisms11020459
Chicago/Turabian StyleKuzina, Ekaterina S., Angelina A. Kislichkina, Angelika A. Sizova, Yury P. Skryabin, Tatiana S. Novikova, Olga N. Ershova, Ivan A. Savin, Olga E. Khokhlova, Alexander G. Bogun, and Nadezhda K. Fursova. 2023. "High-Molecular-Weight Plasmids Carrying Carbapenemase Genes blaNDM-1, blaKPC-2, and blaOXA-48 Coexisting in Clinical Klebsiella pneumoniae Strains of ST39" Microorganisms 11, no. 2: 459. https://doi.org/10.3390/microorganisms11020459
APA StyleKuzina, E. S., Kislichkina, A. A., Sizova, A. A., Skryabin, Y. P., Novikova, T. S., Ershova, O. N., Savin, I. A., Khokhlova, O. E., Bogun, A. G., & Fursova, N. K. (2023). High-Molecular-Weight Plasmids Carrying Carbapenemase Genes blaNDM-1, blaKPC-2, and blaOXA-48 Coexisting in Clinical Klebsiella pneumoniae Strains of ST39. Microorganisms, 11(2), 459. https://doi.org/10.3390/microorganisms11020459





